Abstract
Background
Hypertension is related to autonomic nervous system (ANS) dysfunction, atherosclerosis and chronic inflammation. The stimulation of baroreflex regulation by slow-breathing exercise may improve the interplay among these systems. The objective of this study was to investigate the effect of device-guided slow breathing on ANS, cardiovascular system and chronic inflammation in hypertensive patients.
Methods
We prospectively collected 36 essential hypertension patients who were requested to practice slow-breathing exercise 5 times per day for 3 months. The breathing exercise was guided by a cellphone app with a wearable electrocardiography device and a rhythm of 6 cycles per minute. Cardiovascular indicators including heart rate variability (HRV), blood pressure, pulse wave velocity and baroreflex indexes were sampled 3 times: at the first visit, and 1 month and 3 months after the intervention. The levels of blood inflammatory biomarkers, including tumor necrosis factor-alpha (TNF-α), interleukin-6, interleukin-1 receptor antagonist and C-reactive protein were also collected at all 3 visits. The longitudinal differences in these variables and their correlations were tested.
Results
There was a significant decrease in blood pressure after 1 month of exercise. A significantly continuous decrease in TNF-α was also observed. The baroreflex indexes were significantly increased in the acute intervention of slow-breathing but not in the longitudinal effect. The HRV variables did not show differences with time. There were positive correlations between sympathetic index and TNF-α and galectin-3.
Conclusions
The effect of slow-breathing exercise on blood pressure and chronic inflammation was significant. HRV indexes may also be used to assess chronic inflammation.
Keywords: Atherosclerosis, Autonomic function, Baroreflex, Chronic inflammation, Hypertension, Slow breathing
INTRODUCTION
The World Health Organization estimated that 1.13 billion people worldwide have hypertension, but fewer than 20% people have the problem under control. In Taiwan, a large survey of ~50,000 participants showed that 26.1% of the participants had an elevated pharmacist-measured blood pressure (BP) (≥ 140/90 mm Hg). Moreover, even among those who were receiving treatment for hypertension, 36.9% still had poor BP management.1 In those with coronary heart disease, chronic kidney disease or age over 75 years, a lower target systolic BP (< 120 mm Hg) is suggested.2 In addition to atherosclerosis, desensitization of baroreflex due to sympathetic hyperactivation is one of the most important causes of hypertension,3,4 and it has also been shown to increase variations in blood pressure5-7 and the risk of end-organ damage.8-11 Furthermore, dysfunction of the autonomic nervous system (ANS) in these patients, and especially over activation of the sympathetic system, has been shown to increase the risks of the most fatal cardiovascular diseases, particularly heart attack, myocardial infarction and stroke.12-15
The maladaptation of ANS function can alter the systemic inflammatory response through pro- and anti-inflammatory pathways modulated by the ANS system.16 An increase in acetylcholine (parasympathetic activation) inhibits the secretion of numerous inflammatory cytokines, including tumor necrosis factor (TNF) and interleukins (IL)-1, IL-6 and IL-18,17 while the release of norepinephrine and epinephrine (sympathetic activation) increases the production of TNF18,19 and IL-6.20,21 A chronic low-grade pro-inflammatory state may be caused by altered ANS activity by impairing the reaction of the hypothalamic-pituitary-adrenal axis,22,23 and it has been shown to be independently associated with atherosclerosis in patients with essential hypertension24,25 beyond merely playing an integral part in the pathogenesis of hypertension.26-28 Therefore, the interplay among chronic inflammation, atherosclerosis and ANS function should be measured and considered simultaneously to better understand the progression and severity of hypertension during treatment.
Breathing exercise is a validated nonpharmacological intervention in hypertension control.29-32 Although the underlying mechanisms have yet to be fully elucidated, ANS stimulation of baroreceptors during prolonged inhalation and exhalation may increase baroreflex sensitivity (BRS) and reduce sympathetic activity and chemoreflex activation.33,34 Several studies have shown the effect of slow breathing exercise on BRS, BP and ANS function.33,35,36 However, to the best of our knowledge, no previous study has investigated the effect of breathing exercise on chronic inflammation.
