Abstract
Cell suspension cultures have been studied for decades to produce natural molecules. However, the difficulty in generating stably transformed cell lines has limited their use to produce high value chemicals reproducibly and in elevated quantities.
In this protocol, a method to stably transform and maintain Arabidopsis cell suspension cultures is devised and presented in detail. Arabidopsis cell cultures were directly transformed with A. tumefaciens for the overexpression of the CORONATINE INSENSITIVE 1 (COI1) jasmonate receptor. Cell cultures were established after transformation and continuously maintained and tested for the overexpression of COI1. The protocol was also previously used to silence Arabidopsis peroxidases and allows for long term maintenance of transformed cells. Details on culture maintenance, both in liquid and solid media are provided, alongside with evidence of protein expression to confirm transformation.
The system described provides a powerful tool for synthetic biology to study signaling independent of developmental control and to obtain metabolites of interest for the biotechnological and medical sectors.
Keywords: Arabidopsis, Agrobacterium tumefaciens, Stable plant cell suspension culture, Transformation, Metabolites
Background
Plant cell suspension cultures provide a feasible alternative for the production of plant secondary metabolites and the study of dedifferentiated tissue (Wu and Ge, 2004; Lee et al., 2010 ). Interest in their use has been increasing for decades as they can offer continuous production systems for high-value biotechnological products for human use. There is a wide variety of cell cultures producing high amounts of secondary metabolites of commercial and industrial interest such as anthocyanins, betalains, flavourings, steviosides, among others (Rao and Ravishankar, 2002).
The lack of significant success for plant cell cultures in becoming regularly used for commercial purposes could be attributed in part to the failure of the dedifferentiated cell lines to produce the desired product with the same characteristics found in the organs of parent plants. The use of modern molecular biology and synthetic biology techniques opens the possibility to genetically improve cell lines and plants to enhance metabolite production (Davies and Deroles, 2014).
Methyl jasmonate (MeJA), jasmonyl-isoleucine (JA-Ile) and Jasmonic acid (JA) are collectively referred to as jasmonates (JAs), and they are involved in the regulation of many developmental processes and defense mechanisms in vascular plants ( Pauwels et al., 2008 , Pérez- Salamó et al., 2019 ). JA signaling involves ubiquitination of specific target proteins by the SCFCOI1 complex and their subsequent degradation by the 26S proteasome ( Thines et al., 2007 ).
Bömer et al. (2018) has reported the stably transformed Arabidopsis thaliana cell suspension cultures with an epitope-tagged COI1-overexpressing construct ( Devoto et al., 2002 ) to analyze the effects of JA signaling on cell growth and the production of cell wall proteins and metabolites. In that study, a stable transformation of Arabidopsis cell suspensions was successfully achieved and validated. Changes in the primary metabolism of the transformed cell suspensions were observed. The overexpression of COI1 reinforced its role in mediating defense responses after increasing levels of Oligogalacturonide oxidase 1, beta-Glucosidase/endoglucanases and Polygalacturonase inhibiting protein 2. Furthermore, it affected the availability of metabolites such as beta-alanine, threonic acid, putrescine, glucose and myo-inositol, providing a connection between JA-Inhibited growth and stress responses ( Bömer et al., 2018 ).
The protocol described here was initially devised by O’Brien et al. (2012) to silence Arabidopsis peroxidases adapting a transient transformation system from Ferrando et al. (2000) . The gene insertion was mapped by O’Brien et al. (2012) (two independent lines generated, each with a single insertion, thus homogenous cell cultures were obtained). The construct used in this work was prepared and used by Devoto et al. (2002) to transiently overexpress the CORONATINE INSENSITIVE 1 (COI1) JAs receptor (35S:: COI1::HiA). Stable transformation with the same construct was successfully achieved by Bömer et al. (2018) . Stably transformed Arabidopsis cell cultures, generated by using this protocol, have been maintained since O’Brien et al. (2012) and Bömer et al. (2018) were published. The fully detailed protocol is presented here.
