Abstract
Background and aims
Autoimmune hepatitis presenting as acute on chronic liver failure (AIH-ACLF) is a novel entity with limited data on clinical course and management. We assessed outcomes in patients of AIH-ACLF with no extrahepatic organ dysfunction/failure when administered steroids.
Methods
In this retrospective analysis, clinical data, laboratory parameters, liver biopsy indices and prognostic scores such as model for end-stage liver disease (MELD) and Child–Turcotte–Pugh (CTP) scores at baseline were computed for patients with AIH-ACLF and compared across strata of incident infections and transplant-free survival. The primary outcome was 90-day transplant-free survival. Biochemical remission was assessed, and predictors of end points were identified.
Results
Twenty-nine patients of AIH-ACLF were included with a median follow-up of 4 months. The 90- and 180-day transplant-free survival rates of 55.2 [95% confidence interval (CI): 39.7–76.6]% and 30.2(95% CI: 16.7–54.6)%, respectively, were attained on steroids. Three patients (10.3%) underwent liver transplant while 16 (55.2%) deaths occurred. Infections developed in 12 patients (41.3%), leading to worsening prognostic scores, new onset organ dysfunction/failure and 11 deaths. Seven of ten patients (70%) in the transplant-free survivor group attained biochemical remission on follow-up. The MELD score<24 (sensitivity: 68.4%; specificity: 80%) and CTP<11 (sensitivity: 78.9%; specificity: 90%) had best predictive value for survival, in addition to decrease in the MELD score at 2 weeks (sensitivity: 78.9%; specificity: 70%).
Conclusion
Patients with AIH-ACLF have a morbid disease course despite treatment with steroids. Patients with no extrahepatic organ failure with good baseline prognostic scores may be administered steroids with close monitoring for change in MELD over 2 weeks.
Keywords: autoimmune hepatitis, acute on chronic liver failure, transplant free survival, MELD score, infections
Abbreviations: ACLF, Acute on chronic liver failure; AIH, Autoimmune hepatitis; AKI, Acute kidney injury; ALF, Acute liver failure; ALT, Alanine transaminase; ALP, Alkaline phosphatase; ANA, Antinuclear antibody; APASL, Asian Pacific Association for the Study of the Liver; AS-AIH, Acute severe autoimmune hepatitis; ASMA, Anti-smooth muscle antibody; AST, Aspartate transaminase; AUROC, Area under receiver–operator characteristics curve; CI, Confidence interval; CLIF-OF, Chronic liver failure-organ failure; CTP, Child–Turcotte–Pugh; DILI, Drug-induced liver injury; HAI, Histological activity index; HE, Hepatic encephalopathy; IgG, Immunoglobulin G; INR, International normalised ratio; IQR, Interquartile range; LKM-1, Liver–kidney microsome; LT, Liver transplant; MELD, Model for end-stage liver disease; ROC, Receiver–operator characteristics curve; SBP, Spontaneous bacterial peritonitis; TLC, Total leucocyte count
Autoimmune hepatitis (AIH) is a chronic inflammatory disease of liver characterised by circulating autoantibodies, interface hepatitis on histology and elevated immunoglobulin levels.1 Acute icteric presentation particularly with coagulation abnormalities in the absence of cirrhosis [acute severe AIH (AS-AIH)]2, 3, 4, 5, 6 or with associated encephalopathy [AIH associated acute liver failure (AIH-ALF)]7,8 are distinct entities that requires urgent addressal of treatment. Although AS-AIH has been described from the West3, 4, 5 and by Japanese cohorts,6,8,9 AIH presenting acutely as acute on chronic liver failure (ACLF) is an entity described from many parts of the world.10, 11, 12 The new onset of clinical decompensations such as ascites and/or hepatic encephalopathy (HE) after an acute flare separates AIH-ACLF from other clinical forms of severe AIH.
Management of severe forms of AIH is challenging and depends upon the severity of disease. While the efficacy of corticosteroids, risk of infections and appropriate timing of liver transplant (LT) are established in AS-AIH and in AIH-ALF in multiple studies,3,5,6 limited information exists regarding management of patients who present with ACLF. The association of persistent inflammation, increased predisposition to infections and high propensity for extrahepatic organ dysfunction/failure with an underlying cirrhotic liver makes ACLF a distinct entity from other forms of acute hepatic insults.13 Presently, limited evidence from a single study found steroids to improve the outcome in 75% of patients with AIH-ACLF with less severe disease with no increase in post-treatment infections.12 However, this efficacy needs to be validated in other cohorts before use of steroids in this condition can be recommended. In addition, there is a need to determine predictors of outcome, especially in patients without extrahepatic dysfunction, because this is the subgroup among those presenting as ACLF, which is more likely to benefit from steroids.
The present study evaluated outcomes in patients with AIH who presented as ACLF without evidence of extrahepatic organ dysfunction/failure when administered steroids in terms of transplant-free survival and biochemical remission. In addition, determinants of outcomes on steroids were identified in this relatively homogenous risk group.
Methodology
In this single-centre study from a tertiary care centre, all AIH patients presenting as ACLF as per the Asian Pacific Association for the Study of the Liver (APASL)14 between April 2014–December 2019 were evaluated for inclusion after exclusion of other causes, and those with ACLF without extrahepatic organ dysfunction/failure and given steroids were included. Previously diagnosed and treated patients of AIH (before current presentation) and those diagnosed with cirrhosis in the past were excluded from the study. In addition, patients who 1) had evidence of infection at baseline, 2) had extrahepatic organ dysfunction/failure such as acute kidney injury (AKI), HE, respiratory failure and/or shock and 3) had previous decompensations such as ascites, variceal bleed and/or HE were excluded from the study. This study was a retrospective analysis from this prospectively maintained database of patients following up in our liver clinic. All procedures performed were in accordance with the ethical standards of the institutional ethics committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was waived off in view of the retrospective nature of the study.
