Abstract
Non‐small cell lung cancer (NSCLC) patients harboring EGFR sensitive mutations may benefit from treatment with EGFR TKIs. Osimertinib, which is an irreversible third‐generation EGFR TKI, has demonstrated a convincing efficacy, irrespective of whether it is used in first‐ or second‐line treatment. The acquired resistance mechanisms to osimertinib are highly complicated, and a variety of potential molecular mechanisms have been discovered, including C797S. Here, we determined that ALK rearrangement might be an underlying mechanism contributing to acquired resistance to osimertinib. In our report, a 60‐year‐old female patient with lung adenocarcinoma with an EGFR mutation was administered multiple treatments, including first‐line gefitinib and second‐line osimertinib. According to the next‐generation sequencing (NGS) assay after osimertinib failure, the emergence of an ALK rearrangement was considered to be a potentially acquired resistance mechanism to osimertinib. The combination of osimertinib and crizotinib then maintained a six‐month stable disease. VEGFA amplification was identified after osimertinib plus crizotinib treatment, and chemotherapy plus bevacizumab achieved a continuous stable disease over 21 months. In this study, we also summarized previously reported cases and concluded that ALK rearrangement is a rare but critical resistance mechanism to osimertinib. After failure of combined treatment with osimertinib plus crizotinib, comprehensive molecular profiling should be performed, and chemotherapy plus bevacizumab might be an optimal treatment especially for patients harboring VEGFA amplification.
Keywords: Acquired resistance, ALK rearrangement, EGFR mutation, NSCLC, osimertinib
ALK rearrangement probably serves as a rare molecular mechanism underlying acquired resistance to osimertinib. The dual TKI treatment targeting EGFR and ALK might benefit osimertinib‐resistant NSCLC patients induced by ALK rearrangement. VEGFA amplification might contribute to acquired resistance to combined osimertinib and crizotinib therapy, and chemotherapy plus bevacizumab might be an optimal treatment strategy.
Introduction
Lung cancer is one of the most common malignancies and ranks first as a cause of cancer‐related mortality. 1 Non‐small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases, and the early stage of the disease is usually asymptomatic, which results in difficulties in early diagnosis. 2 With the development of molecular detection technologies, patients harboring sensitive driver gene mutations might benefit from targeted therapy. Epidermal growth factor receptor (EGFR) plays an important role in the carcinogenesis of lung cancer, and approximately 50% of Asian patients harbor EGFR sensitive mutations, mainly including the deletion mutation in EGFR exon 19 (19 del) and the substitution of leucine for arginine (L858R) in EGFR exon 21. 3 , 4 According to the results of the IRESSA Pan‐Asia Study, the first‐generation EGFR tyrosine kinase inhibitor (TKI) caused NSCLC patients harboring EGFR sensitive mutations to have significantly longer progression‐free survival (PFS), which was maintained for 9.5 months (9.5 months vs. 6.3 months) compared with the regular regimen of chemotherapy. 5 However, primary or acquired resistance to first‐generation EGFR TKIs leads to the failure of targeted therapy that targets EGFR sensitive mutations. Therefore, three or more generations of EGFR TKIs were explored in the clinical treatment of NSCLC patients.
The Thr790Met point mutation (T790M) in EGFR exon 20 is a primary contributor to the acquired resistance to first‐generation EGFR TKIs. Osimertinib is an irreversible third‐generation EGFR TKI that is selective for both EGFR sensitizing and T790M resistance mutation. 6 Both primary and metastatic lesions, including metastases in the central nervous system (CNS), respond well to osimertinib treatment. 7 In addition, recent clinical trials have confirmed the role of osimertinib as a first‐line treatment of NSCLC patients harboring EGFR mutations. 8 , 9 Despite the efficacy of osimertinib, its resistance ws unavoidable, and the related mechanisms were demonstrated to be highly heterogeneous. 10 EGFR gene point mutations, MET or HER2 gene amplification 10 , 11 , 12 and the activation of RAS mitogen‐activated protein kinase (MAPK) or RAS phosphatidylinositol‐3 kinase (PI3K) pathways 10 have all been confirmed to be associated with resistance to osimertinib. In addition, a few cases have suggested that an emerging ALK rearrangement was detected after resistance to osimertinib treatment, 13 , 14 , 15 , 16 , 17 but the role of ALK rearrangement in resistance to osimertinib remains unclear. Here, we report an NSCLC patient who experienced osimertinib resistance who was shown to harbor the emerging ALK rearrangement and explored the available treatment strategies for patients after osimertinib resistance mediated by ALK arrangements.
