Abstract
The survival of liver transplantation (LT) recipients has been improved remarkably in short-term. The major causes of mortality in long-term include nonimmunological causes such as cardiovascular, de novo malignancy, chronic kidney disease, and recurrence of primary disease. Rejection-related mortality is rare in the long-term after LT. We discuss nonrejection causes of long-term morbidity/mortality, risk factors, and management strategies in LT recipients. In addition, we discuss osteoporosis, contraception, and pregnancy in LT recipients.
Keywords: cardiovascular disease, de novo malignancy, osteoporosis, pregnancy, recurrence
Abbreviations: AIH, autoimmune hepatitis; BMI, body mass index; CKD, chronic kidney disease; CNI, calcineurin inhibitors; CVD, cardiovascular disease; DDLT, deceased donor liver transplantation; DM, diabetes mellitus; DNM, de novo malignancy; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; HR, hazard ratio; IUCD, Intrauterine contraceptive devices; LDLT, living donor liver transplantation; LT, liver transplantation; MS, metabolic syndrome; MDRD, Modification of Diet in Renal Disease; MMF, mycophenolate; mTORi, Mammalian target of rapamycin inhibitors; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OR, odds ratio; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; PTDM, posttransplantation diabetes mellitus; PTMS, posttransplantation metabolic syndrome; SVR, sustained virological response
The main causes of long-term mortality in liver transplantation (LT) recipients are nonimmunological. The four main categories of long-term morbidity/mortality are cardiovascular, de novo malignancies (DNMs), chronic kidney disease (CKD), and recurrence if pretransplantation disease. Some of risk factors of these issues are modifiable. It is important to identify patients at risk early, so that prevention can be attempted. Osteoporosis and contraception/pregnancy are not related to mortality but are important issues for well-being. We discuss these issues, risk factors, and management strategies in the current review.
Cardiovascular diseases
Although cardiovascular diseases (CVDs) during perioperative or early postoperative period are mainly due to pretransplantation risk factors and/or perioperative complications, CVD events long-term after LT are related to posttransplantation metabolic syndrome (PTMS). CVD is one of major causes of morbidity and mortality after LT.1,2 In a study of 4483 adult primary LT recipients (the United Kingdom transplant database) surviving 1 year or more, cardiac disease contributed to 8.7% of deaths.2 A systemic review of 29 studies (n = 57,493) patients showed that incidence rates of cardiovascular outcomes varied from 1% to 41% at 6 months (or shorter) and 0%–31% for outcomes at > 6 months. Multivariate analyses showed that older age and history of cardiac disease were the most consistent predictors of cardiovascular events after transplantation. However, definitions of cardiovascular outcomes were highly inconsistent across the studies.3 Another meta-analysis of 12 observational studies (n = 4792) with 28,783 person-years follow-up showed that 10-year risk of developing CVD events was 13.6% (pooled estimates). This risk was 4 times more in patients with metabolic syndrome (MS).4 Khurmi et al5 analyzed data from 2002 to 2011 from USA. The authors looked for admissions due to myocardial infarction, stroke, congestive heart failure, dysrhythmias, cardiac arrest, or malignant hypertension. CVD-related hospitalizations increased by 115% in the later period. The authors noted that cerebrovascular accident and myocardial infarction declined over time and congestive heart failure and dysrhythmia increased. A total of 19% of hospitalizations had multiple CVD diagnoses. Fussner et al6 defined CVD as coronary artery disease (clinical diagnosis of angina, or atherosclerotic stenosis >50% in 1 or more major coronary arteries, or myocardial infarction), symptomatic peripheral vascular disease, congestive heart failure, stroke/transient ischemic attack, arrhythmias, and cardiac arrest. The authors retrospectively reviewed details of 455 consecutive LT recipients with 8–12 years of follow-up. There was increase in obesity (23.8% at 4 months to 40.8% at 3 years) and body mass index (BMI) which predicted MS at 1 year after LT. CVD developed in 10.6% at 1-year, 20.7% at 5-years, and 30.3% at 8 years. Age (hazard ratio [HR] per year: 1.03), diabetes (HR: 1.78), prior history of CVD (HR: 2.46), and serum troponin >0.07 ng/mL (HR: 1.98) were independently associated with CVD in the long-term. Presence of renal disease at the time of LT is a risk factor for post-LT CVD-related and all-cause mortality.7 Apart from coronary artery disease, atrial fibrillation also influences mortality after LT. Chokesuwattanaskul et al8 showed that incidence of preexisting atrial fibrillation LT recipients was 5.4%, pooled estimated incidence of atrial fibrillation after LT was 8.5%. Some of the studies in this meta-analysis have shown increased risk of graft failure and mortality in patients with atrial fibrillation.
This increase incidence of CVD is related to increased incidence/prevalence of PTMS. MS is defined as the presence of 3 or more of the following: obesity, (BMI ≥ 30 kg/m2 as surrogate for waist circumference or waist circumference >102 cm (90 cm for Asians) in men and >88 cm (>80 cm in Asians) in women, fasting plasma glucose ≥ 100 mg/dL (or on treatment of diabetes), blood pressure ≥ 130/85 mm Hg (or on treatment of hypertension), serum triglycerides ≥ 150 mg/mL (or on treatment), serum high-density lipoprotein <40 mg/dL in men and <50 mg/dL in women.9,10 The prevalence of posttransplantation MS is 40–60% in LT recipients.11, 12, 13, 14 Laish et al11 noted that MS was more than twice in LT recipients than that reported for the general population. MS was present in 5.4% before LT, which increased to 51.9% after LT. The presence of MS associated with cardiovascular morbidity but not mortality.
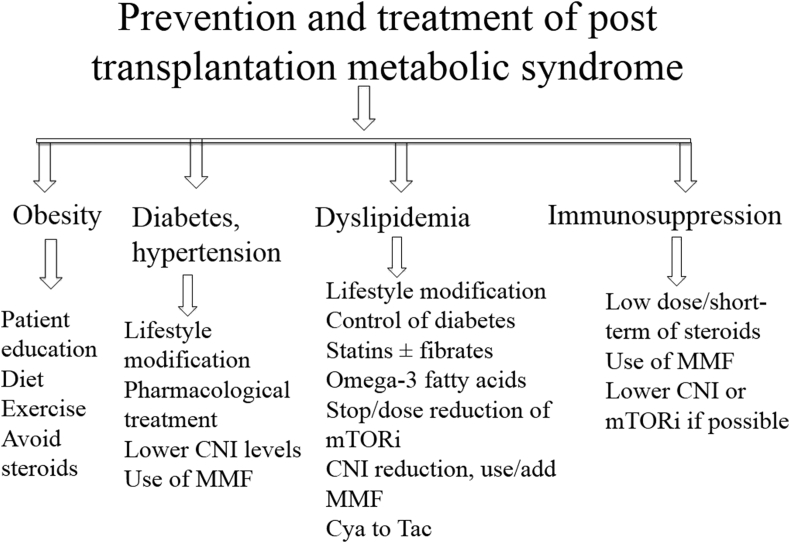
A study from our center at North India showed a 53% prevalence of PTMS. There was a significant rise of BMI and triglycerides after LT, prevalence of hypertension increased from 18% to 39% and prevalence of diabetes increased from 20% to 56% after transplantation.13 Anastácio et al14 evaluated 117 LT recipients at a median period of 3 years and again at a median of 7 years (range: 3–17 years) after LT. The prevalence of MS increased over the years (by international diabetes federation definition, 43.1–53.3%, P = 0.12; and by national cholesterol education program, 34.3–44.8%, P = 0.03). Blood glucose, prevalence of glucose intolerance, waist circumference, and body fat also increased with time. The MS components were associated (P < 0.05) with greater age, family history of diabetes, current and previous (at time of LT) BMI, body fat, corticosteroid use, lack of exercise, as well as greater carbohydrate and fat intake. It is important to note that some of these risk factors are modifiable. Lareya et al12 showed 30% of recipients with PTMS had major cardiovascular events which was significantly higher than recipients without PTMS (8%). Prevention/treatment strategies of PTMS are shown in Figure 1.
Figure 1.
Prevention and treatment strategies of posttransplantation metabolic syndrome. CNI, calcineurin inhibitors; Cya, cyclosporine; MMF, mycophenolate; mTORi, mammalian target of rapamycin inhibitor; Tac, tacrolimus.
Individual components of PTMS
Obesity
Approximately 30–70% of recipients become obese or overweight and most of weight gain happens in first year after LT. Patients older than 50 years and with history of obesity before LT remain at higher risk of obesity. The main causes of this weight gain after LT include effect of steroids, improved appetite due to improvement of chronic disease (cirrhosis), and reversal of the catabolic state of cirrhosis.15 Nair et al16 analyzed UNOS (United Network for Organ Sharing) database and found that 5-year mortality was significantly higher in severe and morbid obese patients, mostly owing to adverse cardiovascular events. Pre-LT obesity is a risk factor for PTMS and diabetes after LT.17,18 Generally patients who are overweight or obese before LT remain so after LT also, approximately one-third of patients with normal BMI before LT become obese after LT. Obesity is present in 20–68% of LT recipient older patients (age > 50 years), pre-LT obesity, and use of high-dose steroids are risk factors for post-LT obesity.15 LT recipients should be educated regarding weight control via diet and exercise, and high doses or prolonged course of steroids should be avoided if feasible in given patients.15 There is not much data on management of post-LT obesity by bariatric surgery. We suggest a low calorie (500 KCal less than required) diet and 30 min brisk walk daily to patients with posttransplantation obesity/MS.
