Abstract
Background
The preoperative predictors of quality of life (QOL) in patients who undergo lung resection for lung cancer are poorly known. Here, we investigated these predictors in such patients using two QOL measures.
Methods
In this single‐institutional prospective cohort study, we administered the EQ‐5D‐5 levels (EQ‐5D‐5L) from January 2015, and the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire with 30 items from April 2015 to April 2018 preoperatively (Pre) and at one month postoperatively (M1), and one year postoperatively (Y1). General health status was measured by the EQ‐5D visual analogue scale (VAS) and EORTC global health status/QOL (GHS) scores. Multivariable linear regression analyses were used to explore the preoperative predictors of QOL at Y1.
Results
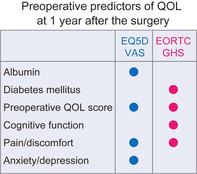
A total of 223 patients were included in the study. The EQ‐5D VAS and EORTC GHS scores, at Pre, M1, and Y1, were 80 ± 15, 77 ± 15, and 84 ± 11; and 74 ± 19, 65 ± 20, and 78 ± 17, respectively. In the multivariable analyses, the albumin level, preoperative VAS score, and preoperative pain/discomfort and anxiety/depression were identified as predictors by the EQ‐5D VAS score. The preoperative EORTC GHS score, absence of diabetes mellitus, preoperative cognitive function score, and preoperative symptom score of pain were identified as predictors by the EORTC GHS score.
Conclusions
The EQ‐5D VAS and EORTC GHS scores traced similar trajectories of QOL. In both QOL measures, preoperative pain was found as a common predictor. These predictors may help improve patient/survivor care in the future.
Keywords: lung cancer, lung resection, preoperative pain, quality of life, survivor care
We investigated the preoperative predictors of QOL in surgical patients with lung cancer. General health status/QOL was measured by EQ‐5D VAS and EORTC GHS scores. Multivariable linear regression analyses were used. Although each QOL measure detected some preoperative predictors, preoperative pain was a common predictor in both, one year after surgery. Predictors detected in this study were possible targets for patient/survivor care.
INTRODUCTION
In patients who undergo lung resection for lung cancer, patient‐reported health‐related quality of life (QOL) is one of the important outcomes, and it is a primary outcome, especially in terms of survivor care. 1 However, reported evidence of QOL in such patients is still sparse. 2 If improvable predictors by intervention of postoperative QOL could be identified, an effective survivor care program could be developed together with the provision of patient care efficiently within limited resources.
Although several factors in patients with lung cancer have been reported to be associated with postoperative QOL decline (age, sex, comorbid conditions, living alone, surgical approach, extent of lung resection, adjuvant therapy), 3 , 4 , 5 , 6 , 7 only two studies exploring predictors in which over 100 patients were prospectively enrolled have been previously reported. 7 , 8 In both studies, the Short Form 36 (SF‐36), 9 which provides physical and mental component summary scores separately, was used as a QOL measure. One of those studies reported that physical decline measured by the physical component summary score was significantly affected by the mental health score, indicating the possibility that physical QOL could be affected by mental or emotional QOL, 8 and vice versa. This finding suggested the necessity of another approach to explore predictors of general health status represented as a single score. Two QOL measures provide general health status as a single score. One measure is the EQ‐5D visual analog scale (VAS) (range 0–100, 99 intervals, interval size = 1). 10 The other measure is the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire 30 items (QLQ‐C30) global health status/QOL (EORTC GHS) score (range 0–100, 12 intervals, interval size = 8.3). 11 We initially assumed that the number of intervals represented the fineness of the scale and that the measure having more intervals as finer measure would be optimal to observe QOL. Based on this assumption, we initiated a pilot assessment with the EQ‐5D alone, and then added the EORTC QLQ‐C30 as a validated measure when the protocol was set.
Moreover, in those studies that explored predictors, 7 , 8 QOL decline was defined in advance as an objective variable, and logistic regression analyses were applied for the predetermined event of QOL decline. Consequently, predictors derived in those studies were bound by the predetermined definition of the QOL decline. Although there is no gold standard for defining clinically important changes in the QOL score, we assumed that a linear regression analysis of a continuous objective variable, such as the EQ‐5D VAS and EORTC GHS scores, would be optimal to explore explanatory variables as predictors without being subjected to the restriction of the definition of QOL decline/improvement.
We hypothesized that the EQ‐5D VAS and EORTC GHS scores would be applicable to patients who underwent surgery for lung cancer in terms of observing their general health status/QOL, and that there would be some preoperative predictors of postoperative QOL. This study aimed to investigate the predictors of postoperative QOL using the EQ‐5D VAS and EORTC GHS scores in these patients.
METHODS
Ethical statements
This study was registered with the UMIN Clinical Trials Registry (UMIN000017594). The study protocol was approved by our institutional review board (approval number: 2015‐4), and inclusion of the three‐month pilot assessment data for analysis was approved. The need for written informed consent was waived because each questionnaire provided the respondent with an opportunity for refusal to answer, and we provided contact information for opting out of the study on our website.
Patient cohort
We performed a prospective QOL assessment in patients with lung cancer who underwent lung resection. Patients were consecutively recruited between January 2015 and April 2018 at Hitachi General Hospital. Of 290 patients, 279 eligible patients were enrolled into this longitudinal cohort. According to the protocol, patients with relapse, those who died due to any reason, those who stopped visiting our outpatient clinic for any reason, those who underwent surgery for multiple lung cancer, and those newly diagnosed as having multiple cancers other than lung, except for noninvasive cancer, were excluded from the assessment. Finally, 223 cases were included in this analysis (Figure 1).
FIGURE 1.
