Abstract
Background and aims
Disparities in timely referral to liver transplantation (LT) evaluation persist. We aim to examine race/ethnicity and insurance-specific differences in the Model for End-Stage Liver Disease (MELD) score at time of waitlist (WL) registration and its impact on WL survival.
Methods
We retrospectively evaluated U.S. adults listed for LT using 2005–2018 United Network for Organ Sharing LT registry. Multiple linear regression methods examined factors associated with MELD at listing, and Fine–Gray competing risks regression were used to analyze WL mortality.
Results
Among 144,163 WL registrants (median age = 56 years, 65.3% male, 56.4% private insurance, 23.3% Medicare, 15.7% Medicaid), mean WL MELD at listing was higher in African Americans versus non-Hispanic whites (2.57 points higher, 95%CI: 2.40–2.74, P < 0.001). Compared with patients with private insurance, adjusted mean WL MELD was higher among those with no insurance, Medicare, or Medicaid (P < 0.001 for all). After correcting for differences in MELD at listing, Asians had lower risk of WL death versus non-Hispanic whites (subhazard ratio (SHR): 0.92, 95% CI: 0.86–1.00, P = 0.04), but no difference was observed in African Americans or Hispanics. Compared with patients with private insurance, higher risk of WL death was observed in patients with no insurance (SHR: 1.33, 95%CI: 1.14–1.56, P < 0.001), Medicare (SHR: 1.20, 95%CI: 1.16–1.25, P < 0.001), or Medicaid (SHR: 1.22, 95%CI: 1.17–1.27, P < 0.001).
Conclusion
Higher MELD scores at listing among African Americans did not translate into increased WL mortality. Patients with Medicare, Medicaid, or uninsured had significantly higher WL mortality than privately insured patients, even after correcting for disparities in MELD scores at listing.
Keywords: waitlist mortality, liver transplantation, insurance, UNOS/OPTN, survival
Abbreviations: BMI, body mass index; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazards ratio; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; NASH, nonalcoholic steatohepatitis; OPTN, Organ Procurement and Transplantation Network; UNOS, United Network for Organ Sharing; WL, waitlist
The Model for End-Stage Liver Disease (MELD) score is a validated tool that accurately predicts short-term mortality in patients with end-stage liver disease.1 In 2002, the United Network for Organ Sharing (UNOS) Organ Procurement and Transplantation Network (OPTN) adopted this system to prioritize patients for liver transplantation (LT), replacing the prior system which relied upon more subjective variables such as severity of ascites and hepatic encephalopathy (HE).1,2 Following implementation of the MELD score, improved equity of organ allocation was observed.3
Despite this, disparities are persistently described in the MELD era, particularly among ethnic minorities4, 5, 6 and patients with public insurance, with prior studies demonstrating more advanced disease severity, reflected by higher MELD scores, at waitlist (WL) registration, which may reflect delays in timely referral for LT evaluation.7 Prior studies have also described lower likelihood of LT in patients with public or no insurance.8,9 Using a comprehensive national LT registry in the U.S., we aim to determine whether race/ethnicity and insurance-specific disparities exist in the MELD score at time of WL registration and to analyze whether these disparities affected the 1-year WL mortality.
Methods
Data Source and Study Population
Using data from the OPTN/UNOS registry, we retrospectively analyzed deidentified, patient-level data on adult (≥18 years) liver transplant WL registrants from January 1, 2005 to June 30, 2018. The OPTN/UNOS registry includes national data on all liver transplant WL registrants and recipients in the U.S. patients with acute liver failure (UNOS status 1A at time of initial wait listing) were excluded from this study, whereas patients with and without hepatocellular carcinoma (HCC) were included in this study. This study was reviewed and determined to qualify the exempt status from the Alameda Health System institutional review board.
Etiology of chronic liver disease leading to WL registration and LT was determined based on the primary diagnosis code and was categorized as hepatitis C virus (HCV), nonalcoholic steatohepatitis (NASH), alcoholic cirrhosis, and other or unknown etiology. The HCV category included patients with HCV and alcoholic cirrhosis. Using methods similar to earlier studies, the NASH category included patients with cryptogenic cirrhosis.10 Race/ethnicity was categorized as white, African American, Hispanic, Asian, and other or unknown. Socioeconomic data, such as the primary projected payment (insurance type/status), was categorized as commercial/private, Medicare, Medicaid, veteran's affairs, other government source, no insurance (self-pay, donation, free care, pending), and unknown. Other clinical and demographic information at the time of WL registration included age, sex, body mass index (kg/m2), blood type (O, A, B, AB), presence of HCC status, and UNOS region. The geographic area for each UNOS region is designated by the Centers for Medicare and Medicaid Services that are served by one organ procurement organization, one or more transplant hospitals, and one or more donor hospitals.
