Abstract
In this retrospective descriptive study, we characterized the clinical, histologic, and immunohistochemical features of 13 cases of canine gallbladder neuroendocrine carcinoma (GB-NEC). Immunohistochemical stains for neuroendocrine (neuron-specific enolase [NSE], chromogranin A, synaptophysin) and gastrin markers were evaluated, and clinicopathologic and follow-up data were obtained for all cases. The average age at diagnosis was 8.9 y, and breeds included 6 Boston Terriers, 2 Bichon Frise, 1 Poodle, 1 English Bulldog, 1 French Bulldog, and 2 mixed-breed dogs. Boston Terriers were overrepresented in this cohort, and therefore a breed predilection is possible. Most dogs were presented with emesis and elevated liver enzyme activities: 13 of 13 had elevated alanine aminotransferase and alkaline phosphatase activities; 8 of 13 had elevated aspartate aminotransferase activity; 7 of 13 had elevated gamma-glutamyl transferase activity. Abdominal ultrasound and/or exploratory surgery revealed a gallbladder mass. All neoplasms had similar histologic features and positive immunoreactivity for NSE, chromogranin A, synaptophysin, and gastrin. Vascular invasion was noted in 8 of 13 neoplasms, and metastasis was present in 6 of 13 cases (4 hepatic and 2 pulmonary metastases). The median survival time was 3.7 y in patients who died; 5 of 8 deaths were directly attributed to the GB-NEC, 3 of which had metastatic spread. GB-NECs have the potential to metastasize; however, surgical excision may be curative in a subset of dogs.
Keywords: canine, gallbladder, immunohistochemistry, neoplasia, neuroendocrine carcinoma
Introduction
Neuroendocrine cells, which make up the diffuse endocrine system, are found throughout the body, predominantly in the gastrointestinal and respiratory tracts.6,10,11 These cells are considered endodermal in origin and can give rise to neuroendocrine carcinomas (NECs). NECs have been observed in the intestines, esophagus, bile duct, gallbladder, liver, nasopharynx, and skin in dogs and cats.11 Hepatobiliary NECs (previously referred to as “carcinoids”) are neoplasms of intrahepatic, bile duct, or gallbladder neuroendocrine cells.4-6,8,9 They are believed to originate from the diffuse neuroendocrine cell population within the biliary epithelium or from hepatic progenitor cells (oval cells).9 Clinically, these tumors often are the cause of signs and biochemical abnormalities consistent with cholestasis, including anorexia, emesis or hematemesis, and elevated hepatic enzyme activities. They can be identified via ultrasound or exploratory surgery, and are removed via cholecystectomy.1,2,8,14
Neuroendocrine cells produce amines and peptides, including secretin, serotonin, cholecystokinin, and adrenocorticotropic hormone.8 Histochemical stains, such as Grimelius stain, have been utilized to establish the neuroendocrine origin of these tumors.5,6,8,9 Immunohistochemically, most neuroendocrine tumors express one or more of the following: neuron-specific enolase (NSE), chromogranin A, and synaptophysin. In a 2005 study, 10 of 10 canine hepatic NECs variably expressed at least 1 of these 3 markers.10 In humans, paraneoplastic gastrin synthesis and secretion by gallbladder NECs (GB-NECs) has been noted, indicating that gastrin may be useful for immunohistochemical (IHC) identification of these tumors in dogs as well.2,4
GB-NECs have been described rarely in domestic animals, including dogs, cats, and cattle, and thus little is known about their biological behavior.2,3,5,8-11,14 Existing literature primarily includes single descriptive case studies highlighting presentation, diagnosis, and treatment of the GB-NEC, with 1 IHC study of 10 dogs with GB-NEC.1,2,5,8,11,14 In humans, NECs account for <1% of all malignant tumors, and primary gallbladder neuroendocrine tumors represent only 0.2% of all neuroendocrine tumors.3,7 A greater understanding of the behavior of this neoplasm may help to guide prognosis and treatment in dogs; the primary objective of our descriptive retrospective study was to characterize the clinical, histologic, and IHC features and outcomes of 13 cases of NEC arising from the gallbladders of dogs.