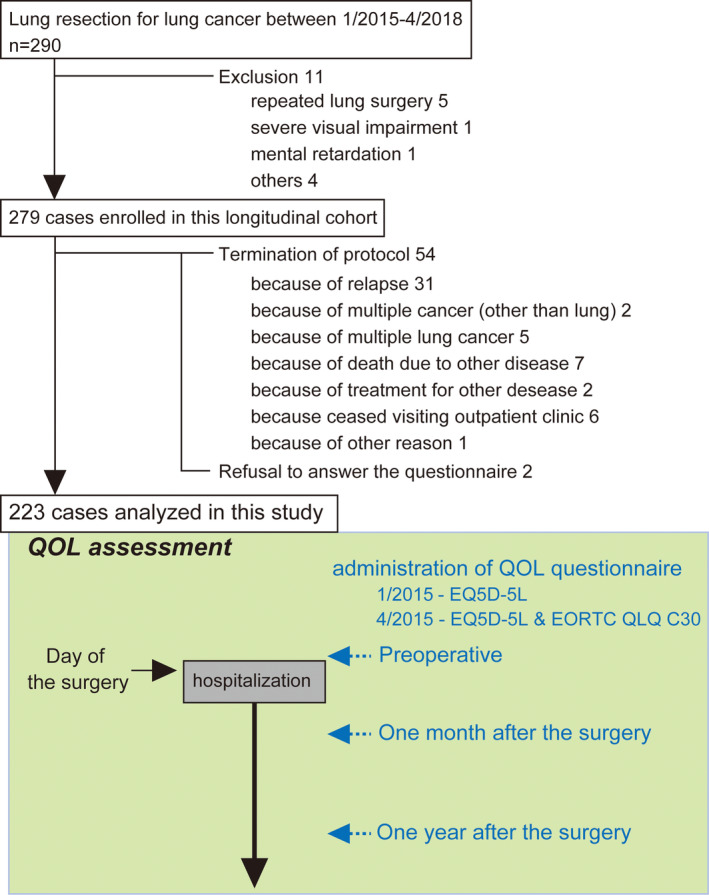
Patient flow diagram and times of questionnaire administration. QOL, quality of life; Pre, preoperative; M1, one month postoperatively; Y1, one year postoperatively
QOL assessment
Based on the findings of previous studies, 12 , 13 , 14 we hypothesized that the QOL score would be significantly lower at one month after the surgery, and would improve significantly and reach a plateau at one year after the surgery compared with that at one month postoperatively. Therefore, the QOL assessment was performed preoperatively (Pre), at one month postoperatively (M1), and at one year postoperatively (Y1) (Figure 1). The thoracic surgeon responsible for the surgery handed the printed questionnaire directly to the patients. The patients were hospitalized on the day before the surgery, and a preoperative (Pre) assessment was performed on the day of hospitalization. M1 and Y1 assessments were performed in the outpatient clinic of the Thoracic Surgery Department. The Y1 assessment was conducted after patients had been informed that there was no any signs of relapse after their work‐up one year after surgery. At the time of the Pre assessment, patients were informed about the longitudinal QOL assessment study with documents stating the aim of the study and a request for their cooperation. At every assessment, patients were asked to return the answered questionnaire to any hospital staff. In this protocol, the QOL assessment was further planned at three and five years after surgery for long‐term evaluation.
We initiated this study using the EQ‐5D‐5 levels (EQ‐5D‐5L) (registration number: 7772) from January 2015 in a pilot study, and added EORTC QLQ‐C30 version 3.0 in Japanese in April 2015. The EQ‐5D‐5L consists of a descriptive system and a VAS. The descriptive system comprises five dimensions, namely, mobility, self‐care, usual activity, pain/discomfort, and anxiety/depression. Each dimension has five levels of possible numerical responses from no problem (1) to extreme problem (5). The VAS was used to determine the patient's self‐reported general health status on a scale of 0 to 100. A score of 100 represented “The best health status I can imagine,” whereas 0 represented “The worst health status I can imagine.” The EORTC QLQ‐C30 had 30 items of questions providing five functional scales (physical/emotional/cognitive/social/role), nine symptom scales (fatigue/nausea and vomiting/pain/dyspnea/insomnia/appetite loss/constipation/diarrhea/financial difficulties), and the global health status/quality of life (GHS) score. Each score was calculated according to the scoring manual.
A total of 37 cases (37/223, 16.6%) had missing items in the EQ‐5D, and 29 cases (29/206, 14.1%) had missing items in the EORTC QLQ‐C30. We did not count the number of uncollected questionnaire sheets, and those were included as missing items. Because all 223 cases could continue to visit our outpatient clinic by themselves without relapse, we did not consider these missing items as “missing data not at random” and conducted analyses with all of the collected data.
Surgical treatment and care
We mainly used three different surgical approaches, namely, video‐assisted thoracic surgery (VATS), axillary mini‐thoracotomy, and the open approach (posterolateral or antero‐axillary and median sternotomy). In our VATS approach, a utility window with a 6–8‐cm incision and three ports were used. In the axillary mini‐thoracotomy approach, one window with a 10–14‐cm incision with metal mini‐rib spreader and two to three ports were used. In this study, we divided the surgical approaches into two categories: less invasive and open approach based on the preliminary analysis of QOL, which indicated that the VAS scores of our VATS and mini‐thoracotomy approach were almost identical (data not shown). Therefore, the less invasive approach included VATS and axillary mini‐thoracotomy in which the surgeon could not insert his/her hand into the thoracic cavity for surgical manipulation. There were six conversions from the initially intended approach (five from VATS to axillary mini‐thoracotomy and one from axillary mini‐thoracotomy to antero‐axillary thoracotomy). The reasons for conversion were as follows: palpation of the lesion (three), and pleural adhesion (three). There was no conversion due to intraoperative complications such as major hemorrhage. All the converted cases were analyzed according to the initially intended approach to determine the effect of the intended surgical approach on postoperative QOL.
