Abstract
Objective: To determine the most appropriate thiamine replacement regimen by evaluating safety and efficacy of the drug specific to alcohol-induced Wernicke’s encephalopathy (WE). Data Sources: A comprehensive literature search was conducted using PubMed, MEDLINE, Scopus, and ProQuest between January and August 2020 using the following keyword and Boolean search terminology: “thiamine” AND “alcohol” AND (encephalopathy OR korsakoff). Study Selection and Data Extraction: Randomized control trials; prospective, observational, and retrospective cohort analyses; and case reports and series were included in this evaluation. A confirmed diagnosis of alcohol-induced WE and treatment with parenteral or intramuscular (IM) thiamine were required for inclusion. Data Synthesis: Six publications composed of 138 patients were evaluated in this review, in which a wide variety of thiamine supplementation strategies were employed. Clinical diagnostic criteria varied significantly between publications. Doses ranged from 100 to 1500 mg intravenous thiamine and up to 300 mg IM thiamine, with no apparent difference in patient outcomes. All patients who received thiamine experienced symptom improvement, and adverse drug events were minimal. Conclusions: Despite the clinical controversy regarding the appropriate thiamine supplementation regimen, the heterogeneity of published works combined with symptom resolution across the gamut of dosing strategies makes a definitive consensus elusive. Clinicians should continue to provide parenteral or IM thiamine in doses of ≥100 mg to patients with confirmed alcohol-induced WE.
Keywords: alcohol intoxication, substance abuse, evidence-based medicine, clinical pharmacy, clinical toxicology
Introduction
Wernicke’s encephalopathy (WE) can be a result of alcohol-induced thiamine deficiency and is a common manifestation of those who suffer from alcohol use disorder (AUD).1 AUD is defined as a brain disease distinguished by compulsive alcohol use, loss of control over alcohol intake, and a negative emotional state when not using alcohol.1 According to the Substance Abuse and Mental Health Services Administration, which conducts the annual National Survey on Drug Use and Health, in 2018, 6.6% of people aged 18 years or older stated that in the past month, they engaged in heavy alcohol use.2 The Centers for Disease Control and Prevention reported that from 2006 to 2010, an estimate of 88 000 people died in the United States due to alcohol-related causes.3 The number of visits to US emergency departments due to alcohol abuse has increased by 50% between the years of 2006 and 2014, according to the National Institute on Alcohol Abuse and Alcoholism.4 Alcohol can negatively influence various aspects of one’s health, whether it be physically or mentally. Chronic alcohol use can result in cardiovascular issues (ie, cardiomyopathy, arrhythmias, stroke, and high blood pressure), hepatic insufficiency (ie, steatosis, alcoholic hepatitis, fibrosis, and cirrhosis), pancreatitis, various types of cancers, and can also weaken the immune system.5 In addition, chronic alcoholism has been associated with motor vehicle accidents, violence, mental health issues, and unemployment.6,7 Alcohol abuse is furthermore commonly associated with thiamine deficiency that can further manifest as WE.8
The classic triad of clinical manifestations associated with WE includes confusion, ataxia, and ophthalmoplegia.8 If the disease continues to progress, Korsakoff syndrome can occur, presenting with chronic cognitive impairment seen as severe memory loss and learning deficits deeming it a medical emergency.9,10 In most cases, those with WE will not experience the entire triad of symptoms, therefore making the diagnosis somewhat challenging and also the classic triad an unreliable tool for diagnosis.11 In WE, the brain undergoes high metabolic demands due to the depletion of thiamine stores.12 The brain experiences oxidative stress and lactic acidosis, and inflammation is also present, leading to the disruption of the blood-brain barrier and alterations in the function of the neurologic system.12 The current treatment for WE, although not consistently approached, includes intravenous (IV) or intramuscular (IM) administration of thiamine followed by a glucose infusion.8