Primary Outcome
Our primary outcome was the biological MELD score at time of LT WL registration. The MELD score was calculated from the total bilirubin (mg/dL), serum creatinine, and international normalized ratio (INR) for prothrombin time at the time of WL registration. The formula to calculate MELD at listing: MELD = 11.2∗loge (INR) + 9.57∗loge (serum creatinine) + 3.78∗ loge (serum total bilirubin) + 6.43, according to its original description.1,11 We excluded patients missing data required to calculate the MELD score.
Secondary Outcomes
Secondary outcomes included waiting time, reason for removal from the WL and outcome data such as WL death, dropout or LT. Waiting time was determined using the first date each patient was placed on the waiting list and the date the patient was removed from the WL. We evaluated 1-year WL mortality/removal because of death, clinical deterioration, or “too sick” (combined into single WL mortality category) with LT as a competing event. Patients removed from the WL for improved condition, loss to follow-up, removal in error, transplant refusal, and “other” were censored.
Statistical Analysis
Clinical and demographic characteristics of the study cohort were presented as frequencies and proportions for categorical variables, and mean ± SD or median (IQR), as appropriate, for continuous variables. Between-group differences in MELD were compared using either the Mann–Whitney U test for paired comparisons or the Kruskal–Wallis test for more than two group comparisons because the MELD score was nonparametric. Multiple linear regression was used to evaluate factors associated with MELD at listing. Transformation of the outcome variable did not yield improved results, and thus, no transformations were performed for regression analysis.
Multivariable competing risks regression was performed using the Fine–Gray subdistribution hazard method.12, 13, 14 WL mortality was modeled with LT as a competing risk. Adjusted cumulative incidence functions were evaluated at specific values of MELD at listing (15, 20, 25, 30, 35) and further stratified by race/ethnicity and insurance. Patients with missing data on waiting time (n = 1) and patients who were removed from the waiting list on the same day they entered (n = 139) were excluded from competing risks regression analysis. All tests of significance were 2-sided, with p-values <0.05 considered statistically significant. All statistical analyses were performed using STATA Version 14.0 (StataCorp, College Station, TX).
Results
Characteristics of the Study Cohort
From 2005 to 2018, among 144,163 WL patients, median age was 56 (IQR: 50–62) years, 65.3% were male, 70.7% were white, and majority of the patients had private insurance (56.4%), followed by Medicare (23.3%) and Medicaid (15.7%) (Table 1). HCC was present for 19.6% of the patients. Among etiologies analyzed, HCV accounted for 35.0%, followed by alcoholic cirrhosis alone (19.3%) and NASH (17.5%). The remaining cohort had other or unspecified etiology of chronic liver disease.
Table 1.
Characteristics of Study Population at the Time of Waitlist Registration (n = 144,163).
| Characteristic | N (%) |
|---|---|
| Age, median (IQR), y | 56 (50–62) |
| Male sex | 94,123 (65.3) |
| Race/ethnicity | |
| White | 101,963 (70.7) |
| African American | 12,447 (8.63) |
| Hispanic | 21,532 (14.9) |
| Asian | 6519 (4.52) |
| Other/unknown | 1702 (1.18) |
| Insurance | |
| Private | 81,337 (56.4) |
| Medicare | 33,622 (23.3) |
| Medicaid | 22,641 (15.7) |
| VA | 3224 (2.24) |
| Other government source | 2189 (1.52) |
| None | 1080 (0.75) |
| Unknown | 70 (0.05) |
| Blood type | |
| O | 66,910 (46.4) |
| A | 54,153 (37.6) |
| B | 17,661 (12.2) |
| AB | 5439 (3.77) |
| Etiology | |
| HCV | 50,490 (35.0) |
| NASH + CC | 25,285 (17.5) |
| Alcoholic cirrhosis | 27,871 (19.3) |
| Other/unknown | 40,517 (28.1) |
| Presence of HCC | 28,212 (19.6) |
| BMI, mean ± SD, kg/m2 | 28.7 ± 5.79 |
| UNOS region | |
| 1 | 6759 (4.69) |
| 2 | 18,344 (12.7) |
| 3 | 18,760 (13.0) |
| 4 | 17,098 (11.9) |
| 5 | 24,213 (16.8) |
| 6 | 3933 (2.73) |
| 7 | 12,225 (8.48) |
| 8 | 9040 (6.27) |
| 9 | 10,466 (7.26) |
| 10 | 11,3–4 (7.84) |
| 11 | 12,021 (8.34) |
BMI, body mass index; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; NASH, nonalcoholic steatohepatitis; UNOS, United Network for Organ Sharing.