In our clinical practice, a patient with a part‐solid ground‐glass nodule (GGN) with a consolidated size less than 25% of the lesion's diameter was considered a candidate for intentional limited surgery. 15
Clinical and social factors
We ascertained and collected the following clinical and social factors: age, sex, performance status (PS), Charlson comorbidity index (CCI), 16 smoking index calculated by the number of cigarettes per day multiplied by the number of years smoking in his/her lifetime, neutrophil‐to‐lymphocyte ratio (NLR), albumin level, C‐reactive protein level, clinical stage according to the seventh edition of the tumor–node–metastasis (TNM) staging system, and predictive postoperative (Ppo) percent vital capacity (%VC). Ppo pulmonary function was calculated according to the planned number of resected segments except for the nonfunctional segments. 17 S1 + 2a + b and S1 + 2c were counted as 1.3 and 0.7, respectively. Ppo forced expiratory volume in 1 s living alone, comorbidities (interstitial pneumonitis [IP], ischemic heart disease, diabetes mellitus [DM], stroke, and chronic obstructive pulmonary disease), preoperative treatment, surgical indication, intended surgical approach, and lung resection mode were also collected. Additional data included the postoperative length of stay, histological type, pathological stage according to the seventh edition of TNM staging system, whether adjuvant therapy was implemented or not, and postoperative adverse events (AEs) occurring within 30 days after the surgery according to Common Terminology Criteria for Adverse Events version 4.0. Among recorded AEs, we further categorized the events according to grades greater than three.
Statistical analysis
Data are presented as means ± standard deviations for continuous variables or numbers (proportions) of patients for categorical and ordinal variables. The paired t‐test was used to compare continuous variables. The Wilcoxon signed‐rank test was used to compare ordinal variables. VAS and EORTC GHS scores at each time point were compared using the mixed effects model for repeated measures including the time variable as a factor with the unstructured covariance matrix. To explore the preoperative predictive factors of the VAS and EORTC GHS scores at Y1, we performed univariable and multivariable linear regression analyses. Considering both statistical and clinical relevance, multivariable analysis was performed in two steps. First, a stepwise regression method (p‐value to enter was set at 0.05, p‐value to remove was set at 0.1) was applied to factors selected using univariable analysis (factors with p < 0.05 using univariable analysis were selected). Second, a stepwise regression analysis (p‐value to enter was set at 0.05, p‐value to remove was set at 0.1) was performed using the union of the set of factors selected in the first step and the set of factors reported to be affecting postoperative QOL 3 , 4 , 5 , 6 , 7 (age, sex, living alone, less invasive surgical approach, sublobar resection as lung resection mode, adjuvant therapy), and postoperative AEs. We used SPSS version 25 (IBM Corp., USA) and SAS version 9.4 (SAS Institute, USA) to perform statistical analyses. A p‐value less than 0.05 was considered statistically significant.
RESULTS
Patient characteristics and missing data
Patient characteristics are summarized in Table 1. The number of patients in each histological type were as follows: adenocarcinoma, 178; squamous cell carcinoma, 40; small cell carcinoma, one; and other,four. The number of patients in each pathological stage were as follows: stage 0, 24; stage I, 151; stage II, 35; stage III, 12; and stage IV, one. Regimens of adjuvant therapy were as follows; oral uracil‐tegafur in 22 cases, cisplatin and vinorelbine with or without radiotherapy in 15 cases, and radiotherapy in three cases. With regard to AEs, there was no bronchopleural fistula and grade 5 in this cohort.
TABLE 1.
Patient characteristics
| Age, years (median, range) | 70, 29–93 |
| Sex (male: female) | 135:88 |
| PS | |
| 0 | 187 (84) |
| 1 | 30 (13) |
| 2 | 6 (3) |
| CCI | |
| 0 | 80 (36) |
| 1 | 49 (22) |
| 2 | 54 (24) |
| >3 | 40 (18) |
| Albumin level (g/dl) | 4.3 ± 0.4 |
| CRP level (mg/dl) | 0.51 ± 2.33 |
| NLR | 2.51 ± 1.96 |
| Ppo %VC | 92 ± 19 |
| Ppo % FEV1.0 | 79 ± 18 |
| Comorbidity, present | |
| Interstitial pneumonitis | 20 (9) |
| Ischemic heart disease | 14 (6) |
| Stroke | 20 (9) |
| Diabetes mellitus | 36 (16) |
| COPD | 63 (28) |
| Smoking index | 580 ± 685 |
| Clinical stage | |
| I | 186 (83) |
| II | 29 (13) |
| III | 8 (4) |
| Living alone | 20 (9) |
| Preoperative treatment | 2 (1) |
| Surgical indication | |
| Intentional limited surgery | 36 (16) |
| Limited surgery for compromised patient | 25 (11) |
| Standard | 162 (73) |
| Surgical approach | |
| Less invasive | 201 (90) |
| Open | 22 (10) |
| Lung resection mode | |
| Wedge | 39 (17) |
| Segmentectomy | 22 (10) |
| Lobectomy | 160 a (72) |
| Pneumonectomy | 2 (1) |
| Postoperative adverse event, present | |
| All grades | 70 (31) |
| ≥Grade 3 | 25 (11) |
| Postoperative length of stay (days) | 10 ± 4 |
| Adjuvant therapy | 40 (18) |
Values are presented as indicated, means ± standard deviations, or number of patients (%).
The total number of patients is 223 unless otherwise indicated.
%FEV1, predicted forced expiratory volume in 1 second; %VC, predicted percent vital capacity; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; NLR, neutrophil‐to‐lymphocyte ratio; Ppo, predictive postoperative; PS, performance status.
Includes five cases of bronchoplasty.