Thiamine, also referred to as vitamin B1, is 1 of the 12 water-soluble vitamins, and it plays a significant role in the maintenance of the nervous system.13 Thiamine also plays a key role in the metabolism of glucose and, therefore, energy production.14 Alcohol consumption alone reduces intestinal absorption of thiamine, rendering oral administration of thiamine insufficient for the treatment of WE.15 As is evident from primary literature and case reports, the administration of oral thiamine alone was insufficient in adequately addressing WE symptoms.16,17 In a case report presented by Chataway and Hardman, a patient was started on 200 mg of PO thiamine for 12 days. Ten weeks later, the patient was readmitted with a diagnosis of WE and 400 mg of oral thiamine was initiated.16 The patient did experience gradual symptomatic improvement, but was left with residual fine lateral nystagmus.16 Another case report by Yoon et al presented a 56-year-old male who had a remarkable past medical history that included AUD. This patient also received strictly oral thiamine supplementation at a dose of 200 mg daily.17 Cognition and balance gradually improved, but the patient was found to have a left-beating horizontal nystagmus halfway through his admission.17 In both of these case reports, findings showed that oral thiamine was not adequate enough to completely stabilize the patient and reverse their symptoms. The transport of oral thiamine across the blood-brain barrier through various mechanisms has been shown to be a slower process when compared with parenteral administration.15 Parenteral thiamine is capable of rapid correction of depleted stores and provides therapeutic plasma levels that assist in reversal of the patient’s neurologic symptoms.15 As for the acute management of WE, it is recommended that IV supplementation, rather than oral supplementation, be provided. For the treatment of WE, the package insert for thiamine recommends administering 100 mg of thiamine intravenously followed by IM doses of 50 to 100 mg until the patient is able to return to a regular diet.18 However, there is not a clear consensus or high-quality evidence that supports this recommendation. Both the British Association of Psychopharmacotherapy and the Royal College of Physicians guidelines recommend that confirmed cases of WE should be treated with parenteral doses of thiamine greater than 500 mg per day for at least 3 to 5 days.15,19 In addition, magnesium supplementation should occur if hypomagnesemia is present as it functions as a cofactor for the conversion of thiamine.15,20 The objective of this literature review was to evaluate effective dosing regimens of thiamine supplementation in WE.
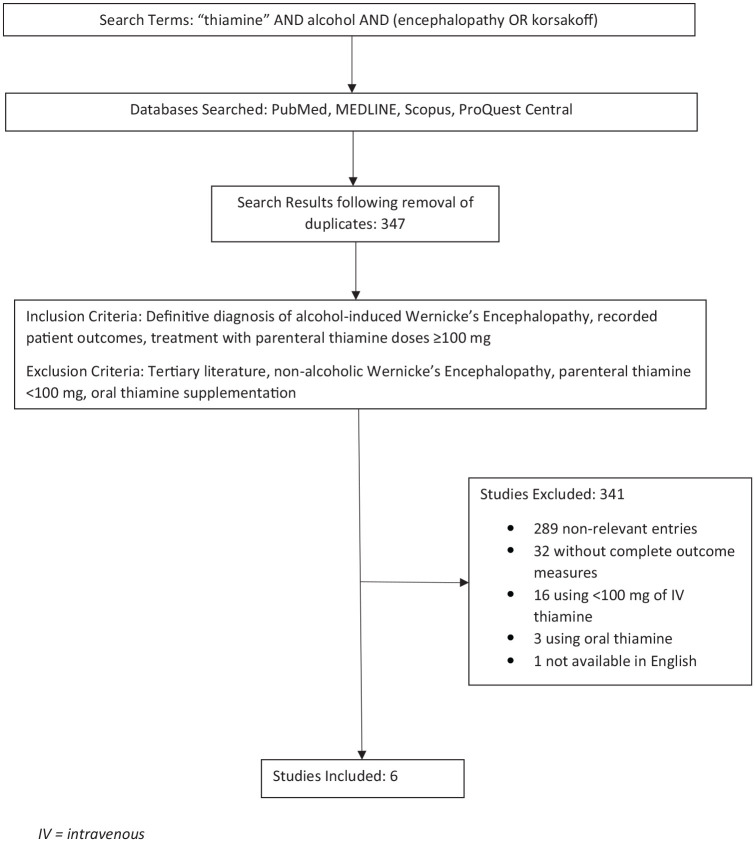
Methods
A comprehensive literature review was conducted by all 4 authors, with assistance from a medical reference librarian, utilizing PubMed, MEDLINE, Scopus, and ProQuest between January and August 2020. Search identifiers were then added to further enhance the relevance of the articles that were pertinent to this review. The search criteria within each database is further described in Figure 1. After assessing whether articles met inclusion and exclusion criteria, the search was narrowed down to 6 articles that were relevant; this process is depicted in Figure 2. Articles were included if participants had a diagnosis of alcohol-induced WE, outcome measures were documented, and if their treatment regimen included parenteral or IM thiamine of doses greater than or equal to 100 mg. Tertiary literature was excluded. Articles were also excluded if they evaluated participants with nonalcoholic WE, if the dose of thiamine was less than 100 mg, or if the participants only received oral thiamine supplementation.