Case report
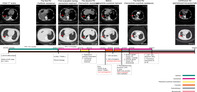
A 60‐year‐old female first presented in March 2015 with symptoms of chest distress for one month. Computed tomography (CT) scan showed a space‐occupying lesion in her right lung combined with pleural effusion. Through percutaneous lung puncture and pathological diagnosis, the patient was diagnosed with stage IV moderately differentiated invasive lung adenocarcinoma (LUAD),. No other metastatic lesions were found after systematic examination. Amplification refractory mutation system‐polymerase chain reaction (ARMS‐PCR) examination of EGFR mutations based on blood samples and immunohistochemistry (IHC) testing of ALK were performed to guide the molecular targeted therapy of this patient. The IHC test suggested a wild‐type ALK protein. Given the confirmation of EGFR 19 del through ARMS‐PCR, treatment with gefitinib was initiated in this patient who experienced a rapid partial response (PR). The detailed treatment regimen for this patient are described in Fig 1. After 18.3 months of gefitinib treatment, a CT scan of this patient revealed progressive disease (PD). The patient suffered serious pleural effusion, and pleural drainage was performed to alleviate the clinical symptoms. A droplet digital‐PCR (dd‐PCR) assay performed on the patient's blood for the EGFR T790M mutation was negative in December 2016. Given the baseline mutation status of EGFR and the false‐negative possibility of ct‐DNA examination, osimertinib was then administered. Fortunately, the patient achieved stable disease (SD) during osimertinib treatment, and pleural effusion gradually decreased. After 11.6 months of treatment in October 2017, the lesions on the right lung enlarged, and the osimertinib response was evaluated as PD. Due to local progression of the primary tumor, the patient was then given radioactive particle implantation treatment of the primary lesion combined with continuation of osimertinib, which maintained SD until December 2017. Although the CT scan demonstrated SD, the concentration of carcinoembryonic antigen (CEA) was significantly elevated from 5.5 to 13.0 ng/mL. Therefore, tissue samples obtained from percutaneous lung puncture before radioactive particle implantation treatment were examined by targeted next‐generation sequencing (NGS) in December 2017, which revealed a de novo ALK rearrangement (EML4‐ALK) in addition to EGFR 19del. Consequently, the patient received osimertinib plus crizotinib for the following treatment since January 2018 according to the NGS assay. During the treatment with dual TKIs, including osimertinib plus crizotinib, the CEA concentration was reduced from 13.0 to 7.5 ng/mL, and the CT scan also demonstrated SD. However, in May 2018, the CEA concentration increased again and reached 11.8 ng/mL. Given the SD condition evaluated by CT scan, the treatment using the original dual TKI regimen was recommended, but the patient opted to try the second‐generation ALK inhibitor brigatinib. Only 12 hours after the first treatment with brigatinib, the patient suffered polypnea and weakness, and 17 hours after treatment, the patient slipped into a coma and was sent to the emergency intensive care unit (EICU). Following CT scan, the patient was diagnosed with serious interstitial pneumonia, most likely induced by brigatinib (Fig 2). After tracheal intubation and treatment with glucocorticoids, on 4 June 2018, the patient recovered from the serious adverse event (SAE) and continued to receive treatment with osimertinib plus crizotinib. During treatment, the CEA concentration increased continuously to 125.9 ng/mL, and the CT scan suggested PD in July 2018. A third biopsy was then obtained, and VEGFA gene amplification was detected through NGS examination in addition to EGFR 19del and ALK arrangement. According to the NGS results, it was thought that VEGFA gene amplification might be involved in the acquired resistance of combination therapy of osimertinib plus crizotinib. The patient was then administered PP regimen chemotherapy (pemetrexed plus carboplatin) combined with bevacizumab and continuation of osimertinib. After treatment with six‐cycle PP regimen chemotherapy plus bevacizumab and osimertinib, the patient maintained SD. Thereafter, pemetrexed plus bevacizumab and osimertinib treatment was maintained, and the last evaluation of the disease follow‐up in October 2019 indicated that the patient was still in SD. Notably, osimertinib treatment was discontinued in December 2018 as a result of the asymptomatic absolute decrease in the left ventricular ejection fraction (LEVF), which was lower than 50% (LVEF = 44%) according to the instructions of osimertinib. The patient is now in good condition and has a good quality of life. The variation trend of carcinoembryonic antigen (CEA) of the patient during the treatment is shown in Fig 3. Detailed information on the molecular detection and the treatment process is presented in Tables 1 and 2, respectively.