With regard to missing items, we performed logistic regression analysis to explore the clinical factors associated with the missing items (Tables S1 and S2). In patients with missing items on the EQ‐5D, advanced clinical stage and symptomatic detection of lung cancer were significant factors. In patients with missing items on the EORTC QLQ‐C30, presence of interstitial pneumonitis was a significant factor associated with the missing items. Frequencies and proportions of the preoperative EQ‐5D‐5L of five dimensions are summarized in Table S3. The proportion of each functional and symptom scale of the EORTC QLQ‐C30 preoperatively is shown in Figures S1 and S2.
Trajectory of QOL and correlation between the EQ‐5D VAS and EORTC GHS scores
General health status was measured as the EQ‐5D VAS and EORTC GHS scores. The VAS scores were 80 ± 15, 77 ± 15, and 84 ± 11 at Pre, M1, and Y1, respectively (Figure 2a). The EORTC GHS scores were 74 ± 19, 65 ± 20, and 78 ± 17 at Pre, M1, and Y1, respectively (Figure 2b). Comparisons of each score between each assessment time showed a significant difference (Figure 2). In terms of the trajectory, both scores decreased at M1 and increased at Y1, more than that at Pre. In correlation analysis, the EQ‐5D VAS and EORTC GHS scores were significantly correlated (Figure 3). Detailed data of QOL measures are shown in Table 2 and in Table S3. In functional scales, scores of physical and role function decreased significantly compared with preoperative scores. Conversely, scores of emotional and social function increased. In symptom scales, deteriorated symptoms compared with preoperative state were fatigue, pain, and dyspnea.
FIGURE 2.
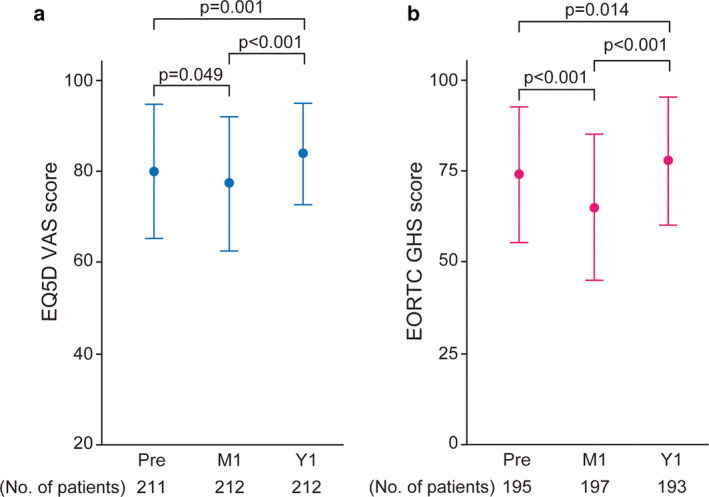
Patient‐reported quality of life (QOL) measured by the EQ‐5D visual analog scale (VAS) scores and the European Organization for Research and Treatment of Cancer (EORTC) global health status/QOL (EORTC GHS) score for patients who underwent surgery for lung cancer. Error bar indicates standard deviation. No. of patients, the number of patients whose data were collected. The p‐value was calculated using the paired t‐test, and data were compared among the assessment points as indicated. Pre, preoperative; M1, one month postoperatively; Y1, one year postoperatively
FIGURE 3.
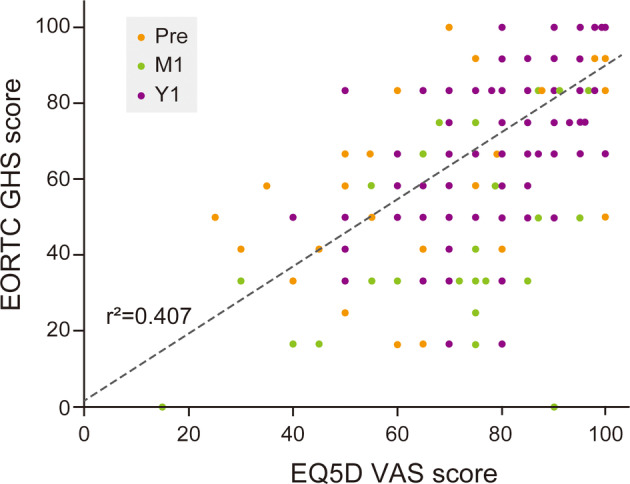
Scatter plot of the EQ‐5D visual analog scale (VAS) score and the European Organization for Research and Treatment of Cancer (EORTC) global health status/QOL (EORTC GHS) score. The regression line is indicated by the dashed line. Colored dots indicate the different assessment points. Pre, preoperative; M1, one month postoperatively; Y1, one year postoperatively
TABLE 2.
Scores of functional and symptom scales at each assessment point
| Pre | M1 | Y1 | p‐value | p‐value | ||
|---|---|---|---|---|---|---|
| Pre vs. M1 | Pre vs. Y1 | |||||
| Functional scales | Physical | 90 ± 14 | 79 ± 15 | 87 ± 15 | <0.001 | <0.001 |
| Role | 92 ± 17 | 70 ± 24 | 89 ± 18 | <0.001 | 0.04 | |
| Emotional | 77 ± 18 | 85 ± 16 | 90 ± 13 | <0.001 | <0.001 | |
| Cognitive | 83 ± 18 | 85 ± 18 | 84 ± 17 | 0.17 | 0.499 | |
| Social | 88 ± 16 | 82 ± 20 | 94 ± 13 | <0.001 | <0.001 | |
| Symptom scales | Fatigue | 20 ± 16 | 32 ± 18 | 24 ± 17 | <0.001 | 0.002 |
| Nausea and vomiting | 1 ± 6 | 2 ± 9 | 2 ± 8 | 0.473 | 0.14 | |
| Pain | 9 ± 15 | 29 ± 20 | 12 ± 15 | <0.001 | 0.016 | |
| Dyspnea | 11 ± 18 | 33 ± 20 | 24 ± 21 | <0.001 | <0.001 | |
| Insomnia | 16 ± 22 | 19 ± 22 | 13 ± 22 | 0.421 | 0.056 | |
| Appetite loss | 8 ± 17 | 15 ± 23 | 7 ± 14 | <0.001 | 0.542 | |
| Constipation | 12 ± 21 | 14 ± 22 | 11 ± 19 | 0.504 | 0.311 | |
| Diarrhea | 4 ± 13 | 5 ± 14 | 6 ± 15 | 0.682 | 0.109 | |
| Financial difficulties | 15 ± 24 | 13 ± 21 | 7 ± 17 | 0.304 | <0.001 |
Pre, preoperative; M1, one month postoperatively; Y1, one year postoperatively.