MELD Score at the Time of WL Registration
Median MELD was higher among women than that in men (Table 2). Compared with whites, median MELD was high among African American and Hispanic patients, and low among Asian patients (Table 2). Patients without insurance had significantly higher MELD at listing than patients with private insurance, Medicare, Medicaid, or other government sources. Patients with alcoholic cirrhosis had the highest median MELD while those with HCV had the lowest.
Table 2.
Differences in MELD at Listing Stratified by Demographic and Clinical Characteristics.
| Characteristic | Median | IQR | P-value |
|---|---|---|---|
| Overall | 15.3 | 11.1–21.6 | – |
| Sex | |||
| Male | 15.2 | 11.0–21.2 | <0.001 |
| Female | 15.5 | 11.2–22.2 | |
| Race/ethnicity | |||
| White | 15.2 | 11.1–21.1 | <0.001 |
| African American | 17.3 | 11.5–24.3 | |
| Hispanic | 15.4 | 11.4–22.2 | |
| Asian | 12.4 | 8.41–20.2 | |
| Insurance | |||
| Private | 15.1 | 10.9–21.3 | <0.001 |
| Medicare | 15.1 | 11.0–20.9 | |
| Medicaid | 16.1 | 11.7–23.5 | |
| VA | 15.3 | 11.4–20.5 | |
| Other government source | 15.9 | 11.8–22.4 | |
| None | 18.7 | 12.7–29.7 | |
| Etiology | |||
| HCV | 13.9 | 10.2–19.2 | <0.001 |
| NASH | 16.0 | 12.2–21.7 | |
| Alcoholic cirrhosis | 17.7 | 13.3–25.2 | |
| Other/unknown | 15.1 | 10.3–22.0 | |
| Presence of HCC | |||
| No | 16.6 | 12.5–23.2 | <0.001 |
| Yes | 10.4 | 8.29–13.9 | |
| BMI, kg/m2 | |||
| <20 | 16.1 | 10.9–22.7 | <0.001 |
| 20-24 | 15.2 | 10.7–21.4 | |
| 25-29 | 15.0 | 10.9–21.0 | |
| 30-34 | 15.2 | 11.1–21.3 | |
| ≥35 | 16.2 | 12.0–23.7 | |
| UNOS region | |||
| 1 | 14.6 | 10.5–20.6 | <0.001 |
| 2 | 14.7 | 10.6–21.2 | |
| 3 | 16.8 | 12.6–22.6 | |
| 4 | 14.7 | 10.6–21.1 | |
| 5 | 14.7 | 10.5–22.1 | |
| 6 | 15.1 | 11.4–20.9 | |
| 7 | 16.5 | 11.7–23.9 | |
| 8 | 15.3 | 11.4–20.8 | |
| 9 | 13.9 | 9.74–20.2 | |
| 10 | 15.0 | 11.3–20.6 | |
| 11 | 16.0 | 12.2–21.3 | |
BMI, body mass index; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; NASH, nonalcoholic steatohepatitis; UNOS, United Network for Organ Sharing; VA, veterans affairs.
On adjusted regression, compared with whites, mean MELD was high among all racial groups, but significantly high among African Americans (2.57 points higher, 95% CI: 2.40–2.74, P < 0.001) (Table 3). Compared with patients with private insurance, mean MELD was considerably high among those with no insurance (4.04 points higher, 95% CI: 3.40–4.69, P < 0.001), while statistically significantly high among those with government sources as well (Table 3). Compared with patients with HCV, patients with alcoholic cirrhosis had high mean MELD at listing by 2.97 points (95% CI: 2.85–3.10, P < 0.001), and those with NASH had high mean MELD by 1.06 points (95% CI 0.94–1.19, P < 0.001) (Table 3).