Definitions
Autoimmune hepatitis was suspected based on constellation of clinical presentation, serology and biopsy features suggestive of AIH. Other aetiologies of liver disease such as alcoholic liver disease, nonalcoholic fatty liver disease, chronic viral hepatitis B or C, Wilson's disease and vascular diseases of liver were excluded based on appropriate history, blood tests, imaging and liver biopsy. As patients may not have positive serologies and/or elevated immunoglobulin G levels when presenting as ACLF, definitive diagnosis was made on conducting liver biopsy. Biopsy findings suggestive of AIH were interface hepatitis, lymphoplasmacytic infiltrate and/or emperipolesis.15 In addition, biopsies were reviewed for features such as centrizonal necrosis, central perivenulitis, lymphoid follicles and intense lymphoplasmacytic infiltrate (as proposed by Stravitz et al.7 for AIH presenting as ALF) as markers of acute injury in AIH.16 Disease activity was calculated via modified histological activity index (HAI)17 for chronic hepatitis with HAI ≥ 4 suggestive of active disease. All biopsies were reviewed for grades of fibrosis.17 ACLF was defined according to APASL definition of ACLF.14 Organ dysfunction/failure was defined as per the ACLF consensus recommendations of APASL.18 The following definitions were used:
-
1)
AKI: renal dysfunction if serum creatinine ≥ 1.1 mg/dL and renal failure if serum creatinine ≥ 1.5 mg/dL
-
2)
HE (as per West Haven criteria): cerebral dysfunction if HE grade is 1 or 2 and cerebral failure if HE grade is 3 or 4;
-
3)
cardiovascular failure: mean arterial pressure < 70 mmHg or requirement of vasopressors to maintain arterial blood pressure and
-
4)
pulmonary failure: ratio of partial pressure of oxygen (mmHg) to fraction of inspired oxygen ratio to be < 400 or requirement of mechanical ventilation for respiratory failure.
Biochemical remission was defined as complete normalisation of liver enzymes in those who had elevated transaminases before treatment.2
Data Collection
All clinical details, laboratory parameters, biopsy reports and treatment-related outcomes were retrieved from a prospectively maintained database. The clinical records were reviewed for duration of presenting symptoms, details of clinical decompensation and any previous treatment received. For evaluating acute precipitants, acute viral hepatitis was diagnosed based on serological records for hepatitis A, B and E. History of use of complementary and alternative medicines and other drugs before the onset of acute flare was assessed for diagnosing drug-induced liver injury. Prognostic scores such as chronic liver failure-organ failure (CLIF-OF),19 the Child–Turcotte–Pugh (CTP) score, and the model for end-stage liver disease (MELD) score were calculated from baseline parameters. The APASL ACLF Research Consortium (AARC) score was not calculated because of the nonavailability of baseline lactate values. Varices were classified in accordance with Baveno VI recommendations.20 Any varix larger than 5 mm in size, presence of red colour signs on varix irrespective of size and presence of varix in Child C cirrhosis were classified as high-risk varices. All other varices were classified as low-risk varices.
Management Strategy
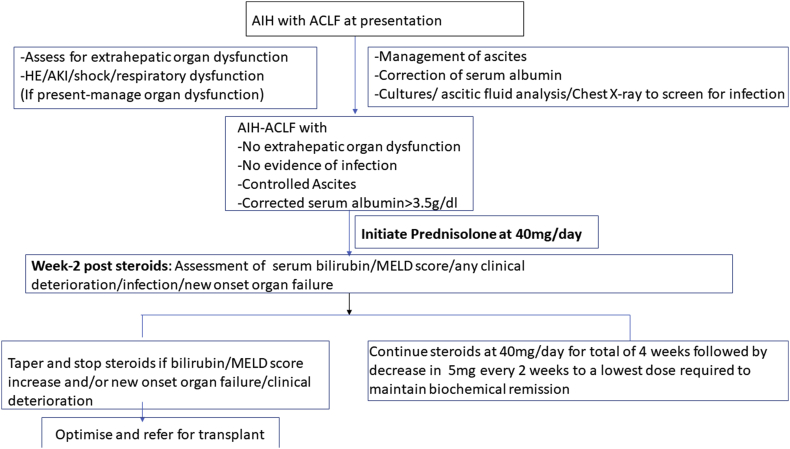
Patients were initially assessed for severity and the type of organ dysfunction/failure associated with acute insult and severity of manifestations of underlying chronic liver disease (CLD). All patients were screened at baseline for evidence of infection21: ascitic fluid was aspirated for ruling out spontaneous bacterial peritonitis (SBP); in addition chest X-ray and blood/urine cultures were obtained. Those who had evidence of extrahepatic organ dysfunction/failure (defined earlier) and/or overt decompensation such as gross ascites despite fluid mobilisation were not given steroids and were listed for LT. Patients with ascites and those with hypoalbuminemia received 20% human albumin twice or thrice a week for fluid mobilisation. In patients with no evidence of infection and after correction of serum albumin (>3.5 g/dl), oral steroids were started at dose of 40 mg per day. None of the patient included in the study was initiated on IV steroids. In addition, along with steroids, oral cephalosporins were given for 7 days. After institution of steroids, patients were closely monitored as inpatients (for first 7 days) and subsequently on a weekly out-patient basis for any evidence of change in clinical condition (improvement/deterioration), biochemical parameters, the MELD score and/or development of infection. Steroids were continued in the same dose for 4 weeks followed by taper off by 5 mg every 2 weeks until a dose of 5–10 mg was attained, which was continued for a prolonged time period. No improvement in the serum bilirubin/MELD score, despite 2 weeks of treatment, was taken as non-response. In patients with non-response, steroids were tapered and discontinued. Deterioration of clinical symptoms, new onset/worsening decompensation, new onset organ failure and/or suspicion of new infection were other indications for discontinuation of steroids.2,22 Listing for transplant was done in case of no response/deterioration on treatment with steroids after control of underlying infection. Patients were followed up till their last follow-up visit or attainment of primary outcome (mortality or LT). An outline of the management protocol has been summarised in Supplementary Figure 1.