Figure 1.
Search criteria.
Figure 2.
Schematic of search methodology. IV, intravenous.
Results
A total of 6 studies were included in this review, the details of which can be viewed in Table 1. Regarding the route of administration of thiamine, only 2 of the studies utilized both oral and parenteral routes.13,21 Each of the studies that were included in this review of literature confirmed that prompt administration of thiamine had an impact on improving symptoms associated with WE. In the 3 studies that had a sample size greater than 1, the onset of resolution of symptoms with higher doses of thiamine compared with lower doses was inconclusive. The duration of therapy and the time to resolution of symptoms for each study varied, demonstrating no correlation between the 2 variables.
Table 1.
Results.
| Study | Study design | Number of participants | Thiamine regimen | Duration of thiamine supplementation | Diagnosis criteria utilized | Effect/primary outcome | Comments |
|---|---|---|---|---|---|---|---|
| Paparrigopoulos et al21 | Case report | 1 | 600 mg PO daily and 300 mg IM daily | 2 months | MMSE | Complete resolution of symptoms | Resolution occurred following 2 months of treatment with this regimen |
| Pandey et al22 | Case report | 1 | 500 mg IV | 1 day | Clinical presentation; MRI findings | Improvement of symptoms | Utility of a single dose of thiamine demonstrated |
| Tjong and Peng23 | Case report | 1 | 500 mg IV followed by 1000 mg IV | 5 days | Caine’s criteria | Complete resolution of symptoms | Concomitant diagnosis of beriberi in addition to WE |
| Nishimoto et at13 | Case series | 11 | 1. 100 mg PO daily, 500 mg IV daily 2. 100 mg PO, 500 mg IV q8hr, 500 mg IV qday, 100 mg PO daily 3. 100 mg PO, 500 mg IV q8hr, 500 mg IV daily 4. 100 mg PO, 500 mg IV BID, 500 mg IV daily 5. 500 mg IV once, 100 mg PO daily 6. 100 mg PO, 500 mg IV q8hr 7. 500 mg IV q8hr, 200 mg IV daily, 100 mg PO daily 8. 500 mg IV q8hr, 100 mg IV daily 9. 500 mg IV q8hr, 100 mg PO daily 10. 500 mg IV TID, 250 mg IV daily 11. 500 mg IV TID, 500 mg IV daily |
1. 3 days 2. 7 days 3. 7 days 4. 8 days 5. 1 day 6. 2 days 7. 3 days 8. 2 days 9. 7 days 10. 3 days 11. 3 days |
Clinical presentation and MRI findings | Complete resolution of symptoms observed in 7/11 patients | Each patient (numbered 1-11) received a different thiamine regimen, some of which included initial oral supplementation. Patients who experienced symptom resolution received at least 3e days of thiamine supplementation as opposed to 2 or fewer days. |
| Alim et al24 | Retrospective cohort | 17 | High dose (n = 12): >100 mg IV (highest dose was 1000 mg) Low dose (n = 5): <100 mg IV |
High doses: 6 to 8 days Low doses: 3 to 5 days |
Clinical presentation | Complete resolution of symptoms | Nonsignificant decrease in time to resolution of symptoms of approximately 0.5 days appreciated between high- and low-dose cohorts. Median dose administered in the high-dose cohort was 400 mg IV. Sixty-five percent of doses were administered daily, while the remaining 35% were administered every 8 hours. |
| Ambrose et al25 | RCT, double blinded | 107 | 1. 5 mg IM daily 2. 20 mg IM daily 3. 50 mg IM daily 4. 100 mg IM daily 5. 200 mg IM daily |
2 days | MMSE | Complete resolution of symptoms demonstrated in all patients; quicker resolution demonstrated for patients in the 100 and 200 mg IM cohorts | The 107 participants were divided into 5 different groups to receive varying thiamine regimens. MMSE examination results were used as a surrogate for formal WE diagnosis. Baseline years of drinking was approximately 17.5, and the mean amount of alcohol consumed was approximately 300 g every 24 hours. Patients in the 200 mg IM cohort performed better on delay alternation tests than those in the 50 mg IM cohort. |
Abbreviations: PO, by mouth; IM, intramuscularly; MMSE, Mini Mental Status Examination; IV, intravenously; MRI, magnetic resonance imaging; WE, Wernicke’s encephalopathy; q8hr, every 8 hours; BID, twice a day; TID, 3 times a day; RCT, randomized control trial.