Diabetes
An international consensus meeting on posttransplantation diabetes mellitus (PTDM) suggested use of the term PTDM in place of new-onset diabetes after transplantation as diabetes is often unrecognized before transplantation. Hyperglycemia is very common in the early period and can occur due to immunosuppression (treatment of rejection in particularly), infections, and other critical conditions.19 Posttransplant hyperglycemia is a risk factor for subsequent PTDM. Other risk factors for PTDM include age, family history of DM, immunosuppression (steroids, tacrolimus), pretransplantation or posttransplantation MS, genetic polymorphisms, preoperative fasting plasma glucose levels, and donor liver steatosis.20, 21, 22, 23, 24, 25, 26, 27 Several studies have shown poor survival in patients with PTDM. Moon et al28 compared 4 groups: pre-LT DM (n = 159), sustained PTDM (equal or more than 6 months, n = 284), transitory PTDM (>1 to 6 months, n = 108), and no DM (n = 227). Patients who had PTDM <1 month due to high-dose steroid were considered as normal. The sustained PTDM group had the worst survival at a median follow-up of 57.2 months, related to infection, chronic rejection, and late onset hepatic artery thrombosis. In another study of 438 patients with PTDM, the authors showed incidence of PTDM as 44.24%, 25.59%, 23.08%, 25.17%, 17.86%, and 18.18%, at 3, 6, 9, 12, 36, and 60 months after LT. The survival was better in non-PTDM group; pre- and/or postoperative fasting plasma glucose levels, tumor recurrence or metastasis, and renal insufficiency were independent risk factors of mortality.27 In SRTR database analysis of 85,194 adult LT, 11.2% had history of pretransplantation DM. In multivariate survival analysis of at least 5 years of cohort follow-up (n = 35,870), independent predictors of mortality included presence of pretransplantation diabetes, PTDM, and donor's history of DM.29 Roccaro et al30 compared incidence of major CV events among patients (n = 994) without DM (39%), with pre-LT DM (24%), post-LT transient DM (16%), and PTDM (20%). Twelve percent of patients experienced major CV event. The presence of sustained PTDM was the only state associated with a significantly increased risk of major cardiovascular events after adjustment of other factors. Patients in sustained PTDM group had a 13% and 27% cumulative incidence of major CV event at 5 and 10 years, respectively. Neal et al31 compared data of 25 LT recipients with stable graft function and conversion of cyclosporine to tacrolimus therapy with a median follow-up of 8 months. The switching from cyclosporine to tacrolimus lead to reduce blood pressure, serum cholesterol, and weight.
Dyslipidemia After LT
Causes of dyslipidemia after LT include the following: genetic predisposition, age, excessive dietary intake of carbohydrates, cholesterol, and saturated fat, weight gain, use of diuretics/beta blockers, use of immunosuppression (cyclosporine, sirolimus, everolimus, steroids, Tacrolimus [possibly]).32 Immunosuppression-related insulin resistance (table 1) leads to more lipolysis from adipose tissue, which leads to more delivery of free fatty acids to the liver. Sirolimus alters insulin signaling causing insulin resistance.33 The free fatty acids are the main substrate for very low-density lipoprotein synthesis. There is an increased conversion of very low-density lipoprotein (VLDL) to low-density lipoprotein(LDL) cholesterol, leading to a rise in LDL levels. In addition steroids increase the activity of 3-hydroxy-3-methylglutaryl coenzyme A, which is the rate-limiting step in the cholesterol synthesis pathway. Cyclosporine interferes with the binding of LDL to the LDL receptor, leading to rise of LDL levels. Cyclosporine also interferes with the enzyme 26 hydroxylase, leading to decreased bile acid synthesis. This also leads to LDL receptor downregulation.34,35
Table 1.
| Class | Adverse events |
|---|---|
| CNIs | Nephrotoxicity, neurotoxicity, diabetes, hyperkalemia, metabolic acidosis, hypertension, hyperlipidemia, hypertension, metabolic syndrome Gingival hyperplasia and hypertrichosis (only with Cyclosporine) |
| MMF | Myelosuppression, gastrointestinal side effects, viral infections (CMV: Cytomegalovirus, HSV: Herpes Simplex Virus), spontaneous abortions in pregnant women |
| Sirolimus | Hyperlipidemia, metabolic syndrome, myelosuppression, proteinuria, poor wound healing, pneumonitis, skin rash |
| Corticosteroids | Diabetes, metabolic syndrome, hypertension, obesity, osteoporosis, avascular necrosis, growth retardation, cushingoid features, psychosis, poor wound healing, adrenal suppression, cataract |
Hypertension
Arterial hypertension occurs due to effect of immunosuppressive medications. Calcineurin inhibitors (CNIs) and corticosteroids are the most strongly implicated, and recent studies have also shown that use of sirolimus is related to post-LT hypertension.15,36,37 CNI cause hypertension via widespread arterial vasoconstriction that increases systemic vascular resistance. The vasoconstriction in the kidney promotes sodium reabsorption and volume expansion.15,38 Post-LT hypertension may be transient (in initial months) or sustained. Two recent studies have shown incidence of HTN as 49% and 53%.36,37 Di Stefano et al36 found that incidence of hypertension increased from 15% in pre-LT to 53% in the post-LT period. The development of sustained hypertension was related to use of mammalian target of rapamycin inhibitors (mTORis), (odds ratio [ORs], 4.02), alcoholic cirrhosis before LT (OR: 3.38), and new-onset hepatic steatosis after LT (OR: 2.13). In a study by Tong et al37, pre-LT systolic blood pressure (OR: 1.04) and use of mTORi (OR: 4.08) predicted hypertension.
Table 1 (references 38,39) and table 2 (references 40, 41, 42) show role of immunosuppression and management of MS-related conditions. The salient features of CVD and PTMS are shown in Text Box 1.
Table 2.
| Parameter | Management | IS modification |
|---|---|---|
| Low-density lipoprotein >100 mg/dL | Life style modification, diet changes Statins ± addition of Ezetimibe if not controlled by diet and lifestyle |
Cyclosporine conversion to Tacrolimus Calcineurin inhibitors reduction and addition of Mycophenolate mTORi discontinuation (weak recommendations) |
| Higher triglycerides | Fibric acid derivatives, fish oils | |
| Diabetes mellitus | Target HbA1c < 7% by lifestyle changes and medications, insulin is preferred when patient is on high doses of steroids | Steroid short-term only/avoidance Tacrolimus to Cyclosporine (if poor glycemic control, (grade 2, level B evidence) |
| Hypertension | Treatment target: blood pressure 130/80 mm Hg Amlodipine/Nifedipine may be preferred as these agents counteract real vasoconstrictor effect of calcineurin inhibitors Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers preferred in presence of chronic kidney disease, diabetes/proteinuria, heart failure with monitoring for hyperkalemia Beta blockers: in patients with coronary artery disease or heart failure |
Minimization of steroids and calcineurin inhibitors |
Text box 1. Metabolic syndrome and cardiovascular disease.
-
•
Weight gain, posttransplant dyslipidemia, diabetes, and hypertension are common
-
•
PTMS is present in 40–60% of recipients
-
•
Cardiovascular diseases are one of the most common causes of morbidity and mortality after LT, risk of cardiovascular disease is more in patients with PTMS
-
•
Prevention of weight gain and optimal use of immunosuppression should be considered
Alt-text: Text box 1
De novo malignancies
LT recipients remain at higher risk of DNMs. The higher risk is attributed to effect of prolonged immunosuppression, which impairs cancer surveillance and patient-related risk factors (age, smoking, etiology of liver disease, oncogenic viruses). Counseling of patients about risk factors and surveillance protocols may help in prevention and diagnosis at early stage. DNMs are one of the main causes of late mortality in LT recipients. The common types of DNMs are skin cancers, solid organ malignancies, and posttransplantation lymphoproliferative disorders.43, 44, 45
Incidence and Types of DNM After LT
In a study of 11,226 LT recipients from French national registry, 1200 (10.7%) developed DNM. The risk of death and standardized incidence ratio (SIR) for all de novo solid organ malignancies was approximately 2 and 2.20, respectively. The risk was higher in men and alcoholics.46 Different studies have shown different incidence rates of DNM due to different population profile (demography, risk factors) and varying follow-up. The incidence of DNMs also varies with follow-up of LT recipients; studies having a higher follow-up have noted higher incidence of DNM. Finkestedt et al47 showed that 96 (12.3%) patients developed 105 malignancies in a cohort of 779 patients. The cumulative risk of DNM was 10% at 5 years, 24% at 10 years, 32% at 15 years, and 42% at 20 years after LT. The patients with skin cancers and solid tumors (diagnosed at early stages) had good outcome. Rademacher et al48 found that DNM developed in 266 of 1616 (16.5%) of LT recipients at a median follow-up of 14.1 years. The probabilities of developing any DNM were 12.9% and 23% at 10 years and 25 years, respectively. A review of literature by Mukthinuthalapati et al.45 showed following SIRs for DNM: nonmelanoma skin cancer (2.1–70), posttransplantation lymphoproliferative disorders (PTLDs) (3.9–21), and solid organ cancers (1.4–3.1). The incidence of DNM ranges from 1% to 16.5% in studies with ≥1000 recipients.48,49 The types of DNMs in a given LT population reflect common malignancies prevalent in the given non-LT population. Thus skin cancer is the most common malignancy in the western world,50,51 whereas aerodigestive malignancies are the most common form of DNM in Asia.49,52 In the series of 1952 LT recipients from Korea, 2.3% developed DNM. The stomach cancer (25%) was the most common form of DNM followed by colorectal (20%). In a study of 2100 adult LT recipients from our center, 7 of 21 (33%) had oropharyngeal malignancies.49
Risk Factors and Prognosis After Development of DNM
Patients on cyclosporine and azathioprine are at higher risk for development of skin cancers after transplantation.53 The risk of PTLD is more in pediatric population, likely secondary to absence of previous Epstein-Barr virus exposure. PTLD occurs in 1–3% of adult population.54,55 Common solid organs DNM include head and neck malignancies, gastrointestinal malignancies, lung tumors, genitourinary, breast, and pancreatic malignancies. Alcohol and smoking are important risk factors for head and neck, as well as lung malignancies.54,55 Mangus et al56 showed that risk of DNM was higher for current and previous smokers, compared with nonsmokers, and in addition survival was worse for current smokers. Watt et al57 showed that increased age, history of smoking, primary sclerosing cholangitis (PSC), and alcoholic liver disease were important risk factors on multivariate analysis for development of solid organ DNM. The prognosis of solid organ malignancies remains poor in long-term unless diagnosed early. The probability of death after diagnosis of hematologic malignancy was 44.0% at 1 year and 57.6% at 5 years in the study by Watt et al.57 The probabilities of death in patients with solid organ DNM at 1 and 5 years were 38% and 53.1%, respectively. Another important risk factor for oral malignancies is use of smokeless tobacco, which is the common cause of these malignancies in south Asia.58
Impact of Screening Protocols
There are not much data on impact of surveillance strategies on outcomes of DNM after LT. It is necessary to maintain a high level of suspicion and consider surveillance strategies. A suggested protocol is given as table 3. Two studies analyzed impact of surveillance strategies. Herrero et al59 compared outcomes of active screening in 9 patients of 24 patients, where a diagnosis of DNM was based on symptoms or incidental. The authors excluded skin, hepatobiliary carcinomas, and lymphoproliferative diseases. All 9 patients were alive at a median follow-up of 25 months, and all were free of tumor in active screening group; 18 of 24 died with a median survival of 13.5 months in the other group. The difference in survival was significant, P = 0.002. Finkestedt et al47 also noted improved diagnosis of DNM after introduction of an intensified surveillance protocol. The rate of diagnosis increased from 4.9% to 13%, and in addition more DNM could be diagnosed at early stage. The authors noted improved survival in the intensive group, and the salient features of DNM are shown in Text Box 2.