Outcome
The primary outcome in this study was assessment of 90-day transplant-free survival in patients with AIH-ACLF treated with steroids. Secondary outcomes were 1) 180-day transplant-free survival, 2) assessment of predictors of transplant-free survival in patients treated with steroids, 3) occurrence of infections and its impact on outcomes, 4) changes in serum liver functions and the MELD score at 2 weeks of treatment and 5) incidence of biochemical remission in patients with transplant-free survival.
Statistical analysis
The baseline data were recorded as number (%) or mean ± standard deviation or median (interquartile range) as appropriate, based on the normalcy of distribution. Baseline parameters were compared using the chi-square test/Fischer's exact test for categorical variables and Student's t-test for continuous variables with normal distribution. Continuous variables with non-normal distribution were compared using independent samples Mann-Whitney U test. For all statistical tests, a P value < 0.05 was considered statistically significant. Three-month and 6-month transplant-free survival rates were computed using Kaplan-Meier analysis, for overall cohort and across strata of whether or not incident infections occurred. Survival was compared using the log-rank test, with Kaplan-Meier plots to represent survival across strata.
Linear mixed effects models were constructed using infection status and time as fixed effects and patient ID as the random effect for assessment of change in various biochemical parameters and prognostic indices over the first month of treatment with steroids. Results were represented using profile plots with significance of time and different strata represented for each comparison.
The role of prognostic scores such as MELD and CTP for predicting survival was further assessed using receiver–operator characteristics (ROC) curve, and optimal cut-offs for each predictor were identified using Youden's index. Performance characteristics of optimal cut-offs and area under ROC (AUROC) of respective predictors were reported for comparison of accuracy.
The data were entered using Microsoft Excel 2011 and were analysed using Rstudio. In addition to the base packages in R, plotROC, OptimalCutpoints, Survival, survminer, lme4, car and Tidyverse packages were used.
Results
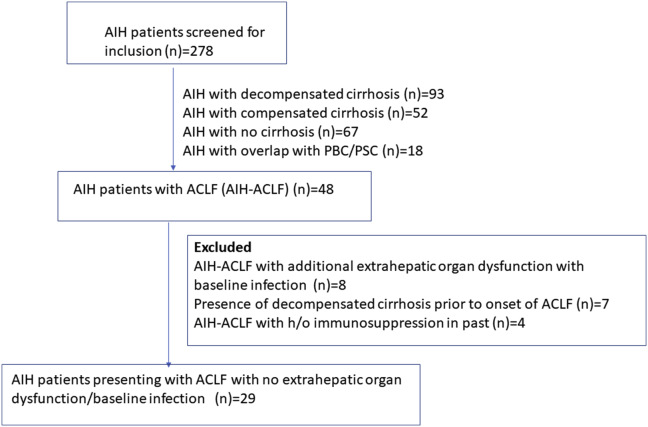
A total of 278 patients with AIH were screened for the purpose of inclusion in this study. Of these, 48 patients had ACLF according to APASL definition. Twenty-nine of these patients with AIH-ACLF without extrahepatic organ dysfunction and with no evidence of baseline infection were administered steroids and were included in this study (Supplementary Figure 2).
Baseline Characteristics and Outcome of the Cohort (Table 1)
Table 1.
Baseline Characteristics and Outcomes in Patients With AIH-ACLF Stratified by Transplant-Free Survival Status.
| Variable | Survivors (n = 10) | Death/transplant (n = 19) | Significance |
|---|---|---|---|
| Age | 28.6 ± 9.6 | 40.6 ± 17.6 | 0.024 |
| Men | 4 (40%) | 6 (31.6%) | 0.65 |
| Duration of jaundice | 45 (30–66) | 40 (25–60) | 0.604 |
| Ascites at presentation | 10 (100%) | 19 (100%) | – |
| EGD | |||
| No varices | 3 (30%) | 0 (0%) | 0.024 |
| Low risk | 5 (50%) | 6 (31.6%) | |
| High risk | 2 (20%) | 13 (68.5%) | |
| Biochemical parameters | |||
| Bilirubin (mg/dl) | 8.2 (6.7–10) | 12 (8–18) | 0.126 |
| AST (IU/dl) | 148 (68–175) | 200 (120–280) | 0.126 |
| ALT (IU/dl) | 88 (78–120) | 164 (120–246) | 0.04 |
| ALP (IU/dl) | 205 (170–280) | 244 (205–300) | 0.247 |
| Albumin (g/dl) | 3 ± 0.3 | 2.7 ± 0.3 | 0.042 |
| Creatinine (mg/dl) | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.236 |
| INR | 1.9 ± 0.6 | 2.3 ± 0.6 | 0.086 |
| Haemoglobin (g/dl) | 9.5 ± 1.4 | 9.6 ± 1.7 | 0.851 |
| TLC (mm3) | 6600 (4800–9300) | 5500 (4200–5600) | 0.062 |
| Platelet (X1000/mm3) | 88 (80–120) | 80 (78–96) | 0.429 |
| ANA titre (1:80 or more) | 2 (20%) | 13 (68.4%) | 0.013 |
