Abstract
Background
Improved outcome in tobacco smoking patients with non‐small cell lung cancer (NSCLC) following immunotherapy has previously been reported. However, little is known regarding this association during first‐line immunotherapy in patients with high PD‐L1 expression. In this study we compared clinical outcomes according to the smoking status of two large multicenter cohorts.
Methods
We compared clinical outcomes according to the smoking status (never smokers vs. current/former smokers) of two retrospective multicenter cohorts of metastatic NSCLC patients, treated with first‐line pembrolizumab and platinum‐based chemotherapy.
Results
A total of 962 NSCLC patients with PD‐L1 expression ≥50% who received first‐line pembrolizumab and 462 NSCLC patients who received first‐line platinum‐based chemotherapy were included in the study. Never smokers were confirmed to have a significantly higher risk of disease progression (hazard ratio [HR] = 1.49 [95% CI: 1.15–1.92], p = 0.0022) and death (HR = 1.38 [95% CI: 1.02–1.87], p = 0.0348) within the pembrolizumab cohort. On the contrary, a nonsignificant trend towards a reduced risk of disease progression (HR = 0.74 [95% CI: 0.52–1.05], p = 0.1003) and death (HR = 0.67 [95% CI: 0.45–1.01], p = 0.0593) were reported for never smokers within the chemotherapy cohort. After a random case–control matching, 424 patients from both cohorts were paired. Within the matched pembrolizumab cohort, never smokers had a significantly shorter progression‐free survival (PFS) (HR = 1.68 [95% CI: 1.17–2.40], p = 0.0045) and a nonsignificant trend towards a shortened overall survival (OS) (HR = 1.32 [95% CI: 0.84–2.07], p = 0.2205). On the contrary, never smokers had a significantly longer PFS (HR = 0.68 [95% CI: 0.49–0.95], p = 0.0255) and OS (HR = 0.66 [95% CI: 0.45–0.97], p = 0,0356) compared to current/former smoker patients within the matched chemotherapy cohort. On pooled multivariable analysis, the interaction term between smoking status and treatment modality was concordantly statistically significant with respect to ORR (p = 0.0074), PFS (p = 0.0001) and OS (p = 0.0020), confirming the significantly different impact of smoking status across the two cohorts.
Conclusions
Among metastatic NSCLC patients with PD‐L1 expression ≥50% receiving first‐line pembrolizumab, current/former smokers experienced improved PFS and OS. On the contrary, worse outcomes were reported among current/former smokers receiving first‐line chemotherapy.
Keywords: immunotherapy, non‐small cell lung cancer, pembrolizumab, smoking, tobacco
Improved outcome in tobacco smoking NSCLC patients following treatment with immune checkpoint inhibitors (ICIs) has previously been reported. Little is known regarding this association during first‐line immunotherapy in patients with high PD‐L1 expression. Clinical outcomes according to the smoking status of two large multicenter cohorts were compared in this study. Smokers with high PD‐L1 expression ≥ 50% experienced improved progression‐free survival (PFS) and overall survival (OS) with first‐line pembrolizumab. The opposite trend was found in NSCLC patients treated with first‐line platinum‐based chemotherapy.
INTRODUCTION
Programmed death 1 (PD‐1) checkpoint inhibitors have become the backbone of the treatment algorithm of nononcogene addicted non‐small cell lung cancer (NSCLC) patients. 1 Tobacco use is known to be the main risk factor for lung cancer development and is related to a high all‐cause morbidity and mortality overall. 2 Nevertheless, smoking of tobacco has been associated with improved outcomes in NSCLC patients receiving checkpoint inhibitors across different lines and regardless of programmed death‐ligand 1 (PD‐L1) tumor expression. 3 Intriguingly, a meta‐analysis has also suggested that checkpoint inhibitors significantly improve survival over chemotherapy in smoker patients only. 4
We recently published a large (1016 patients) real‐world multicenter study of patients with metastatic NSCLC with PD‐L1 expression ≥50% who received first‐line single agent pembrolizumab at 34 European institutions, aimed at investigating the clinicopathological correlates of efficacy. 5 , 6 Multivariable analysis determined that former smokers (but not current smokers) experienced significantly prolonged progression‐free survival (PFS) and overall‐survival (OS) compared to never smokers. 5 We subsequently gathered a cohort of metastatic NSCLC patient treated with first‐line platinum‐based doublet chemotherapy for the external validation of the role of BMI in the same study population. 7
In order to further assess the role of the baseline smoking status during first‐line single agent immunotherapy in NSCLC patients with high PD‐L1 tumor expression, we compared the clinical outcomes analyses according to the smoking status between the above mentioned two cohorts.