Preoperative predictive factors of QOL at one year postoperatively using the EQ‐5D VAS score
In univariable linear regression analysis of the VAS score at Y1 (Table 3), an increase in the albumin level, Ppo %VC, and preoperative EQ‐5D VAS score significantly increased the VAS score at Y1. The absence of IP and less invasive surgical approach also increased the EQ‐5D VAS score at Y1. Conversely, an increase in PS, CCI, clinical stage, and all five dimensions of the EQ‐5D significantly decreased the EQ‐5D VAS score at Y1. In multivariable analysis (Table 3) (factors with P < 0.05 were entered), the albumin level, preoperative EQ‐5D VAS score, preoperative pain/discomfort, and anxiety/depression were identified as significant predictive factors of QOL. The predicted VAS score at Y1 was calculated as follows: VAS at Y1 = 57.125 + 0.142 (VAS score at Pre) + 6.231 (albumin level) – 6.095 (preoperative pain/discomfort) – 2.261 (preoperative anxiety/depression). The adjusted coefficient of determination R2 of this equation was 0.285. Addition of age, sex, living alone, adjuvant therapy, and presence of postoperative AEs in the multivariable analysis did not change the significance of the four factors.
TABLE 3.
Univariable and multivariable regression analyses of the EQ‐5D VAS score at one year postoperatively
| Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|
| B | SE | p‐value | B | SE | p‐value | ||
| Age | −0.079 | 0.083 | 0.338 | ||||
| Sex: female (ref: male) | −1.122 | 1.59 | 0.481 | ||||
| Smoking index | −0.002 | 0.001 | 0.08 | ||||
| NLR | −0.237 | 0.63 | 0.708 | ||||
| Alb level (g/dl) | 9.186 | 2.068 | <0.001 | 6.231 | 1.878 | 0.001 | |
| CRP level (mg/dl) | −0.152 | 0.327 | 0.643 | ||||
| PS | −5.945 | 1.718 | 0.001 | ||||
| CCI | −1.155 | 0.502 | 0.023 | ||||
| Comorbidity | w/o IP (ref: present) | 5.77 | 2.765 | 0.038 | |||
| w/o ischemic heart disease (ref: present) | 2.343 | 3.242 | 0.471 | ||||
| w/o stroke (ref: present) | 3.91 | 2.65 | 0.142 | ||||
| w/o DM (ref: present) | 2.814 | 2.139 | 0.19 | ||||
| w/o COPD (ref: present) | −2.034 | 1.723 | 0.239 | ||||
| Ppo % VC | 0.093 | 0.041 | 0.026 | ||||
| Ppo % FEV1.0 | 0.060 | 0.042 | 0.158 | ||||
| Clinical stage | −4.938 | 1.606 | 0.002 | ||||
| Social factors | Living alone (ref: living with someone) | −0.806 | 2.726 | 0.768 | |||
| w/o preoperative treatment | 8.976 | 8.031 | 0.265 | ||||
| Surgical indication | Intentional limited surgery (ref: other) | 3.287 | 2.136 | 0.125 | |||
| Surgical approach | Less invasive approach (ref: open) | 7.057 | 2.619 | 0.008 | |||
| Resection mode | Sublobar resection (ref: more than lobar resection) | 1.693 | 1.773 | 0.341 | |||
| Preoperative QOL | VAS score | 0.263 | 0.051 | <0.001 | 0.142 | 0.05 | 0.005 |
| Dimensions of EQ‐5D | |||||||
| Mobility | −7.114 | 1.75 | <0.001 | ||||
| Self‐care | −11.2 | 2.309 | <0.001 | ||||
| Usual activity | −8.072 | 1.567 | <0.001 | ||||
| Pain/discomfort | −7.971 | 1.204 | <0.001 | −6.095 | 1.169 | <0.001 | |
| Anxiety/depression | −4.224 | 1.04 | <0.001 | −2.261 | 0.968 | 0.02 | |
%FEV1, predicted forced expiratory volume in 1 second; %VC, predicted percent vital capacity; Alb, albumin; B, regression coefficient; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; DM, diabetes mellitus; IP, interstitial pneumonitis; NLR, neutrophil‐to‐lymphocyte ratio; Ppo, predictive postoperative; PS, performance status; QOL, quality of life; ref, reference; SE, standard error; VAS, visual analog scale; w/o, without. Bold values represent a P‐value <0.05 among subgroups.
Preoperative predictive factors of QOL at one year postoperatively using the EORTC GHS score
In univariable regression analysis of the EORTC GHS score at Y1 (Table 4), an increase in the albumin level, preoperative EORTC GHS score, all preoperative functional scores in the EORTC QLQ‐C30, and absence of DM significantly increased the EORTC GHS score at Y1. Conversely, an increase in NLR, PS, CCI, and symptom scores of fatigue/pain/dyspnea/appetite loss/diarrhea/financial difficulties significantly decreased the EORTC GHS score at Y1. In multivariable analysis (Table 4) (factors with p < 0.05 were entered), absence of DM, preoperative EORTC GHS score, preoperative cognitive function score, and preoperative symptom score of pain were identified as significant predictive factors of QOL at Y1. The predicted EORTC GHS score at Y1 was calculated as follows: EORTC GHS at Y1 = 37.496 + 0.256 (EORTC GHS score at Pre) + 0.217 (cognitive function score at Pre) – 0.241 (preoperative symptom score of pain) + 6.256 (absence of DM = 1, present = 0). The adjusted coefficient of determination R2 of this equation was 0.333. Addition of age, sex, living alone, less invasive surgical approach, sublobar resection as lung resection mode, adjuvant therapy, and presence of postoperative AEs in the multivariable analysis did not alter the significance of the four factors.