Materials and methods
Case selection and clinicopathologic data
We identified 12 cases of canine GB-NEC from 2007 to 2018 in the Penn Veterinary Diagnostic Laboratories (Philadelphia, PA) database, and 1 additional case was provided by Colorado State University Veterinary Diagnostic Laboratory (Fort Collins, CO). Questionnaires regarding those cases were completed by primary and referring veterinarians and/or through provided medical records. These questionnaires included data on signalment (age, breed, sex, and spay/castration status), presentation (clinical signs and age at diagnosis), diagnostic testing (imaging and laboratory results including alanine aminotransferase [ALT], alkaline phosphatase [ALP], aspartate aminotransferase [AST]; gamma-glutamyl transferase [GGT], total bilirubin, and other parameters), treatment, and follow-up data (current survival status, recurrence of clinical signs following treatment, association of euthanasia or natural death with GB-NEC or other disease, and overall survival time post-diagnosis).
Histopathologic and IHC analysis
Hematoxylin and eosin–stained slides for all cases were reviewed by 2 veterinary pathologists (AC Durham, MD Sánchez). Gallbladder neoplasms and non-tumoral gallbladder tissue were reviewed; liver tissue was also examined in 7 cases. Morphologic features recorded for each case included mitotic count, nuclear and cellular pleomorphism, intratumoral necrosis, presence of vascular invasion, and lesions in adjacent gallbladder or liver. Mitotic counts were performed in cellular regions with the most mitotic activity, avoiding areas of hemorrhage and necrosis, and defined as the total number of mitotic figures in 10 contiguous, non-overlapping 2.37-mm2 fields. Formalin-fixed, paraffin-embedded (FFPE) tissues were retrieved for IHC staining. For immunohistochemistry, 5-µm thick paraffin sections were mounted on slides (ProbeOn; Thermo Fisher Scientific). The immunostaining procedure was performed using an automated platform (Valent; Biocare Medical) combined with the ENVISION + kit (Agilent). Briefly, after dewaxing and rehydration, sections for synaptophysin staining were pretreated with heat-induced epitope retrieval (pH 6.0, 40 min, 95°C). Endogenous peroxidase was inactivated with 3% H2O2 (10 min, room temperature [RT]). Monoclonal primary antibodies against chromogranin A (mouse; Immunostar), synaptophysin (rabbit; Dako), NSE (mouse; Dako), and gastrin (rabbit; GeneTex) were used at concentrations of 1:1,500, 1:20, 1;2,000, and prediluted/ready-to-use, respectively. All of the antibodies were incubated on the sections for 30 min at RT. A biotin-free polymeric IHC detection system consisting of horseradish peroxidase–conjugated anti-rabbit or anti-mouse IgG was then applied for 15 min at RT. Immunoreactivity was revealed with the diaminobenzidine chromogen reaction. Slides were counterstained with hematoxylin, dehydrated in an ethanol series, cleared in xylene, and coverslipped. Positive controls of tissues known to express the markers included normal canine pancreas (chromogranin A, synaptophysin, NSE) and stomach (gastrin); negative test tissue controls (via omission of primary antibody) were also performed.
The semi-quantitative system utilized for IHC expression was adapted from one used in a previous study.10 Scores 0, 1+, 2+, and 3+ were assigned based on the degree of IHC expression in neoplastic cells of each marker of interest: 0 = no observable staining; 1+ = <25% of cells are positive; 2+ = 25–50% of cells are positive; 3+ >50% of cells are positive.10
Results
We included 13 dogs in our study: 10 males and 3 females. Breeds included 6 Boston Terriers, 2 Bichon Frise, 1 Poodle, 1 English Bulldog, 1 French Bulldog, and 2 mixed-breed dogs. The average age at diagnosis was 8.9 y (median: 9 y). Clinical and hematologic signs included emesis (10 of 13), hematemesis (4 of 13), and elevated liver enzyme activities (ALT: 13 of 13; ALP: 13/13; AST: 8 of 13; GGT: 7 of 13; Suppl. Table 1). Abdominal ultrasound and/or exploratory surgery revealed a gallbladder mass in all patients, and all were treated by cholecystectomy.
Histologically, all neoplasms were characteristic of neuroendocrine neoplasms and consisted of packets of neoplastic cells supported by a fine fibrovascular stroma. Neoplastic cells were round-to-polygonal, densely packed, and exhibited marked pleomorphism with karyomegaly. These cells had distinct cell borders, abundant granular amphophilic-to-basophilic cytoplasm with round nuclei, and variably distinct nucleoli (Fig. 1). The mean mitotic count was 8 mitoses per 10 2.37-mm2 fields (median: 9; range: 2–13).