| IgG levels (upto 1.6 g/dl) | 2.3 ± 0.6 | 2.2 ± 0.5 | 0.796 |
| Acute precipitant | 0.373 | ||
|
8 (80%) | 17 (89.5%) | |
|
1 (10%) | 1 (5.3%) | |
|
1 (10%) | 1 (5.3%) | |
| Baseline prognostic scores | |||
| CLIF-OF score | 7 (7–8) | 8 (7–10) | 0.031 |
| CTPscore | 10 (9–10) | 11 (11–12) | 0.001 |
| MELD score | 21.5 (18–23) | 25 (22–29) | 0.008 |
| MELD sodium score | 22 (18–25) | 27 (24–30) | 0.005 |
| Liver biopsy findings | |||
| Modified HAI | 7 (6–8) | 7 (5–8) | 0.982 |
| Bile duct injury | 7 (70%) | 10 (52.6%) | 0.367 |
| Intracytoplasmic/intracanalicular cholestasis | 4 (40%) | 8 (42.1%) | 0.913 |
| F4 fibrosis | 10 (100%) | 19 (100%) | |
| Outcomes | |||
| Duration of follow-up/event (days) | 180 (120–360) | 60 (60–140) | <0.001 |
| Liver transplant | 0 (0%) | 3 (15.8%) | |
| Infections | 1 (10%) | 11 (57.9%) | 0.013 |
|
0 (0%) | 1 (5.3%) | |
|
0 (0%) | 2 (10.5%) | |
|
1 (10%) | 8 (42.1%) | |
| Biochemical remission | 7 (70%) | 1 (5.3%) | <0.001 |
Data is presented as mean ± standard deviation or median (interquartile range) for quantitative variables and n (%) for qualitative variables unless otherwise specified. List of abbreviations: ACLF, acute on chronic liver failure; AKI, acute kidney injury; ANA, antinuclear antibody; ASMA, anti-smooth muscle antibody; ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate transaminase; CLIF-OF, chronic liver failure-organ failure; CTP, Child–Turcotte–Pugh; GI bleed, gastrointestinal bleed; HAI, histological activity index; HE, hepatic encephalopathy; IgG, immunoglobulin G; INR, international normalised ratio; LKM-1, liver–kidney microsome; MELD, model for end-stage liver disease; SBP, spontaneous bacterial peritonitis; TLC, total leucocyte count.
Patients were predominantly women (19/29, 65.5%) with an average age of 36.5 ± 16.1 years. The median duration of symptoms (jaundice) before presentation was 45 (25–66) days. Auto-antibody positivity was seen in only 51.2% of patients. Flare of underlying AIH was the most common acute precipitant identified in 25 (86.2%) patients. Two patients had history of anti-tubercular therapy use, whereas remaining two patients had positive serology for IgM HEV. All patients including these four with different acute precipitants were confirmed to have AIH on liver biopsy with active disease. Liver biopsies were done via the transjugular route. On biopsy, the median modified HAI score was 7 (6–8). Histological evidence of bile duct injury and intracanalicular/intracytoplasmic cholestasis was present in 58.6% and 41.3% patients, with two patients having bridging necrosis. All the included patients had cirrhosis (F4 fibrosis) on liver biopsy. Of 29 patients started on steroids, 20 required discontinuation of steroids over the period of follow-up. Most common indication for discontinuation was infection in 12 patients followed by no improvement/deterioration in clinical/biochemical parameters in eight patients.
Primary Outcome
The primary outcome of the 90-day transplant-free survival rate was 55.2 (95% CI: 39.7–76.6)%. In addition, 30.2 (95% CI: 16.7–54.6) % patients had survived by 6 months.
Secondary Outcomes
Comparison of Transplant-free Survivors and Non-Survivors/Patients Undergoing LT
Transplant-free survivors were more likely to be younger (mean age: 28.6 versus 40.6 years; P = 0.024), have higher serum albumin (mean albumin: 3.0 g/dl versus 2.7 g/dl; P = 0.042) and less frequent antinuclear antibody positivity (20% versus 68.4%; P = 0.013). They were also likely to have less frequent high-risk varices, a lower CTP score (median: 10 versus 11; P = 0.001) and a lower MELD score (median: 21.5 versus 25; P = 0.008) at presentation. Rest of the baseline parameters including serum bilirubin and international normalised ratio were statistically similar in both groups. On comparison of outcomes with respect to presence/absence of varices, all three patients without varices had survived whereas seven of 26 patients with varices survived at 90 days and at 180 days.