METHODS
Study design
We compared the clinical outcomes analyses according to the smoking status (never vs. current/former smokers) of two real‐world retrospective multicenter cohorts: a cohort of metastatic NSCLC patients with PD‐L1 expression ≥50%, consecutively treated with first‐line pembrolizumab monotherapy, from January 2017 to October 2019, at 34 institutions (Supplementary file 1), and a cohort of metastatic epidermal growth factor receptor (EGFR) wild‐type NSCLC patients treated with platinum‐based doublet chemotherapy in clinical practice from January 2013 to January 2020, at 10 institutions among the abovementioned. 5 , 6 , 7 The measured clinical outcomes were objective response rate (ORR), PFS and OS. Methods regarding clinical outcomes estimation in the two cohorts have been previously reported. 5 , 6 , 7
A fixed multivariable regression model was used to estimate clinical outcomes (ORR, PFS and OS) according to the smoking status (current/former smokers vs. never smokers) in both pembrolizumab and chemotherapy cohorts. 8 , 9 , 10 The key covariates were: age (<70 vs. ≥70 years old), 11 gender (male vs. female), Eastern Cooperative Oncology Group–Performance Status (ECOG‐PS) (0–1 vs. ≥ 2), central nervous system (CNS) metastases (yes vs. no), bone metastases (yes vs. no) and liver metastases (yes vs. no).
Considering the different sample size, a random case–control matching was also performed to better compare the results across the cohorts. All the cases from the chemotherapy cohort and controls from the pembrolizumab cohort were randomly paired on the basis of the smoking status (current/former smokers vs. never smokers) and those characteristics which were significantly unbalanced between the cohorts: ECOG‐PS (0–1 vs. 2), age (< 70 vs. ≥ 70 years old), and baseline BMI according to the World Health Organization categories (underweight, BMI < 18.5; normal‐weight, 18.5 ≤ BMI ≤24.9; overweight, 25 ≤ BMI ≤29.9; obese, BMI ≥30). 7
Lastly, to take into account the potential role of all baseline characteristics, we performed a pooled analysis, using a multivariable regression model (inclusive of the previously selected covariates plus primary tumor histology [squamous vs. nonsquamous] and baseline BMI) including the interaction term between the smoking status and the treatment modality (pembrolizumab vs. chemotherapy), used as covariates.
All patients provided their written, informed consent to treatment with immunotherapy. The procedures followed were in accordance with the precepts of Good Clinical Practice and the declaration of Helsinki. The study was approved by the respective local ethical committees on human experimentation of each institution, after previous approval by the coordinating center (Comitato Etico per le provice di L'Aquila e Teramo, verbale N.15 del 28 Novembre 2019).
Median PFS and median OS were evaluated using the Kaplan–Meier method. Median period of follow‐up was calculated according to the reverse Kaplan–Meier method. χ2 test was used for the univariable analysis of ORR, logistic regression was used for the fixed multivariable analyses of ORR. Cox proportional hazards regression was used for the univariable analysis of PFS and OS and for the fixed multivariable analyses. The alpha level for all analyses was set to p < 0.05. Adjusted hazard ratios (HRs) and adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Forest plot graphs were used to compare HRs between the pembrolizumab and chemotherapy cohorts. After the random case–control matching, clinical outcomes of the two cohort were compared with univariable analyses. Considering the sample size of the pembrolizumab cohort (more than twice the chemotherapy cohort) a caliper width of <1 for the standard deviation was used for the random case–control matching. 12 All statistical analyses were performed using MedCalc Statistical Software version 18.11.3 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2019).
RESULTS
A total of 962 patients and 426 patients were included in the pembrolizumab and chemotherapy cohorts, respectively. Patient characteristics of the two cohorts have already been previously reported, 7 a summary of which is available in Table S2. A total of 864 patients (89.8%) and 378 patients (88.7%) were former/current smokers in the pembrolizumab and chemotherapy cohorts, respectively, and 249 patients (58.5%) within the chemotherapy cohort had received a further treatment with either PD‐1 or PD‐L1 checkpoint inhibitors at the data cutoff.
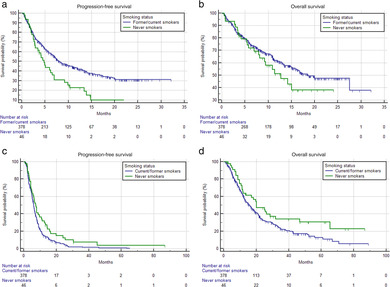
Table 1 summarizes the univariable analysis of ORR, PFS and OS. Never smokers had a significantly lower ORR (p = 0.0367), significantly shorter PFS (HR = 1.74 [95% CI: 1.36–2.23], p < 0.0001) (Figure 1(a)) and OS (HR = 1.59 [95% CI: 1.19–2.13], p = 0.0015) (Figure 1(b)) compared to former/current smokers within the pembrolizumab cohort. In the chemotherapy cohort the smoking status was not significantly related to the ORR (p = 0.0919), whilst significantly longer PFS (HR = 0.70 [95% CI: 0.51–0.96], p = 0.0296) and OS (HR = 0.66 [95% CI: 0.45–0.96], p = 0.0339) were reported for never smokers.
TABLE 1.