Figure 1.
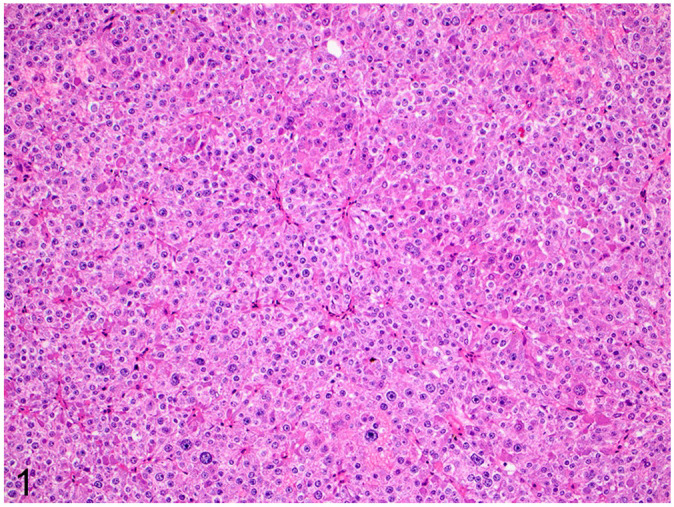
Histology of a neuroendocrine carcinoma in the gallbladder of a dog. Densely cellular packets are supported by a fine fibrovascular stroma and composed of round-to-polygonal cells with marked pleomorphism and karyomegaly. H&E stain.
Histologic lesions (Table 1, Suppl. Table 2) included vascular invasion in 8 of 13 neoplasms; 12 of 13 cases had clean margins. Hemorrhage (11 of 13) and necrosis (7 of 13) were present in most cases. Associated gallbladder lesions included cholecystitis, cystic mucosal hyperplasia, inspissated bile, choleliths, mineralization, mucocele, and mural fibrosis. Lesions in the adjacent liver were bile duct hyperplasia and ductal bile stasis with periductal fibroplasia or fibrosis, indicative of biliary obstructive disease, nodular hyperplasia, steroid hepatopathy, and portal fibrosis.
Table 1.
Histologic features of gallbladder neuroendocrine carcinoma in neoplastic and non-neoplastic tissue from gallbladder and liver of 13 canine cases.
| Mitotic count (per 10 hpf) | |
| Range | 2–13 |
| Mean | 8 |
| Median | 9 |
| Pleomorphism | |
| Severe | 7/13 |
| Moderate | 4/13 |
| Mild | 2/13 |
| Vascular invasion | 8/13 (62%) |
| Necrosis | 7/13 (54%) |
| Hemorrhage | 11/13 (85%) |
| Margins | 12/13 (92%) clean |
| Gallbladder (non-tumor) | |
| Cholecystitis | 7/13 |
| Cystic mucosal hyperplasia | 6/13 |
| Inspissated bile | 5/13 |
| Choleliths | 5/13 |
| Mineralization | 2/13 |
| Mucocele | 1/13 |
| Fibrosis | 1/13 |
| Liver | |
| Cholangitis | 7/13 |
| Bile duct hyperplasia | 4/13 |
| Bile duct stasis | 3/13 |
| Periductal fibroplasia/ fibrosis | 3/13 |
| Nodular hyperplasia | 2/13 |
| Steroid hepatopathy | 2/13 |
| Portal fibrosis | 1/13 |
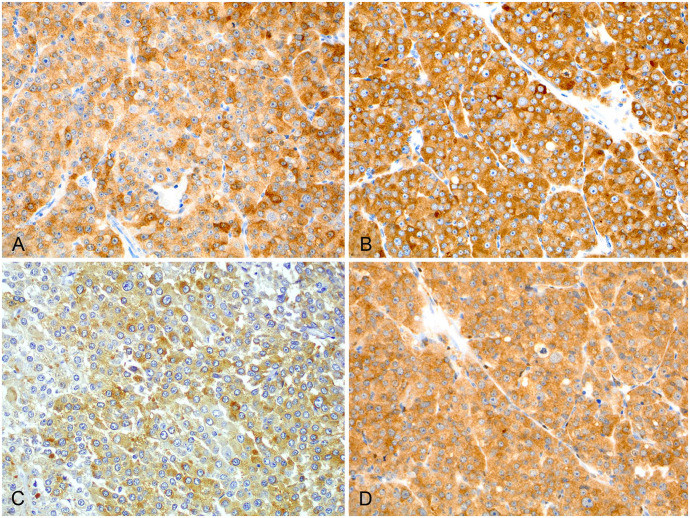
Selected slides from each case were immunohistochemically labeled (Table 2). All 13 neoplasms were positive for all neuroendocrine markers, except 1 case in which NSE results were unavailable. Chromogranin A immunolabeling was generally lower than that for the other neuroendocrine markers (Fig. 2). Of interest, all 6 deceased dogs had the lowest (1+) chromogranin A immunolabeling, and 4 of these 6 dogs had the lowest NSE immunolabeling score (1+ or 2+). All neoplasms immunolabeled positive for gastrin.