A case report by Paparrigopoulos et al presented a 52-year-old male who had a 10-year history of alcohol abuse.21 He was admitted for symptoms of WE and initially received 100 mg of thiamine intramuscularly daily.21 He was then given aggressive treatment with thiamine 600 mg/day orally and 300 mg/day intramuscularly.21 The patient’s symptoms began improving within 3 weeks, and after a yearly checkup, the patient was reported to be functioning normally.21 The article recommended that higher doses should be utilized for a longer duration, for most treatment regimens are subtherapeutic due to the fact that treatment must be initiated immediately to prevent irreversible damage.21
Pandey et al reported a 45-year-old female who had a history of alcohol abuse who presented after 4 days of nausea, vomiting, dizziness, and falls.22 She displayed impaired cognition, appeared disordered, slightly pale, and was foul smelling; there was no mention of other comorbidities.22 The patient was given 500 mg of IV thiamine, and afterward, the patient’s symptoms dramatically improved.22
Another case report by Tjong and Peng presented a 30-year-old male who had predisposed beriberi and developed WE 2 months after a single heavy drinking session, which resulted in a neurologic emergency deeming prompt treatment.23 The patient was clearly malnourished, due to his inability to tolerate solid foods and the previous diagnosis of beriberi.23 The patient was given 500 mg of IV thiamine and did not respond appropriately; another 500 mg was given.23 It took 10 times the recommended dose to achieve clinical resolution, in which all neurologic symptoms were reversed within hours.23
A case series by Nishimoto et al reported that of the 11 patients who were admitted with possible WE, all were treated with doses of thiamine >500 mg per day.13 Patients were enrolled in the case series through International Classification of Diseases, Ninth Revision (ICD-9) review.9 There was no mention of additional comorbid states amid the study population, although this information could have been useful. All the cases were treated with different protocols of dosing; some of the individuals were given oral thiamine first followed by parenteral treatment of 500 mg while others were started on parenteral treatment immediately.9 The majority of the cases had different time to resolution of symptoms based on the duration of high-dose thiamine given; there were some cases that were refractory to the thiamine treatment.9
A retrospective cohort conducted by Alim et al included both low-dose (defined as doses <100 mg) and high-dose (defined as doses >100 mg) IV thiamine prescribing patterns.24 Of the 141 patients, only 17 of them were prescribed thiamine due to suspicion of WE.24 For those suspected to have WE, 12 patients received high-dose thiamine.24 Other comorbidities that were reported among the study population included the following types of liver dysfunction: cirrhosis, nonalcoholic fatty liver disease, alcoholic hepatic steatosis, hepatocellular carcinoma, alcoholic hepatitis, and viral hepatitis.24 The study revealed that of the 141 patients receiving either high-dose or low-dose thiamine, no patient showed significant differences in time of resolution of the triad of symptoms.24 For confusion, ataxia, and ocular abnormalities, 13 participants, 6 participants, and 7 participants in the high-dose groups experienced resolution of symptoms, respectively.24
A randomized control trial by Ambrose et al was conducted in which 107 participants who were detoxifying from alcohol were split into 5 different groups to receive varying doses of parenteral thiamine treatment.25 The groups received the following daily doses of IM thiamine for 2 consecutive days: 5 mg, 20 mg, 50 mg, 100 mg, and 200 mg.25 Each subject had an average of 17 years of alcohol consumption, but there was no mention of confounding disease states among any of the patients.25 Each of the groups performed similarly on the Mini Mental Status Examination (MMSE) at baseline, indicating that there was no significant difference in the mental cognition of each participant.25 The results of this trial concluded that increased doses corresponded with higher working memory performances as quantified by the delayed alternation method, a test conducted by a psychologist to determine the delay in responses to verbal stimuli.25
Discussion
Results from the selected literature demonstrated the efficacy of thiamine doses greater than or equal to 100 mg, in alignment with the product package insert, both intravenously and intramuscularly at various dosing intervals in the treatment of alcohol-induced WE.18 Due to the similar responses seen with the varying thiamine regimens among each study, it is imperative that treatment of alcohol-induced WE be patient specific, with a dose and duration of thiamine sufficient to mitigate the patients’ symptoms. Factors that affect dosing decisions are numerous, but may include the duration of alcohol exposure, the time since last alcoholic beverage consumption, the presence of malnutrition markers including hyponatremia, the rapidity of onset of WE, and severity of present symptoms.