Materials and Reagents
Petri Dishes (90 mm), Thermo ScientificTM SterilinTM Standard (Fisher Scientific, catalog number: 11389273)
FisherbrandTM L-Shaped Cell Spreaders (Fisher Scientific, catalog number: 15615467)
MicrospecTM Sterile Plastic Inoculation Loops (Fisher Scientific, catalog number: 15792105)
Micropore 1.25 cm x 10 cm tape rolls (Medical World Group, catalog number: MA1530-125)
15 ml sterile Falcon tube (Sarstedt, catalog number: 64554002)
50 ml sterile Falcon tubes (Sarstedt, catalog number: 62547004)
Spectrophotometer 10 x 10 x 45 cuvettes (Greiner Bio-One, catalog number: 61301)
200 ml glass Erlenmeyer flask covered with doubly folded aluminium cap
200 µl yellow, ultra-point graduated tip (StarLab, catalog number: S1113-1006)
1,000 µl blue, ultra-point graduated tip (StarLab, catalog number: S1111-6001)
45 µm sterile disposable filters (Sarstedt, catalog number: 831826)
5 ml sterile disposable syringe (BD Emerald, catalog number: 307731)
1.5 ml Eppendorf microfuge tubes (Greiner Bio-One, catalog number: 616 201)
Light tubing (Polylux XL, catalog number: F30W/840)
Arabidopsis ecotype Landsberg erecta (Ler) cell suspension cultures (obtained originally from the laboratory of the late Prof Tony Slabas and now maintained by the Devoto laboratory at RHUL and by Dr Steve Chivasa laboratory, University of Durham)
Agrobacterium tumefaciens GV3101 containing vector pBin19PLUS (Van Engelen et al., 1995 ) with the intron-tagged COI1::HiA construct ( Devoto et al., 2002 )
Tryptone. Peptone from casein (Merck, catalog number: VM944113 749)
Yeast extract (VWR, catalog number: 1.03753.0500)
CaCl2 (Sigma, catalog number: C1016-100G)
NaOH (Sigma, catalog number: 221465-500G)
Agar-agar ultrapure (VWR, catalog number: 1.01613.1000)
Phyto-agar (Melford, catalog number: P1003)
Gentamicin sulfate salt (Sigma, catalog number: G1264-250MG)
Kanamycin sulphate from Streptomyces kanamyceticus (Sigma, catalog number: K1377)
Rifampicin (Sigma, catalog number: R8626-1G)
Timentin [Ticarcillin/Potassium Clavulanate Mixture 15:1 Ratio], 5 Grams (Melford Laboratories Ltd., catalog number: T36000-5.0)
LS Media & Vitamins (Duchefa, catalog number: L0230.0050)
Sucrose (Sigma, catalog number: 84100)
Naphtalene acetic acid (Sigma, catalog number: N1641)
Kinetin (Sigma, catalog number: K3253)
96-99% Ethanol (VWR, catalog number: 20821.330)
LS media (see Recipes)
TY media and agar (see Recipes)
1 M NaOH (see Recipes)
Gentamicin 50 mg/ml (see Recipes)
Kanamycin 50 mg/ml (see Recipes)
Rifampicin 25 mg/ml (see Recipes)
Timentin 160 mg/ml (see Recipes)
Equipment
200 µl Micropipette (Gilson)
1,000 µl Micropipette (Gilson)
10 ml beaker
Erlenmeyer flasks
Magnetic stirring bar
Plant Shaker Incubator (Bottmingen Infurs, model: CH-4103)
Plant Incubator (Sanyo, model: MLR-352-PE)
Bacterial Shaker Incubator (New Brunswick Scientific, model: Innova 4000)
Bacterial Incubator (Memmert)
Biophotometer (Eppendorf, model: 6131 01139)
Centrifuge (Eppendorf, model: 5810R)
Vortex (Spinmix, model: SGP-205-010X)
Laminar flow hood (Bassaire, model: AH191)
pH meter (HANNA Instruments, model: pH210)
Magnetic stirrer (Nickel Electro Ltd., model: MSU-1)
Procedure
-
Transformation and establishment of cell suspension cultures
-
Arabidopsis Ler suspension cultures (see Materials and Reagents for source details) are maintained at 22 °C in constant light (80 μmol m-2s-1 intensity in constant agitation (100 rpm) in LS media and sub-cultured every 7 days as described in Section B.