Table 2.
Results of immunohistochemistry in 13 dogs with gallbladder neuroendocrine carcinoma.
| Case | Neuron-specific enolase | Synaptophysin | Chromogranin A | Gastrin |
|---|---|---|---|---|
| 1 | 2+ | 3+ | 1+ | 2+ |
| 2 | 1+ | 3+ | 1+ | 3+ |
| 3 | 3+ | 3+ | 3+ | 3+ |
| 4 | 1+ | 3+ | 1+ | 3+ |
| 5 | 3+ | 3+ | 1+ | 3+ |
| 6 | 3+ | 3+ | 1+ | 3+ |
| 7 | 2+ | 3+ | 1+ | 3+ |
| 8 | 3+ | 3+ | 3+ | 3+ |
| 9 | 3+ | 3+ | 3+ | 3+ |
| 10 | 3+ | 3+ | 3+ | 3+ |
| 11 | 3+ | 3+ | 3+ | 3+ |
| 12 | 3+ | 3+ | 2+ | 3+ |
| 13 | NA | 3+ | 3+ | 3+ |
NA = not available. Scores 0, 1+, 2+, and 3+ were assigned based on the degree of IHC expression in neoplastic cells of each marker of interest: 0 = no observable staining; 1+ = <25% of cells are positive; 2+ = 25–50% of cells are positive; 3+ >50% of cells are positive.10
Figure 2.
Immunohistochemical staining of a primary gallbladder neuroendocrine carcinoma in a dog. The neoplasm has positive expression for all 4 markers: A. neuron-specific enolase, B. synaptophysin, C. chromogranin A, D. gastrin.
Metastasis was reported in 6 of 13 cases, including 4 hepatic, 2 pulmonary, and 1 mesenteric metastases; 1 case of hepatic metastasis was confirmed via biopsy at the time of surgery; the remainder were diagnosed or suspected postoperatively via abdominal ultrasound and clinical signs. Eight dogs are deceased, with a median survival time (MST) of 1,358 d (3.7 y). Of these 8 dogs, metastasis was present in 5 of 8, vascular invasion was present in 6 of 8, and 8 of 8 had clean margins on the original excisional biopsy. Five of these deaths were directly attributed to the GB-NEC as reported by the veterinarian on the clinical questionnaire; all of these dogs had continued clinical signs, and 3 had documented metastatic disease. Of the patients still alive, the diagnostic interval ranges from 507–1,834 d, which is below the calculated median survival time (Table 3).
Table 3.
Clinical signs and outcomes of 13 cases of gallbladder neuroendocrine carcinoma in dogs.
| Case | Presenting clinical signs | Alive/dead | Cause of death | Metastasis | Overall survival time (d) |
|---|---|---|---|---|---|
| 1 | Emesis, hematemesis, abdominal distention | Dead | Euthanasia (GB-NEC) | Liver; mesentery | 1,926 |
| 2 | Emesis, hematemesis, abdominal distention | Dead | Euthanasia (pulmonary masses) | Lung (radiographs) | 2,191 |
| 3 | Emesis | Dead | Euthanasia (GB-NEC) | Lung (radiographs) | 1,021 |
| 4 | Emesis, hematemesis | Dead | Euthanasia (GB-NEC) | Liver | 432 |
| 5 | Emesis, diarrhea | Dead | Euthanasia (GB-NEC) | No | 1,537 |
| 6 | Hematuria, hematemesis | Dead | Euthanasia (GB-NEC) | No | 1,785 |
| 7 | None | Dead | Euthanasia (lost to follow-up) | Liver | 1,174 |
| 8 | Emesis, pain, anorexia | Dead | Euthanasia (cardiac mass) | No | 1,178 |
| 9 | None | Alive | No | 1,834* | |
| 10 | Emesis | Alive | No | 1,151* | |
| 11 | Emesis | Alive | Liver | 989* | |
| 12 | Emesis, diarrhea | Alive | No | 518* | |
| 13 | Emesis, anorexia | Alive | No | 507* | |
| Mean | 1,406 | ||||
| Median | 1,358 |
Days alive at last known assessment.