Univariate analyses of objective response rate (ORR), progression‐free survival (PFS) and overall survival (OS) according to smoking status
| Pembrolizumabcohort | Chemotherapycohort | |||||
|---|---|---|---|---|---|---|
| Smoking status | Response/ratio | ORR (95% CI) | χ2 test | Response/ratio | ORR (95% CI) | χ2 test |
| Former/current smokers | 344/760 | 45.3% (40.6–50.3) | p = 0.0367 | 158/373 | 42.4% (36.0–49.5) | p = 0.0919 |
| 26/47 | 55.3% (36.1–81.1) | |||||
| Never smokers | 28/84 | 33.3% (22.1–48.2) | ||||
| PFS (95% CI) (events) | HR (95%CI) | PFS (95% CI) (events) | HR (95% CI) | |||
| Former/current smokers | 9.1 months (7.5–10.7) (486) | 1.74 (1.36–2.23); p < 0.0001 | 6.0 months (5.6–6.4) (344) | 0.70 (0.51–0.96); | ||
| Never smokers | 4.1 months (2.7–5.7) (73) | 7.5 months (4.7–10.8) (43) | p = 0.0296 | |||
| OS (95% CI) (censored) | HR (95%CI); p ‐ value | OS (95%CI) (censored) | HR (95% CI) | |||
| Former/current smokers | 19.9 months (16.9–27.5) (522) | 1.59 (1.19–2.13); p = 0.0015 | 15.8 months (13.2–18.3) (119) | 0.66 (0.45–0.96); p = 0.0339 | ||
| 20.0 months (11.8–31.8) (17) | ||||||
| Never smokers | 9.4 months (6.9–15.0) [45] | |||||
FIGURE 1.
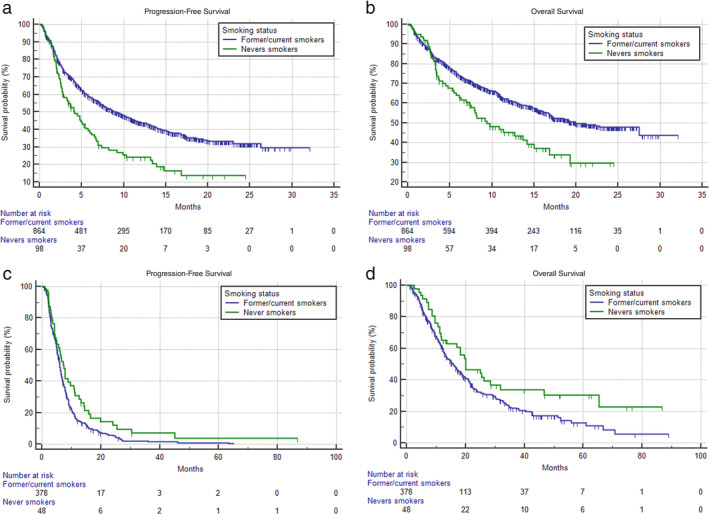
Kaplan–Meier survival curves according to smoking status. Pembrolizumab cohort (a) progression‐free survival (PFS); and (b) overall survival (OS); chemotherapy cohort (c) PFS and (d) OS. See Table 1 for survival estimations
FIGURE 2.
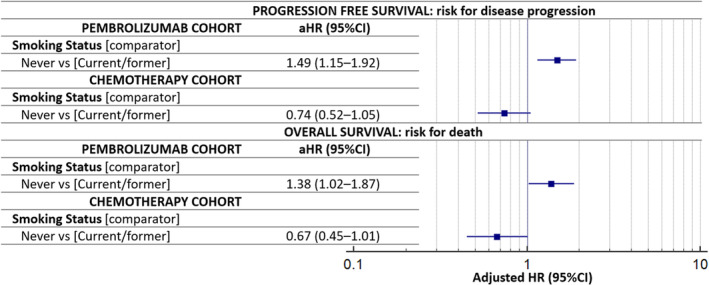
Forest plot graph for adjusted hazard ratios (aHRs) for disease progression (progression‐free survival [PFS]) and death (overall survival [OS]) according to smoking status
FIGURE 3.
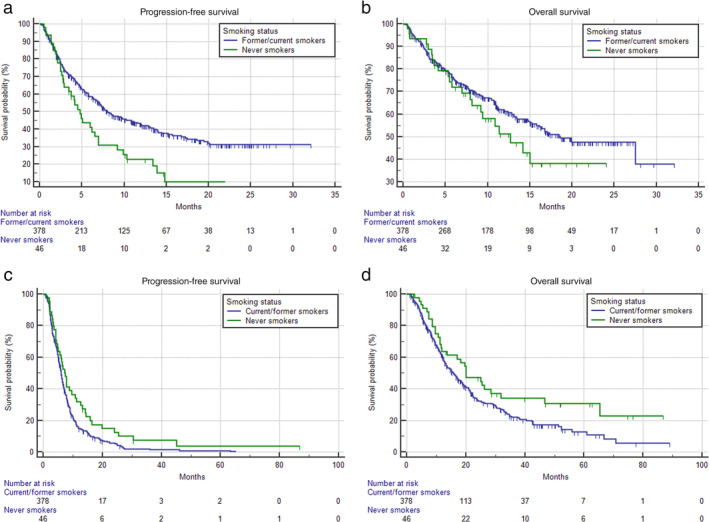
Kaplan–Meier survival curves according to smoking status within the randomly matched cohorts; Pembrolizumab cohort PFS. (a) Never smokers 4.7 months (95% CI: 2.8–6.9; 35 progression events), current/former smokers 8.0 months (95% CI: 8.9–10.8; 217 progression events) (p = 0.0045). OS. (b) Never smokers 12.7 months (95% CI: 7.9–15.0; 24 censored patients), current/former smokers 18.6 months (95% CI:15.2–27.4; 227 censored patients) (p = 0.2205); PFS. (c) Never smokers 7.4 months (95% CI: 5.1–10.8; 41 progression events), current/former smokers 6.0 months (95% CI: 5.6–6.4; 344 progression events) (p = 0.0255). OS. (d) Never smokers 20.1 months (95% CI: 11.6–31.8; 16 censored patients), current/former smokers 15.8 months (95% CI: 13.2–18.4; 119 censored patients) (p = 0.0255). PFS, progression‐free survival; OS, overall survival