Figure 1.
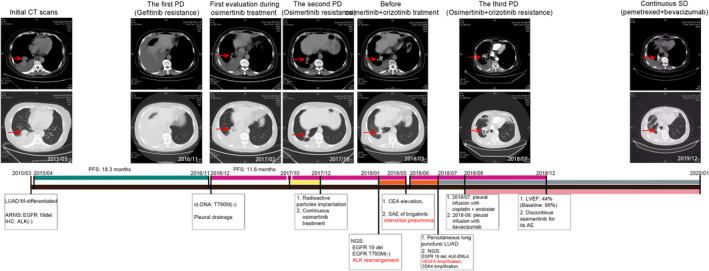
Treatment history of the patient in the study.  , Gefitinib;
, Gefitinib;  , Osimertinib;
, Osimertinib;  , Radioactive particles implantation;
, Radioactive particles implantation;  , Crizotinib;
, Crizotinib;  , Chemotherapy;
, Chemotherapy;  , Bevacizumab.
, Bevacizumab.
Figure 2.
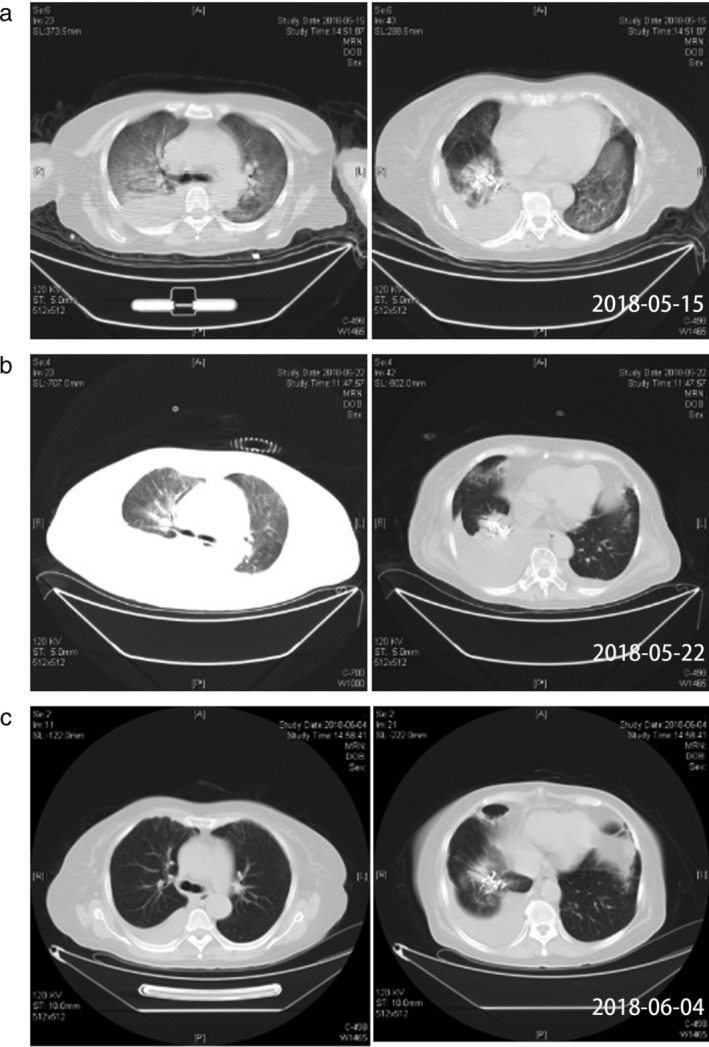
Computed tomography (CT) scan evolution of the patient who suffered from interstitial pneumonia caused by brigatinib. (a) 17 hours after brigatinib treatment. (b) 1 week after brigatinib treatment. (c) the patient recovered from the SAE induced by brigatinib.