As is the case in clinical practice, numerous different dosing regimens are described in the results of this review. While all trials reported efficacy of thiamine ≥100 mg administered in a route other than orally, perhaps the most poignant argument for administering high doses of thiamine can be derived from Ambrose et al.25 The 5 dosing regimens employed in this trial ranged from 5 mg to 200 mg IM thiamine administered daily, and the patients who received the highest doses experienced the most rapid resolution of symptoms and demonstrated higher mental acuity.25 This trend aligns with other subsequently conducted evaluations included in this review, in that patients who received high doses of thiamine experienced a shorter duration of symptoms associated with WE.21-23,25
While the assessment of doses and routes of administration of supplemental thiamine deserves significant attention, perhaps equally important is the duration of therapy. In the trial conducted by Nishomoto et al, 4 of 11 patients did not experience a resolution of symptoms despite receiving high-dose thiamine, defined by the authors as >500 mg IV daily.13 In a review of the patients whose symptoms resolves in comparison to those whose did not, the duration of therapy becomes significant: patients who experienced symptoms resolution were treated with the high-dose thiamine regimen for 3 days, as opposed to being administered for 2 days in the patients who did not experience a resolution of symptoms.13 While the patient evaluated by Pandey et al experienced improvement in symptoms after a single 500 mg IV dose of thiamine, it may be reasonable to consider a minimum of 72 hours of treatment with a high-dose thiamine strategy to achieve a complete resolution of symptoms.13,22
Visual assessment of patients in conjunction with a thorough physical examination are important in the diagnosis of WE; however, the symptoms associated with this syndrome may mimic other pathologies, making a definitive diagnosis difficult. In an effort to elucidate WE-specific symptomatology, the Caine criteria were developed, though based on the results of this review, are only sporadically employed.22,23 The Caine criteria can be viewed in Table 2 26. Three of the 6 studies that we analyzed did not specify an objective method of assessing symptom improvement, and relied on clinical observational reports. Though unvalidated for this indication, the MMSE was utilized in 2 of the studies as a means of assessing treatment efficacy and the diagnosis of WE.21,25
Table 2.
Caine Criteria.26
| Requires 2 of the following 4 for a diagnosis of WE: |
| 1. Dietary deficiencies |
| 2. Oculomotor abnormalities |
| 3. Cerebral dysfunction |
| 4. Either an altered mental state or mild memory impairment |
Abbreviation: WE, Wernicke’s encephalopathy.
Alcohol-induced WE is commonly underdiagnosed due to the nonspecific patient presentation, which makes the evaluation in the clinical trial setting difficult. Additionally, specific treatment regimens are not well defined within the literature, as is evidenced in the wide variety of options contained within this review. Although there was a resolution of symptoms seen with lower doses of thiamine, the heterogeneity among the selected studies makes it difficult to identify a conclusive dose of thiamine supplementation. Based on the outcomes of the data collected throughout our literature search and both the British Association of Psychopharmacotherapy and the Royal College of Physicians guidelines, in conjunction with the results of this review, a reasonable strategy would be the regimen presented in Table 3.15,19 Because of current knowledge on bioavailability and increased absorption via the parenteral route in patients who present with alcoholic WE, oral supplementation should not be considered in this patient population. Additionally, magnesium supplementation along with thiamine should be considered because magnesium is a cofactor for the transketolase and pyruvate decarboxylase reactions, and is important for the conversion of thiamine into its active form.15,20
Table 3.