Table 2 summarizes the multivariable analysis of ORR. The smoking status was not confirmed to be associated with ORR in both the pembrolizumab (OR = 0.66 [95% CI: 0.40–1.09], p = 0.1070), and chemotherapy (OR = 1.83 [95% CI: 0.94–3.70], p = 0.0751) cohorts. Table 3 summarizes the multivariable analysis of PFS. Never smokers were confirmed to have a significantly shorter PFS compared to current/former smokers in the pembrolizumab cohort (HR = 1.49 [95% CI: 1.15–1.92], p = 0.0022). On the other hand, the opposite association was not confirmed within the chemotherapy cohort (HR = 0.74 [95% CI: 0.52–1.05], p = 0.1003) (Figure 2). Similarly, never smokers were confirmed to have a significantly shorter OS compared to current/former smokers in the pembrolizumab cohort (HR = 1.38 [95% CI: 1.02–1.87], p = 0.0348), while a nonsignificant trend of a prolonged OS was reported for never smokers within the chemotherapy cohort (HR = 0.67 [95% CI: 0.45–1.01], p = 0.0593) (Table 4) (Figure 2).
TABLE 2.
Summary of the objective response rate (ORR) multivariable analysis in the pembrolizumab and chemotherapy cohorts
| Pembrolizumab cohort Objective response rate | Chemotherapy cohort Objective response rate | |||||
|---|---|---|---|---|---|---|
| Variable (comparator) | Coefficient | Standard error | OR (95% CI); p‐value | Coefficient | Standard error | OR (95% CI); p‐value |
|
Smoking status (never vs. current/former) |
0.411 | 0.255 | 0.66 (0.40–1.09); p = 0.1070 | −0.606 | 0.340 | 1.83 (0.94–3.57); p = 0.0751 |
|
Gender (male vs. female) |
0.006 | 0.155 | 0.99 (0.73–1.34); p = 0.9651 | 0.131 | 0.229 | 0.88 (0.56–1.37); p = 0.5672 |
|
Age (elderly vs. non‐elderly) |
0.034 | 0.145 | 0.96 (0.72–1.28); p = 0.8108 | 0.547 | 0.210 | 0.58 (0.38–0.87); p = 0.0093 |
|
CNS metastases (yes vs. no) |
0.031 | 0.188 | 0.97 (0.67–1.40); p = 0.8665 | −0.015 | 0.279 | 1.02 (0.58–1.75); p = 0.9545 |
|
Bone metastases (yes vs. no) |
0.662 | 0.161 | 0.51 (0.37–0.71); p < 0.0001 | 0.683 | 0.244 | 0.50 (0.31–0.81); p = 0.0050 |
|
Liver metastases (yes vs. no) |
0.364 | 0.211 | 0.69 (0.45–1.05); p = 0.0853 | 0.593 | 0.317 | 0.55 (0.29–1.03); p = 0.0616 |
| ECOG PS ≥2 vs. (0–1) | 0.942 | 0.216 | 0.39 (0.26–0.59); p = 0.0038 | 0.176 | 0.405 | 0.83 (0.37–1.85); p = 0.6632 |
Abbreviations: CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; OR, odds ratio.
TABLE 3.
Summary of the progression‐free survival (PFS) multivariable analysis in the pembrolizumab and chemotherapy cohorts
| Pembrolizumab cohort Progression‐free survival | Chemotherapy cohortProgression‐free survival | |
|---|---|---|
| Variable (comparator) | HR (95% CI); p‐value | HR (95% CI); p‐value |
| Smoking status (never vs. current/former) | 1.49 (1.15–1.92); p = 0.0022 | 0.74 (0.52–1.05); p = 0.1003 |
| Gender (male vs female) | 0.99 (0.83–1.19); p = 0.9574 | 1.21 (0.96–1.54); p = 0.1018 |
| Age (elderly vs. nonelderly) | 1.07 (0.90–1.27); p = 0.4282 | 1.17 (0.95–1.44); p = 0.1345 |
| CNS metastases (yes vs. no) | 1.21 (0.98–1.50); p = 0.0733 | 1.08 (0.81–1.44); p = 0.5611 |
| Bone metastases (yes vs. no) | 1.60 (1.33–1.91); p < 0.0001 | 1.32 (1.05–1.65); p = 0.0160 |
| Liver metastases (yes vs. no) | 1.75 (1.41–2.16); p < 0.0001 | 1.37 (1.02–1.83); p = 0.0338 |
| ECOG PS ≥2 vs (0–1) | 2.42 (1.98–2.94); p < 0.0001 | 2.16 (1.46–3.21); p = 0.0001 |
Abbreviations: CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio.