Table 3.
Model Examining Factors Associated with MELD at Listinga.
| Coef. | 95% CI | P | |
|---|---|---|---|
| Race/ethnicity | |||
| White | [Ref.] | – | – |
| African American | 2.57 | 2.40–2.74 | <0.001 |
| Hispanic | 0.67 | 0.54–0.80 | <0.001 |
| Asian | 0.30 | 0.07–0.53 | 0.010 |
| Insurance | |||
| Private | [Ref.] | – | – |
| Medicare | 0.39 | 0.29–0.50 | <0.001 |
| Medicaid | 0.99 | 0.86–1.12 | <0.001 |
| VA | 0.55 | 0.27–0.83 | <0.001 |
| Other government source | 0.65 | 0.29–1.00 | <0.001 |
| None | 4.04 | 3.40–4.69 | <0.001 |
| Etiology | |||
| HCV | [Ref.] | – | – |
| NASH + CC | 1.06 | 0.94–1.19 | <0.001 |
| Alcoholic cirrhosis | 2.97 | 2.85–3.10 | <0.001 |
| Presence of HCC | |||
| No | [Ref.] | – | – |
| Yes | (-6.21) | (-6.31) – (-6.12) | <0.001 |
BMI, body mass index; Coef., coefficient HCC, hepatocellular carcinoma; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; NASH, nonalcoholic steatohepatitis; Ref., reference; UNOS, United Network for Organ Sharing; VA, veterans affairs.
Adjusted for age, sex, BMI, and UNOS region.
Waitlist Mortality
Of the patients registered on the waiting list included in the competing risks analysis (n = 144,023), 21.6% died while waiting, and 53.3% underwent LT. On adjusted competing risks regression, for every unit increase in MELD at listing, the relative incidence of 1-year WL mortality increased by 5.6% (subhazard ratio (SHR): 1.06, 95% CI: 1.05–1.06, P < 0.001) (Table 4). Asians were at lower risk of death on the WL than whites (SHR: 0.92, 95% CI: 0.86–1.00, P = 0.04) (Table 4). Patients with no insurance (SHR: 1.33, 95% CI: 1.14–1.56, P < 0.001), and patients with Medicare (SHR: 1.20, 95% CI: 1.16–1.25, P < 0.001) or Medicaid (SHR: 1.22, 95% CI: 1.17–1.27, P < 0.001) were at increased risk of WL death compared with patients with private insurance (Table 4).
Table 4.
Competing Risk Model Evaluating Waitlist Mortality among Patients Listed for Liver Transplantation (n = 144,023).
| Proportion died on waiting list (%) | Proportion received LT (%) | Multivariable subhazard ratioa (95% CI) | P-value | |
|---|---|---|---|---|
| MELD at listing | 1.056 (1.054–1.057) | <0.001 | ||
| Race/ethnicity | ||||
| White | 21.1 | 54.0 | 1 [Ref.] | – |
| African American | 21.0 | 57.7 | 0.971 (0.922–1.022) | 0.259 |
| Hispanic | 24.8 | 47.9 | 1.002 (0.962–1.044) | 0.925 |
| Asian | 19.2 | 51.5 | 0.924 (0.858–0.995) | 0.037 |
| Insurance | ||||
| Private | 19.6 | 55.2 | 1 [Ref.] | – |
| Medicare | 25.3 | 50.4 | 1.203 (1.162–1.246) | <0.001 |
| Medicaid | 23.2 | 50.0 | 1.217 (1.169–1.268) | <0.001 |
| VA | 23.5 | 53.3 | 1.036 (0.939–1.142) | 0.482 |
| Other government source | 18.7 | 56.7 | 1.077 (0.955–1.215) | 0.228 |
| None | 21.5 | 61.8 | 1.333 (1.143–1.555) | <0.001 |
CI, confidence interval; HCC, hepatocellular carcinoma; MELD, Model for End-Stage Liver Disease; UNOS, United Network for Organ Sharing; VA, veterans affairs.
Adjusted for age, sex, blood type, presence of HCC, etiology and UNOS region.