Figure 3.
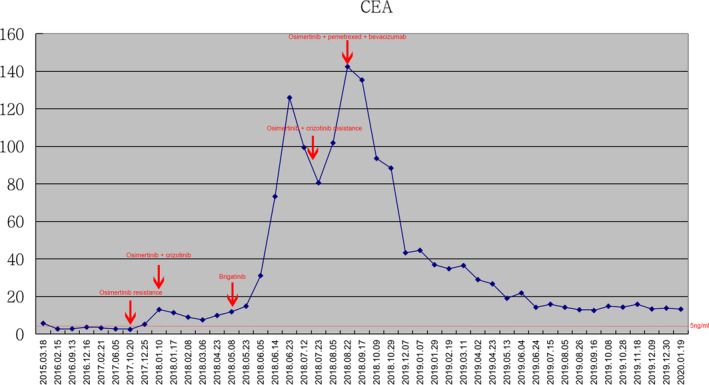
Dynamic change in carcinoembryonic antigen (CEA) of the patient during various treatments.  , CEA.
, CEA.
Table 1.
Molecular detection during treatment of the patient in the study
| Detection time | Samples | Methods | Mutation (abundance %) |
|---|---|---|---|
|
03/2015 First diagnosis |
Blood | ARMS‐PCR | EGFR 19 del |
| Tumor tissue | IHC | ALK (−) | |
|
12/2016 Gefitinib resistance |
Blood | dd‐PCR | EGFR T790M (−) |
|
10/2017 Osimertinib resistance |
Tumor tissue | NGS | EGFR 19 del (16.69%); ALK rearrangement (22.29%); CDK4 amplification (CN = 16.67); NOTCH1 missense mutation (12.88%) |
| Blood | NGS | NA | |
|
07/2018 Osimertinib + crizotinib resistance |
Tumor tissue | NGS | EGFR 19 del (15.85%); ALK rearrangement (27.79%); VEGFA amplification (CN = 3.95); CDK4 amplification (CN = 12.94) |
| Blood | NGS | NA | |
|
05/2019 Post pemetrexed + bevacizumab treatment |
Blood | NGS | NA |
Table 2.
Patient treatment information
| Treatment methods | Regimen | PFS (months) | Adverse events (Grade) |
|---|---|---|---|
| First generation EGFR TKI | Gefitinib | 18.3 | Rash (II) |
| Third generation EGFR TKI | Osimertinib | 11.6 | Rash (II); Skin hyperpigmentation (I) |
| Third generation EGFR TKI + radioactive particles implantation | Osimertinib + particles implantation | 2.1 | Rash (II) |
| Third generation EGFR TKIs + first generation ALK TKIs | Osimertinib + crizotinib | 6.0 | Rash (II); paronychia (II) |
| Third generation EGFR TKIs + second generation ALK TKIs | Osimertinib + brigatinib | NA | Interstitial penumonia (IV) |
| Third generation EGFR TKIs + chemotherapy + antiangiogenic therapy | Osimertinib + pemetrexed/carboplatin + bevacizumab | 4.2 | Rash (II); Asymptomatic, absolute decrease in LEVF (44%) |
| Chemotherapy + antiangiogenic therapy | Pemetrexed/carboplatin + bevacizumab | 16.8 (last CT scan: SD) | Anemia (II) |
Discussion
EGFR and ALK are both key driver genes contributing to the carcinogenesis of NSCLC. According to previous studies, EGFR mutations and ALK rearrangements share the same downstream signaling pathways, such as the PI3K/AKT pathway, JAK/STAT pathway and MAPK pathway, through the phosphorylation of tyrosine kinase. 18 , 19 , 20 Therefore, targeted TKIs demonstrate convincing efficacy in NSCLC patients harboring EGFR mutations or ALK rearrangements. However, the mutual downstream signaling pathways might also explain the mechanisms of TKI resistance induced by the activation of the other driven genes. Previous studies clarified the role of EGFR bypass pathway activation in resistance to ALK TKIs in vitro, 21 , 22 but whether emerging ALK rearrangement is involved in acquired resistance to EGFR TKIs, especially osimertinib, remains unknown. In Table 3, we summarize seven cases that might indicate the novel mechanism of ALK rearrangement involvement in acquired resistance to osimertinib. According to NGS testing after osimertinib resistance, all these patients harbored an emerging ALK rearrangement. The dual TKI treatment, including EGFR TKI plus crizotinib or crizotinib monotherapy, had a therapeutic effect on patients. As a result, our report suggests that ALK rearrangement participates in acquired resistance to osimertinib, although this only occupies a small fraction.