| Dose and frequency | Route | Duration |
|---|---|---|
| Thiamine 500 mg every 8 hours PLUS Magnesium sulfate 35-50 mmol, if hypomagnesemia is present |
Intravenous | Minimum of 3 days |
Several limitations exist that warrant discussion. As with any review of the literature, the search methodology or criteria may have inadvertently excluded articles that may have been of benefit to this report. The overall paucity of randomized clinical trials for the evaluation of thiamine dosing for the management of WE severely diminishes the external validity and generalizability of our findings, especially those derived from case reports and case series. The heterogeneity of trial design, dosing regimens, routes of administration of thiamine, and outcome measures similarly add difficulty in making a definitive consensus on an appropriate thiamine regimen for the treatment of alcohol-induced WE. As the largest comprehensive health organization that provides specific recommendations for the treatment of alcohol-induced WE, the Royal College of Physicians guidelines should be utilized until further conclusive research has been conducted.
Conclusions
Inconclusive data exist with regard to a specific thiamine dosing regimen for the treatment of alcohol-induced WE. Based on the information available from clinical trials, it may be reasonable to treat patients with a high-dose thiamine regimen including 500 mg IV thiamine every 8 hours for at least 3 days, in addition to the supplementation of magnesium if appropriate given patient serum levels. Further clinical investigations are warranted in which the diagnosis of WE, and the dosing and duration of thiamine supplementation is consistently defined among all study participants.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Justin P. Reinert  https://orcid.org/0000-0003-0321-5608
https://orcid.org/0000-0003-0321-5608
References
- 1. National Institute on Alcohol Abuse and Alcoholism. Alcohol use disorder. Accessed March 11, 2020. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-use-disorders
- 2. SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health. Section 2 PE Tables. Results from the 2018 National Survey on Drug Use and Health: Detailed Tables, Sections 1-3. Accessed March 11, 2020. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2018R2/NSDUHDetTabsSect2pe2018.htm#tab2-1b
- 3. Esser MB, Sherk A, Liu Y, et al. Deaths and Years of Potential Life Lost From Excessive Alcohol Use — United States, 2011–2015. MMWR Morb Mortal Wkly Rep 2020;69:981-987. doi:10.15585/mmwr.mm6930a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Institute on Alcohol Abuse and Alcoholism. NIH study shows steep increase in rate of alcohol-related ER visits. Published January 12, 2018. Accessed February 5, 2020. https://www.niaaa.nih.gov/news-events/news-releases/nih-study-shows-steep-increase-rate-alcohol-related-er-visits
- 5. National Institute on Alcohol Abuse and Alcoholism. Alcohol’s effects on the body. Accessed March 11, 2020. https://www.niaaa.nih.gov/alcohols-effects-body
- 6. World Health Organization. Global status report on alcohol and health 2018. Accessed March 11, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/en/
- 7. Smith GS, Branas CC, Miller TR. Fatal nontraffic injuries involving alcohol: a meta-analysis. Ann Emerg Med. 1999;33:659-668. doi: 10.1016/s0196-0644(99)80004-3 [DOI] [PubMed] [Google Scholar]
- 8. Kanny D, Brewer RD, Mesnick JB, Paulozzi LJ, Naimi TS, Lu H. Vital signs: alcohol poisoning deaths—United States, 2010-2012. MMWR Morb Mortal Wkly Rep. 2015;63:1238-1242. [PMC free article] [PubMed] [Google Scholar]
- 9. Castaneda R, Sussman N, Westreich L, Levy R, O’Malley M. A review of the effects of moderate alcohol intake on the treatment of anxiety and mood disorders. J Clin Psychiatry. 1996;27:207-212. [PubMed] [Google Scholar]