Notes:
All instruments should be kept clean and sterile to minimize cross contamination.
All steps listed in the current protocol are performed under the laminar flow hood to maintain aseptic conditions throughout the process.
-
Streak on TY media agar plates containing the appropriate antibiotics the A. tumefaciens GV3101 strain containing the pBin19PLUS vector (Van Engelen et al., 1995 ) with the gene (or construct) of interest (Figure 1A). In this case, the intron-tagged COI1::HiA construct was used ( Devoto et al., 2002 ; Bömer et al., 2018 ) with the following antibiotics:
Gentamycin (50 µg/ml).
Rifampicin (25 µg/ml).
Kanamycin (50 µg/ml) for plasmid selection.
Incubate the plates at 28 °C for 2-3 days by placing them lid down in the incubator, to avoid condensation.
-
Streak one single colony of A. tumefaciens from plate and grow for ~48 h in shaker incubator (28 °C 180 rpm in the dark) in 50 ml sterile Falcon tubes containing 7 ml TY media containing:
Gentamycin (50 µg/ml).
Rifampicin (25 µg/ml).
Kanamycin (50 µg/ml) for plasmid selection.
After 48 h or when O.D.600 nm = 0.8-1.0, take 1 ml of the Agrobacterium bacterial suspension, and add it to a 200 ml Erlenmeyer flask, in a final volume of 50 ml of TY media added with antibiotics as in Step A2 (Figure 1A).
-
When O.D.600 nm = 0.8-1.0 (after ~12 h), the bacterial pellet is recovered by transferring the culture from the Erlenmeyer flask into 50 ml falcon tubes, centrifugation (3,100 x g for 10 min at room temperature) and resuspension in 5 ml sterile fresh TY media. This culture is ready for infection of Arabidopsis cells.
Note: After 12 h of culture, measure O.D.600 nm every 1-2 h, depending on the rate of growth of A. tumefaciens. Bacteria is ready for infection when O.D.600 nm = 0.8-1.0 (O’Brien et al., 2012).
-
On the same day of infection, fresh Arabidopsis cells must be sub-cultured in new Erlenmeyer flasks (Figure 1B).
To a previously sterilized 200 ml Erlenmeyer flask containing 45 ml of fresh LS media.
Add 5 ml of a 6-7 days old homogeneous Ler cell suspension culture (Figure 1E). Density is measured using packed cell volume (PCV) as described by Bömer et al.(2018 ) and detailed at Note 5.
Keep it shaking at 100 rpm, 22 °C, continuous light regime 80 μmol m-2s-1 intensity, until transformation is performed.
Approximately 1 x 1010 (2 ml) A. tumefaciens cells should be added to 50 ml of a freshly sub-cultured Arabidopsis Ler (WT) cell cultures.
Immediately after adding A. tumefaciens containing the plasmid of interest, suspensions should be left to stand in the dark with no agitation, for about 2 h.
Afterwards, Erlenmeyer flasks containing transformed cell cultures, referred to as COV (COI1 over expressor), must be kept in the dark under constant agitation 100 rpm at 22 °C.
Three days after cocultivation, remove COV cell suspensions from the shaker for 5-10 min to allow the cells to sediment or spin them down at room temperature, 18 x g (800-1,000 rpm) in 50 ml Falcon tubes, for 3-5 min.