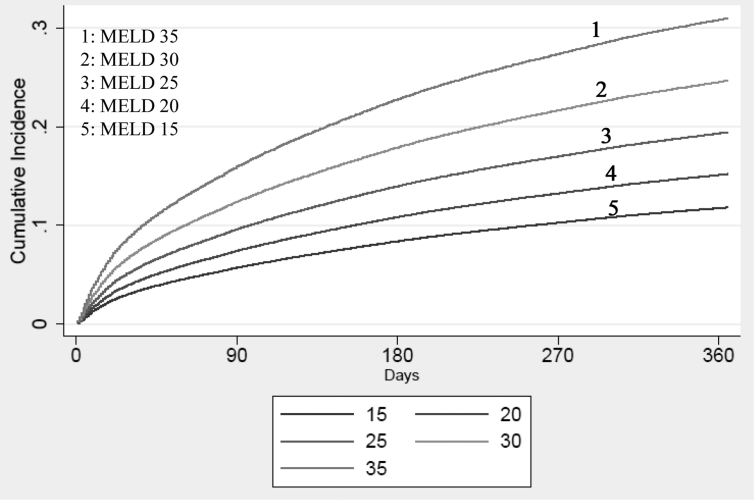
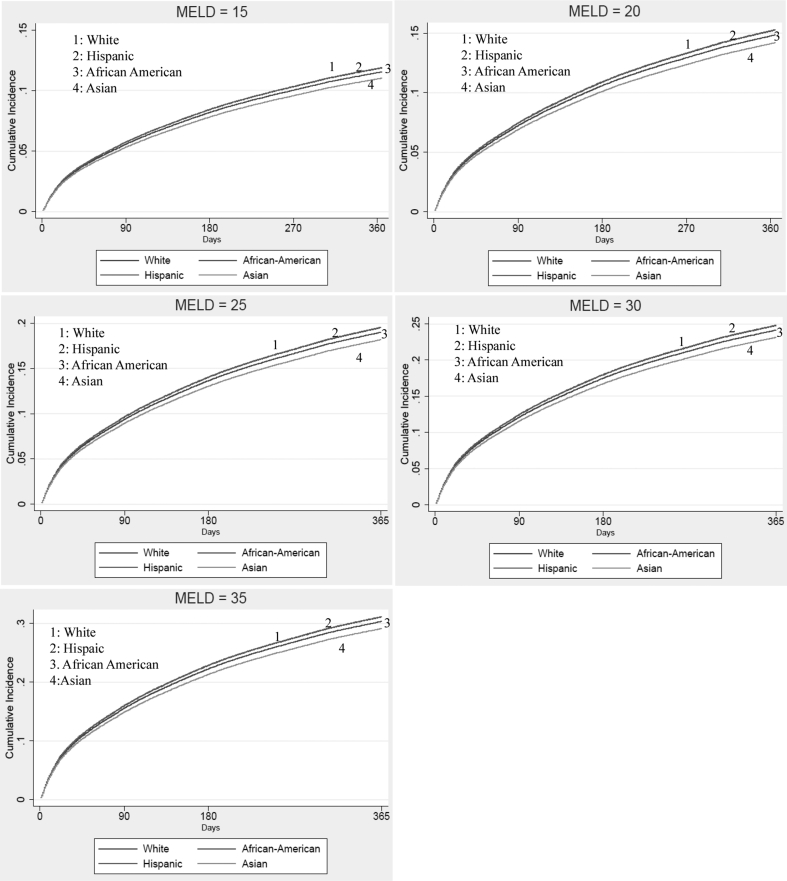
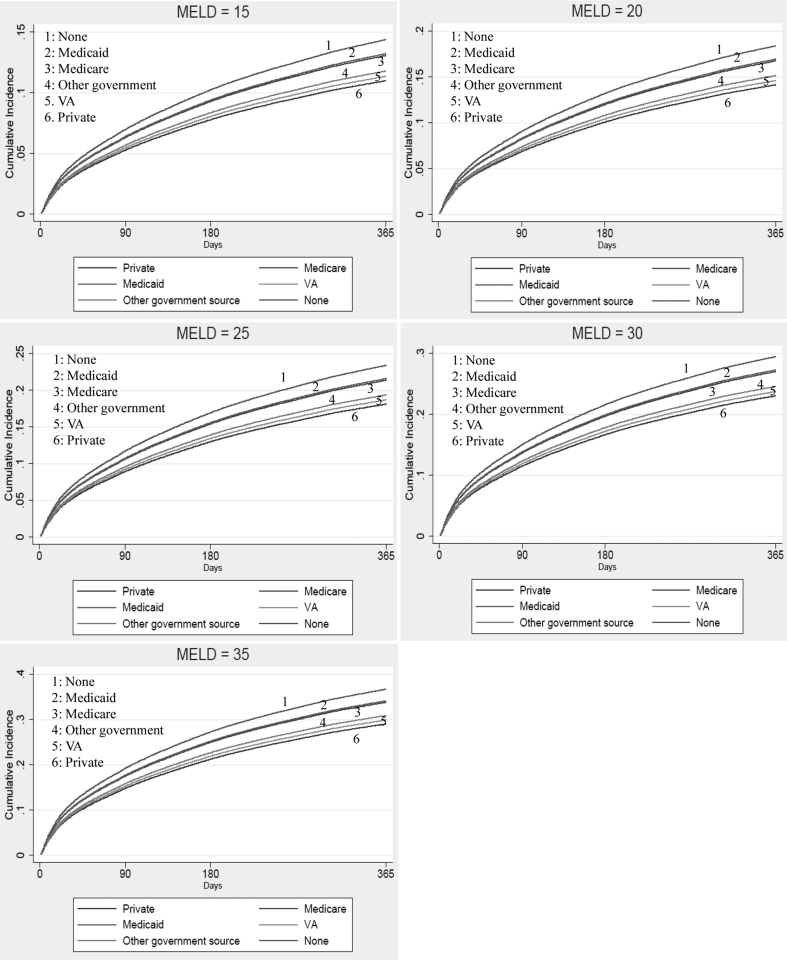
Differences in the 1-year cumulative incidence of WL mortality at specified values of MELD at listing, in the presence of LT as a competing event, are depicted in Figure 1. When stratified by race, increasing MELD was associated with increased cumulative incidence of WL mortality, and Asians remained at the lowest risk at all specified values of MELD (Supplemental Figure S1). Similarly, when stratified by insurance, patients with no insurance had the highest cumulative incidence of WL mortality at all specified values of MELD (Supplemental Figure S2).
Figure 1.
Cumulative incidence of 1-year waitlist mortality by MELD at listing (15, 20, 25, 30, 35). MELD, Model for End-Stage Liver Disease.
Discussion
This analysis of 14 years of MELD-era U.S. national LT registry data, including 144,163 WL registered patients, revealed significant disparities in MELD score at time of WL registration. African Americans, Medicare, Medicaid, uninsured patients, and those with a diagnosis of alcoholic cirrhosis had relatively higher MELD scores at time of listing. Furthermore, patients with no insurance, Medicare, or Medicaid were at increased risk of WL death compared to patients with private insurance.
Prior studies have noted worse WL outcomes in uninsured patients or those with Medicaid; however, most have focused on those WL patients with HCC.8,9 A 2019 single center study by Gutin.9 found that among HCC WL patients with public insurance, there was a nearly 70% increased risk of WL dropout (owing to liver-related death or HCC tumor progression) when compared with patients having private (Kaiser Permanente) insurance (HR, 1.69 [95% CI, 1.17–2.43]), despite having similar tumor characteristics. Prior studies have also noted that HCC patients with Medicare or Medicaid were less likely to undergo LT and surgical resection than patients with private insurance.8,15 Our current study observed significantly higher risk of WL mortality among uninsured patients or patients with Medicare or Medicaid when compared with those with private insurance. This higher risk of WL mortality persisted even after adjusting for presence of HCC and the insurance-specific disparities in the MELD score at time of WL registration.