Discussion
Brachycephalic breeds, particularly Boston Terriers, were overrepresented in this cohort; therefore, a breed predilection is possible. Subjects were middle-aged to senior, with an average age of 8.9 y. A majority of dogs were presented with emesis or hematemesis (11 of 13) and all had elevated liver enzyme activities (including elevated ALT and ALP in all cases), consistent with cholestasis. Emesis and hematemesis may have also been related to paraneoplastic gastrin secretion, identified immunohistochemically in all neoplasms; however, gastrin positivity in cells is not directly correlated with secretion of the hormone. Abdominal ultrasound was a diagnostic modality of choice in all cases for identification of the lesion and indication for surgical intervention (cholecystectomy).
IHC studies of GB-NECs in dogs have rarely been performed since 1988, apart from a few single case reports and a small case series.2,3,8-11,14 Although NSE, chromogranin A, and synaptophysin are standard IHC markers used for confirming neuroendocrine origin, NECs have also been associated with inducing various endocrine paraneoplastic syndromes resulting from secretion of hormones, such as gastrin-releasing factor, which stimulates the release of gastrin from G cells in the stomach.2,12,13 It is theorized that variable differentiation from hepatic progenitor cells may subsequently lead to variable expression of different hormones by NECs; these hormones then affect the clinical signs observed. Gastrin production is reported in humans with gastrointestinal NECs. A majority of our cases were presented with gastrointestinal signs potentially induced by gastrin secretion—emesis (9 of 13) and hematemesis (4 of 13)—and therefore we looked for staining for gastrin in the tumors.12,13 Hematemesis in particular may indicate a bleeding gastric ulcer as a result of this paraneoplastic syndrome. If gastrin secretion by GB-NECs indeed induces vomiting, this may also contribute to a better prognosis (MST 3.7 y) compared to hepatic NECs (MST 3 d) as a result of earlier identification, and therefore treatment, of obvious clinical signs.10
In our study, 13 of 13 neoplasms were positive for all 4 IHC markers used (NSE, chromogranin A, synaptophysin, and gastrin), with the exception of 1 case that could not be assessed for NSE because of unavailability of the tissue block (Table 2). This IHC staining demonstrates the neuroendocrine origin of the tumor as well as the paraneoplastic expression of gastrin previously seen in human studies and supports the use and reliability of these IHC markers in the diagnosis of GB-NECs.2,10
GB-NECs have the potential to metastasize; however, in our patients, cholecystectomy was most often curative (7 of 13 cases did not metastasize). Additionally, the MST was extremely long (3.7 y) compared to studies of hepatic NEC in dogs, which have a MST of 3 d.9,10 Of note, 5 patients were still alive at the conclusion of our study, with follow-up data ranging from 507 to 1,834 d, below the calculated MST. In human cases of GB-NEC, the MST is 9.8 mo.4
Statistical analysis of our cases is limited because of our small sample size. Therefore, more cases of GB-NEC must be identified to assess the association of clinicopathologic, histopathologic, or IHC features with survival outcome. Additionally, in a few cases, it is unclear whether the patient’s GB-NEC was the cause of death; these patients died from a cardiac mass, suspected lung metastases, or were brought to the hospital deceased from unknown causes. Also, given that referring veterinarians were sent a comprehensive questionnaire, our data are based on their individual reports or medical records, which is a limitation of the study.
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_1040638720978172 for Clinical, histopathologic, and immunohistochemical features of 13 cases of canine gallbladder neuroendocrine carcinoma by Kevin M. O’Brien, Braelyn J. Bankoff, Peri K. Rosenstein, Daphne C. Clendaniel, Melissa D. Sánchez and Amy C. Durham in Journal of Veterinary Diagnostic Investigation
Acknowledgments
Our research was made possible through the generous sharing of tissue blocks from dogs with canine GB-NEC, including Penn Vet Diagnostic Laboratories, School of Veterinary Medicine, University of Pennsylvania; and School of Veterinary Medicine, Colorado State University. We thank our contributing veterinarians for providing biopsies for analysis and participating in data collection.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Peri K. Rosenstein  https://orcid.org/0000-0003-3703-3749
https://orcid.org/0000-0003-3703-3749
Amy C. Durham  https://orcid.org/0000-0002-2245-6872
https://orcid.org/0000-0002-2245-6872
Supplementary material: Supplementary material for this article is available online.