Infections
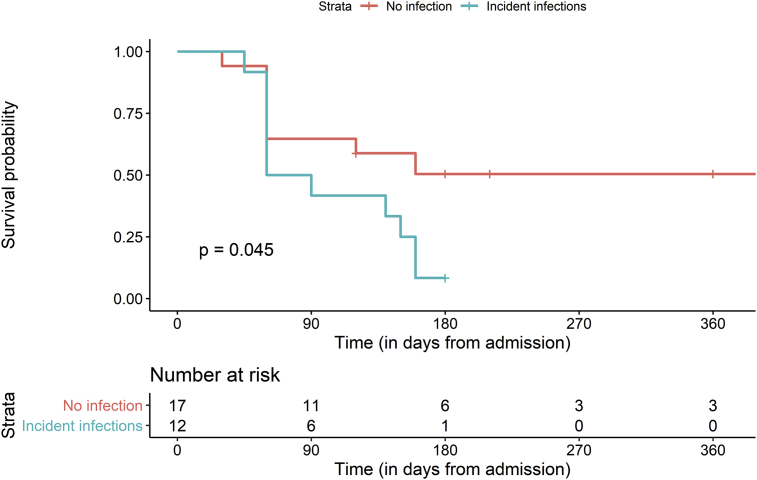
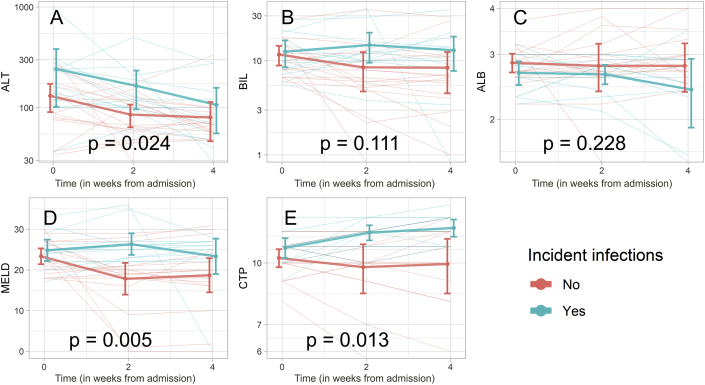
Infections developed in 12 of 29 patients (41.3%) of AIH-ACLF over a median duration of 60 (50–145) days. Nine of these 12 patients (75%) had bacterial pneumonia, two were diagnosed with SBP and one patient developed bacteraemia in the absence of any localisation. Infections developed less frequently in transplant-free survivors [1 infection (10%) in transplant-free survivors versus 11 (57.9%) in those who succumbed/required transplant, P = 0.013]. Overall outcomes were significantly worse in patients developing incident infections (Table 2 and Figure 1), with 90- and 180-day survival of 41.6% and 8.3% among those with incident infections in comparison with 64.7% and 50.4% among those without them (log-rank test; P = 0.045). Refractory sepsis with multiorgan dysfunction was the cause of death in 68.7% (11 of 16) patients who succumbed. Patients who developed infections were essentially similar at presentation in comparison with those who did not, with the exception of lower platelet counts (median: 80,000/mm3 in the infected and 90,000/mm3 in the non-infected group; P = 0.021) and a trend towards worse liver function parameters (Table 3). Despite statistically similar values at baseline, linear mixed-effects models of follow-up data(Figure 2) after treatment with steroids showed significant differences in the MELD score (P = 0.005), CTP score (P = 0.014) and alanine transaminase (P = 0.025) over time in those who developed incident infections when compared with those who did not. Steroids were stopped in all patients who developed intercurrent infections.
Table 2.
Survival at 90-Day and 180-DayFollow-Up in Overall Cohort of Patients With AIH-ACLF and in Strata of Whether or Not Incident Infections Occurred. Significance was Estimated Using The Log-Rank Test.
| Group | 90-day survival | 180-day survival | Significance |
|---|---|---|---|
| Overall cohort (n = 29) | 55.2% (39.7%–76.6%) | 30.2% (16.7%–54.6%) | |
| Infection strata | 0.045 | ||
| Incident infections (n = 12) | 41.6% (21.3%–81.4%) | 8.33% (1.3%–54.4%) | |
| No infections (n = 17) | 64.7% (45.5%–91.9%) | 50.4% (30.6%–83.1%) |
Figure 1.
Kaplan–Meier plots demonstrating transplant-free survival in patients with AIH-ACLF stratified by whether or not incident infections occurred. Time on x-axis is in days from steroid initiation.
Table 3.
Baseline Characteristics and Outcomes in Patients With AIH-ACLF Stratified by Whether or Not Incident Infections Occurred.
| Variables | No incident infections (n = 17) | Incident infections (n = 12) | P value |
|---|---|---|---|
| Age (years) | 35.4 ± 17.2 | 38 ± 15.1 | 0.672 |
| Males | 8 (47.1%) | 2 (16.7%) | 0.09 |
| Duration of jaundice (days) | 45 (20–66) | 40 (30–60) | 0.602 |
| Ascites at presentation | 17 (100%) | 12 (100%) | – |
| EGD | 0.088 | ||
|
3 (17.6%) | 0 (0%) | |
|
7 (41.2%) | 4 (33.3%) | |
|
7 (41.2%) | 8 (66.7%) | |
| Biochemical parameters | |||
| Bilirubin (mg/dl) | 10 (8–15) | 9.7 (7.6–17.5) | 0.948 |
| AST (IU/dl) | 142 (112–175) | 242.5 (135–353.5) | 0.059 |
| ALT (IU/dl) | 120 (78–164) | 199 (110–246) | 0.088 |
| ALP (IU/dl) | 220 (180–286) | 242 (218.5–300) | 0.37 |
| Albumin (g/dl) | 2.8 ± 0.3 | 2.7 ± 0.3 | 0.195 |
| INR | 2.1 ± 0.6 | 2.3 ± 0.7 | 0.655 |
| Haemoglobin (g/dl) | 9.6 ± 1.7 | 9.5 ± 1.6 | 0.934 |
| TLC (mm3) | 5600 (4300–6600) | 5550 (4340–6200) | |
| Platelet (X1000/mm3) | 90 (80–120) | 80 (63–85) | 0.021 |
| ANA (≥1:80) | 7 (41.2%) | 8 (66.7%) | 0.176 |
| IgG (g/dl) | 2.2 ± 0.5 | 2.4 ± 0.4 | 0.317 |
| Acute variceal bleed | 2 (11.8%) | 1 (8.3%) | 0.765 |
| Acute precipitant | 0.286 | ||
|
16 (94.1%) | 9 (75%) | |
|
1 (5.9%) | 1 (8.3%) | |
|
0 (0%) | 2 (16.7%) | |
| Baseline prognostic scores | |||
| CLIF-OF | 8 (7–9) | 8 (7–9.5) | 0.647 |
| CTPscore | 10 (10–11) | 11 (10–11.5) | 0.211 |
| MELD | 23 (20–27) | 24.5 (22–27.5) | 0.471 |
| Liver biopsy findings | |||
| HAI | 8 (7–8) | 6 (0–8) | 0.117 |
| Bile duct injury | 11 (64.7%) | 6 (50%) | 0.426 |
| Intracytoplasmic/intracanalicular Cholestasis |
7 (41.2%) | 5 (41.7%) | 0.979 |
| Outcomes | |||
| Duration of follow-up (days) | 120 (60–180) | 75 (60–155) | 0.283 |
| Transplant-free survival | 9 (52.9%) | 1 (8.3%) | 0.029 |
| Transplants | 2 (11.8%) | 1 (8.3%) | 0.43 |
| Biochemical remission | 7 (41.2%) | 1 (8.3%) | 0.051 |
Data is presented as mean ± standard deviation or median (interquartile range) for quantitative variables and n (%) for qualitative variables unless otherwise specified. List of abbreviations: ACLF, acute on chronic liver failure; AKI, acute kidney injury; ANA, antinuclear antibody; ASMA, anti-smooth muscle antibody; ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate transaminase; CLIF-OF, chronic liver failure-organ failure; CTP, Child–Turcotte–Pugh; GI bleed, gastrointestinal bleed; HAI, histological activity index; HE, hepatic encephalopathy; IgG, immunoglobulin G; INR, international normalised ratio; LKM-1, liver–kidney microsome; MELD, model for end-stage liver disease; SBP, spontaneous bacterial peritonitis; TLC, total leucocyte count.