Table 3.
Summary of osimertinib acquired resistant NSCLC patients induced by ALK rearrangement
| First‐line treatment | Second‐line treatment | Third‐line treatment | Ongoing treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Contributors | Case | Gender | Age | Molecular detection; treatment | PFS (months) | Molecular detection; treatment | PFS (months) | Molecular detection; treatment | PFS (months) | Molecular detection; treatment | PFS (months) |
| Zhang, et al. 13 | #1 | Male | 66 | EGFR 19 del; gefitinib | 18 | EGFR T790M (+); osimertinib | 14 | EGFR 19del, T790M(+), C797G in cis(+), EML4‐ALK; crizotinib | 1 | No molecular detection; pemetrexed + bevacizumab | NA # |
| Zhou, et al. 14 | #2 | Male | 42 | EGFR 19 del; gefitinib | 10 | EGFR T790M (+); osimertinib | 5 | EGFR19del, T790M(−), STRN‐ALK; gefitinib+crizotinib | 6 | — | — |
| Hou, et al | #3 | Female | 60 | EGFR 19 del; gefitinib | 18.3 | EGFR T790M (−); osimertinib | 11.6 |
EGFR 19del, T790M (−), CDK4 Amp, EML4‐ALK; osimertinib + crizotinib |
6 | EML4‐ALK, EGFR 19del, VEGFA Amp; osimertinib* + chemotherapy + bevacizumab | Over 17 months |
| Schrock et al. 15 | #4 | Female | 70 | EGFR L858R; erlotinib | 12 | EGFR T790M (+); afatinib | 2 | EGFR T790M (+); osimertinib | 10 | EGFR L858R, EGFR T790M (+), PLEKHA7‐ALK; osimertinib + alectinib | 6 |
| Offin et al. 16 | #5 | Female | 65 | EGFR 19 del; erlotinib | 19 | EGFR T790M (+); osimertinib + necitumumab | 9 | EGFR 19del, T790M(+), EML4‐ALK; osimertinib + crizotinib | NA # | — | — |
| Offin et al. 16 | #6 | Female | 68 | EGFR L858R; erlotinib | 15 | EGFR T790M (+); osimertinib | 6 | EGFR L858R, T790M (+), EML4‐ALK; alectinib | NA # | — | — |
| Liang et al. 17 | #7 | Female | 46 | EGFR 19 del; erlotinib | 4 | No molecular detection; pemetrexed + bevacizumab | 0 | No molecular detection; osimertinib (followed 2‐cycle GP regimen chemotherapy) | 2 | EGFR 19 del, EGFR L747S, EML4‐ALK; osimertinib + crizotinib/brigatinib* | NA # |
Case 3: Osimertinib was discontinued as a result of its cardiac toxicity as shown in Table 2.
Case 7 was evaluated as progressive disease after two months of osimertinib plus crizotinib treatment and the regimen was changed to osimertinib plus brigatinib.
NA, not available. Patients maintained a continuous stable disease.
It has been previously reported that approximately 1.3% to 1.6% of NSCLC patients harbor concomitant EGFR mutations and ALK rearrangements. 23 , 24 For patients with concomitant alterations at baseline, a previous study suggested a standard therapy based on the phosphorylation level of both EGFR and ALK. Previous research reported that only 0.13% of NSCLC patients harboring EGFR mutations acquired EGFR TKI resistance mediated by emerging ALK rearrangement. 25 Nevertheless, no studies have established a standard treatment regimen for NSCLC patients harboring EGFR mutations and EGFR TKI resistance induced by adaptive ALK rearrangement. Here, for the first time, our report reveals an underlying regimen for this group of patients. As listed in Table 3, the regimen of osimertinib combined with crizotinib achieved a more satisfactory efficacy than monotherapy. This result suggested that dual TKI treatment might benefit EGFR TKI‐acquired resistant NSCLC patients induced by ALK rearrangement rather than ALK inhibitor alone (Cases No. 2, 3, 4, 5 and 7 in Table 3), which warrants further study.