Remove the old media and add fresh sterile LS media to the sedimented infected cells for a total volume of 50 ml.
-
Add the following concentration of antibiotics on every subculture, for selection and to eliminate A. tumefaciens from cultures. Keep the cells at 100 rpm, 22 °C, continuous light regime 80 μmol m-2s-1 intensity.
Kanamycin (50 µg/ml) for plasmid selection.
Timentin (200 µg/ml).
To maintain stable COV cell lines, 15 ml of COV cells should be sub-cultured weekly in 35 ml fresh sterile LS media for a total volume of 50 ml, containing the same final concentration of antibiotics as in Step A13.
-
Once the cells are stabilized and reach homogeneous consistency (Figures 1E and 2A, see also Note 5) the culture volume is gradually increased to 100 ml (the timeline to increase is between 1 or 2 months, depending on the growth rate of the culture, generally in incremental steps of 15 ml (e.g., from 50 ml to 65 ml to 80 ml, up to 100 ml).
Micropipette: view image, Falcon tube: https://contacts.google.com/?hl=en&tab=iC&authuser=0
-
-
Calli induction for establishment of single colony cell suspension cultures
-
From a 6-7 old days stably transformed cell suspension (Figures 1E and 2A), 1-2 ml of dense culture (density evaluated by PCV, as described by Bömer et al. (2018) and detailed at Note 5 and Figure 2B) are spread evenly onto LS-agar media plates, where cell aggregates formed from single transformed cells, will be able to grow, with antibiotics as follows:
Kanamycin (50 µg/ml) for plasmid selection.
Timentin (200 µg/ml).
Seal the edge of plates with micropore tape.
Place the plates under normal growth conditions: 22 °C, in a continuous light regime 80 μmol m-2s-1 intensity.
Calli will grow to confluence within 3-4 weeks (Figure 2C), and transfer to new plates should be done periodically, every 3-4 weeks depending on the callus.
After 4-5 cycles, when calli have been continuously sub-cultured and maintained, pick a subset of cells ‘microcolony’; (~5 mm diameter) from the established callus (Figure 2C) to obtain stable transformed cell cultures coming from a reduced number of cells to reduce heterogeneity.
Place microcolonies to fresh LS-agar media and grow them following the same parameters and conditions as in Steps B3 and B4.
After 4-5 cycles, transfer a microcolony from an established plate to a new Erlenmeyer flask with fresh sterile LS media, and keep at 100 rpm, 22 °C, continuous light regime 80 μmol m-2s-1 intensity.
Notes:
Restart the culture from a callus microcolony in a smaller volume of LS media, e.g., 35 ml. The calli will start proliferating after ~6-7 days. This will become apparent as portions of the friable callus mass will start detaching from the original one and producing new independent callus mass (Figure 2A). After ~3-4 weeks, the culture will reach confluence and density for subculturing as described at Section C and Note 5.
The callus stage is necessary to select microcolonies to make the cultures genetically homogeneous and it is advantageous, in the long term, as it reduces liquid culture handling providing longer intervals to subculturing.
-
-
Sub-culturing of Arabidopsis cell suspension
Remove aluminum foil cap from a 200 ml glass Erlenmeyer flask (Flask 1) containing 85 ml of fresh sterile LS media and from flask containing 7 days old cell culture (Flask 2).
Take 15 ml of cells from flask 2 and add to flask 1 containing fresh media.
If appropriate, add antibiotics (Step A13).
Close with foil cap.
Place cell suspensions back on to shaker.
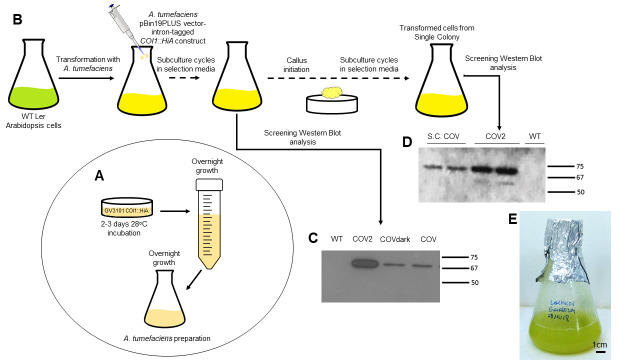
Figure 1. Experimental scheme of the direct stable transformation of Arabidopsis Ler cell suspension cultures.