Table 3.
Suggested Protocol for Surveillance of de Novo Malignancies in Adult LDLT Recipients (Reference 49,53).
| Site specific | Tests | Applicable to transplant recipients |
|---|---|---|
| Skin cancer | Annual examination, early in patients with risk factors | All recipients |
| Colon cancer | Annual colonoscopy with random surveillance biopsy | Patients with inflammatory bowel disease, primary sclerosing cholangitis |
| Lung cancer | Annual chest X-ray, yearly CT chest if chronic symptoms or active smoking | All |
| Oropharyngeal/laryngeal cancer | Annual otolaryngology evaluation by specialist | Ethanol, current or ex-smokers, tobacco chewing |
| Cervical cancer | Pelvic examination with gynecologist and Papanicolaou smear | Female recipients |
| Breast cancer | Annual mammography starting at the age of 45 years | Female recipients |
| Prostate cancer | Annual prostate-specific antigen testing starting at the age of 50 years | Male recipients |
| RCC | Annual USG abdomen |
Text box 2. De novo malignancy.
-
•
Important cause of late morbidity/mortality
-
•
Higher risk in alcoholic, smokers, patients transplanted for PSC
-
•
Intense surveillance should help in early detection, thus improving survival
Alt-text: Text box 2
Chronic kidney disease
Importance and Prevalence
CKD is common after LT and results in significant morbidity and mortality in long-term. As model for end-stage liver disease score is heavily influenced by serum creatinine values, patients with some form of kidney disease get LT and remain at higher risk of CKD after LT. Sharma et al60 found that CKD was 15% higher in the model for end-stage liver disease era. Ojo et al61 performed Scientific Registry of Transplant Recipients data analysis of solid (nonrenal) organ transplantation. The cumulative incidence of CKD (defined as estimated glomerular filtration rate [eGFR] < 29 ml per minute per 1.73 M2 of body-surface area) was 18% at 5 years in LT recipients. The risk of CKD was more in intestine transplants followed by LT recipients and heart or lung transplantations (combined or isolated). Most of the studies have calculated eGFR, and there is limited data on measured GFR. Cohen et al62 showed that measured GFR at 1-year identified patients with subsequent renal dysfunction. The cumulative incidence of renal failure was 6.25% at 7 years and 10% at 10 years in the study cohort. Watt et al1 analyzed data of 798 transplantation recipients. The renal failure contributed to 6% deaths. Patients with older age, diabetes, and renal insufficiency were at highest risk of poor survival.
Guisto et al63 found CKD (defined as eGFR <60 ml/min) in 45% patients at 5 years in a cohort of 179 patients. The estimated GFR at LT, development of hypertension, episodes of severe infection were prognostic factors at the Cox regression time-dependent analysis. Burra et al64 showed that hepatitis C status and serum creatinine levels before transplantation were independent predictors of renal function one year after LT, whereas pretransplantation creatinine was more important in long-term. In a long-term follow-up study of 1211 LT recipients, 54% died, 9% underwent kidney transplantation, whereas 21% and 18% had measured GFR 59–30 and < 30 ml/min, respectively. The risk of death increased when measured GFR (by iothalamate clearance) decreased < 30 ml/min (HR = 2.67 or < 15 ml/min (HR = 5.47). The eGFR underestimated mortality risk as compared with measured GFR. The decline in GFR after transplantations occurred in 2 phases. The first phase, seen in the initial 4 months represented a steep decline in GFR, which was attributed to the nephrotoxicity of immunosuppression. This early decline of GFR highlights the importance of prevention or timely reversal of perioperative/early kidney injury.65 The second phase is of gradual decline of GFR.65,66
Factors Causing CKD After LT
The kidney dysfunction in LT recipients is multifactorial and related to preexisting renal impairment, pretransplantation and perioperative acute kidney injury events, nephrotoxic effect of immunosuppressive medications, and comorbidities such as DM and hypertension. It is important to identify kidney impairment early as few interventions impact the course of progression once serum creatinine is significantly elevated. CNI (tacrolimus and cyclosporine) induced nephrotoxicity contributes to both short- and long-term renal function deterioration. The acute component is mediated by afferent arteriolar vasoconstriction, which is caused by imbalance of vasoconstrictors and vasodilators. This afferent arteriolar vasoconstriction causes dose-related acute and reversible decrease in glomerular filtration rate, renal blood flow, and urine output. The chronic CNI exposure affects renal vessels (arteriolar hyalinosis), tubulointerstitium (presence of tubular atrophy and interstitial fibrosis), and glomeruli (thickening and fibrosis of Bowman's capsule and glomerular sclerosis). The mechanisms of chronic CNI nephrotoxicity include hemodynamic changes and possible direct effects on tubular epithelial cells.67 Although CNI toxicity is considered a major contributor, the studies have shown that other risk factors for CKD (perioperative acute kidney injury, DM, hypertension) play a significant role.68,69 Pillebout et al68 examined renal biopsies of 26 recipients with chronic renal failure, at a mean of 5 years after LT. Twelve patients were diabetic and 25 were hypertensive. Histology revealed interstitial fibrosis and glomerular sclerosis (mean 45%), as well as severe arteriosclerosis in all biopsies. There were four main diagnosis as follows: chronic CNI arteriolopathy, typical diabetic nephropathy, acute or chronic thrombotic microangiopathy (attributed to CNI or alpha-interferon), and tubular changes due to administration of hydroxyethylstarch. In a study by Kim et al69, 81 LT recipients with impaired kidney function or new proteinuria underwent kidney biopsy at a mean time of 4.8 years. The baseline parameters at the time of biopsy were as follows: mean serum creatinine 2.0 mg/dL, Modification of Diet in Renal Disease (MDRD) eGFR 38.7 mL/min, and 24-h urine protein 1.37 g. A total of 42% biopsies showed primary glomerular diseases, and 16% had evidence of CNI toxicity. Eight of these patients progressed to end-stage renal disease at a mean follow-up of 20 months. Israni et al70 have described a prediction equation. The variables in the 6-month to 5-year equation included race, diabetes, hepatitis C status, albumin, bilirubin, and serum creatinine.
Management of CKD After LT
The effect of CNIs on kidney function can be reversed or minimized with reduced exposure to CNIs.71 It should be noted that the eGFR should be calculated by the CKD Epidemiology Collaboration or the MDRD equations, which performs better than other creatinine-based equations in solid organ transplantation recipients when measured GFR is calculated from urinary clearance of iothalamate or plasma clearance of iohexol.72
The strategy of short-term induction therapy with delayed CNI introduction in patients at risk has been shown to be associated with better renal outcomes. This strategy avoids synergistic effects of vasoconstriction by CNIs with other perioperative risk factors.73,74 The use of everolimus has been shown to improve GFR, particularly if used early (first year).75 A multicenter randomized study of 3 groups (everolimus + reduced exposure tacrolimus, everolimus + tacrolimus elimination [TAC elimination], or standard exposure tacrolimus) showed better renal outcomes in everolimus and reduced dose tacrolimus arm. The TAC elimination arm was prematurely terminated due to a higher rate of biopsy-proven acute rejection.76 It should be noted that MTORis cause proteinuria and use of mTORis is not feasible in patients with significant baseline proteinuria. A very early use also impairs wound healing.38 The long-term renal function maintenance can be achieved by CNI reduction at early course (1–12 months) in combination with other non-nephrotoxic immunosuppressive agents.71 The other immunosuppressive agents that can be used include mycophenolate (MMF) and everolimus. These strategies may not work if used too late.71 In the study by Kornberg et al77, use of full-dose MMF and the early conversion were independent predictors of persistent renal function improvement. Pageaux et al78 showed that introduction of MMF combined with the reduction of at least 50% of CNI dose was associated with significantly improved renal function at 1 year without rejection episodes or other significant secondary effects. Although some studies of late conversion with mTORi have shown a benefit, not all studies have found the same.79,80 Abdelmalek et al showed higher rates of biopsy-proven acute cellular rejection in sirolimus arm with no improvement if GFR in a cohort with late conversion. De Simone et al81 also found similar results. Although LT recipients converted early to everolimus demonstrated increase of eGFR at 12 months after conversion, patients converted at >1 year after LT had no GFR change. It has been suggested that MMF with concurrent reduction in CNI therapy may results in improvement of renal function even if performed at > 1 year after LT (grade of recommendation 2B), whereas there is no substantial evidence of improved renal functions in favor of mTORi with reduction, or elimination of CNI therapy improves renal function when performed >1 year after LT (grade of recommendation 2C).71 It is important to control of diabetes and hypertension, in addition to modification of immunosuppression. The exposure to nephrotoxins (medications with known nephrotoxicity such as aminoglycosides, amphotericin B, nonsteroidal anti-inflammatory agents) and contrast agents should be avoided if possible. When possible, reducing or holding CNI therapy should be considered with a temporary increase of non-nephrotoxic immunosuppressive medications as permissible by immunologic risk. Angiotensin II receptor blocker or angiotensin-converting enzyme inhibitor therapy should be used in diabetic patients with urine albumin excretion 30–300 mg/24 h and in nondiabetic adults with CKD and urine albumin excretion >300 mg/24 h (or equivalent) (grade of recommendation 1B).71
The important features of CKD are shown in Text Box 3.