TABLE 4.
Univariable and multivariable regression analyses of the EORTC GHS score at one year postoperatively
| Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|
| B | SE | p‐value | B | SE | p‐value | ||
| Age | −0.060 | 0.134 | 0.654 | ||||
| Sex: female (ref: male) | −1.32 | 2.559 | 0.607 | ||||
| Smoking index | −0.003 | 0.002 | 0.126 | ||||
| NLR | −1.335 | 0.634 | 0.037 | ||||
| Alb level (g/dl) | 10.257 | 3.383 | 0.003 | ||||
| CRP level (mg/dl) | 0.258 | 0.505 | 0.610 | ||||
| PS | −5.656 | 2.784 | 0.044 | ||||
| CCI | −2.060 | 0.786 | 0.009 | ||||
| Comorbidity | w/o IP (ref: present) | 4.014 | 4.433 | 0.306 | |||
| w/o ischemic heart disease (ref: present) | 1.488 | 5.023 | 0.767 | ||||
| w/o stroke (ref: present) | 6.233 | 4.107 | 0.131 | ||||
| w/o DM (ref: present) | 7.493 | 3.3 | 0.024 | 6.256 | 2.906 | 0.033 | |
| w/o COPD (ref: present) | −1.162 | 2.837 | 0.682 | ||||
| Ppo % VC | 0.094 | 0.067 | 0.158 | ||||
| Ppo % FEV1.0 | 0.08 | 0.068 | 0.238 | ||||
| Clinical stage | −2.943 | 2.714 | 0.28 | ||||
| Social factors | Living alone (ref: living with someone) | −3.486 | 4.56 | 0.445 | |||
| w/o preoperative treatment | −1.493 | 12433 | 0.905 | ||||
| Surgical indication | Intentional limited surgery (ref: other) | 6.462 | 3.353 | 0.055 | |||
| Surgical approach | Less invasive approach (ref: open) | 5.776 | 4.11 | 0.162 | |||
| Resection mode | Sublobar resection (ref: more than lobar resection) | 0.358 | 2.856 | 0.9 | |||
| Preoperative QOL | EORTC GHS score | 0.434 | 0.061 | <0.001 | 0.256 | 0.069 | <0.001 |
| EORTC functional scales | |||||||
| Physical | 0.407 | 0.09 | <0.001 | ||||
| Role | 0.279 | 0.07 | <0.001 | ||||
| Emotional | 0.338 | 0.067 | <0.001 | ||||
| Cognitive | 0.406 | 0.067 | <0.001 | 0.217 | 0.07 | 0.002 | |
| Social | 0.285 | 0.077 | <0.001 | ||||
| EORTC symptom scales | |||||||
| Fatigue | −0.378 | 0.065 | 0.000 | ||||
| Nausea and vomiting | −0.368 | 0.204 | 0.073 | ||||
| Pain | −0.491 | 0.076 | 0.000 | −0.241 | 0.081 | 0.003 | |
| Dyspnea | −0.304 | 0.069 | 0.000 | ||||
| Insomnia | −0.111 | 0.059 | 0.061 | ||||
| Appetite loss | −0.256 | 0.073 | 0.001 | ||||
| Constipation | −0.124 | 0.063 | 0.051 | ||||
| Diarrhea | −0.209 | 0.1 | 0.037 | ||||
| Financial difficulties | −0.183 | 0.053 | 0.001 | ||||
%FEV1, predicted forced expiratory volume in 1 second; %VC, predicted percent vital capacity; Alb, albumin; B, regression coefficient; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; DM, diabetes mellitus; EORTC, European Organization for Research and Treatment of Cancer; GHS, global health status; IP, interstitial pneumonitis; NLR, neutrophil‐to‐lymphocyte ratio; Ppo, predictive postoperative; PS, performance status; QOL, quality of life; ref, reference; SE, standard error; w/o, without. Bold values represent a P‐value <0.05 among subgroups.
DISCUSSION
The main findings of this study were as follows. First, the EQ‐5D VAS and EORTC GHS scores could trace the hypothesized trajectory of the postoperative general health status/QOL in patients with lung cancer. Second, there were some preoperative predictors of QOL at one year after the surgery according to linear regression analysis in which those QOL scores were applied. Third, preoperative pain was identified as one of the common significant predictors in both QOL measures.
With regard to the QOL measure, although there is as yet no specifically validated QOL measure for surgical patients with lung cancer, 18 the EQ‐5D VAS and EORTC GHS scores revealed the hypothesized trajectory in our setting (time of assessment, mode of administration), indicating the responsiveness of both scores. The EQ‐5D‐5L also provides another single score originally called the EQ‐5D index value, which is derived from the descriptive system of five dimensions using the EQ‐5D value sets. The EQ‐5D index value has been reported to be inappropriate for patients with early and advanced lung cancer. 19 , 20 In contrast, the EQ‐5D VAS score could yield the same result with other validated QOL measures in advanced lung cancer 19 and be used with other validated measures in the surgical setting. 3 Our result reconfirmed the applicability of the EQ‐5D VAS score in surgical patients with lung cancer. The EORTC QLQ‐C30 provides another single score called the summary score which has been reported to be more sensitive than the EORTC GHS score for assessing the QOL decline during the three months after surgery for lung cancer. 21 Although evaluation and development of invasive treatment requires sensitive QOL measures to detect patients' symptoms, a single score such as the EQ‐5D VAS and EORTC GHS that represents the patient's subjective perspective about their health status may be an option in the context of patient/survivor care, because these include the concepts of eudaimonic (sense of purpose) and hedonic (life satisfaction) well‐being, in addition to health‐related functions and symptoms.
