Abstract
Due to the emergence of immune checkpoint inhibitors, the abscopal effect has gained more attention. We report a case of extracranial abscopal effect after whole‐brain irradiation therapy due to brain metastasis. After the initial abscopal effect was confirmed, a second abscopal effect was confirmed following radiation therapy for bone metastases. This case confirms the reproducibility of the abscopal effect. Moreover, the abscopal effect was not observed in metastatic lesions with low immunogenicity, even in the same patient.
Keywords: abscopal effect, atezolizumab, lung cancer, whole‐brain irradiation
Once an initial abscopal effect is observed, the occurrence of a second effect is likely. There may be a correlation between the duration of the first and the second effect.
INTRODUCTION
The abscopal effect was reported in 1953 as a radiotherapy effect that induces tumor shrinkage at nonirradiated, distant tumor sites. 1 Local radiation therapy induces immunostimulatory cell death, triggering the host immune response. 2 Due to the emergence of immune checkpoint inhibitors, reports on abscopal effects have increased. 3 We report a case wherein the abscopal effect was observed twice in the same patient. The first abscopal effect was confirmed after whole‐brain irradiation, and the second was confirmed after irradiation to bone metastases with atezolizumab.
CASE REPORT
A 42‐year‐old Asian female patient was diagnosed with adenocarcinoma in the left upper lobe of the lung (cT4N3M1c stage IVB). Multiple lung metastasis, bone metastasis, and brain metastasis were observed as distant metastases (Figure 1). Real‐time polymerase chain reaction revealed the presence of epidermal growth factor receptor (EGFR) mutation (Ex 21 L858R). The expression rate of programmed cell death ligand 1 (PD‐L1) in primary lung cancer was 1%. Osimertinib monotherapy (80 mg) was introduced. Although a partial response was observed, increasing size of the primary lesion was confirmed on the 468th day after starting osimertinib. Osimertinib was continued because the patient only wanted to be treated with an EGFR tyrosine kinase inhibitor; however, worsening of multiple brain metastases was confirmed on the 554th day after the start of osimertinib (Figure. 2(a)). Although there was exacerbation of the lung lesions, the patient did not experience any symptoms other than nausea and did not develop airway stenosis. She was treated with betamethasone (3.3 mg twice daily) and glycerin, but her nausea persisted. Therefore, she received a dose of 30 Gy (10 fractions) irradiation for brain metastases. Then, reduction of the primary lesion was observed 4 days after whole‐brain irradiation was initiated (Figure 2(b)). After completing whole‐brain irradiation, the primary lesion shrunk from 82 × 64 mm to 53 × 47 mm (Figure 2(c)). Other metastatic lesions remained stable. Her CYFRA and KL levels decreased following whole‐brain irradiation from 12.2 to 5.6 ng/mL and 901 to 779 U/mL, respectively. Her CEA level did not rise consistently following diagnosis and did not reflect the disease progress. However, 45 days after whole‐brain radiotherapy, exacerbation of the primary lesion was observed (Figure 2(d)). Carboplatin (area under the curve, 5 mg/ml·min) plus pemetrexed (500 mg/m2) therapy was introduced on day 1 of the 21‐day cycle, with the patient's consent. After two courses, all lesions were reduced. After three courses, exacerbation of lung and bone metastatic lesions was observed on day 137 after whole‐brain irradiation (Figure 3(a), yellow arrow). Atezolizumab (1200 mg) was administered at 3‐weekly intervals; however, the back pain caused by thoracic spine metastasis worsened, therefore radiation therapy for bone metastasis was performed. On day 6 after radiotherapy for bone metastases (day 167 after whole‐brain irradiation), primary lesion shrinkage was confirmed again (Figure 3(b)). However, on day 10 after radiotherapy for bone metastases (day 171 after whole‐brain irradiation), she lost consciousness and had convulsions in the upper left limb. Edema due to brain metastasis was observed in the right temporal lobe (Figure 3(c), yellow circle). Radiation therapy was discontinued, and she was treated with betamethasone 4 mg twice daily and glycerin. Irradiation was continued at a dose of 36 Gy (12 fractions), and an abscopal effect on the intracranial metastatic lesion was expected. On day 14 after radiotherapy for bone metastases (day 175 after whole‐brain irradiation), abscopal effect on the lung metastases was confirmed (Figure 3(d), red circle). However, she died on day 183 after whole‐brain irradiation. Postmortem computed tomographic scan confirmed that the lung lesions remained shrunken at the time of death. On the other hand, worsening of brain metastasis and appearance of a new liver metastasis were observed (Figure 3(e)).
FIGURE 1.