The dual TKI treatment achieved a six‐month PFS in the patient reported here as the third‐line treatment. Through NGS examination, VEGFA amplification was identified in a rebiopsy sample, which might contribute to resistance to osimertinib plus crizotinib treatment. VEGF was indicated to be upregulated after EGFR TKI resistance, and the combination treatment of anti‐VEGF therapy plus TKIs has been previously reported to demonstrate a synergetic antitumor efficacy. 26 , 27 In our report, the patient achieved a satisfactory exceptional response by treatment with osimertinib plus chemotherapy and bevacizumab. In addition, as shown in Table 3, two previously reported patients (Cases 1 and 3) were administered chemotherapy plus bevacizumab, and all three achieved continuous SD even without VEGFA amplification (Case 1), which indicated that chemotherapy plus bevacizumab might serve as the optimal regimen for EGFR TKI plus ALK inhibitor treatment‐resistant lung cancer patients.
The AEs or even SAEs of targeted therapy in this patient are worth noting. Regardless of the grade II AEs, including rash, paronychia and grade I skin hyperpigmentation induced by EGFR TKIs, grade IV interstitial pneumonia induced by brigatinib must be noted. From the results of the previous study, ALK TKIs demonstrated significant respiratory toxicity. A total of 5.09% and 4.29% of patients might suffer pneumonia and dyspnea/respiratory failure induced by brigatinib, respectively. 2 In addition, the risk of cardiac toxicity induced by long‐term use of osimertinib might be involved in the evaluation, especially the asymptomatic decrease in LVEF or even congestive heart failure.
In conclusion, ALK rearrangement probably serves as a rare molecular mechanism underlying acquired resistance to osimertinib. The dual TKI treatment targeting EGFR and ALK might benefit osimertinib‐resistant NSCLC patients induced by ALK rearrangement. VEGFA amplification might contribute to acquired resistance to combined osimertinib and crizotinib therapy, and chemotherapy plus bevacizumab might be an optimal treatment strategy.
Disclosure
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors thank Burning Rock Biotech for providing assistance in NGS analysis in this study. This report was funded by the Special Funding for Qilu Sanitation and Health Leading Talents Cultivation Project to Helei Hou and Taishan Scholar Foundation of Shandong Province (No. tshw201502061 to Xiaochun Zhang).
Contributor Information
Helei Hou, Email: houhelei@qdu.edu.cn.
Dantong Sun, Email: sundantongerik@163.com.
Chuantao Zhang, Email: 87563551@qq.com.
Dong Liu, Email: sdsgld@163.com.
Xiaochun Zhang, Email: zxc9670@qdu.edu.cn.
References
- 1. Allemani C, Matsuda T, Carlo VD, et al. Global surveillance of trends in cancer survival: Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers during 2000–2014 from 322 population‐based registries in 71 countries (CONCORD‐3). Lancet. 2018;391(10125):1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hou HL, Sun DT, Liu KW, et al. The safety and serious adverse events of approved ALK inhibitors in malignancies: A meta‐analysis. Cancer Manag Res. 2019;11:4109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L. Making the first move in EGFR‐driven or ALK‐driven NSCLC: First‐generation or next‐generation TKI? Nat Rev Clin Oncol. 2018;15(11):694–708. [DOI] [PubMed] [Google Scholar]
- 4. Castellanos E, Feld E, Horn L. Driven by mutations: The predictive value of mutation subtype in EGFR‐mutated non‐small cell lung cancer. J Thorac Oncol. 2017;12(4):612–23. [DOI] [PubMed] [Google Scholar]
- 5. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. [DOI] [PubMed] [Google Scholar]
- 6. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med. 2017;376(7):629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M‐positive advanced non‐small‐cell lung cancer: Data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36(26):2702–9. [DOI] [PubMed] [Google Scholar]
- 8. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378(2):113–25. [DOI] [PubMed] [Google Scholar]