Contributor Information
Kevin M. O’Brien, Department of Pathobiology, University of Pennsylvania, School of Veterinary Medicine, Philadelphia, PA.
Braelyn J. Bankoff, Department of Pathobiology, University of Pennsylvania, School of Veterinary Medicine, Philadelphia, PA.
Peri K. Rosenstein, Department of Pathobiology, University of Pennsylvania, School of Veterinary Medicine, Philadelphia, PA
Daphne C. Clendaniel, Mid-Atlantic Veterinary Specialists, Malvern, PA
Melissa D. Sánchez, Antech Diagnostics, Framingham, MA
Amy C. Durham, Department of Pathobiology, University of Pennsylvania, School of Veterinary Medicine, Philadelphia, PA.
References
- 1. Bhandal J, et al. Use of color flow doppler ultrasonography to diagnose a bleeding neuroendocrine tumor in the gallbladder of a dog. J Am Vet Med Assoc 2009;235:1326–1329. [DOI] [PubMed] [Google Scholar]
- 2. Birettoni F, et al. Primary neuroendocrine carcinoma of the gallbladder in dog. Vet Res Commun 2008;32(Suppl 1):239–242. [DOI] [PubMed] [Google Scholar]
- 3. Chen C, et al. Gallbladder neuroendocrine carcinoma: report of 10 cases and comparison of clinicopathologic features with gallbladder adenocarcinoma. Int J Clin Exp Pathol 2015;8:8218–8226. [PMC free article] [PubMed] [Google Scholar]
- 4. Eltawil KM, et al. Neuroendocrine tumors of the gallbladder: an evaluation and reassessment of management strategy. J Clin Gastroenterol 2010;44:687–695. [DOI] [PubMed] [Google Scholar]
- 5. Johnson LK, et al. Neuroendocrine carcinoma of the liver and gallbladder in a cow. J Comp Pathol 2008;138:165–168. [DOI] [PubMed] [Google Scholar]
- 6. Kulve MH, Mayer RJ. Carcinoid tumors. N Engl J Med 1999;340:858–868. [DOI] [PubMed] [Google Scholar]
- 7. Mezi S, et al. Neuroendocrine tumors of the gallbladder: a case report and review of the literature. J Med Case Rep 2011;5:334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrell CN, et al. A carcinoid tumor in the gallbladder of a dog. Vet Pathol 2002;39:756–758. [DOI] [PubMed] [Google Scholar]
- 9. Patnaik AK, et al. Canine hepatic carcinoids. Vet Pathol 1981;18:445–453. [DOI] [PubMed] [Google Scholar]
- 10. Patnaik AK, et al. Canine hepatic neuroendocrine carcinoma: an immunohistochemical and electron microscopic study. Vet Pathol 2005;42:140–146. [DOI] [PubMed] [Google Scholar]
- 11. Patnaik AK, et al. Hepatobiliary neuroendocrine carcinoma in cats: a clinicopathologic, immunohistochemical, and ultrastructural study of 17 cases. Vet Pathol 2005;42:331–337. [DOI] [PubMed] [Google Scholar]
- 12. Scott N, et al. Gastrin releasing peptide and gastrin releasing peptide receptor expression in gastrointestinal carcinoid tumours. J Clin Pathol 2004;57:189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vinik A, et al. Carcinoid tumors. In: De Groot LJ, et al., eds. Endotext [Internet]. MDText.com, 2000. [cited 2018 Feb 5]. https://www.ncbi.nlm.nih.gov/books/NBK279162/
- 14. Willard MD, et al. Neuroendocrine carcinoma of the gallbladder in a dog. J Am Vet Med Assoc 1988;192:926–928. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_1040638720978172 for Clinical, histopathologic, and immunohistochemical features of 13 cases of canine gallbladder neuroendocrine carcinoma by Kevin M. O’Brien, Braelyn J. Bankoff, Peri K. Rosenstein, Daphne C. Clendaniel, Melissa D. Sánchez and Amy C. Durham in Journal of Veterinary Diagnostic Investigation