Although the insurance-specific disparities in WL mortality observed are likely multifactorial, the significantly higher MELD score at WL registration among these groups may have reflected more advanced disease that resulted in potential delays in timely referral and evaluation for LT. However, it is important to note that although statistically significant, the magnitude of differences in adjusted mean WL MELD scores in Medicare and Medicaid patients compared with privately insured patients were less than 1 point difference, making it unlikely from a clinical perspective that such would be a major contributor to overall WL mortality. Other studies have also cited greater WL dropout in public insurance populations due to inadequate social support, nonadherence, or being lost to follow-up.9 Another potential explanation is that patients with public insurance are more likely to have lower socioeconomic status or have more medical comorbidities than those with private insurance, which may not only lead to delays or barriers to LT while on the WL, but may also contribute to increased mortality.16 Potential explanations in the HCC population for WL dropout may be due to not receiving treatments that may have helped bridge them to transplantation or survive longer.17 Sobotka et al.15 observed that Medicaid patients with HCC are less likely to undergo ablation than patients with private insurance, and patients with other forms of insurance were less likely to undergo transarterial chemoembolization compared with those with private insurance. These “bridging” therapies may be particularly important to ensure HCC is managed within Milan criteria to be eligible for LT.15
The current analysis also observed significant race/ethnicity-specific disparities in MELD scores at time of WL registration, particularly for African Americans. The observed higher MELD at WL among African Americans is consistent with a retrospective study by Jesse et al.,18 which identified 1968 patients with end-stage liver disease who underwent LT evaluation from 2004 to 2012 to determine whether listing for LT was influenced by socioeconomic status and/or race/ethnicity. They observed that African Americans, even when adjusted for medical and socioeconomic factors, had 26% lower odds of being listed for LT and longer delays to listing when than all other patients. In the current study, although African Americans had significantly higher MELD at time of listing, this did not translate into increased WL mortality for African Americans in the competing risks model. Nevertheless, higher MELD at listing itself was significantly associated with higher risk of WL death. Although inconsistent with prior studies,4, 5, 6 the current study's findings is similar to a study by Volk et al.19 which showed no differences in receipt of LT or WL death between white, African American, or Hispanic patients when accounting for unequal distribution of racial/ethnic groups within the U.S. Our study also observed lower MELD scores at listing in Asians. When the insurance status and etiology was stratified by race/ethnicity, we did not observe significant differences in proportion of Asians with private insurance, but African Americans had lower proportion of private insurance (48.2% vs. 59.8%, P < 0.01) and higher proportion of Medicaid (20.4% vs. 12.4%, P < 0.01), which could partly explain higher MELD among African Americans. While HCV and alcoholic cirrhosis accounted for the majority of liver disease patients in whites, African Americans, and Hispanics, over 60% of Asians had other diagnosis, which is primarily represented by HBV. HBV patients can develop HCC without significant hepatic decompensation, and thus, the lower MELD among Asians may be partly explained by compensated HBV patients with HCC. Furthermore, African Americans had higher proportion of HCV (46.9%) than whites (32.6%) or Hispaics (36.4%), whereas Hispanics and whites had significantly higher proportion of patients with alcoholic cirrhosis. Although the proportion of patients with private insurance was similar across liver disease etiologies, NASH patients had higher proportion of Medicare compared to HCV and alcoholic cirrhosis patients, and both HCV and alcoholic cirrhosis patients had higher proportion of patients with Medicaid. These baseline differences may shed light on some of potential factors contributing to MELD score variation at time of listing. However, even after adjusting for these variables, differences persisted in our multivariate regression models.
The inclusion of 14 years of MELD era data using a national LT registry provides a comprehensive assessment of LT outcomes. Limitations of registry data, including potential for misclassification, must be balanced with the strength and generalizability of a large nationally representative sample cohort. One key limitation is the lack of detailed data before LT WL registration, including timing of referral and potential barriers or delays, which would shed more light on the etiology of our MELD score differences. Although not the main focus of our current study, UNOS also lacks specific data about treatments for the underlying liver disease (e.g. antiviral therapies for HCV and HBV) and for complications of cirrhosis (e.g. treatment of ascites, HE). While the results of the competing risks regression may not be as generalizable to other transplant programs, the use of MELD as an indicator of the risk of WL mortality has been previously established.20 Another limitation is that competing risk estimates require that censoring be independent of outcome. However, although this assumption may not always hold true, analysis of risk of WL death while accounting for nondeath events allows representation of the experience pertinent to patients on the WL, for whom both transplantation and death are real possibilities. We acknowledge there may persist unmeasured confounders that affected the associations observed in this study. Furthermore, there likely exists multiple factors affecting MELD at listing and WL outcomes originating from the period before WL registration (e.g. timely referral to liver transplant evaluation, coordination of care and follow-up, insurance or system-specific barriers in timely access to diagnostic testing or treatment). These data were not available in the UNOS data set for inclusion in our analyses.