- 9. Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib as first‐line treatment of EGFR mutation‐positive advanced non‐small‐cell lung cancer. J Clin Oncol. 2018;36(9):841–9. [DOI] [PubMed] [Google Scholar]
- 10. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR‐mutated non‐small cell lung cancer. Br J Cancer. 2019;121(9):725–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Li L, Han R, Jiao L, Zheng J, He Y. Clinical analysis by next‐generation sequencing for NSCLC patients with MET amplification resistant to osimertinib. Lung Cancer. 2018;118:105–10. [DOI] [PubMed] [Google Scholar]
- 12. Ortiz‐Cuaran S, Scheffler M, Plenker D, et al. Heterogeneous mechanisms of primary and acquired resistance to third‐generation EGFR inhibitors. Clin Cancer Res. 2016;22(19):4837–47. [DOI] [PubMed] [Google Scholar]
- 13. Zhang L, Li Y, Zhang S, Gao C, Nie K, Ji Y. Primary resistance to crizotinib treatment in a non‐small cell lung cancer patient with an EML4‐ALK rearrangement: A case report. Cancer Biol Med. 2018;15(2):178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou C, Zeng L, Zhang Y, Yang N. Responder of gefitinib plus crizotinib in osimertinib failure EGFR‐mutant NSCLC‐resistant with newly identified STRN‐ALK by next‐generation sequencing. J Thorac Oncol. 2019;14(7):e143–4. [DOI] [PubMed] [Google Scholar]
- 15. Schrock AB, Zhu VW, Hsieh WS, et al. Receptor tyrosine kinase fusions and BRAF kinase fusions are rare but actionable resistance mechanisms to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2018;13(9):1312–23. [DOI] [PubMed] [Google Scholar]
- 16. Offin M, Somwar R, Rekhtman N, et al. Acquired ALK and RET gene fusions as mechanisms of resistance to osimertinib in EGFR‐mutant lung cancers. JCO Precis Oncol. 2018;2:1–12. 10.1200/PO.18.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang W, He Q, Chen Y, et al. Metastatic EML4‐ALK fusion detected by circulating DNA genotyping in an EGFR‐mutated NSCLC patient and successful management by adding ALK inhibitors: A case report. BMC Cancer. 2016;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK‐rearranged lung carcinomas. Am J Surg Pathol. 2011;35:1226–34. [DOI] [PubMed] [Google Scholar]
- 19. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature. 2007;448:561–6. [DOI] [PubMed] [Google Scholar]
- 20. Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non‐small cell lung cancer. Clin Cancer Res. 2008;14:2895–9. [DOI] [PubMed] [Google Scholar]
- 21. Miyawaki M, Yasuda H, Tani T, et al. Overcoming EGFR bypass signal‐induced acquired resistance to ALK tyrosine kinase inhibitors in ALK‐translocated lung cancer. Mol Cancer Res. 2017;15(1):106–14. [DOI] [PubMed] [Google Scholar]
- 22. Yamaguchi N, Lucena‐Araujo AR, Nakayama S, et al. Dual ALK and EGFR inhibition targets a mechanism of acquired resistance to the tyrosine kinase inhibitor crizotinib in ALK rearranged lung cancer. Lung Cancer. 2014;83(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ulivi P, Chiadini E, Dazzi C, et al. Non‐small‐cell lung cancer patients who carry a double mutation of EGFR, EML4‐ALK or KRAS: Frequency, clinical‐pathological characteristics, and response to therapy. Clin Lung Cancer. 2016;17:384–90. [DOI] [PubMed] [Google Scholar]
- 24. Yang JJ, Zhang XC, Su J, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: Diverse responses to EGFR‐TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res. 2014;20:1383–92. [DOI] [PubMed] [Google Scholar]
- 25. Xu H, Shen J, Xiang J, et al. Characterization of acquired receptor tyrosine‐kinase fusions as mechanisms of resistance to EGFR tyrosine‐kinase inhibitors. Cancer Manag Res. 2019;11:6343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masuda C, Yanagisawa M, Yorozu K, et al. Bevacizumab counteracts VEGF‐dependent resistance to erlotinib in an EGFR‐mutated NSCLC xenograft model. Int J Oncol. 2017;51(2):425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li H, Takayama K, Wang S, et al. Addition of bevacizumab enhances antitumor activity of erlotinib against non‐small cell lung cancer xenografts depending on VEGF expression. Cancer Chemother Pharmacol. 2014;74(6):1297–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