Figure 2.
Profile plots demonstrating changes in (A) ALT, (B) bilirubin, (C) albumin, (D) MELD and (E) CTP based on development of incident infections. Weighted lines represent mean and standard deviation (error bars) of parameters at different time points. Time on x-axis is in weeks from steroid initiation. Significance is provided for association of infection with respective parameters. ALB, albumin; ALT, alanine transaminase; BIL, bilirubin; CTP, Child–Turcotte–Pugh; MELD, model for end-stage liver disease.
New Onset Organ Failure
Overall new organ dysfunction/failure developed in 16 (59.2%) patients over the period of follow-up, grade-III/IV HE being the most common (14/16 patients; 87.7%). Five patients developed AKI, and four patients developed circulatory failure. Infection was identified as the cause of organ failure in 12 (75%) of these patients while it was attributed to variceal bleed in 2 patients. In the remaining two patients, no cause could be determined.
Biochemical Remission
Patients who survived beyond three months were assessed for biochemical remission. Biochemical remission occurred in 7 of 10 patients (70%) in the transplant-free survivor group over a follow-up duration of 6 (4–12) months. One patient in the other group also developed biochemical remission. However, that patient developed infection resulting in new onset organ failure and succumbed to the illness.
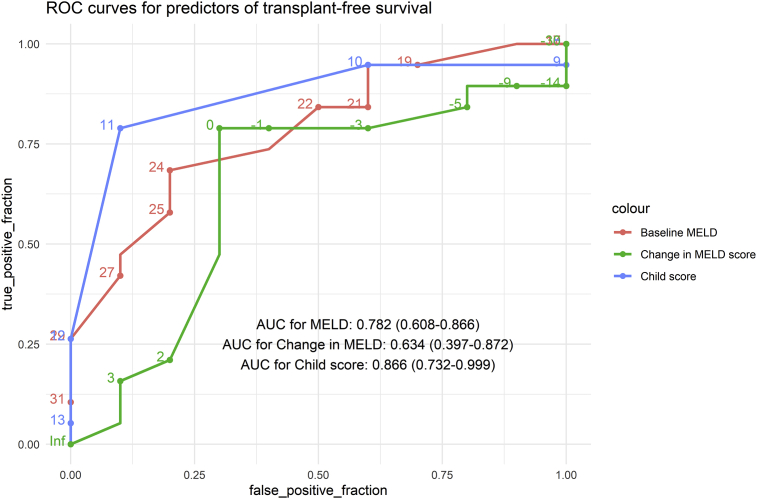
Determinants of Outcomes (Figure 3)
Figure 3.
Receiver–operator characteristics curve demonstrating the role of the baseline MELD (red), baseline CTP score (blue) and change in MELD at 2 weeks (green) for predicting survival. Area under curves and 95% confidence intervals are provided. ACLH, acute on chronic liver failure; AIH, autoimmune hepatitis; AKI, acute kidney injury; CTP, Child–Turcotte–Pugh; HE, hepatic encephalopathy; MELD, model for end-stage liver disease.
Performance of different scores for predicting transplant-free survival in steroid-treated AIH patients with ACLF was assessed by plotting ROC curves. The AUROC for the baseline CTP score and MELD score for predicting survival was 0.866 (0.732–0.999) and 0.782 (0.608–0.955), respectively. A cut-off of the MELD score of 24 had 68.4 (43.4–87.4) % sensitivity and 80.0 (44.4–97.4) % specificity for predicting mortality. Similarly, the CPT score of 11 had a sensitivity of 78.9 (54.4–93.9) % and specificity of 90.0 (55.5–99.7) % for predicting mortality.
For assessment of dynamic parameters, change in the MELD score at 2 weeks was also included, which had an AUROC of 0.634 (0.397–0.872). Worsening of the MELD score at 2 weeks had 78.9 (54.4–93.9) % sensitivity and 70.0 (34.7–93.3) % specificity for predicting death/need for LT.
Discussion
Presentation of AIH as ACLF is a relatively new entity, increasingly being described from this part of the world.10, 11, 12 The complex interplay of persistent inflammation and increased predisposition to infection in a background of a previously compromised liver makes management of acute flare of AIH with consequent ACLF a challenge, with a need to balance expected benefits of corticosteroids with the risks of infection. In the present study of AIH patients presenting with ACLF without extrahepatic organ dysfunction/failure, transplant-free survival rates of 55% and 30% were realized at 90 days and 180 days, respectively, upon treatment with steroids. Along with baseline severity of liver disease (higher CPT and MELD scores), no change/worsening of prognostic scores at 2 weeks was a major determinant of poor outcome upon treatment with steroids.