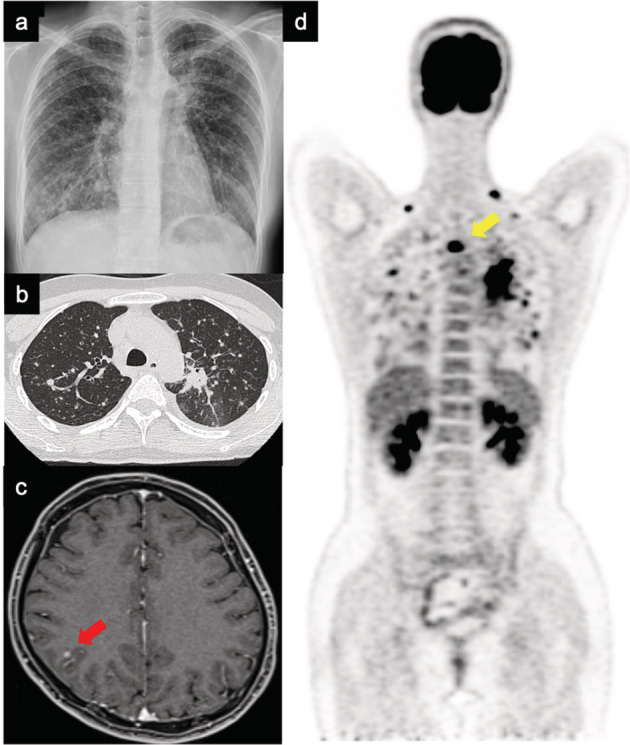
Images at the time of lung cancer diagnosis. (a) Chest X‐ray and (b) chest computed tomography showing primary lesion in the upper left lobe and multiple lung metastases. (c) Brain magnetic resonance image. The red arrow points to the brain metastasis. (d) Whole‐body scan using positron emission tomography. The yellow arrow points to the bone metastatic lesion
FIGURE 2.
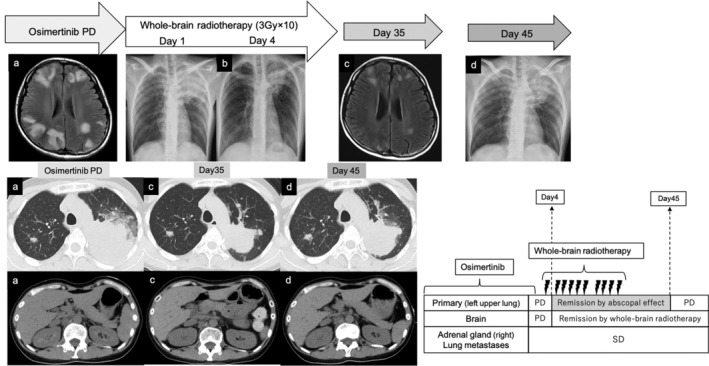
Flow from the first confirmation of the abscopal effect to the disappearance of the effect. (a) Before the initiation of radiation therapy. (b) Date of abscopal effect confirmation. (c) Follow‐up after radiation. (d) Date of withdrawal of abscopal effect. SD, stable disease; PD, progressive disease
FIGURE 3.
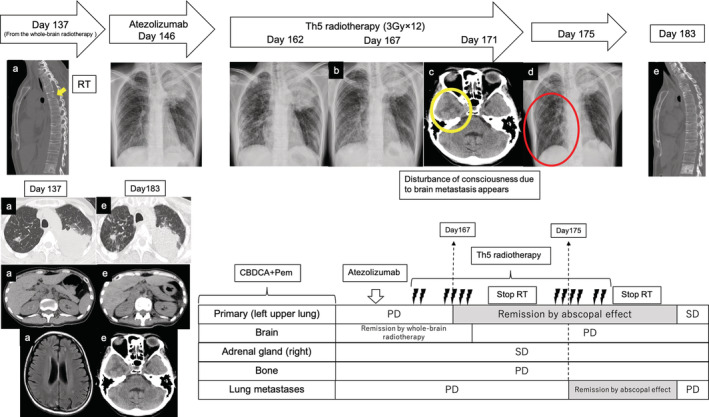
Flow from the second confirmation of the abscopal effect to the disappearance of the effect. (a) Before the initiation of radiation therapy (yellow arrow; bone metastatic lesion). (b) Date of abscopal effect confirmation. (c) Confirmed exacerbation of brain metastases (yellow circle; edema). (d) Date of abscopal effect confirmation to lung metastasis (red circle; right lung metastasis). (e) Date of withdrawal of abscopal effect. CBDCA, carboplatin; Pem, pemetrexed; RT, radiation therapy; SD, stable disease; PD, progressive disease
DISCUSSION
This case features a patient who developed the abscopal effect twice, proving its reproducibility. On both occurrences, the abscopal effect was prominently observed in the primary lesion, although its effects on metastatic organs were insufficient. This was a valuable case wherein immunogenic heterogeneity was assumed. In previous reports, the abscopal effect was caused only by radiation therapy for lung cancer. 4 , 5 , 6 , 7 , 8 Only two cases from whole‐brain irradiation have been reported. 6 , 7 In recent years, abscopal effects caused by immune checkpoint inhibitors have been reported. 9 In this case, the first abscopal effect was confirmed after whole‐brain irradiation, and the immune response assumably worked after passing through the blood–brain barrier. When the second abscopal effect was confirmed—and despite the addition of the checkpoint inhibitor—no effects on metastatic brain lesions were observed. Also, lesions with low immunogenicity were detected. It is highly possible that the no immune response was mounted against the brain lesions at all, and the lesions were affected purely by whole‐brain irradiation.