Text box 3. Chronic kidney disease.
-
•
Patients with pretransplantation renal impairment remain at higher risk
-
•
One of important causes of late morbidity and mortality
-
•
Generally multifactorial (pretransplantation injury, effect of immunosuppression, diabetes, hypertension)
-
•
Early modification of immunosuppression is better than late modification
Alt-text: Text box 3
Recurrence of disease
Alcohol
Alcohol relapse (any amount) has been shown in 16%–33.8% of LT recipients in various studies, and harmful drinking is shown to be in 10%–18% of LT recipients.82 Harmful alcohol relapse is associated with poor survival.83 The various predictors of relapse include absence of structured program, length of sobriety (range from <3 months to < 1 year), other substance abuse/psychiatric comorbidities, lack of social support, younger age, low motivation for alcohol treatment, active smoking, poor stress management skills, criminal history, and history of complications in post-LT course, as well as continued engagement in social activities with alcohol present.82, 83, 84, 85, 86 Active involvement of psychiatry team may decrease risk of relapse. Björnsson et al87 demonstrated relapse rate of 48% (19/40) compared to 22% in structured management program group. DiMartini et al88 also found a lower relapse rate in recipients managed by alcohol addiction unit within transplantation center. Clinical scores have been suggested to predict risk of alcohol relapse. De Gottardi et al89 suggested a risk score comprising duration of abstinence of less than 6 months, presence of psychiatric comorbidities, and high-risk alcoholism relapse scale score higher than 3. Alcohol relapse was 5% in recipients with no risk factors, 18% in presence of 1 risk factor, 64% in presence of 2, and 100% in presence of all risk factors. Smoking is common in alcoholic liver disease recipients and is one of the predictor of long-term outcomes.90 The patients should be counseled for the same.
Recurrent and de novo Nonalcoholic Fatty Liver Disease
Recurrent or de novo nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH) is common finding in LT recipients. It is called recurrent when baseline disease was NASH-related cirrhosis and de novo when pretransplantation etiology was other than NASH. There is rise in metabolic risk factors after LT. MS is present in a significant number of recipients as discussed earlier. Recurrent NAFLD is more common than de novo NAFLD. There is suggestion of accelerated fibrosis progression in some of these patients as compared with slow progression of fibrosis in patients with non-LT NAFLD. Studies have shown 24%–100% prevalence of recurrent NAFLD, with majority of patients showing presence of NAFLD in long term.17,38,42,91,92 The development of recurrent or de novo NAFLD is related to weight gain, impact of immunosuppression agents (causing insulin resistance, hypertension, dyslipidemia as shown in table 1) combined with diet, lifestyle, and genetic susceptibility of a patient. All these factors lead to a higher incidence of MS after LT, which is commonly associated with NAFLD.42 It should be noted that MMF is devoid of these metabolic side effects.38
A meta-analysis of 12 studies including 2166 patients showed a 26% pooled (range: 14.7–52%) prevalence of de novo NAFLD. Fibrosis was present in 3.3%–52% of patients, and NASH was presented in 1–32% patients (not reported in all studies).93
The available data have not shown any clear association between recurrent or de novo NAFLA/NASH and patient or graft survival; however quality of evidence is low, and strength of recommendation is weak.40 As NAFLD is independently (of metabolic risk factors) associated with CVD and fibrosis to cirrhosis progression is slow,94 the analysis of long-term graft and patient survival and additional clinical end points such as cardiovascular events, renal disease, and malignancy should be more meaningful. The current impression of limited clinical significance of recurrent/de novo NAFLD may be a biased statement as most of studies have short follow-up.
Recently three studies presented long-term follow-up of post-LT NAFLD. Bhati et al95 studied 103 patients of recurrent NASH. Biopsy (n = 34) showed recurrent NAFLD in 88.2%, NASH in 41.2%, and bridging fibrosis in 20.6% patients. Fifty six patients had transient elastography at median follow-up of 75 (40–146) months, which was suggestive of advanced fibrosis in 26.8% and cirrhosis in 5.4%. The leading cause of mortality (n = 32) was cancer and infections (25% each) followed by CVD in 21.9% and cirrhosis in 9%. Narayanan et al96 analyzed 588 patients transplanted from 1999 to 2006. Recurrent NAFLD was present in 77.6% and de novo NAFLD was present in 44.7% recipients at 10 years. The risk factors for post-LT NAFLD included female gender, hepatitis C, and time-dependent BMI. The presence of NASH was associated with cardiovascular events (HR of 2.04). Gitto et al97 compared 43 recipients with de novo NAFLD to 151 recipients without NAFLD. The patients with de novo NAFLD had significantly higher prevalence of DM (51.2% versus 30.5%), obesity (48.8% versus 9.9%), and MS (74.4%vs 29.8%). Cardiovascular events (34.9% versus 7.9%) and de novo solid malignancies (30.2%vs 11.9%) occurred more commonly in de novo NAFLD group. The presence of de novo NAFLD was independent predictor of mortality.
Table 2 describes immunosuppression modification for MS/NAFLD after LT. Steroids avoidance and minimization of CNI may reduce the risk of PTMS and thus NAFLD.98,99 Dietary interventions and exercise should be implemented early after LT to avoid weight gain.
Hepatitis C
Treatment of hepatitis C virus (HCV) recurrence was associated with poor sustained virological response (SVR) and significant adverse events in the peginterferon era.100,101 With availability of sofosbuvir-based direct acting antiviral agents, SVR rates have improved considerably without significant side effects.102,103 A systemic review of 22 (n = 1730 patients) showed a pooled SVR rate of 90.1%. The SVR 12 rate was higher in recipients with mild fibrosis as compared with advanced fibrosis or cirrhosis. The pooled discontinuation rate because of adverse events was 3.3%.103 Thus, recurrence of HCV causing cirrhosis should be very rare in sofosbuvir era.
Recurrence of Autoimmune Diseases
Autoimmune disease (autoimmune hepatitis [AIH], PSC, primary biliary cholangitis [PBC]) may recur after LT, however, despite recurrence prognosis remains good, and a 5-year survival >70% is reported in majority of studies.104, 105, 106 Recurrence rates, risk factors for recurrence, and diagnostic criteria are shown in table 4. The following strategies are suggested to decrease risk of recurrence: treatment of active cirrhosis, near normalization of transaminases before LT and continuation of steroids in AIH; treatment of active colitis before LT and maintaining remission/colectomy if poor response to medical treatment in patients with PSC; ursodeoxycholic acid and use of cyclosporine in patients with PBC.106 Recurrence of PSC needs exclusion of the following events: hepatic artery thrombosis or stenosis, chronic rejection, anastomotic strictures alone, nonanastomotic strictures <90 days after LT, and ABO incompatible graft.107 Duct to duct reconstruction versus Roux-en-Y reconstitution in patients with PSC have similar outcomes in terms of strictures after LT.108 One study from Japan showed that recurrence of PSC may be more in patients with genetically related living donors; 109 however, a study from our center (20% recurrence) and a large study involving both decreased and living donors have not found higher recurrence in living donor liver transplantation (LDLT).110,111 Gordon et al111 compared 241 LDLT recipients and 65 deceased donor LT (DDLT) recipients and found that there was no difference in PSC recurrence among the two groups of patients, the 5- and 10-year PSC recurrence rates were 9.4% versus 9.5% and 36.9% versus 21.1% in DDLT and LDLT, respectively. There is no specific medical therapy for PSC recurrence, a re-LT for PSC recurrence is associated with good results as compared with retransplantation for other etiologies requiring re-LT.112 It is important to understand that pretransplantation criteria to diagnose AIH and PBC are not applicable in posttransplantation setting; antibodies are not needed to make a diagnosis of recurrent AIH or PBC. In addition, pretransplantation criteria are not validated for posttransplantation population.113, 114, 115 There is limited data on recurrence of overlap syndrome. A small study showed a recurrence of 53% and 69% at 5 and 10 years, respectively, which was significantly more as compared with other AI diseases, although survival was similar.116
Table 4.
| Recurrent disease | Recurrence reported, risk factors | Diagnosis of recurrent disease | Management, prognosis |
|---|---|---|---|
| AIH | 18–68% at 5-years, HLA mismatching at transplant, severe inflammation on histology, and abnormal AST/ALT at LT, lower levels of immunosuppression, rapid tapering/discontinuation of steroids |
The characteristic histological findings of recurrent AIH are interface hepatitis and mononuclear inflammatory infiltrates with plasma cells, exclusion of other causes |
Steroids, azathioprine, addition of mycophenolate, mTORi, higher level of immunosuppression needed |
| PBC | 9–34% Younger/older age LT, HLA-DR locus, use of tacrolimus, severe cholestasis at 3 months, and ALT >50 at 6 months after LT, gender mismatch |
Biopsy suggestive of PBC exclusion of other causes | UDCA (few studies have not shown a benefit) |
| PSC | 10–37%, recurrent acute cellular rejection, intact colon, active IBD, younger recipient age, genetically related donors, donor-recipient gender mismatch, older donor age | PSC before LT, cholangiogram showing intrahepatic and/or extrahepatic biliary structuring, beading, and irregularity occurring > 90 days post-LT and/or biopsy showing fibrous cholangitis and/or fibro-obliterative lesions with or without ductopenia, biliary fibrosis, or biliary cirrhosis |
Antibiotics for cholangitis, endoscopic management for dominant stricture, No proven medical therapy for recurrent PSC, eventually retransplantation needed |
mTORi, mammalian target of rapamycin inhibitors.