In terms of the trajectory, both scores were better at one year after the surgery than preoperatively. This trajectory was also observed in other studies. 3 , 12 We speculated that the anxiety experienced by patients about surgery and postoperative daily life caused a decrease in the preoperative scores. The patient's recovery from lung surgery, a big event in their life, and being able to return to their daily living activities without relapse at one year after the surgery caused the score to increase. We also speculate that these increased scores were partly attributable to the response shift. 22 Moreover, post‐traumatic growth may play a role in the trajectories of QOL. 23 These are important subjects in QOL research, and elucidation of the underling mechanisms of this trajectory may help improve patient care. However, this is beyond the scope of our study and further studies are needed.
We found several preoperative predictors in addition to the preoperative QOL scores, namely, the albumin level, preoperative anxiety/depression, absence of DM, cognitive function, and preoperative pain. Notably, preoperative pain was extracted as a significant predictor by both the EQ‐5D VAS and EORTC GHS scores. Pompili et al. also reported that preoperative pain was a significant predictor of QOL decline at three months after surgery using the SF‐36. 8 It is important that three different QOL measures found the same factor as a preoperative predictor. Moreover, our result could indicate preoperative pain as a predictor of QOL recovery at one year after the surgery. In this cohort, only 14 patients were recognized as symptomatic, and they mostly had respiratory symptoms (cough, sputum, and dyspnea). Only two patients had chest pain, and their preoperative computed tomography scans showed pleural involvement. However, most patients who rated their preoperative pain/discomfort as more than two (50/223, 22%) in the EQ‐5D (Table S3) and those whose preoperative symptom score of pain was more than 0 (56/206, 27%) in the EORTC QLQ‐C30 (Figure S2) were recognized as being asymptomatic. Taken together, their pain could be regarded as chronic pain, although we did not have data about the site of pain.
Our approach of using linear regression analysis to explore predictors was the first attempt in surgical patients with lung cancer. Using this approach, we did not need to define QOL decline/improvement. In two previous studies, the authors defined QOL decline and applied logistic regression analysis. One study defined QOL decline as a 10% reduction of the summary score, although the rationale for this was not described. 7 Another study defined QOL decline by using Cohen's effect size. An effect size greater than 0.8 was used to dichotomize the patients with or without large QOL decline. 8 Although there is no gold standard to interpret or determine change of the QOL score, further research is needed to clarify this issue. Therefore, we initiated another prospective study to determine the minimally important difference in change of the QOL score (UMIN000037864) in surgically treated patients with lung cancer.
Although we do not know whether these predictors can be improved by interventions, these predictors would be possible targets for patient/survivor care. There is no room for intervention for presence of comorbidity. However, for instance, mindfulness‐based cognitive therapy has been reported to be effective in patients with cancer 24 and with chronic pain. 25 Such a therapy could be tested in surgical patients with lung cancer.
The study was limited in certain aspects. First, it had a relatively small sample size and was single‐centered, thus limiting the applicability of findings in other centers. Second, according to the predetermined protocol, the thoracic surgeon responsible for the surgery handed all the printed questionnaires directly to the patients, and the survey was administered at Y1 just after patients were informed that there had been no findings indicative of relapse. Patients with relapse were excluded, and only patients followed up at our outpatient clinic were included. All these factors might have influenced the patients' responses resulting in better estimation of QOL than that of the actual patient's condition. If resources permit, the protocol should be improved in future to minimize possible external factors that would affect patients' responses. Third, our data lacked validation for extrapolation and generalization. Because QOL might vary in different clinical settings, cultural backgrounds, and public health insurance systems, predictors of postoperative QOL should be evaluated in each environment. However, the significant finding that the same predictor (preoperative pain) was identified in two different cultural backgrounds cannot be ignored. 8
In conclusion, in this prospective study, EQ‐5D VAS and EORTC GHS scores identified albumin level, preoperative anxiety/depression, cognitive function, and preoperative pain (assumed to be chronic pain) as some of the preoperative predictors of recovery of postoperative QOL. These predictors could be possible targets for improvement of patient/survivor care.
Supporting information
Figure S1. Bar plots showing the percentage distribution of respondents at each functional score of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire 30 items (QLQ‐C30).
Figure S2. Bar plots showing the percentage distribution proportion of respondents at each symptom score of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire 30 items (QLQ‐C30).
Table S1. Univariate logistic regression analyses for missing items on the EQ‐5D.
Table S2. Univariate logistic regression analyses for missing items on the EORTC QLQ‐C30.
Table S3. Distribution of EQ‐5D‐5L† dimensions responses at each assessment point.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.jp) for English language editing. This study was partly presented in 2020 at a Poster session of the 73rd Annual Scientific Meeting of the Japanese Association for Thoracic Surgery, convened as Web conference, Japan.