Recent improvements in wearable devices which provide heart rate variability (HRV) analysis, atrial fibrillation detection and blood pressure monitoring may provide real-time biofeedback on the effectiveness of breathing exercise. Electrocardiography (ECG)-based analysis of HRV has been shown to be able to noninvasively quantify the function of ANS, and some HRV parameters have been suggested to be correlated with inflammatory factors such as C-reactive protein (CRP) and white blood cells.37-39 Properly integrating information of autonomic systems could provide an alternative, noninvasive way to assess neural-regulated immunity. Therefore, in this study, we investigated the longitudinal effect of (1) device-guided breathing exercise (DGSB) on BP, ANS function, baroreflex activity, vascular stiffness, and chronic inflammation, and (2) the correlation between device-derived HRV and blood inflammatory markers in hypertensive patients.
Hypothesis
• Three-month DGSB exercise may induce significant BP changes in subjects with essential hypertension.
• There might be changes in autonomic modulation, such as HRV indicators, pulse wave velocity (PWV) and BRS in subjects with essential hypertension before and after 3 months of DGSB exercise.
• There might also be differences in inflammatory biomarkers before and after 3 months of DGSB exercise in these subjects with essential hypertension.
METHODS
Subject selection
This was a prospective observational study. We recruited 46 essential hypertension patients who visited the outpatient clinic at National Taiwan University Hospital between May 2015 and June 2016. The inclusion criteria were patients who received medications and had good lifestyle control. The ages of the patients ranged from 35 to 75 years. Patients with recent medication titration related to BP or ANS function within 2 weeks, with recent major surgery or admission within 1 year, who had a history of anemia, asthma, thyroid dysfunction or autonomic neuropathy, or lived at high altitudes or had recently visited high mountainous areas for more than 1 week were excluded from this study. All subjects agreed and were willing to practice DGSB exercise for 3 months and visit the clinic 3 times for data collection. All subjects were requested to keep a routine diet, lifestyle and drugs during the 3-month study period.
DGSB exercise
The subjects were requested to perform DGSB five times per day over the 3 months. At the first visit, the subjects were instructed to perform the DGSB exercise using a wearable ECG wrist band (MiCor A100, MiTAC Corp., Taiwan) which was later brought home by the subjects. The respiratory frequency was 6 breathes per minutes and lasted for 3 minutes. During the breathing exercise, the rhythm of inhalation and exhalation was visually guided by a smartphone app, in which an interface of a six-petal flower was presented (corresponding to the 6 cycles of breathing per minute). The length of the petal grew with corresponding cardiopulmonary coupling, i.e. the respiratory sinus arrhythmias that induced rhythmic changes in heartbeat intervals were synchronized with the guided respiratory rhythm (Figure 1). The larger the amplitude of the respiratory sinus arrhythmia, the longer the length of the petal.
Figure 1.
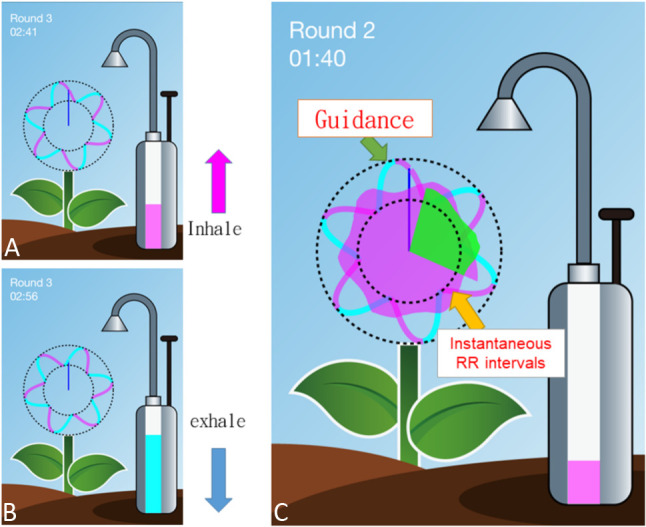
The graphical user interface of our Device Guided Slow Breathing (DGSB). During the breathing exercise, a pump at the right of the screen with an increasing/decreasing bar indicating inhale (a)/exhale (b). The length of the petal (c) changes with respect to the instantaneous changes in heartbeat interval (RR) due to respiration.