Only one case of EGFR mutation‐positive lung adenocarcinoma has been reported, 8 in which the abscopal effect persisted over time. However, this was a postoperative recurrence case, and the situation at the time of radiational intervention was different from our case. In this case, the primary and lung lesions showed a good abscopal effect, although the extrathoracic lesion was unresponsive. The abscopal effect lasted for 1 month, which was shorter than previously reported effects. 10 EFGR mutation‐positive lung cancer is less likely to benefit from immune checkpoint‐inhibitors. 11 The EGFR activation pathway suppresses the immune response by infiltrating regulatory T cells locally into the cancer. An alternative cause could be the decrease in the proportion of PD‐L1‐positive and CD8‐positive tumor‐infiltrating lymphocytes. 11 , 12 Tumor‐infiltrating lymphocyte deficiency and tumor mutation profiles are noninflamed phenotypes. This may make it difficult to maintain the abscopal effect.
To summarize, reproducible abscopal effects were elicited. In this case, no additional effect was observed on addition of immune checkpoint inhibitors. These findings will help determine effective radiotherapy strategies.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This treatment is supported by the Shimane University Radiation Therapy Department.
Hotta T, Okuno T, Nakao M, Amano Y, Isobe T, Tsubata Y. Reproducible abscopal effect in a patient with lung cancer who underwent whole‐brain irradiation and atezolizumab administration. Thorac Cancer. 2021;12:985–988. 10.1111/1759-7714.13875
REFERENCES
- 1. Mole R. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–41. [DOI] [PubMed] [Google Scholar]
- 2. Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation‐induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T‐cell killing. Oncotarget. 2014;5:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siva S, Callahan J, MacManus MP, Martin O, Hicks RJ, Ball DL. Abscopal [corrected] effects after conventional and stereotactic lung irradiation of non‐small‐cell lung cancer. J Thorac Oncol. 2013;8:e71–2. [DOI] [PubMed] [Google Scholar]
- 5. Hamilton AJ, Seid J, Verdecchia K, Chuba P. Abscopal effect after radiosurgery for solitary brain metastasis from non‐small cell lung cancer. Cureus. 2018;10:e3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chuang CH, Hsu JF, Shen YT, Yang CJ. Regression of a metastatic lung mass after receiving whole brain irradiation: Can the abscopal effect cross the blood–brain barrier? Asia Pac J Clin Oncol. 2018;14:e548–50. [DOI] [PubMed] [Google Scholar]
- 7. Katayama K, Tamiya A, Koba T, Fukuda S, Atagi S. An abscopal response to radiation therapy in a patient with metastatic non‐small lung cancer: A case report. J Cancer Sci Ther. 2017;9:365–7. [Google Scholar]
- 8. Kuroda A, Tabuchi T, Iwami E, Sasahara K, Matsuzaki T, Nakajima T, et al. Abscopal effect of radiation on multiple lung metastases of lung adenocarcinoma: A case report. BMC Cancer. 2019;19:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garelli E, Rittmeyer A, Putora PM, Glatzer M, Dressel R, Andreas S. Abscopal effect in lung cancer: Three case reports and a concise review. Immunotherapy. 2019;11:1445–61. [DOI] [PubMed] [Google Scholar]
- 10. Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dong ZY, Zhang JT, Liu SY, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD‐1 blockade in non‐small cell lung cancer. Onco Targets Ther. 2017;6:e1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin R, Liu C, Zheng S, Wang X, Feng X, Li H, et al. Molecular heterogeneity of anti‐PD‐1/PD‐L1 immunotherapy efficacy is correlated with tumor immune microenvironment in east Asian patients with non‐small cell lung cancer. Cancer Biol Med. 2020;17:768–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