TABLE 4.
Summary of the overall survival (OS) multivariable analysis in the pembrolizumab and chemotherapy cohorts
| Pembrolizumab cohort Overall survival | Chemotherapy cohortOverall survival | |
|---|---|---|
| Variable (comparator) | HR (95% CI); p‐value | HR (95% CI); p‐value |
| Smoking status (never vs. current/former) | 1.38 (1.02–1.87); p = 0.0348 | 0.67 (0.45–1.01); p = 0.0593 |
| Gender (male vs. female) | 1.11 (0.89–1.39); p = 0.3131 | 1.05 (0.80–1.39); p = 0.6918 |
| Age (elderly vs. nonelderly | 1.10 (0.90–1.35); p = 0.3298 | 1.22 (0.96–1.55); p = 0.1005 |
| CNS metastases (yes vs. no) | 1.15 (0.89–1.48); p = 0.2743 | 1.27 (0.92–1.76); p = 0.1396 |
| Bone metastases (yes vs. no) | 1.68 (1.36–2.07); p < 0.0001 | 1.38 (1.06–1.80); p = 0.0144 |
| Liver metastases (yes vs. no) | 1.69 (1.32–2.16); p < 0.0001 | 1.23 (0.86–1.75); p = 0.2427 |
| ECOG PS ≥2 vs (0–1) | 2.95 (2.36–6.69); p < 0.0001 | 2.44 (1.65–3.63); p < 0.0001 |
Abbreviations: CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group.
After the case–control random matching, 424 patients from the pembrolizumab and chemotherapy cohorts were perfectly paired, with no statistically significant differences between the characteristics of matched subjects; 378 (89.2%) current/former smoker patients were included in both matched cohorts. In the matched pembrolizumab cohort, the ORR for current/former smokers and never smokers was 33.2% (95% CI: 27.5–39.8) and 30.9% (95% CI: 16.5–52.9) (p = 0.7658), respectively; among the matched chemotherapy cohort the ORR for current/former smokers and never smokers was 42.4% (95% CI: 36.0–49.5) and 55.6% (95% CI: 35.9–82.0) (p = 0.0923), respectively. Never smokers had a significantly shorter PFS (HR = 1.68 [95% CI: 1.17–2.40], p = 0.0045) (Figure 3a) and a nonsignificant trend towards a shortened OS (HR = 1.32 [95% CI: 0.84–2.07], p = 0.2205) within the matched pembrolizumab cohort (Figure 3b). On the contrary, never smokers had a significantly longer PFS (HR = 0.68 [95% CI: 0.49–0.95], p = 0.0255) (Figure 3c) and OS (HR = 0.66 [95% CI: 0.45–0.97], p = 0,0356) (Figure 3d)I' compared to current/former smoker patients within the matched chemotherapy cohort.
Table 5 summarizes the multivariable regression analyses from the pooled population for ORR, PFS and OS including all the baseline patient characteristics. At the pooled analysis, the interaction term between the smoking status and treatment modality was concordantly statistically significant with respect to ORR (p = 0.0074), PFS (p = 0.0001) and OS (p = 0.0020), confirming the significantly different impact of smoking status across the two cohorts.
TABLE 5.
Pooled multivariable analysis including the interaction term between treatment modality and smoking status
| Objective response rate | Progression‐free survival | Overall survival | |
|---|---|---|---|
| Variable (comparator) | OR (95% CI); p–value | HR (95% CI); p–value | HR (95% CI); p‐value |
| Treatment modality (chemotherapy vs. pembrolizumab) | 0.79 (0.61–1.03); p = 0.0799 | 1.93 (1.67–2.23); p < 0.0001 | 1.27 (1.07–1.51); p = 0.0055 |
| Smoking status (never vs. current/former) | 0.68 (0.41–1.12); p = 0.1236 | 1.71 (1.32–2.25); p < 0.0001 | 1.51 (1.12–2.04); p = 0.0060 |
| Interaction smoking status*treatment modality | p = 0.0074 | p = 0.0001 | p = 0.0020 |
| ECOG PS (≥ 2 vs. 0–1) | 0.46 (0.31–0.67); p = 0.0001 | 2.39 (2.01–2.85); p < 0.0001 | 2.88 (2.37–3.49); p < 0.0001 |
| Gender (male vs. female) | 0.98 (0.75–1.26); p = 0.8317 | 1.04 (0.90–1.21); p = 0.5111 | 1.12 (0.94–1.33); p = 0.1966 |
| Age (elderly vs. nonelderly) | 0.83 (0.66–1.06); p = 0.1295 | 1.08 (0.94–1.23) p = 0.2531 | 1.15 (0.99–1.35); p = 0.0650 |
| CNS metastases (yes vs. no) | 0.99 (0.72–1.35); p = 0.9193 | 1.17 (0.99–1.39); p = 0.0611 | 1.19 (0.97–1.45); p = 0.0861 |
| Liver metastases (yes vs. no) | 0.64 (0.45–0.91); p = 0.0124 | 1.63 (1.37–1.93); p < 0.0001 | 1.51 (1.24–1.85); p < 0.0001 |
| Bone metastases (yes vs. no) | 0.51 (0.38–0.66); p < 0.0001 | 1.53 (1.33–1.77); p < 0.0001 | 1.57 (1.33–1.85); p < 0.0001 |
| BMI | |||
| Normal weight (comparator) | |||
| Underweight | 0.53 (0.27–1.01); p = 0.0520 | 1.26 (0.91–1.74); p = 0.1619 | 0.97 (0.65–1.44); p = 0.9062 |
| Overweight | 0.78 (0.59–1.02); p = 0.0612 | 0.98 (0.85–1.14); p = 0.8728 | 0.91 (0.76–1.08); p = 0.3176 |
| Obese | 1.41 (0.98–2.04); p = 0.0665 | 0.81 (0.66–1.01); p = 0.0620 | 0.89 (0.69–1.14); p = 0.3776 |
| Histology | |||
| Nonsquamous vs. squamous | 1.07 (0.81–1.42); p = 0.6202 | 0.85 (0.73–0.99); p = 0.0483 | 0.94 (0.78–1.14); p = 0.5765 |
Abbreviations: CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio.