ACLF can have varied presentations.14 Patients presenting with higher grades of ACLF often have multiple organ dysfunction and associated infections.21 Involvement of the extrahepatic organ is a critical event as it substantially increases mortality,23 and steroids are unlikely to have significant impact on such advanced disease, with such patients best managed with supportive treatment. On the other hand, patients with organ failure limited to the liver are most likely to benefit from steroids, and this defines a relatively homogenous risk group which was included in this study. Our study shows that 90-day transplant-free survival in these patients was seen in only 55% of patients, with transplant-free survivors having significantly lower MELD and CTP scores at presentation. MELD less than 24 and CTP less than 11 at presentation, along with improvement in the MELD score at 2 weeks, had best accuracy to predict transplant-free survival. Our results are slightly different from the only other study on impact of steroids in AIH-ACLF outcomes, where 90-day survival was seen in about 75% patients.12 While patients in that study had a higher MELD score and associated HE (excluded in present study), cirrhosis (F4 disease) on liver biopsy was seen in only 39% of their patients compared with 100% of patients in the present study. This difference in underlying liver histology may be responsible for the difference in the survival in these two studies.
Post-treatment infections developed in 41.3% of patients receiving steroids despite administration of prophylactic antibiotics. Overall outcomes were significantly worse in those who developed infection with more frequent multi-organ dysfunction and lower 90-day survival. Patients who did not develop post-treatment infections had better outcomes, with significant decline in MELD and aminotransferase levels with steroids compared with those who developed infections. However, this improvement in the MELD score was evident as early as 2 weeks after treatment initiation, whereas most post-treatment infections developed after 2 months. Therefore, it is plausible that infections may be a consequence to the lack of improvement of liver disease despite treatment rather than being directly responsible for poor response. Similar experience has been seen with treatment with steroids in severe alcoholic hepatitis.24 Previous studies evaluating risk of infections with corticosteroids in severe forms of AIH had increased risk of sepsis in steroids treated patients in AS-AIH,3,5 but not in AIH-ACLF.12 From our study, it can be concluded that development of infection signalled poor response to steroids. However, in the absence of the control group of untreated patients of AIH-ACLF, the definite role of steroids in predisposition to infection cannot be ascertained.
Long-term outcomes such as biochemical remission become important in those patients who survive the initial phase of disease, and its attainment is important in AIH-ACLF to prevent subsequent flares. In this regard, biochemical remission was seen in 70% of patients who survived, indicating definite benefit in those who tolerate steroids well in the initial phase.
The findings of our study bring us back to the question whether steroids should be administered in AIH-ACLF. While the earlier study supported the use of steroids in patients with less severe disease (MELD<27) similar to our cohort (MELD>24; poor outcome), we realized much lower survival rates compared with their study. Based on our results, we suggest that the decision to treat with steroids should be based on baseline disease severity, and in addition, close observation should be continued for change in indices of severity of liver disease. Poor responders can be identified as early as 2 weeks after treatment with a need to stop steroids and consider them for early LT. For those who respond, a large fraction of them achieve biochemical remission.
This study had some important limitations. First, this is a retrospective study, and it is possible that few outcomes may have been missed. Second, in the absence of a control group of patients with similar disease profile who are not administered steroids, survival advantage and role of steroids in predisposition to infection cannot be conclusively demonstrated. We did not include the untreated ACLF cohort as a definitive control group because of presence of extrahepatic organ failure and sepsis in them. Third, we had defined ACLF according to APASL definition but used the ACLF-specific score (CLIF-OF) defined using EASL-CLIF definition owing to inadequate data for the AARC score. However, there is limited consensus in the literature regarding the definition and prognostic scores in ACLF, and MELD/CTP scores may have good prognostic value in these patients. Fourth, in view of a small sample size, no definite baseline predictors for post-treatment infection could be identified, and multivariable adjusted analysis could not be conducted. Fifth, it is possible that patients with ACLF may harbour infection even in absence of positive culture. With the present manuscript, we cannot decipher the role of baseline infections in determining the outcome to steroids in AIH-ACLF. Sixth, we used empirical antibiotics with of initiation of steroids. This was done as infections are difficult to diagnose in patients with ACLF and can lead to rapid deterioration after initiation of steroids. Antibiotics were initiated as per our management protocol of AIH-ACLF to cover for any infection. However, this practice is unsupported by evidence, and recommendation for this algorithm cannot be proposed based on the current evidence. Similarly, albumin was given in the management of ascites as per our protocol for management of ascites in ACLF before initiation of steroids. Although evidence is emerging regarding role of albumin in management of uncomplicated ascites,25 its administration currently is not the standard of care and, therefore, from the present manuscript its role in ACLF cannot be recommended. From our study, it is not clear how many patients would have had elevation in serum albumin, as only long-term albumin administration has been associated with increase in serum albumin.25, 26, 27, 28 The definitive role of short-term albumin administration (as in the present study) besides raising the oncotic pressure is unclear.
Further studies with larger sample size in the presence of a control group are required to address these issues.
In conclusion, in AIH-ACLF patients without extrahepatic organ dysfunction or baseline infections, a trial of steroids can be attempted, with relatively good outcomes in patients with better baseline prognostic scores (MELD < 24, CTP<11). These patients should be closely monitored, and early LT should be considered in patients who have no improvement in the MELD score at 2 weeks after treatment. Risk of infections remains a major concern in patients who do not respond to steroids.
Ethical clearance
Obtained.
CRediT authorship contribution statement
Sanchit Sharma: Formal analysis, interpretation of data, statistical analysis, Writing - original draft, acquisition of data, Samagra Agarwal, Formal analysis, interpretation of data, statistical analysis, Writing - original draft, acquisition of data, Srikant Gopi, Formal analysis, interpretation of data, acquisition of data, Writing - original draft, Abhinav Anand, acquisition of data, Writing - original draft, Srikant Mohta, acquisition of data, Writing - original draft, Deepak Gunjan, Supervision, Writing - original draft, acquisition of data, Rajni Yadav, acquisition of data, Writing - original draft, Anoop Saraya, Conceptualization, Methodology, acquisition of data, Writing - review & editing, Supervision.