Data collection
The time points of the three visits were: (1) baseline, when the patient first visited the clinic and agreed to participate, (2) 1 month after baseline (noted as 1M), and (3) 3 months after baseline (noted as 3M). The subjects were asked to refrain from alcohol and caffeine intake within 24 hours before the visit for data collection. During each visit, the following data were collected by trained nursing personnel: (1) basic characteristics: body height, body weight and body mass index (BMI); (2)BP, including systolic (SBP), diastolic (DBP) and mean artery pressure (MAP); (3) three ECG recordings, each for 2 minutes, for heart rate and HRV analysis using the same type of ECG wristband (MiCor A100, MiTAC Corp., Taiwan); (4) brachial-ankle pulse wave velocity (baPWV) measurement in the supine position using a Colin VP-2000 system (Omron Inc., Japan) on both the right side and left side of the body; (5) BRS, measured by continuous BP and heartbeat interval recordings monitored using a Nexfin system (BMEYE Co., Ltd., USA). The BRS was recorded in two situations: spontaneous breathing session at rest, and during DGSB. The subjects were asked to rest for the first 3 minutes, and then the BRS was measured for 5 minutes during spontaneous breathing. Afterwards, the subjects were asked to perform DGSB exercise, during which the BRS was measured simultaneously for 5 minutes; the low-frequency components (0.1 Hz) at the frequency spectrum of BP and RRI were defined as BPLF and RRLF, respectively. BRS was calculated as the ratio of BPLF and RRLF. (6) Whole venous blood samples (3-5 cm3) were obtained, and 1~2 cm3 was taken for biochemical analysis. The levels of blood inflammatory markers, including TNF-α, IL-6 and IL-1 receptor antagonists (IL-1ra/IL-1F3), galectin-3 and CRP were analyzed according to the standard protocol at the laboratory of the recruiting hospital.
HRV analysis
ANS function was measured using HRV analysis derived from the ECG recordings from the wearable device at each visit. The built-in protocol for HRV analysis was 2 minutes long. Three consecutive measurements were made in each patient, and the average was used for analysis. The ECG recordings were sent to the smartphone immediately after each measurement and saved to the cloud server provided by the device manufacturer. We retrieved the raw data of ECG recordings and performed HRV analysis offline using a standard protocol which was described in detail in our previous article.40 In brief, the R-peak was identified by an automatic algorithm. Artifacts or ectopic beats were replaced by interpolated beats derived from the nearest valid data. If the proportion of artifacts or ectopic beats was > 5%, the recording was excluded from study. HRV indexes were performed on these RR intervals (RRI). Time domain analysis included standard deviation of RRI (SDNN), proportion of RRI that was > 50 msec (pNN50), and root mean square standard deviation (RMSSD). Frequency domain analysis was conducted by summing the power spectrum of RRI within the low frequency range 0.04-0.15 Hz (LFP) and high frequency range 0.15-0.4 Hz (HFP). SDNN, pNN50, RMSSD, and HFP are indicators of the activity of parasympathetic nervous system, while the ratio between HFP and LFP defines low-high ratio (LF/HF ratio) and indicates sympathetic nervous system (SNS) activity.
Statistical analysis
Measurements of BP, baPWV, HRV indexes and blood inflammatory biomarkers were compared among the three visits. In all the statistical tests, normality was assessed using the Shapiro-Wilk test. If the data were normally distributed, repeated measures ANOVA was performed for each of the measurements, and post hoc comparisons among baseline and 1M/3M were performed using the Tukey HSD test. Otherwise, Friedman repeated-measures analysis of variance on ranks was used, with the Student-Newman-Keuls test for post hoc comparisons between baseline and 1M/3M. In addition, for the outcome variables that had significant intervention effects, two-way repeated measures ANOVA was used to explore the effects of comorbidities (either coronary artery disease, hyperlipidemia or diabetes) on the intervention. For BRS, two-way repeated measures ANOVA was performed to test the effect of treatment time, test conditions (resting or DGSB session) and their interactions.
Ethics
Written informed consent was obtained from each patient before enrollment. All procedures were approved by the Ethical Committee of National Taiwan University Hospital (20150406RINB).