DISCUSSION
The primary aim of this analysis was to further evaluate the opposite role of the smoking status according to the first‐line treatment modality in NSCLC patients. The fixed multivariable analyses confirmed that never smokers had significantly shortened PFS and OS compared to current/former smokers among NSCLC patients with PD‐L1 expression ≥50% receiving first‐line pembrolizumab. On the contrary, a trend towards prolonged PFS and OS was reported for never smoker patients receiving first‐line platinum‐based chemotherapy. Of note, never smokers achieved a prolonged OS within the chemotherapy cohort, despite 58.5% of patients receiving PD‐1/PD‐L1 checkpoint inhibitors as a later line of treatment. Even though a significantly lower ORR was reported for never smokers on the univariable analysis in the pembrolizumab cohort, no further significant associations between smoking habit and ORR were found.
The random case–control matching strengthened our findings with regard to PFS. Never smokers had a significantly shorter PFS and a trend towards a shortened OS within the matched pembrolizumab cohort. Conversely, significantly longer PFS and OS were reported for never smokers, compared to current/former smokers, within the matched chemotherapy cohort. Finally, the concordantly statistically significant interaction term between the treatment modality (pembrolizumab vs. chemotherapy) and smoking status with respect to ORR, PFS and OS at the pooled analysis, further confirmed the differential role of the smoking status between the cohorts, regardless of any other baseline characteristics.
The tumor mutational burden (TMB) has been already proposed as an agnostic predictive biomarker for PD‐1 checkpoint inhibitors across different malignancies, even though its applicability in the real‐life context is still controversial. 13 , 14 , 15 Nevertheless, the TMB could have its own complementary and independent role from PD‐L1 immunohistochemical evaluation. 16 , 18 It has been reported that smoking‐induced carcinogenesis is associated with a higher TMB, 18 to such an extent that it has been assumed that smoking‐related lung cancer is more likely to be immunogenic. 19 Interestingly, Rizvi et al. reported that a smoking‐associated genomic signature, characterized by high frequency of transversion, was significantly associated to improved ORR and PFS among 34 advanced NSCLC patients treated with pembrolizumab, whilst the self‐reported smoking history did not significantly predict the clinical outcome within the same population. 20 Recently, Gainor et al. reported that among NSCLC patients with PD‐L1 expression ≥50% receiving first‐line single agent pembrolizumab, heavy smokers experienced numerically better outcomes compared to never/light smokers. 21 Moreover, they confirmed that the TMB was higher within heavy smoker patients, compared to light/never smokers, while no significant differences were found between light and never smokers. 21 In addition, we have to consider that tobacco smoking exposure has been also associated with increasing in vivo and in vitro intratumoral PD‐L1 expression. 22 Concordantly, we previously reported a significant trend towards an increased PD‐L1 expression according to the smoking status (never, former and current smokers) within our study population. 6
In the context of single‐agent pembrolizumab, current/former smokers have already been confirmed to experience improved ORR and prolonged survival within the phase I Keynote 001 trial population. 23 , 24 Similarly, the subgroup analysis of the Keynote 024 trial revealed that the survival benefit for single agent pembrolizumab over chemotherapy in NSCLC patients with high PD‐L1 expression was greater for former smokers, compared to current and never smokers. 25 On the contrary, in the Keynote 189 trial, the subgroup analysis showed no significant differences according to smoking status. However, the survival benefit for the experimental arm (chemotherapy/pembrolizumab) over the control arm (chemotherapy/placebo) was greater for never smokers (HR for death 0.23), compared to current/former smokers (HR for death 0.54), appearing that the addition of chemotherapy had flattened the smoking‐related effects on immunotherapy. 26 Intriguingly, the TMB was not significantly associated with efficacy in both arms of the same trial population. 27
From this perspective, considering the smoking status as an easily available surrogate for the underlying TMB, it might be used to assist clinicians in the decision‐making process for first‐line treatment. With that in mind, a combinational approach, rather than single agent pembrolizumab, might be taken into consideration with greater solicitude in never smoker patients with high PD‐L1 expression, compared to former/current smokers.