Bone disease
A significant number of patients with decompensated cirrhosis remain suffering from osteoporosis. The risk factors for bone loss in cirrhosis include cholestatic liver diseases, malabsorption, hypogonadism, vitamin D deficiency, reduced physical activity, gut dysbiosis, and history of steroid treatment (in patients with AIH).117,118 There is accelerated bone mineral loss in initial several months after LT primarily secondary to use of steroids, and there is gain in bone density later. There is increased risk of fractures after LT. Vertebral fractures are the most common form of fractures. The following risk factors for fractures have been identified in patients receiving a liver: advanced age, pretransplantation vertebral fractures/bone mineral density, chronic cholestatic liver diseases, and dose of glucocorticoids.119, 120, 121 EASL guidelines have suggested yearly bone mineral density screening in patients with preexisting osteoporosis/osteopenia and in every 2–3 years in patients with normal bone mineral density. Patients should be encouraged for regular weight-bearing exercise and should receive calcium and vitamin D supplementation. In patients with osteoporosis or recurrent fractures, bisphosphonate should be considered (grade II-2).122
Pregnancy and contraception
Return of Fertility and Contraception
Women with decompensated cirrhosis often have amenorrhea, and pregnancies are rare.123 Impaired fertility happens in patients with decompensated cirrhosis is due to dysregulation in the hypothalamic-pituitary-ovarian axis, that resolves after transplantation. The majority of women resume regular menstrual cycles within 1 year after transplantation, although ovulation may resume as early as within the first postoperative months.124,125 Mass et al125 showed that 95% of women with age <46 years had menstrual bleeding within the first year after LT, and it was not related to liver function tests which were severely abnormal in 19%. This fact highlights importance of discussing contraception with LT female recipients of childbearing age to avoid accidental pregnancies very early after LT.124
There is not much good quality data regarding contraception. Coitus interrupts is not recommended due to significant risk of unplanned pregnancies.126 Diaphragms and condoms are widely used and devoid of drug interactions are avoided; however efficacy is not 100%. Condoms prevent sexual transmissible diseases as well. The use of diaphragms increases the risk of urinary tract infections. This increased risk of infection can be a matter of concern in the LT recipients.127 Intrauterine contraceptive devices (IUCDs) work by producing local effects, mainly the following: inflammation secondary to foreign body reaction (Copper devices) or by local effects of levonorgestrel (glandular atrophy, stromal decidualization, and foreign body reaction) released by IUCD. IUCDs may be less efficacious in LT recipients theoretically due to reduced local inflammatory response secondary to immunosuppressive medications; however, later reports have not shown IUD failure. Although an early report showed IUCD failure, a large series have not shown pregnancy in IUCD group.128, 129, 130, 131 Although hormonal contraception has risk of systemic side effects and drug interaction, limited data show that it is effective.132 There are risks of thrombosis, hypertension, and cholestatic drug–induced liver injury due to estrogen component of combined estrogen and progesterone pills, only progesterone pills should be associated with less risk of drug-induced liver injury.124
Pregnancy After LT
It is important to explain to recipients of childbearing age that they should plan pregnancy after 1–2 years, in presence of stable graft functions.133 A recent systemic review of 19 studies (1290 pregnancies in 885 women) noted 1014 live births. The incidence of spontaneous abortions was 0.5%–33.3%. Thirty two percent pregnancies resulted in preterm births; preeclampsia was reported in 16% of pregnancies (range: 2–33%). Cesarean section rates reported as 20%–67.9%. Thus pregnant LT recipients remain at higher risk than the general population and close monitoring is required.134 Available evidence suggests an increased incidence of pregnancy-related complications (preterm delivery, fetal growth retardation, systemic hypertension/preeclampsia, and gestational diabetes) in pregnancy after LT.134
The women LT recipients of childbearing age group should be counseled to discuss with primary team before planning pregnancy, so that immunosuppression can be adjusted. The FDA categories of immunosuppression are as following: steroids (B), tacrolimus, cyclosporine, sirolimus (C), MMF, and azathioprine (D),135 although sirolimus a category C drug, it is generally avoided. The preferred immunosuppression agents are tacrolimus and steroids.
Immunosuppression and drug interactions
It is important to know about drug interactions with immunosuppressive agents. Drug interactions are shown in table 5 (references 41,136, 137, 138). In addition to interactions given in table 5, statins have an important drug interaction. When statins are used with cyclosporine and sirolimus, there is increased risk of statin toxicity.139
Table 5.
| Drug | CNIs | mTOR inhibitors | Mycophenolate |
|---|---|---|---|
| Fluoroquinolones (primarily ofloxacin > ciprofloxacin | Increased levels | – | – |
| Macrolides (erythromycin > clarithromycin > azithromycin) | Markedly increased levels | Markedly increased levels | – |
| Rifamycins (rifampin > rifabutin | Markedly decreased levels | Markedly decreased levels | – |
| Linezolid | – | Increased myelosuppression | Increased myelosuppression, thrombocytopenia |
| Triazoles (ketoconazole/voriconazole/posaconazole > itraconazole/fluconazole) | Increased levels | Increased levels (voriconazole contraindicated) | – |
| Ganciclovir/valganciclovir | – | Increased myelosuppression | Increased myelosuppression |
| Miscellaneousa | Increased levels-Verapamil Diltiazem Nicardipine amiodarone; cimetidine; danazol; fluvoxamine; protease inhibitors (HIV and HCVa) decreased levels-phenytoin; phenobarbital; carbamazepine; St. John's wort; Isoniazid |
Increased levels-verapamil; amiodarone; cimetidine; danazol; fluvoxamine; protease inhibitors (HIV and HCVa) decreased levels-phenytoin; phenobarbital; carbamazepine; St. John's wort; Isoniazid |
Magnesium or aluminum containing products (decreased absorption); antacids, cholestyramine, cyclosporine (↓enterohepatic recirculation); antibiotics (↓enterohepatic recirculation); Renal dysfunction – incrased immunosuppreasion |
Some of newer direct acting antivirals have potential of interaction; sofosbuvir, ledipasvir and velpatasvir are free of interactions.
The long-term survival after LT is mainly affected by CVD, DNMs, CKD, and recurrence of primary disease. Many of risk factors to these diseases are modifiable. It is important to identify patients at risk and manage accordingly.
CRediT authorship contribution statement
Narendra S. Choudhary: Conceptualization, Writing - original draft. Neeraj Saraf: Conceptualization, Writing - original draft. Sanjiv Saigal: Writing - review & editing. Arvinder S. Soin: Writing - review & editing.
Conflicts of interest
The authors have none to declare.
References
- 1.Watt K.D., Pedersen R.A., Kremers W.K., Heimbach J.K., Charlton M.R. Evolution of causes and risk factors for Mortality post-liver transplant: results of the NIDDK long term follow-up study. Am J Transplant. 2010;10:1420–1427. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelson W., Hoare M., Dawwas M.F., Vowler S., Gibbs P., Alexander G. The pattern of late mortality in liver transplant recipients in the United Kingdom. Transplantation. 2011;91:1240–1244. doi: 10.1097/TP.0b013e31821841ba. [DOI] [PubMed] [Google Scholar]
- 3.Konerman M.A., Fritze D., Weinberg R.L., Sonnenday C.J., Sharma P. Incidence of and risk assessment for adverse cardiovascular outcomes after liver transplantation: a systematic review. Transplantation. 2017;101:1645–1657. doi: 10.1097/TP.0000000000001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madhwal S., Atreja A., Albeldawi M., Lopez R., Post A., Costa M.A. Is liver transplantation a risk factor for cardiovascular disease? A meta-analysis of observational studies. Liver Transplant. 2012;18:1140–1146. doi: 10.1002/lt.23508. [DOI] [PubMed] [Google Scholar]
- 5.Khurmi N.S., Chang Y.H., Eric Steidley D. Hospitalizations for cardiovascular disease after liver transplantation in the United States. Liver Transplant. 2018;24:1398–1410. doi: 10.1002/lt.25055. [DOI] [PubMed] [Google Scholar]
- 6.Fussner L.A., Heimbach J.K., Fan C. Cardiovascular disease after liver transplantation: when, what, and who is at risk. Liver Transplant. 2015;21:889–896. doi: 10.1002/lt.24137. [DOI] [PubMed] [Google Scholar]
- 7.VanWagner L.B., Montag S., Zhao L. Cardiovascular disease outcomes related to early stage renal impairment after liver transplantation. Transplantation. 2018;102:1096–1107. doi: 10.1097/TP.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chokesuwattanaskul R., Thongprayoon C., Bathini T. Liver transplantation and atrial fibrillation: a meta-analysis. World J Hepatol. 2018;10:761–771. doi: 10.4254/wjh.v10.i10.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone N.J., Bilek S., Rosenbaum S. Recent national cholesterol education program adult treatment panel III update: adjustments and options. Am J Cardiol. 2005;96:53E–59E. doi: 10.1016/j.amjcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Misra A., Chowbey P., Makkar B.M. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Phys India. 2009;57:163. [PubMed] [Google Scholar]
- 11.Laish I., Braun M., Mor E., Sulkes J., Harif Y., Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transplant. 2011;17:15–22. doi: 10.1002/lt.22198. [DOI] [PubMed] [Google Scholar]