Conflicts of interest
The authors have none to declare.
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2020.08.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
Algorithm for management of AIH-ACLF patients treated with steroids
Supplementary Figure 2.
The consort diagram showing the overall AIH patients screened for inclusion (n = 278) and patients with AIH-ACLF included in the study (n = 29) and reasons for exclusion.
References
- 1.Czaja A.J. Diagnosis and management of autoimmune hepatitis: current status and future directions. Gut Liver. 2016;10:177–203. doi: 10.5009/gnl15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mack CL, Adams D, Assis DN, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the study of liver diseases. Hepatology. n/a(n/a). doi:10.1002/hep.31065. [DOI] [PubMed]
- 3.Ichai P., Duclos-Vallée J.-C., Guettier C. Usefulness of corticosteroids for the treatment of severe and fulminant forms of autoimmune hepatitis. Liver Transplant. 2007;13:996–1003. doi: 10.1002/lt.21036. [DOI] [PubMed] [Google Scholar]
- 4.Mendizabal M., Marciano S., Videla M.G. Fulminant presentation of autoimmune hepatitis: clinical features and early predictors of corticosteroid treatment failure. Eur J Gastroenterol Hepatol. 2015;27:644–648. doi: 10.1097/MEG.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 5.Yeoman A.D., Westbrook R.H., Zen Y. Prognosis of acute severe autoimmune hepatitis (AS-AIH): the role of corticosteroids in modifying outcome. J Hepatol. 2014;61:876–882. doi: 10.1016/j.jhep.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Yasui S., Fujiwara K., Yonemitsu Y., Oda S., Nakano M., Yokosuka O. Clinicopathological features of severe and fulminant forms of autoimmune hepatitis. J Gastroenterol. 2011;46:378–390. doi: 10.1007/s00535-010-0316-3. [DOI] [PubMed] [Google Scholar]
- 7.Stravitz R.T., Lefkowitch J.H., Fontana R.J. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. 2011;53:517–526. doi: 10.1002/hep.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara K., Yasui S., Yokosuka O. Autoimmune acute liver failure: an emerging etiology for intractable acute liver failure. Hepatol Int. 2013;7:335–346. doi: 10.1007/s12072-012-9402-3. [DOI] [PubMed] [Google Scholar]
- 9.Joshita S., Yoshizawa K., Umemura T. Clinical features of autoimmune hepatitis with acute presentation: a Japanese nationwide survey. J Gastroenterol. 2018;53:1079–1088. doi: 10.1007/s00535-018-1444-4. [DOI] [PubMed] [Google Scholar]
- 10.null Shalimar, Kedia S., Mahapatra S.J. Severity and outcome of acute-on-chronic liver failure is dependent on the etiology of acute hepatic insults: analysis of 368 patients. J Clin Gastroenterol. 2017;51:734–741. doi: 10.1097/MCG.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 11.Sonthalia N., Rathi P.M., Jain S.S. Natural history and treatment outcomes of severe autoimmune hepatitis. J Clin Gastroenterol. 2017;51:548–556. doi: 10.1097/MCG.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 12.Anand L., Choudhury A., Bihari C. Flare of autoimmune hepatitis causing acute on chronic liver failure: diagnosis and response to corticosteroid therapy. Hepatology. 2019;70:587–596. doi: 10.1002/hep.30205. [DOI] [PubMed] [Google Scholar]
- 13.Sarin S.K., Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131–149. doi: 10.1038/nrgastro.2015.219. [DOI] [PubMed] [Google Scholar]
- 14.Sarin S.K., Kedarisetty C.K., Abbas Z. Acute-on-chronic liver failure: consensus recommendations of the asian pacific association for the study of the liver (APASL) 2014. Hepatol Int. 2014;8:453–471. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 15.Balitzer D., Shafizadeh N., Peters M.G., Ferrell L.D., Alshak N., Kakar S. Autoimmune hepatitis: review of histologic features included in the simplified criteria proposed by the international autoimmune hepatitis group and proposal for new histologic criteria. Mod Pathol. 2017;30:773–783. doi: 10.1038/modpathol.2016.267. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara K., Fukuda Y., Yokosuka O. Precise histological evaluation of liver biopsy specimen is indispensable for diagnosis and treatment of acute-onset autoimmune hepatitis. J Gastroenterol. 2008;43:951–958. doi: 10.1007/s00535-008-2254-x. [DOI] [PubMed] [Google Scholar]
- 17.Ishak K., Baptista A., Bianchi L. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 18.Sarin S.K., Choudhury A., Sharma M.K. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalan R., Saliba F., Pavesi M. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 20.de Franchis R., Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 21.null Shalimar, Rout G., Jadaun S.S. Prevalence, predictors and impact of bacterial infection in acute on chronic liver failure patients. Dig Liver Dis. 2018;50:1225–1231. doi: 10.1016/j.dld.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Manns M.P., Czaja A.J., Gorham J.D. AASLD Pract Guidein. 2010;51:31. [Google Scholar]
- 23.Bajaj J.S., O'Leary J.G., Reddy K.R. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250–256. doi: 10.1002/hep.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louvet A., Wartel F., Castel H. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541–548. doi: 10.1053/j.gastro.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 25.Caraceni P., Riggio O., Angeli P. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417–2429. doi: 10.1016/S0140-6736(18)30840-7. [DOI] [PubMed] [Google Scholar]
- 26.Solà E., Solé C., Simón-Talero M. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250–1259. doi: 10.1016/j.jhep.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Fernández J., Clària J., Amorós A. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157:149–162. doi: 10.1053/j.gastro.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Bernardi M., Angeli P., Claria J. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut. 2020;69:1127–1138. doi: 10.1136/gutjnl-2019-318843. [DOI] [PMC free article] [PubMed] [Google Scholar]