A. A. tumefaciens containing the plasmid of interest is streaked on selection media for 2-3 days and then grown overnight. A final 50 ml culture is inoculated with 1 ml of the overnight culture and used to inoculate the freshly sub-cultured Arabidopsis cells (see Procedure Steps A2-A5). B. Diagrammatic chronological representation of cell culture transformation starting with the freshly sub-cultured cells ready to be transformed. Transformation with A. tumefaciens containing vector pBin19PLUS with the intron-tagged COI1::HA construct is then performed and subsequent sub-culturing cycles performed. When the cell culture is homogeneous, calli induction is conducted. After several sub-culturing cycles calli microcolonies are isolated to reduce heterogeneity and inoculated in fresh sterile LS media (see Procedure Steps A7-A15 and B1-B7). C and D. Screening of transformed cells by SDS-Page and Western Blot analyses. C. The expression of the tagged COI1 protein (COI1::HiA) grown in light (COV and COV2, two independently transformed cell lines) and dark (COV dark) conditions is detected, to confirm the occurrence of transformation (~69 kDa). Note the absence of the tagged protein in wild-type (WT) cells. Different COI protein expression levels can be observed in COV and COV2. D. Screening of transformed cell cultures expressing COI1::HiA, originating from selected calli microcolonies (S.C: selected microcolony). E. Stabilized cell culture ~7 days after subculture (DAS).
Figure 2. Cell cultures and calli growth.

A. Propagation of cell culture from callus microcolonies during time. The cells in suspension cultures grow initially as small aggregates. Then, due to the agitation the aggregate splits giving a homogeneous culture. Left, Centre and Right panels: ~1, 2, 3-4 weeks; refer to Section B Notes for a detailed description. B. Established, confluent callus grown on agar plate after 3-4 weeks. C. Packed cell volume (PCV) of cell culture at day 0 (right) and 7 (left).
Notes
Sterilization of Erlenmeyer flasks containing media is performed at 15 psi and 121 °C for 15 min in the autoclave.
Micropipette tips used in this protocol must be clean and sterile in all steps.
200 ml Erlenmeyer flasks should be sterilized before performing the sub culturing, containing 45 ml of the LS media already prepared and with pH adjusted to 5.7-5.8. A doubly folded foil aluminum cap was used to cover the Erlenmeyer flasks opening and neck. All work was performed under a laminar flow hood.
After a few weeks the cells can be sub-cultured as described in Step A7 and Section C to grow under continuous light regime 80 μmol m-2s-1 intensity, 22 °C and 100 rpm.
Packed cell volume (PCV) is used to evaluate appropriate cell density (Figure 2B). To determine PCV and gather information on the growth rate of the cell culture: 1). Take 8 ml of cell culture (at 0 and 7 days after sub-culture (DAS) and transfer it to a 15 ml sterile Falcon tube; 2). Let the cells sediment in stationary conditions at room temperature for 1 h; 3). Determine the volume before and after sedimentation. Typically, at 7 DAS, the PCV of a WT cell suspension culture (from 8 ml) will be ~2.5 ml or 30% of the culture volume.
WT cell suspension cultures are maintained in 200 ml Erlenmeyer flasks containing 85 ml of sterile LS media (see Note 1 for specific conditions) with pH adjusted to 5.7-5.8. A doubly folded foil aluminium cap was used to cover the Erlenmeyer flasks opening neck. 15 ml of a 6-7days old homogeneous Ler cell suspension culture is added and kept shaking at 100 rpm, 22 °C, continuous light regime 80 μmol m-2s-1 intensity and 100 rpm.