- 12.Laryea M., Watt K.D., Molinari M. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transplant. 2007;13:1109–1114. doi: 10.1002/lt.21126. [DOI] [PubMed] [Google Scholar]
- 13.Choudhary N.S., Saigal S., Saraf N. Sarcopenic obesity with metabolic syndrome: a newly recognized entity following living donor liver transplantation. Clin Transplant. 2015;29:211–215. doi: 10.1111/ctr.12505. [DOI] [PubMed] [Google Scholar]
- 14.Anastácio L.R., Diniz K.G., Ribeiro H.S. Prospective evaluation of metabolic syndrome and its components among long-term liver recipients. Liver Int. 2014;34:1094–1101. doi: 10.1111/liv.12495. [DOI] [PubMed] [Google Scholar]
- 15.Luca L.D., Westbrook R., Tsochatzis E.A. Metabolic and cardiovascular complications in the liver transplant recipient. Ann Gastroenterol. 2015;28:183–192. [PMC free article] [PubMed] [Google Scholar]
- 16.Nair S., Verma S., Thuluvath P.J. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi G., Marchesini G., Marzocchi R., Pinna A.D., Zoli M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transplant. 2008;14:1648–1654. doi: 10.1002/lt.21588. [DOI] [PubMed] [Google Scholar]
- 18.Beckmann S., Drent G., Ruppar T., Nikolić N., De Geest S. Body weight parameters are related to morbidity and mortality after liver transplantation: a systematic review and meta-analysis. Transplantation. 2019;103:2287–2303. doi: 10.1097/TP.0000000000002811. [DOI] [PubMed] [Google Scholar]
- 19.Sharif A., Hecking M., de Vries A.P. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14:1992–2000. doi: 10.1111/ajt.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakkera H.A., Knowler W.C., Devarapalli Y. Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus. Clin J Am Soc Nephrol. 2010;5:1669–1675. doi: 10.2215/CJN.09481209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caillard S., Eprinchard L., Perrin P. Incidence and risk factors of glucose metabolism disorders in kidney transplant recipients: role of systematics screening by oral glucose tolerance test. Transplantation. 2011;91:757–764. doi: 10.1097/TP.0b013e31820f0877. [DOI] [PubMed] [Google Scholar]
- 22.Sharif A., Baboolal K. Risk factors for new onset diabetes after transplantation. Nat Rev Nephrol. 2010;6:415–423. doi: 10.1038/nrneph.2010.66. [DOI] [PubMed] [Google Scholar]
- 23.Israni A.K., Snyder J.J., Skeans M.A. Clinical diagnosis of metabolic syndrome: predicting new-onset diabetes, coronary heart disease and allograft failure late after transplant. Transpl Int. 2012;25:748–757. doi: 10.1111/j.1432-2277.2012.01488.x. [DOI] [PubMed] [Google Scholar]
- 24.Porrini E., Delgado P., Alvarez A. The combined effect of pre-transplant triglyceride levels and the type of calcineurin inhibitor in predicting the risk of new onset diabetes after renal transplantation. Nephrol Dial Transplant. 2007;23:1436–1441. doi: 10.1093/ndt/gfm762. [DOI] [PubMed] [Google Scholar]
- 25.Hjelmesaeth J., Hartmann A., Kofstad J. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation. 1997;64:979–983. doi: 10.1097/00007890-199710150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Tavira B., Coto E., Torres A. Association between a common KCNJ11 polymorphism (rs5219) and new-onset posttransplant diabetes in patients treated with tacrolimus. Mol Genet Metabol. 2012;105:525–527. doi: 10.1016/j.ymgme.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Lv C., Zhang Y., Chen X. New-onset diabetes after liver transplantation and its impact on complications and patient survival. J Diabetes. 2015;7:881–890. doi: 10.1111/1753-0407.12275. [DOI] [PubMed] [Google Scholar]
- 28.Moon J.I., Barbeito R., Faradji R.N., Gaynor J.J., Tzakis A.G. Negative impact of new-onset diabetes mellitus on patient and graft survival after liver transplantation: long-term follow up. Transplantation. 2006;82:1625–1628. doi: 10.1097/01.tp.0000250361.60415.96. [DOI] [PubMed] [Google Scholar]
- 29.Younossi Z.M., Stepanova M., Saab S. The impact of type 2 diabetes and obesity on the long-term outcomes of more than 85 000 liver transplant recipients in the US. Aliment Pharmacol Ther. 2014;40:686–694. doi: 10.1111/apt.12881. [DOI] [PubMed] [Google Scholar]
- 30.Roccaro G.A., Goldberg D.S., Hwang W.T. Sustained posttransplantation diabetes is associated with long-term major cardiovascular events following liver transplantation. Am J Transplant. 2018;18:207–215. doi: 10.1111/ajt.14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neal D.A., Gimson A.E., Gibbs P., Alexander G.J. Beneficial effects of converting liver transplant recipients from cyclosporine to tacrolimus on blood pressure, serum lipids, and weight. Liver Transplant. 2001;7:533–539. doi: 10.1053/jlts.2001.24637. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal A., Prasad G.V.R. Post-transplant dyslipidemia: mechanisms, diagnosis and management. World J Transplant. 2016;6:125–134. doi: 10.5500/wjt.v6.i1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrisett J.D., Abdel-Fattah G., Hoogeveen R. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res. 2002;43:1170–1180. [PubMed] [Google Scholar]
- 34.Princen H.M., Meijer P., Wolthers B.G., Vonk R.J., Kuipers F. Cyclosporin A blocks bile acid synthesis in cultured hepatocytes by specific inhibition of chenodeoxycholic acid synthesis. Biochem J. 1991;275(Pt 2):501–505. doi: 10.1042/bj2750501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hricik D.E. Hyperlipidemia in renal transplant recipients. Graft. 2000;3:177–184. [Google Scholar]
- 36.Di Stefano C., Vanni E., Mirabella S. Risk factors for arterial hypertension after liver transplantation. J Am Soc Hypertens. 2018;12:220–229. doi: 10.1016/j.jash.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Tong M.S., Chai H.T., Liu W.H. Prevalence of hypertension after living-donor liver transplantation: a prospective study. Transplant Proc. 2015;47:445–450. doi: 10.1016/j.transproceed.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 38.Choudhary N.S., Saigal S., Shukla R., Kotecha H., Saraf N., Soin A.S. Current status of immunosuppression in liver transplantation. J Clin Exp Hepatol. 2013;3:150–158. doi: 10.1016/j.jceh.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pillai A.A., Levitsky J. Overview of immunosuppression in liver transplantation. World J Gastroenterol. 2009;15:4225–4233. doi: 10.3748/wjg.15.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Germani G., Laryea M., Rubbia-Brandt L. Management of recurrent and de novo NAFLD/NASH after liver transplantation. Transplantation. 2018 Oct 17 doi: 10.1097/TP.0000000000002485. [DOI] [PubMed] [Google Scholar]
- 41.Lucey M.R., Terrault N., Ojo L. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transplant. 2013;19:3–26. doi: 10.1002/lt.23566. [DOI] [PubMed] [Google Scholar]
- 42.Choudhary N.S., Saigal S. Preventive strategies for nonalcoholic fatty liver disease after liver transplantation. J Clin Exp Hepatol. 2019;9:619–624. doi: 10.1016/j.jceh.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pruthi J., Medkiff K.A., Esrason K.T. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transplant. 2001;7:811–815. doi: 10.1053/jlts.2001.27084. [DOI] [PubMed] [Google Scholar]
- 44.Baccarani U., Adani G.L., Serraino D. De novo tumors are a major cause of late mortality after orthotopic liver transplantation. Transplant Proc. 2009;41:1303–1305. doi: 10.1016/j.transproceed.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 45.Mukthinuthalapati P.K., Gotur R., Ghabril M. Incidence, risk factors and outcomes of de novo malignancies post liver transplantation. World J Hepatol. 2016;8:533–544. doi: 10.4254/wjh.v8.i12.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sérée O., Altieri M., Guillaume E. Longterm risk of solid organ de novo malignancies after liver transplantation: a French national study on 11,226 patients. Liver Transplant. 2018;24:1425–1436. doi: 10.1002/lt.25310. [DOI] [PubMed] [Google Scholar]
- 47.Finkenstedt A., Graziadei I.W., Oberaigner W. Extensive surveillance promotes early diagnosis and improved survival of de novo malignancies in liver transplant recipients. Am J Transplant. 2009;9:2355–2361. doi: 10.1111/j.1600-6143.2009.02766.x. [DOI] [PubMed] [Google Scholar]
- 48.Rademacher S., Seehofer D., Eurich D. The 28-year incidence of de novo malignancies after liver transplantation: a single-center analysis of risk factors and mortality in 1616 patients. Liver Transplant. 2017;23:1404–1414. doi: 10.1002/lt.24795. [DOI] [PubMed] [Google Scholar]
- 49.Tiwari A., Saigal S., Choudhary N.S. De Novo Malignancy after Living Donor Liver Transplantation: A Large Volume Experience. J Clin Exp Hepatol. 2020 doi: 10.1016/j.jceh.2020.02.001. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saigal S., Norris S., Muiesan P., Rela M., Heaton N., O'Grady J. Evidence of differential risk for posttransplantation malignancy based on pretransplantation cause in patients undergoing liver transplantation. Liver Transplant. 2002;8:482–487. doi: 10.1053/jlts.2002.32977. [DOI] [PubMed] [Google Scholar]
- 51.Chatrath H., Berman K., Vuppalanchi R. De novo malignancy post liver transplantation: a single center, population controlled study. Clin Transplant. 2013;27:582–590. doi: 10.1111/ctr.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park H.W., Hwang S., Ahn C.S. De novo malignancies after liver transplantation: incidence comparison with the Korean cancer registry. Transplant Proc. 2012;44:802–805. doi: 10.1016/j.transproceed.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 53.Burra P., Shalaby S., Zanetto A. Long-term care of transplant recipients: de novo neoplasms after liver transplantation. Curr Opin Organ Transplant. 2018;23:187–195. doi: 10.1097/MOT.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 54.Mumtaz K., Faisal N., Marquez M. Posttransplant lymphoproliferative disorder in liver recipients: characteristics, management, and outcome from a single-centre experience with >1000 liver transplantations. Chin J Gastroenterol Hepatol. 2015;29:417–422. doi: 10.1155/2015/517359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chak E., Saab S. Risk factors and incidence of de novo malignancy in liver transplant recipients: a systematic review. Liver Int. 2010;30:1247–1258. doi: 10.1111/j.1478-3231.2010.02303.x. [DOI] [PubMed] [Google Scholar]