Ichimura H, Kobayashi K, Gosho M, et al. Preoperative predictors of restoration in quality of life after surgery for lung cancer. Thoracic Cancer. 2021;12:835–844. 10.1111/1759-7714.13819
REFERENCES
- 1. Vijayvergia N, Shah PC, Denlinger CS. Survivorship in non‐small cell lung cancer: challenges faced and steps forward. J Natl Compr Canc Netw. 2015;13:1151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poghosyan H, Sheldon LK, Leveille SG, Cooley ME. Health‐related quality of life after surgical treatment in patients with non‐small cell lung cancer: a systematic review. Lung Cancer. 2013;81:11–26. [DOI] [PubMed] [Google Scholar]
- 3. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video‐assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17:836–44. [DOI] [PubMed] [Google Scholar]
- 4. Ostroff JS, Krebs P, Coups EJ, Burkhalter JE, Feinstein MB, Steingart RM, et al. Health‐related quality of life among early‐stage, non‐small cell, lung cancer survivors. Lung Cancer. 2011;71:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balduyck B, Hendriks J, Lauwers P, Van Schil P. Quality of life after lung cancer surgery: a prospective pilot study comparing bronchial sleeve lobectomy with pneumonectomy. J Thorac Oncol. 2008;3:604–8. [DOI] [PubMed] [Google Scholar]
- 6. Barlési F, Doddoli C, Loundou A, Pillet E, Thomas P, Auquier P. Preoperative psychological global well‐being index (PGWBI) predicts postoperative quality of life for patients with non‐small cell lung cancer managed with thoracic surgery. Eur J Cardiothorac Surg. 2006;30:548–53. [DOI] [PubMed] [Google Scholar]
- 7. Möller A, Sartipy U. Predictors of postoperative quality of life after surgery for lung cancer. J Thorac Oncol. 2012;7:406–11. [DOI] [PubMed] [Google Scholar]
- 8. Pompili C, Brunelli A, Xiumé F, Refai M, Salati M, Sabbatini A. Predictors of postoperative decline in quality of life after major lung resections. Eur J Cardiothorac Surg. 2011;39:732–7. [DOI] [PubMed] [Google Scholar]
- 9. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 10. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- 12. Balduyck B, Hendriks J, Lauwers P, Sardari Nia P, Van Schil P. Quality of life evolution after lung cancer surgery in septuagenarians: a prospective study. Eur J Cardiothorac Surg. 2009;35:1070–5. [DOI] [PubMed] [Google Scholar]
- 13. Kenny PM, King MT, Viney RC, Boyer MJ, Pollicino CA, McLean JM, et al. Quality of life and survival in the 2 years after surgery for non‐small‐cell lung cancer. J Clin Oncol. 2008;26:233–41. [DOI] [PubMed] [Google Scholar]
- 14. Brunello A, Soccer L, Refax M, Salami M, Xiamen F, Sabbatini A. Quality of life before and after major lung resection for lung cancer: a prospective follow‐up analysis. Ann Thorac Surg. 2007;84:410–6. [DOI] [PubMed] [Google Scholar]
- 15. Suzuki K, Koike T, Asakawa T, Kusumoto M, Asamura H, Nagai K, et al. A prospective radiological study of thin‐section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol. 2011;6:751–6. [DOI] [PubMed] [Google Scholar]
- 16. Lüchtenborg M, Jakobsen E, Krasnik M, Linklater KM, Mellemgaard A, Møller H. The effect of comorbidity on stage‐specific survival in resected non‐small cell lung cancer patients. Eur J Cancer. 2012;48:3386–95. [DOI] [PubMed] [Google Scholar]
- 17. Sawabata N, Nagayasu T, Kadota Y, Goto T, Horio H, Mori T, et al. Risk assessment of lung resection for lung cancer according to pulmonary function: republication of systematic review and proposals by Guideline Committee of the Japanese Association for Chest Surgery 2014. Gen Thorac Cardiovasc Surg. 2015;63:14–21. [DOI] [PubMed] [Google Scholar]
- 18. Pompili C. Quality of life after lung resection for lung cancer. J Thorac Dis. 2015;7:S138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reck M, Brahmer J, Bennett B, Taylor F, Penrod JR, DeRosa M, et al. Evaluation of health‐related quality of life and symptoms in patients with advanced non‐squamous non‐small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur J Cancer. 2018;102:23–30. [DOI] [PubMed] [Google Scholar]
- 20. Bejjani J, Fiore JF Jr, Lee L, Kaneva P, Mata J, Ncuti A, et al. Validity of the EuroQol‐5 dimensions as a measure of recovery after pulmonary resection. J Surg Res. 2015;194:281–8. [DOI] [PubMed] [Google Scholar]
- 21. Pompili C, Koller M, Velikova G, Franks K, Absolom K, Callister M, et al. EORTC QLQ‐C30 summary score reliably detects changes in QoL three months after anatomic lung resection for Non‐Small Cell Lung Cancer (NSCLC). Lung Cancer. 2018;123:149–54. [DOI] [PubMed] [Google Scholar]
- 22. Friedrich M, Zenger M, Hinz A. Response shift effects of quality of life assessments in breast cancer survivors. Eur J Cancer Care (Engl). 2019;28:e12979. [DOI] [PubMed] [Google Scholar]
- 23. Morrill EF, Brewer NT, O'Neill SC, Lillie SE, Dees EC, Carey LA, et al. The interaction of post‐traumatic growth and post‐traumatic stress symptoms in predicting depressive symptoms and quality of life. Psychooncology. 2008;17:948–53. [DOI] [PubMed] [Google Scholar]
- 24. Compen F, Bisseling E, Schellekens M, Donders R, Carlson L, van der Lee M, et al. Face‐to‐face and internet‐based mindfulness‐based cognitive therapy compared with treatment as usual in reducing psychological distress in patients with cancer: a multicenter randomized controlled trial. J Clin Oncol. 2018;36:2413–21. [DOI] [PubMed] [Google Scholar]
- 25. la Cour P, Petersen M. Effects of mindfulness meditation on chronic pain: a randomized controlled trial. Pain Med. 2015;16:641–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Bar plots showing the percentage distribution of respondents at each functional score of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire 30 items (QLQ‐C30).
Figure S2. Bar plots showing the percentage distribution proportion of respondents at each symptom score of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire 30 items (QLQ‐C30).
Table S1. Univariate logistic regression analyses for missing items on the EQ‐5D.
Table S2. Univariate logistic regression analyses for missing items on the EORTC QLQ‐C30.
Table S3. Distribution of EQ‐5D‐5L† dimensions responses at each assessment point.