In conclusion, significantly race/ethnicity-specific disparities in the MELD score at time of WL registration were observed particularly for African Americans. However, these differences did not translate into higher risk of WL mortality among African Americans. Our study also observed significantly higher MELD scores at listing in uninsured patients and those with Medicare or Medicaid insurance when compared to private insured patients. However, even after adjusting for these disparities in MELD score at listing, significantly higher risk of WL death was observed in uninsured patients and those with Medicare or Medicaid insurance.
CREDIT AUTHORSHIP CONTRIBUTION
Ann Robinson: Study concept and design, Analysis and interpretation of data, Writing of article, Critical revision of the manuscript. Grishma Hirode: Study concept and design, Analysis and interpretation of data, Statistical analysis, Critical revision of the manuscript. Robert Wong: Study concept and design, Analysis and interpretation of data, Statistical analysis, Critical revision of the manuscript, Study supervision, Full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Conflicts of interest
No relevant conflicts of interests or disclosures for all authors.
Grants and financial support
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2020.07.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Wiesner R., Edwards E., Freeman R. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 2.Kamath P.S., Wiesner R.H., Malinchoc M. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 3.Moylan C.A., Brady C.W., Johnson J.L. Disparities in liver transplantation before and after introduction of the MELD score. J Am Med Assoc. 2008;300:2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn J., Liu B., Bhuket T. Race/ethnicity-specific outcomes among chronic hepatitis C virus patients listed for liver transplantation. Dig Dis Sci. 2017;62:1051–1057. doi: 10.1007/s10620-017-4469-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y. The impact of the share 35 policy on racial and ethnic disparities in access to liver transplantation for patients with end stage liver disease in the United States: an analysis from UNOS database. Int J Equity Health. 2017;16:55. doi: 10.1186/s12939-017-0552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaswala D.H., Zhang J., Liu A. A comprehensive analysis of liver transplantation outcomes among ethnic minorities in the United States. J Clin Gastroenterol. 2020;54:263–270. doi: 10.1097/MCG.0000000000001236. [DOI] [PubMed] [Google Scholar]
- 7.Kemmer N., Zacharias V., Kaiser T.E. Access to liver transplantation in the MELD era: role of ethnicity and insurance. Dig Dis Sci. 2009;54:1794–1797. doi: 10.1007/s10620-008-0567-5. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Ha J., Lopez A. Medicaid and uninsured hepatocellular carcinoma patients have more advanced tumor stage and are less likely to receive treatment. J Clin Gastroenterol. 2018;52:437–443. doi: 10.1097/MCG.0000000000000859. [DOI] [PubMed] [Google Scholar]
- 9.Gutin L., Yao F., Dodge J.L. Comparison of liver transplant wait-list outcomes among patients with hepatocellular carcinoma with public vs private medical insurance. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong R.J., Aguilar M., Cheung R. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Malinchoc M., Kamath P.S., Gordon F.D. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 12.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13.Lau B., Cole S.R., Gange S.J. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trieu J.A., Bilal M., Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31:84–89. doi: 10.20524/aog.2017.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sobotka L.A., Hinton A., Conteh L.F. Insurance status impacts treatment for hepatocellular carcinoma. Ann Hepatol. 2019;18:461–465. doi: 10.1016/j.aohep.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Virnig B.A. Associating insurance status with cancer stage at diagnosis. Lancet Oncol. 2008;9:189–191. doi: 10.1016/S1470-2045(08)70043-3. [DOI] [PubMed] [Google Scholar]
- 17.Graziadei I.W., Sandmueller H., Waldenberger P. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transplant. 2003;9:557–563. doi: 10.1053/jlts.2003.50106. [DOI] [PubMed] [Google Scholar]
- 18.Jesse M.T., Abouljoud M., Goldstein E.D. Racial disparities in patient selection for liver transplantation: an ongoing challenge. Clin Transplant. 2019;33 doi: 10.1111/ctr.13714. [DOI] [PubMed] [Google Scholar]
- 19.Volk M.L., Choi H., Warren G.J. Geographic variation in organ availability is responsible for disparities in liver transplantation between Hispanics and Caucasians. Am J Transplant. 2009;9:2113–2118. doi: 10.1111/j.1600-6143.2009.02744.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim W.R., Therneau T.M., Benson J.T. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology. 2006;43:345–351. doi: 10.1002/hep.21025. [DOI] [PubMed] [Google Scholar]