- 56.Mangus R.S., Fridell J.A., Kubal C.A. Worse long-term patient survival and higher cancer rates in liver transplant recipients with a history of smoking. Transplantation. 2015;99:1862–1868. doi: 10.1097/TP.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 57.Watt K.D., Pedersen R.A., Kremers W.K., Heimbach J.K., Sanchez W., Gores G.J. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137:2010–2017. doi: 10.1053/j.gastro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asthana S., Labani S., Kailash U., Sinha D.N., Mehrotra R. Association of smokeless tobacco use and oral cancer: a systematic global review and meta-analysis. Nicotine Tob Res. 2019;21:1162–1171. doi: 10.1093/ntr/nty074. [DOI] [PubMed] [Google Scholar]
- 59.Herrero J.I., Alegre F., Quiroga J. Usefulness of a program of neoplasia surveillance in liver transplantation. A preliminary report. Clin Transplant. 2009;23:532–536. doi: 10.1111/j.1399-0012.2008.00927.x. [DOI] [PubMed] [Google Scholar]
- 60.Sharma P., Schaubel D.E., Guidinger M.K., Goodrich N.P., Ojo A.O., Merion R.M. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant. 2011;11:2372–2378. doi: 10.1111/j.1600-6143.2011.03703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ojo A.O., Held P.J., Port F.K. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 62.Cohen A.J., Stegall M.D., Rosen C.B. Chronic renal dysfunction late after liver transplantation. Liver Transplant. 2002;8:916–921. doi: 10.1053/jlts.2002.35668. [DOI] [PubMed] [Google Scholar]
- 63.Giusto M., Berenguer M., Merkel C. Chronic kidney disease after liver transplantation: pretransplantation risk factors and predictors during follow-up. Transplantation. 2013;95:1148–1153. doi: 10.1097/TP.0b013e3182884890. [DOI] [PubMed] [Google Scholar]
- 64.Burra P., Senzolo M., Masier A. Factors influencing renal function after liver transplantation. Results from the MOST, an international observational study. Dig Liver Dis. 2009;41:350–356. doi: 10.1016/j.dld.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 65.Allen A., Kim W.R., Therneau T.M., Larson J.J., Heimbach J.K., Rule A.D. Chronic kidney disease and associated mortality after liver transplantation- A time dependent analysis using measured glomerular filtration rate. J Hepatol. 2014;61:286–292. doi: 10.1016/j.jhep.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez E.Q., Melton L.B., Chinnakotla S. Predicting renal failure after liver transplantation from measured glomerular filtration rate: review of up to 15 years of follow-up. Transplantation. 2010;89:232–235. doi: 10.1097/TP.0b013e3181c42ff9. [DOI] [PubMed] [Google Scholar]
- 67.Duvoux C., Pageaux G.P. Immunosuppression in liver transplant recipients with renal impairment. J Hepatol. 2011;54:1041–1054. doi: 10.1016/j.jhep.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Pillebout E., Nochy D., Hill G. Renal histopathological lesions after orthotopic liver transplantation (OLT) Am J Transplant. 2005;5:1120–1129. doi: 10.1111/j.1600-6143.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- 69.Kim J.Y., Akalin E., Dikman S. The variable pathology of kidney disease after liver transplantation. Transplantation. 2010;89:215–221. doi: 10.1097/TP.0b013e3181c353e5. [DOI] [PubMed] [Google Scholar]
- 70.Israni A.K., Xiong H., Liu J. Predicting end-stage renal disease after liver transplant. Am J Transplant. 2013;13:1782–1792. doi: 10.1111/ajt.12257. [DOI] [PubMed] [Google Scholar]
- 71.Levitsky J., O'Leary J.G., Asrani S. Protecting the kidney in liver transplant recipients: practice-based recommendations from the American society of transplantation liver and intestine community of practice. Am J Transplant. 2016;16:2532–2544. doi: 10.1111/ajt.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaffi K., Uhlig K., Perrone R.D. Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am J Kidney Dis. 2014;63:1007–1018. doi: 10.1053/j.ajkd.2014.01.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshida E.M., Marotta P.J., Greig P.D. Evaluation of renal function in liver transplant recipients receiving daclizumab (Zenapax), mycophenolate mofetil, and a delayed, low-dose tacrolimus regimen vs. a standard-dose tacrolimus and mycophenolate mofetil regimen: a multicenter randomized clinical trial Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver. Transplantation Society. 2005;11:1064–1072. doi: 10.1002/lt.20490. [DOI] [PubMed] [Google Scholar]
- 74.Neuberger J.M., Mamelok R.D., Neuhaus P. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: the ‘ReSpECT’ study. Am J Transplant. 2009;9:327–336. doi: 10.1111/j.1600-6143.2008.02493.x. [DOI] [PubMed] [Google Scholar]
- 75.De Simone P., Nevens F., De Carlis L. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008–3020. doi: 10.1111/j.1600-6143.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fischer L., Saliba F., Kaiser G.M. Three-year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: follow-up results from a randomized, multicenter study. Transplantation. 2015;99:1455–1462. doi: 10.1097/TP.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 77.Kornberg A., Kupper B., Thrum K. Sustained renal response to mycophenolate mofetil and CNI taper promotes survival in liver transplant patients with CNI-related renal dysfunction. Dig Dis Sci. 2011;56:244–251. doi: 10.1007/s10620-010-1386-z. [DOI] [PubMed] [Google Scholar]
- 78.Pageaux G.P., Rostaing L., Calmus Y. Mycophenolate Mofetil in Combination with Reduction of Calcineurin Inhibitors for Chronic Renal Dysfunction after Liver Transplantation. Liver Transpl. 2006;12:1755–1760. doi: 10.1002/lt.20903. [DOI] [PubMed] [Google Scholar]
- 79.Abdelmalek M.F., Humar A., Stickel F. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: a randomized trial. Am J Transplant. 2012;12:694–705. doi: 10.1111/j.1600-6143.2011.03919.x. [DOI] [PubMed] [Google Scholar]
- 80.Castroagudin J.F., Molina E., Romero R., Otero E., Tome S., Varo E. Improvement of Renal Function after the Switch from a Calcineurin Inhibitor to Everolimus in Liver Transplant Recipients with Chronic Renal Dysfunction. Liver Transpl. 2009;15:1792–1797. doi: 10.1002/lt.21920. [DOI] [PubMed] [Google Scholar]
- 81.De Simone P., Metselaar H.J., Fischer L. Conversion from a Calcineurin Inhibitor to Everolimus Therapy in Maintenance Liver Transplant Recipients: A Prospective, Randomized, Multicenter Trial. Liver Transpl. 2009;15:1262–1269. doi: 10.1002/lt.21827. [DOI] [PubMed] [Google Scholar]
- 82.Choudhary N.S., Kumar N., Saigal S., Rai R., Saraf N., Soin A.S. Liver transplantation for alcohol-related liver disease. J Clin Exp Hepatol. 2016;6:47–53. doi: 10.1016/j.jceh.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rustad J.K., Stern T.A., Prabhakar M., Musselman D. Risk factors for alcohol relapse following orthotopic liver transplantation: a systematic review. Psychosomatics. 2015;5:21–35. doi: 10.1016/j.psym.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Tandon P., Goodman K.J., Ma M.M. A shorter duration of pre-transplant abstinence predicts problem drinking after liver transplantation. Am J Gastroenterol. 2009;104:1700–1706. doi: 10.1038/ajg.2009.226. [DOI] [PubMed] [Google Scholar]
- 85.Rodrigue J.R., Hanto D.W., Curry M.P. The Alcohol Relapse Risk Assessment: a scoring system to predict the risk of relapse to any alcohol use after liver transplant. Prog Transplant. 2013;23:310–318. doi: 10.7182/pit2013604. [DOI] [PubMed] [Google Scholar]
- 86.Kitajima T., Nagai S., Segal A. Posttransplant complications predict alcohol relapse in liver transplant recipients. Liver Transplant. 2019 doi: 10.1002/lt.25712. [DOI] [PubMed] [Google Scholar]
- 87.Björnsson E., Olsson J., Rydell A. Long-term follow-up of patients with alcoholic liver disease after liver transplantation in Sweden: impact of structured management on recidivism. Scand J Gastroenterol. 2005;40:206–216. doi: 10.1080/00365520410009591. [DOI] [PubMed] [Google Scholar]
- 88.DiMartini A., Day N., Dew M.A. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transplant. 2006;12:813–820. doi: 10.1002/lt.20688. [DOI] [PubMed] [Google Scholar]
- 89.De Gottardi A., Spahr L., Gelez P. A simple score for predicting alcohol relapse after liver transplantation: results from 387 patients over 15 years. Arch Intern Med. 2007;167:1183–1188. doi: 10.1001/archinte.167.11.1183. [DOI] [PubMed] [Google Scholar]
- 90.Pungpapong S., Manzarbeitia C., Ortiz J. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transplant. 2002;8:582–587. doi: 10.1053/jlts.2002.34150. [DOI] [PubMed] [Google Scholar]
- 91.Yalamanchili K., Saadeh S., Klintmalm G.B. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transplant. 2010;16:431–439. doi: 10.1002/lt.22004. [DOI] [PubMed] [Google Scholar]
- 92.Dureja P., Mellinger J., Agni R. NAFLD recurrence in liver transplant recipients. Transplantation. 2011;91:684–689. doi: 10.1097/TP.0b013e31820b6b84. [DOI] [PubMed] [Google Scholar]
- 93.Losurdo G., Castellaneta A., Rendina M., Carparelli S., Leandro G., Di Leo A. Systematic review with meta-analysis: de novo nonalcoholic fatty liver disease in liver-transplanted patients. Aliment Pharmacol Ther. 2018;47:704–714. doi: 10.1111/apt.14521. [DOI] [PubMed] [Google Scholar]
- 94.Choudhary N.S., Duseja A. Screening of cardiovascular disease in nonalcoholic fatty liver disease. J Clin Exp Hepatol. 2019 doi: 10.1016/j.jceh.2019.02.005. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhati C., Idowu M.O., Sanyal A.J. Long-term outcomes in patients undergoing liver transplantation for nonalcoholic steatohepatitis-related cirrhosis. Transplantation. 2017;101:1867–1874. doi: 10.1097/TP.0000000000001709. [DOI] [PubMed] [Google Scholar]