RESULTS
General characteristics of the participants
Initially, 46 patients with essential hypertension were enrolled in this study, of whom seven were lost to follow-up, two were excluded owing to refractory arrhythmias, and one withdrew because of poor compliance to self-practice DGSB exercise with blood pressure measurement protocol. A total of 36 subjects (male/female = 22/14) finished the 3-month follow-up protocol and completed data collection. The average age of these 36 subjects was 59.4 ± 9.0 years, and the BMI was 25.9 ± 3.1 kg/m2. The summary of clinical characteristics of these 36 subjects is shown in Table 1. During the 3-month follow-up period of DGSB training, none of the subjects changed their prescriptions of hypertension medication. In addition, fasting glucose, triglycerides, high-density lipoprotein and low-density lipoprotein in the blood biochemistry profiles were all well controlled in the subjects.
Table 1. Clinical characteristics of 36 subjects who finished the 3-month self-practice DGSB exercise training.
| Variable | Mean ± SD (percentage) |
| Age | 59.4 ± 9.0 |
| Gender: male | 22 (61.1%) |
| Body mass index (kg/m2) | 25.9 ± 3.1 |
| Comorbidity | |
| Coronary artery disease | 20 (55.6%) |
| Hyperlipidemia | 18 (50.0%) |
| Diabetes | 10 (27.8%) |
| Current medications | |
| Statin | 19 (52.8%) |
| Beta-blockers | 24 (66.7%) |
| Calcium channel blockers | 16 (44.4%) |
| ACEI/ARB | 23 (63.9%) |
| Biochemistry sampling | |
| Triglyceride (mg/dL) | 143.3 ± 69.6 |
| Total cholesterol (mg/dL) | 172.6 ± 33.3 |
| HDL-cholesterol (mg/dL) | 46.2 ± 10.5 |
| LDL-cholesterol (mg/dL) | 96.1 ± 35.4 |
| Fasting serum glucose (mg/dL) | 102.7 ± 22.8 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; DGSB, Device Guided Slow Breathing; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
Blood pressure and baPWV
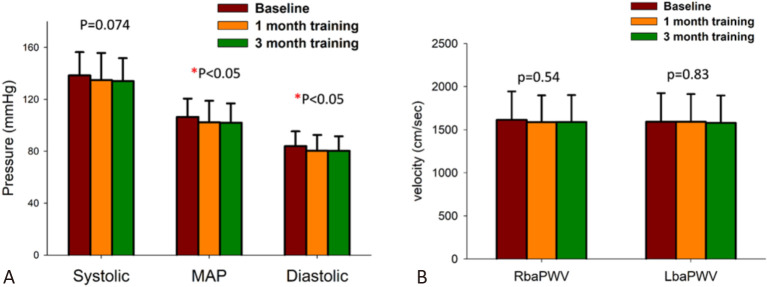
Figure 2(A) demonstrates the changes in BP in the non-dominant hands of the subjects during the self-practice DGSB exercise training period. There was no significant difference between the SBP measurements at baseline, 1M and 3M. However, the DBP and MAP showed significant decreases between the measurements at baseline, 1M and 3M (p < 0.05). A post-hoc analysis showed significant changes in DBP and MAP (both p < 0.05) from baseline to 1M and baseline to 3M; the change from 1M to 3M was not significant. The effects of DGSB training on MAP and DBP were not significantly different between the patients with or without comorbidities (all p > 0.05). There were no significant differences in right baPWV or left baPWV in any of the subjects during the 3-month follow-up period (Figure 2(B)).
Figure 2.
Changes in blood pressures (A) and brachial-ankle pulse wave velocity (baPWV) (B) during the 3-month period of device-guided slow breathing. MAP, mean artery pressure. RbaPWV, baPWV of the right side; LbaPWV, baPWV of the left side.
Baroreflex sensitivity
On the three visits during the experiment (baseline, 1M and 3M), BPLF and RRLF, which are the low-frequency components (0.1 Hz) of BP and heart rate, respectively, increased significantly during the DGSB intervention compared to spontaneous breathing sessions. This significant difference was observed in all three visits (Figure 3). However, the differences in BPLF and RRLF between DGSB exercise and resting did not change during the study period, i.e. the effect of the interaction between time and session was not significant (Figure 3). There was no significant time effect on these two variables. BRS did not show a significant difference between resting and DGSB sessions, nor in the three different visits.