Normal growth conditions: both cell suspension cultures and plated calli are maintained at 22 °C and continuous light regime 80 μmol m-2s-1 intensity and continuous shaking (100 rpm). However, for other research purposes, both the cell cultures and plates with calli were grown under dark conditions.
Confirmation of transformation was performed by SDS-Page and Western blot as described by Devoto et al. (2002) .
Recipes
-
LS media
4.43 g LS Media & Vitamins
30 g sucrose
Dissolve in 900 ml water
Add 500 µl naphthalene acetic acid
Add 50 µl 1 mg/ml kinetin
Adjust pH 5.7-5.8 with 1 M NaOH
Top up to 1 L with ddH2O
If needed, add 1 g/100 ml of Phyto-agar
-
TY media and agar
5 g Tryptone
3 g Yeast extract
0.671 g CaCl2
Top volume up to 1 L with ddH2O
If needed, add 9 g of Agar-agar/L
-
1 M NaOH
2 g NaOH
50 ml with ddH2O
-
Gentamicin 50 mg/ml
500 mg of Gentamicin in a 15 ml sterile Falcon tube
Add 10 ml of ddH2O
Vortex vigorously until solution looks clear and transparent
In the laminar flow hood pour Gentamicin solution in a 10 ml beaker
Using a 5 ml sterile disposable syringe, suck the Gentamicin solution
Place a 45 µm sterile disposable filter and pour 1 ml into 10, 1.5 ml Eppendorf microfuge tubes.
Keep the microfuge tubes containing the Gentamicin solution at -20 °C. Avoid defrosting it more than 2 times
-
Kanamycin 50 mg/ml
500 mg of Kanamycin in a 15 ml sterile Falcon tube
Add 10 ml of ddH2O
Vortex vigorously until solution looks clear and transparent
In the laminar flow hood pour Kanamycin solution in a 10 ml beaker
Using a 5 ml sterile disposable syringe, suck the Kanamycin solution
Place a 45 µm sterile disposable filter and pour 1 ml into 10, 1.5 ml Eppendorf microfuge tubes
Keep the microfuge tubes containing the Kanamycin solution at -20 °C. Avoid defrosting it more than 2 times
-
Rifampicin 25 mg/ml
250 mg of Rifampicin in a 15 ml sterile Falcon tube
Add 10 ml of 96-99% Ethanol
Vortex vigorously until lumps are completely dissolved and color of solution looks uniform
In the laminar flow hood pour Rifampicin solution in a 10 ml beaker
Using a 5 ml sterile disposable syringe, suck the Rifampicin solution
Place a 45 µm sterile disposable filter and pour 1 ml into 10, 1.5 ml Eppendorf microfuge tubes
Keep the microfuge tubes containing the Rifampicin solution at -20 °C. Avoid defrosting it more than 2 times
-
Timentin 160 mg/ml
1.600 g of Timentin in a 15 ml sterile Falcon tube
Add 10 ml of ddH2O
Vortex vigorously until lumps are completely dissolved and color of solution looks clear and uniform
In the laminar flow hood pour Timentin solution in a 10 ml beaker
Using a 5 ml sterile disposable syringe, suck the Timentin solution
Place a 45 µm sterile disposable filter and pour 1 ml into 10, 1.5 ml Eppendorf microfuge tubes
Keep the microfuge tubes containing the Timentin solution at -20 °C. Avoid defrosting it more than 2 times
Acknowledgments
Kel Liu is acknowledged for technical help with cell culture and calli maintenance and photographs. This work was supported by BBSRC (BB/E003486/1) to A.D; E.D was supported by CONACyT (the Mexican Council of Science and Technology, scholarship No. 472007); J.A.O was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) Nº 1181358 and BBSRC grant BB/E021166; I.P.S. was supported by WestFocus PARK SEED FUND INVESTMENT AWARD and H2020-MSCA-IF-2015 #705427; J.K. was supported by a BBSRC DTP Studentship (BB/J014574/1 – 100609005) to RHUL, M.B. was supported by SWAN (South West London Alliance Network) grant to A.D.