Certainly, we are a long way from minimizing the strong negative role of smoking overall. In fact, in this study population we already confirmed that former smokers experienced the best outcome, compared to current and never smokers, 6 suggesting the presence of an underlying global/functional benefit from smoking cessation, without impairing the TMB‐gain related to the smoking habit.
Several study limitations have to be acknowledged beyond the retrospective design and consequent selection biases. The biggest flaw in the study was the lack of information regarding quantification of the smoking status. For a proper estimation of its effect, it should have been classified in a more quantitative way (e.g., pack per year), as has already been determined in other studies. 21 Moreover, we were not able to separately assess former/current smokers within the chemotherapy cohort because this analysis was not preplanned. Additionally, the chemotherapy cohort was not powered to detect significant findings according to smoking categories and being a historic cohort we did not have data regarding PD‐L1 expression. However, considering the real‐world prevalence of PD‐L1 expression in NSCLC, we assumed that one third of the patients in the chemotherapy cohort had a PD‐L1 expression ≥50%. 28 TMB is not routinely assessed in clinical practice in Europe, and therefore we were unable to perform a correlation analyses. Moreover, we should also consider the true incidence of oncogene addiction in NSCLC beyond EGFR, ALK and ROS‐1, which are regularly evaluated as it is known that oncogene addiction is inversely related with smoking status and immunotherapy efficacy. 29 Additional limitations include the lack of available data regarding comorbidities which might have been affected by smoking habit.
In conclusion, our study confirmed that current/former smoker NSCLC patients with PD‐L1 expression ≥50% receiving first‐line single agent pembrolizumab experienced improved PFS and OS compared to never smokers, whilst the opposite trend was found within NSCLC patients treated with first‐line platinum‐based chemotherapy. The random case–control matching and the pooled analysis further strengthened our results on the opposite role of smoking during immunotherapy and chemotherapy. The putative predictive role of the smoking status in this setting needs to be assessed in prospective controlled trials.
CONFLICT OF INTEREST
Dr Alessio Cortellini received speaker fees and grant consultancies by Astrazeneca, MSD, BMS, Roche, Novartis, Istituto Gentili and Astellas. Dr Alessandro Leonetti received speaker fees by Astrazeneca. Dr Raffaele Giusti received speaker fees and grant consultancies by Astrazeneca and Roche. Dr Alex Friedlaender received grant consultancies by Roche, Pfizer, Astellas and BMS. Dr Alfredo Addeo received grant consultancies by Takeda, MSD, BMJ, Astrazeneca, Roche and Pfizer. Dr Rita Chiari received speaker fees by BMS, MSD, Takeda, Pfizer, Roche and Astrazeneca. Dr Carlo Genova received speaker fees/grant consultancies by Astrazeneca, BMS, Boehringer‐Ingelheim, Roche and MSD.
Supporting information
Table S1 List of the oncological institutions of the study.
Table S2 Patient characteristics. CNS: central nervous system; BMI: body mass index; ECOG‐PS: Easter Cooperative Oncology Group‐Performance Status).
ACKNOWLEDGMENTS
A special thanks to the “Consorzio Interuniversitario Nazionale per la Bio‐Oncologia” for their support in this study.
Cortellini A, De Giglio A, Cannita K, et al. Smoking status during first‐line immunotherapy and chemotherapy in NSCLC patients: A case–control matched analysis from a large multicenter study. Thoracic Cancer. 2021;12:880–889. 10.1111/1759-7714.13852
Alessio Cortellini and Andrea De Giglio equally contributed.
[Correction added on 10 February 2021, after initial publication on 2 February 2021: the 6th author's surname was corrected from ‘Baldesarri’ to ‘Baldessari’.]
REFERENCES
- 1. Remon J, Passiglia F, Ahn MJ, Barlesi F, Forde PM, Garon EB, et al. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. 2020;15(6):914–947. 10.1016/j.jtho.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 2. Ashraf‐Uz‐Zaman M, Bhalerao A, Mikelis CM, Cucullo L, German NA. Assessing the current state of lung cancer chemoprevention: a comprehensive overview. Cancers (Basel). 2020;12(5):1265. 10.3390/cancers12051265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El‐Osta H, Jafri S. Predictors for clinical benefit of immune checkpoint inhibitors in advanced non‐small‐cell lung cancer: a meta‐analysis. Immunotherapy. 2019;11(3):189–99. [DOI] [PubMed] [Google Scholar]
- 4. Kim JH, Kim HS, Kim BJ. Prognostic value of smoking status in non‐small‐cell lung cancer patients treated with immune checkpoint inhibitors: a metaanalysis. Oncotarget. 2017;8(54):93149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cortellini A, Tiseo M, Banna GL, Cappuzzo F, Aerts JGTV, Barbieri F, et al. Clinicopathologic correlates of first‐line pembrolizumab effectiveness in patients with advanced NSCLC and a PD‐L1 expression of ≥50%. Cancer Immunol Immunother. 2020;69:2209–2221. 10.1007/s00262-020-02613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cortellini A, Friedlaender A, Banna GL, Porzio G, Bersanelli M, Cappuzzo F, et al. Immune‐related adverse events of Pembrolizumab in a large real‐world cohort of patients with NSCLC with a PD‐L1 expression ≥50% and their relationship with clinical outcomes. Clin Lung Cancer. 2020;21(6):498–508. 10.1016/j.cllc.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 7. Cortellini A, Ricciuti B, Tiseo M, Bria E, Banna GL, Aerts JGJV, et al. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD‐L1 expression ≥ 50%: a multicenter study with external validation. J Immunother Cancer. 2020;8(2):e001403. 10.1136/jitc-2020-001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woolley KK. How variables uncorrelated with the dependent variable can actually make excellent predictors: the important suppressor variable case. Southwest Educational Research Association Annual Meeting proceedings. 1997. https://eric.ed.gov/?id=ED407420. Accessed April 20, 2020.