- 10. Booth BM, Feng W. The impact of drinking and drinking consequences son short-term employment outcomes in at-risk drinkers in six southern states. J Behav Health Serv Res. 2002;29:157-166. doi: 10.1007/bf02287702 [DOI] [PubMed] [Google Scholar]
- 11. Leonard KE, Rothbard JC. Alcohol and the marriage effect. J Stud Alcohol Suppl. 1993;13:139-146. doi: 10.15288/jsas.1999.s13.139 [DOI] [PubMed] [Google Scholar]
- 12. Flynn A, Macaluso M, D’Empaire I, Troutman MM. Wernicke’s encephalopathy: increasing clinician awareness of this serious, enigmatic, yet treatable disease. Prim Care Companion CNS Disord. 2015;17:10.4088/PCC.14r01738. doi: 10.4088/PCC.14r01738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishimoto A, Usery J, Winton JC, Twilla J. High-dose parenteral thiamine in treatment of Wernicke’s encephalopathy: case series and review of the literature. In Vivo. 2017;31:121-124. doi: 10.21873/invivo.11034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nikolakaros G, Ilonen T, Kurki T, Paju J, Papageorgiou SG, Vataja R. Non-alcoholic Korsakoff syndrome in psychiatric patients with a history of undiagnosed Wernicke’s encephalopathy. J Neurol Sci. 2016;370:296-302. doi: 10.1016/j.jns.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 15. Lingford-Hughes AR, Welch S, Peters L; British Association for Psychopharmacology, Expert Reviewers Group. BAP updated guidelines: evidence-based guidelines for the pharmacological management of substance abuse, harmful use, addiction and comorbidity: recommendations from BAP. J Psychopharmacol. 2012;26:899-952. doi: 10.1177/0269881112444324 [DOI] [PubMed] [Google Scholar]
- 16. Chataway J, Hardman E. Thiamine in Wernicke’s syndrome—how much and how long? Postgrad Med J. 1995;71:249. doi: 10.1136/pgmj.71.834.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoon C, Gedzior J, DePry D. Wernicke-Korsakoff syndrome: focus on low-threshold diagnosis and prompt treatment in the primary care setting. Int J Psychiatry Med. 2019;54:172-180. doi: 10.1177/0091217419832771 [DOI] [PubMed] [Google Scholar]
- 18. DailyMed. Thiamine hydrochloride injection, solution. Accessed February 5, 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12986d0e-b8bf-4338-9d85-07f973b35439
- 19. Thomson AD, Cook CCH, Touquet R, Henry JA; Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke’s encephalopathy in the accident and emergency department. Alcohol Alcohol. 2002;37:513-521. doi: 10.1093/alcalc/37.6.513 [DOI] [PubMed] [Google Scholar]
- 20. Traviesa DC. Magnesium deficiency: a possible cause of thiamine refractoriness in Wernicke-Korsakoff encephalopathy. J Neurol Neurosurg Psychiatry. 1974;37:959-962. doi: 10.1136/jnnp.37.8.959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paparrigopoulos T, Tzavellas E, Karaiskos D, Kouzoupis A, Liappas I. Complete recovery from undertreated Wernicke-Korsakoff syndrome following aggressive thiamine treatment. In Vivo. 2010;24:231-233. [PubMed] [Google Scholar]
- 22. Pandey D, Kuhn JL, Tejero H, Banks JS. Alcohol induced Wernicke encephalopathy with atypical MRI findings. Cureus. 2019;11:e5203. doi: 10.7759/cureus.5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tjong E, Peng YY. Gastrointestinal beriberi and Wernicke’s encephalopathy triggered by one session of heavy drinking. Case Rep Neurol. 2019;11:124-131. doi: 10.1159/000499601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alim U, Bates D, Langevin A, et al. Thiamine prescribing practices for adult patients admitted to an internal medicine service. Can J Hosp Pharm. 2017;70:179-187. doi: 10.4212/cjhp.v70i3.1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ambrose ML, Bowden SC, Whelan G. Thiamin treatment and working memory function of alcohol-dependent people: preliminary findings. Alcohol Clin Exp Res. 2001;25:112-116. doi: 10.1111/j.1530-0277.2001.tb02134.x [DOI] [PubMed] [Google Scholar]
- 26. Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62:51-60. doi: 10.1136/jnnp.62.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]