Authors contributions:
A.D. Original idea conception, designed the research, funding acquisition, performed the research, analyzed the data, wrote, and edited the paper.
J.A.O Original idea conception, designed the research, performed the research, analysed the data, edited the paper.
E.D performed the research, analyzed the data, wrote, and edited the paper.
I.P.S, J.K., M.B. performed the research, analyzed the data, edited the paper.
Competing interests
No financial, personal, or professional interests have influenced the work.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Bömer M., O'Brien J. A., Perez-Salamo I., Krasauskas J., Finch P., Briones A., Daudi A., Souda P., Tsui T. L., Whitelegge J. P., Paul Bolwell G. and Devoto A.(2018). COI1-dependent jasmonate signalling affects growth, metabolite production and cell wall protein composition in Arabidopsis . Ann Bot 122(7): 1117-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies K. M. and Deroles S. C.(2014). Prospects for the use of plant cell cultures in food biotechnology. Curr Opin Biotechnol 26: 133-140. [DOI] [PubMed] [Google Scholar]
- 3. Devoto A., Nieto-Rostro M., Xie D., Ellis C., Harmston R., Patrick E., Davis J., Sherratt L., Coleman M. and Turner J. G.(2002). COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis . Plant J 32(4): 457-466. [DOI] [PubMed] [Google Scholar]
- 4. Ferrando A., Farras R., Jasik J., Schell J. and Koncz C.(2000). Intron-tagged epitope: a tool for facile detection and purification of proteins expressed in Agrobacterium-transformed plant cells . Plant J 22(6): 553-560. [DOI] [PubMed] [Google Scholar]
- 5. Lee E. K., Jin Y. W., Park J. H., Yoo Y. M., Hong S. M., Amir R., Yan Z., Kwon E., Elfick A., Tomlinson S., Halbritter F., Waibel T., Yun B. W. and Loake G. J.(2010). Cultured cambial meristematic cells as a source of plant natural products. Nat Biotechnol 28(11): 1213-1217. [DOI] [PubMed] [Google Scholar]
- 6. O'Brien J. A., Daudi A., Finch P., Butt V. S., Whitelegge J. P., Souda P., Ausubel F. M. and Bolwell G. P.(2012). A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense . Plant Physiol 158(4): 2013-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pauwels L., Morreel K., De Witte E., Lammertyn F., Van Montagu M., Boerjan W., Inze D. and Goossens A.(2008). Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells . Proc Natl Acad Sci U S A 105(4): 1380-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pérez-Salamó I., Krasauskas J., Gates S., Díaz-Sánchez E. K. and Devoto A.(2019). An Update on Core Jasmonate Signalling Networks, Physiological Scenarios, and Health Applications. Ann Plant Review Online 2: 1-65. [Google Scholar]
- 9. Rao S. R. and Ravishankar G. A.(2002). Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol Adv 20(2): 101-153. [DOI] [PubMed] [Google Scholar]
- 10. Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S. Y., Howe G. A. and Browse J.(2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448(7154): 661-665. [DOI] [PubMed] [Google Scholar]
- 11. Van Engelen F. A., Molthoff J. W., Conner A. J., Nap J. P., Pereira A. and Stiekema W. J.(1995). pBINPLUS: an improved plant transformation vector based on pBIN19. Transgenic Res 4(4): 288-290. [DOI] [PubMed] [Google Scholar]
- 12. Wu J. and Ge X.(2004). Oxidative burst, jasmonic acid biosynthesis, and taxol production induced by low-energy ultrasound in Taxus chinensis cell suspension cultures. Biotechnol Bioeng 85(7): 714-721. [DOI] [PubMed] [Google Scholar]