- 96.Narayanan P., Mara K., Izzy M. Recurrent or de novo allograft steatosis and long-term outcomes after liver transplantation. Transplantation. 2018 doi: 10.1097/TP.0000000000002317. [DOI] [PubMed] [Google Scholar]
- 97.Gitto S., de Maria N., di Benedetto F. De-novo nonalcoholic steatohepatitis is associated with long-term increased mortality in liver transplant recipients. Eur J Gastroenterol Hepatol. 2018;30:766–773. doi: 10.1097/MEG.0000000000001105. [DOI] [PubMed] [Google Scholar]
- 98.Segev D.L., Sozio S.M., Shin E.J. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transplant. 2008;14:512–525. doi: 10.1002/lt.21396. [DOI] [PubMed] [Google Scholar]
- 99.Castedal M., Skoglund C., Axelson C., Bennet W. Steroid-free immunosuppression with low-dose tacrolimus is safe and significantly reduces the incidence of new-onset diabetes mellitus following liver transplantation. Scand J Gastroenterol. 2018;53:741–747. doi: 10.1080/00365521.2018.1463390. [DOI] [PubMed] [Google Scholar]
- 100.Guillouche P., Feray C. Systematic review: anti-viral therapy of recurrent hepatitis C after liver transplantation. Aliment Pharmacol Ther. 2011;33:163–174. doi: 10.1111/j.1365-2036.2010.04505.x. [DOI] [PubMed] [Google Scholar]
- 101.Saigal S., Choudhary N.S., Saraf N. Genotype 3 and higher low-density lipoprotein levels are predictors of good response to treatment of recurrent hepatitis C following living donor liver transplantation. Indian J Gastroenterol. 2015;34:305–309. doi: 10.1007/s12664-015-0578-z. [DOI] [PubMed] [Google Scholar]
- 102.Choudhary N.S., Saigal S., Gautam D. Efficacy and safety of sofosbuvir based regimens for treatment of hepatitis C recurrence after living donor liver transplantation: an experience from India. J Clin Exp Hepatol. 2018;8:121–124. doi: 10.1016/j.jceh.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qu Y., Guo Y., Li T. Efficacy and safety of sofosbuvir-based interferon-free therapies for hepatitis C in liver transplant recipients. J Gastroenterol Hepatol. 2017;32:740–748. doi: 10.1111/jgh.13614. [DOI] [PubMed] [Google Scholar]
- 104.Gautam M., Cheruvattath R., Balan V. Recurrence of autoimmune liver disease after liver transplantation: a systematic review. Liver Transplant. 2006;12:1813–1824. doi: 10.1002/lt.20910. [DOI] [PubMed] [Google Scholar]
- 105.Lim N., Lake J. Recurrent disease after liver transplantation. Curr Hepat Rep. 2020;19:54–62. [Google Scholar]
- 106.Montano-Loza A.J., Bhanji R.A.1, Wasilenko S., Mason A.L. Systematic review: recurrent autoimmune liver diseases after liver transplantation. Aliment Pharmacol Ther. 2017;45:485–500. doi: 10.1111/apt.13894. [DOI] [PubMed] [Google Scholar]
- 107.Graziadei I.W., Wiesner R.H., Batts K.P. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology. 1999;29:1050–1056. doi: 10.1002/hep.510290427. [DOI] [PubMed] [Google Scholar]
- 108.Pandanaboyana S., Bell R., Bartlett A.J., McCall J., Hidalgo E. Meta-analysis of Duct-to-duct versus Roux-en-Y biliary reconstruction following liver transplantation for primary sclerosing cholangitis. Transpl Int. 2015;28:485–491. doi: 10.1111/tri.12513. [DOI] [PubMed] [Google Scholar]
- 109.Egawa H., Taira K., Teramukai S. Risk factors for recurrence of primary sclerosing cholangitis after living donor liver transplantation: a single center experience. Dig Dis Sci. 2009;54:1347–1354. doi: 10.1007/s10620-009-0773-9. [DOI] [PubMed] [Google Scholar]
- 110.Choudhary N.S., Saigal S., Thummala S. Good long-term outcomes in patients with Primary Sclerosing Cholangitis undergoing living donor liver transplantation. J Clin Exp Hepatol. 2020 doi: 10.1016/j.jceh.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gordon F.D., Goldberg D.S., Goodrich N.P. Recurrent primary sclerosing cholangitis in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study: comparison of risk factors between living and deceased donor recipients. Liver Transplant. 2016;22:1214–1222. doi: 10.1002/lt.24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Henson J.B., Patel Y.A., King L.Y., Zheng J., Chow S.C., Muir A.J. Outcomes of liver retransplantation in patients with primary sclerosing cholangitis. Liver Transplant. 2017;23:769–780. doi: 10.1002/lt.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duclos-Vallee J.C., Sebagh M. Recurrence of autoimmune disease, primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis after liver transplantation. Liver Transplant. 2009;15(suppl 2):S25–S34. doi: 10.1002/lt.21916. [DOI] [PubMed] [Google Scholar]
- 114.Manns M.P., Czaja A.J., Gorham J.D. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 115.Liberal R., Zen Y., Mieli-Vergani G., Vergani D. Liver transplantation and autoimmune liver diseases. Liver Transplant. 2013;19:1065–1077. doi: 10.1002/lt.23704. [DOI] [PubMed] [Google Scholar]
- 116.Bhanji R.A., Mason A.L., Girgis S., Montano-Loza A.J. Liver transplantation for overlap syndromes of autoimmune liver diseases. Liver Int. 2013;33:210–219. doi: 10.1111/liv.12027. [DOI] [PubMed] [Google Scholar]
- 117.Choudhary N.S., Tomar M., Chawla Y.K. Hepatic osteodystrophy is common in patients with noncholestatic liver disease. Dig Dis Sci. 2011;56:3323–3327. doi: 10.1007/s10620-011-1722-y. [DOI] [PubMed] [Google Scholar]
- 118.Jeong H.M., Kim D.J. Bone diseases in patients with chronic liver disease. Int J Mol Sci. 2019;20:4270. doi: 10.3390/ijms20174270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guadalix Iglesias S., Martínez Díaz-Guerra G., Hawkins Carranza F. Bone disease following liver transplant. Rev Osteoporos Metab Miner. 2010;2 1:37-46. [Google Scholar]
- 120.Leidig-Bruckner G., Hosch S., Dodidou P. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet. 2001;357:342–347. doi: 10.1016/S0140-6736(00)03641-2. [DOI] [PubMed] [Google Scholar]
- 121.Guichelaar M.M., Schmoll J., Malinchoc M., Hay J.E. Fractures and avascular necrosis before and after orthotopic liver transplantation: long-term follow-up and predictive factors. Hepatology. 2007;46:1198–1207. doi: 10.1002/hep.21805. [DOI] [PubMed] [Google Scholar]
- 122.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu. EASL Clinical Practice Guidelines: liver transplantation. J Hepatol. 2016;64:433–485. [Google Scholar]
- 123.Jabiry-Zieniewicz Z., Kaminski P., Bobrowska K. Menstrual function in female liver transplant recipients of reproductive age. Transplant Proc. 2009;41:1735–1739. doi: 10.1016/j.transproceed.2009.03.073. [DOI] [PubMed] [Google Scholar]
- 124.Sarkar M., Bramham K., Moritz M.J., Coscia L. Reproductive health in women following abdominal organ transplant. Am J Transplant. 2018;18:1068–1076. doi: 10.1111/ajt.14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mass K., Quint E.H., Puch M.R., Merion R.M. Gynecological and reproductive function after liver transplantation. Transplantation. 1996;62:476–479. doi: 10.1097/00007890-199608270-00009. [DOI] [PubMed] [Google Scholar]
- 126.Lessan-Pezeshki M., Ghazizadeh S., Khatami M.R. Fertility and contraceptive issues after kidney transplantation in women. Transplant Proc. 2004;36:1405–1406. doi: 10.1016/j.transproceed.2004.04.090. [DOI] [PubMed] [Google Scholar]
- 127.Fihn S.D., Latham R.H., Roberts P., Running K., Stamm W.E. Association between diaphragm use and urinary tract infection. J Am Med Assoc. 1985;254:240–245. [PubMed] [Google Scholar]
- 128.Estes C.M., Westhoff C. Contraception for the transplant patient. Semin Perinatol. 2007;31:372–377. doi: 10.1053/j.semperi.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 129.Zerner J., Doil K.L., Drewry J., Leeber D.A. Intrauterine contraceptive device failures in renal transplant patients. J Reprod Med. 1981;26:99–102. [PubMed] [Google Scholar]
- 130.Ortiz M.E., Croxatto H.B. Copper-T intrauterine device and levonorgestrel intrauterine system: biological bases of their mechanism of action. Contraception. 2007;75:S16–S30. doi: 10.1016/j.contraception.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 131.Huguelet P.S., Sheehan C., Spitzer R.F., Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception. 2017;95:378–381. doi: 10.1016/j.contraception.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 132.Jabiry-Zieniewicz Z., Bobrowska K., Kaminski P. Low-dose hormonal contraception after liver transplantation. Transplant Proc. 2007;39:1530–1532. doi: 10.1016/j.transproceed.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 133.Sivaprasadan S., Mathew J.S., Surendran S., Padma U.D. Pregnancy after liver transplantation: outcomes from a single-center experience. J Clin Exp Hepatol. 2019 doi: 10.1016/j.jceh.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prodromidou A., Kostakis I.D., Machairas N. Pregnancy outcomes after liver transplantation: a systematic review. Transplant Proc. 2019;51:446–449. doi: 10.1016/j.transproceed.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 135.Jabiry-Zieniewicz Z., Dabrowski F.A., Pietrzak B. Pregnancy in the liver transplant recipient. Liver Transplant. 2016;22:1408–1417. doi: 10.1002/lt.24483. [DOI] [PubMed] [Google Scholar]
- 136.Malat G., Culkin C. The ABCs of immunosuppression: a primer for primary care physicians. Med Clin. 2016;100:505–518. doi: 10.1016/j.mcna.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 137.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu; European association for the study of the liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 138.Hussaini T., Erb S., Yoshida E.M. Immunosuppressive pharmacotherapy in liver transplantation. AME Med J. 2018;3:18. [Google Scholar]
- 139.Zhelyazkova-Savova M., Gancheva S., Sirakova V. Potential Statin-Drug Interactions: Prevalence and Clinical Significance. Springer Plus. 2014;3:168. doi: 10.1186/2193-1801-3-168. [DOI] [PMC free article] [PubMed] [Google Scholar]