- 9. Thompson FT, Levine DU. Examples of easily explainable suppressor variables in multiple regression research. Multi Lin Regres Viewpoints. 1997;24:11–3. [Google Scholar]
- 10. “Stopping stepwise: Why stepwise selection is bad and what you should use instead”. On towardsdatascience.com website. Available at: https://towardsdatascience.com/stopping-stepwise-why-stepwise-selection-is-bad-and-what-you-should-use-instead-90818b3f52df. Accessed March 29, 2020.
- 11. Gridelli C, Balducci L, Ciardiello F, di Maio M, Felip E, Langer C, et al. Treatment of elderly patients with non‐small‐cell lung cancer: results of an international expert panel meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer. 2015;16(5):325–33. [DOI] [PubMed] [Google Scholar]
- 12. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–61. 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willis C, Fiander M, Tran D, Korytowsky B, Thomas J‐M, Calderon F, et al. Tumor mutational burden in lung cancer: a systematic literature review. Oncotarget. 2019;10(61):6604–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Subbiah V, Solit DB, Chan TA, Kurzrock R. The FDA approval of pembrolizumab for adult and pediatric patients with tumor mutational burden (TMB) ≥10: a decision centered on empowering patients and their physicians. Ann Oncol. 2020;31(9):1115–8. 10.1016/j.annonc.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 15. Prasad V, Addeo A. The FDA approval of pembrolizumab for patients with TMB >10 Mut/Mb: was it a wise decision? No. Ann Oncol. 2020;31(9):1112–4. [DOI] [PubMed] [Google Scholar]
- 16. Ricciuti B, Mahadevan N, Umeton R. 246 Clinicopathologic and genomic correlates of tumor mutational burden and its impact on PD‐(L)1 inhibition efficacy in non‐small cell lung cancer according to different PD‐L1 expression subgroups. Journal for ImmunoTherapy of Cancer. 2020;8. 10.1136/jitc-2020-SITC2020.0246. [DOI] [Google Scholar]
- 17. Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez‐Vega F, Ahuja A, et al. Genomic features of response to combination immunotherapy in patients with advanced non‐small‐cell lung cancer. Cancer Cell. 2018;33(5):843–52.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier‐Valette C, et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alexandrov LB, Nik‐Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science (New York, NY). 2015;348(6230):124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gainor JF, Rizvi H, Jimenez Aguilar E, Skoulidis F, Yeap BY, Naidoo J, et al. Clinical activity of programmed cell death 1 (PD‐1) blockade in never, light, and heavy smokers with non‐small‐cell lung cancer and PD‐L1 expression ≥50. Ann Oncol. 2020;31(3):404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang GZ, Zhang L, Zhao XC, Gao SH, Qu LW, Yu H, et al. The aryl hydrocarbon receptor mediates tobacco‐induced PD‐L1 expression and is associated with response to immunotherapy. Nat Commun. 2019;10(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leighl NB, Hellmann MD, Hui R, Carcereny E, Felip E, Ahn MJ, et al. Pembrolizumab in patients with advanced non‐small‐cell lung cancer (KEYNOTE‐001): 3‐year results from an open‐label, phase 1 study. Lancet Respir Med. 2019;7(4):347–57. [DOI] [PubMed] [Google Scholar]
- 24. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372(21):2018–28. [DOI] [PubMed] [Google Scholar]
- 25. Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375(19):1823–33. [DOI] [PubMed] [Google Scholar]
- 26. Gandhi L, Rodríguez‐Abreu D, Gadgeel S, Esteban E, Felip E, de Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 27. Garassino M, Rodriguez‐Abreu D, Gadgeel S, Esteban E, Felip E, Speranza G, et al. OA04.06 evaluation of TMB in KEYNOTE‐189: Pembrolizumab plus chemotherapy vs placebo plus chemotherapy for nonsquamous NSCLC. J Thorac Oncol. 2019;14(10):S216–7. [Google Scholar]
- 28. Dietel M, Savelov N, Salanova R, Micke P, Bigras G, Hida T, et al. Real‐world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non‐small‐cell lung cancer: the global, multicenter EXPRESS study. Lung Cancer. 2019;134:174–9. [DOI] [PubMed] [Google Scholar]
- 29. Smolle E, Leithner K, Olschewski H. Oncogene addiction and tumor mutational burden in non‐small‐cell lung cancer: clinical significance and limitations. Thorac Cancer. 2020;11(2):205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 List of the oncological institutions of the study.
Table S2 Patient characteristics. CNS: central nervous system; BMI: body mass index; ECOG‐PS: Easter Cooperative Oncology Group‐Performance Status).