Figure 3.
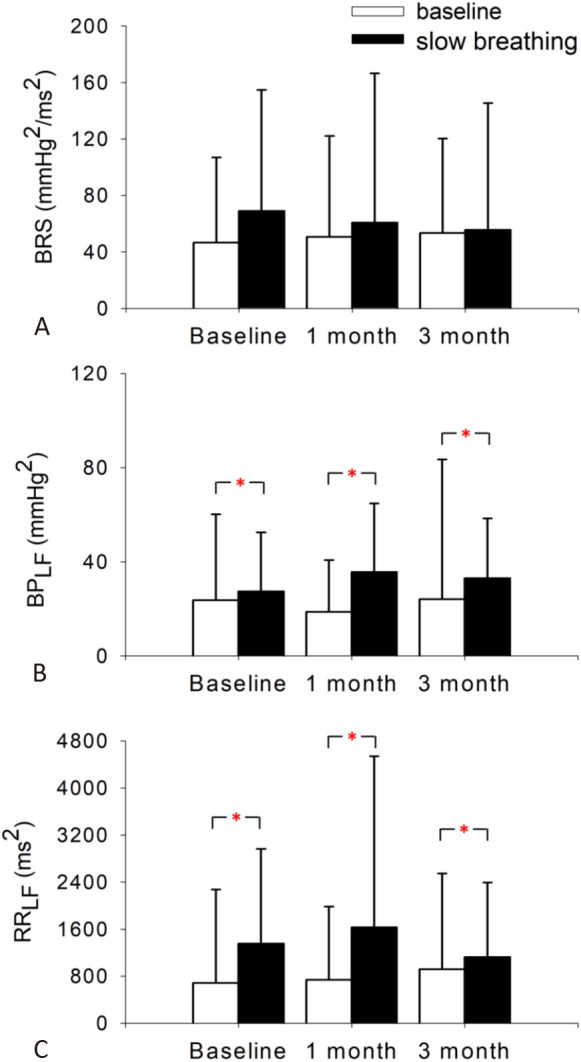
The effect of device-guided slow breathing exercise on baroreflex sensitivity related indexes between resting session and slow-breathing session during the 3-month period. (A) Baroreflex sensitivity. (B) The low-frequency component (0.1 Hz) of blood pressures (BPLF). (C) The low-frequency component (0.1 Hz) of heartbeat intervals (RRLF). * p < 0.05.
Heart rate variability
Table 2 shows the HRV parameters of SDNN, pNN50, RMSSD, HFP and LF/HF ratio of the subjects at baseline, 1M and 3M during the period of 3-month DGSB exercise training. There were no significant differences in the three visits in any of the HRV parameters.
Table 2. Changes in heart rate variability (HRV) parameters during the 3-month period of DGSB.
| Variable | Baseline | 1 month | 3 month |
| SDNN (ms) | 28.68 ± 13.70 | 26.83 ± 15.62 | 27.29 ± 16.00 |
| pNN50 (%) | 7.65 ± 15.22 | 5.22 ± 12.92 | 5.45 ± 14.97 |
| RMSSD (ms) | 22.91 ± 17.13 | 19.64 ± 18.73 | 19.06 ± 18.64 |
| HFP (ms2) | 219.21 ± 322.10 | 179.74 ± 424.17 | 76.95 ± 350.79 |
| LF/HF ratio (ms2) | 2.708 ± 3.124 | 1.213 ± 4.307 | 0.848 ± 3.657 |
DGSB, Device Guided Slow Breathing; HFP, high frequency power (0.15-0.4 Hz) of heartbeat series; LF/HF ratio, the ratio between HFP and LFP [low frequency power (0.04-0.15 Hz) of heartbeat series]; pNN50, proportion of heartbeat intervals that are larger than 50 msec; RMSSD, root mean square standard of heart rate intervals; SDNN, standard deviation of heartbeat intervals.
Blood inflammatory biomarkers
Figure 4 demonstrates the changes in blood inflammatory biomarkers during the experiment. TNF-α showed a significant continuous decline during the 3-month training period: the difference was significant after 1 month of DGSB exercise training (p < 0.05), and it declined further after 3 months of training (p < 0.05). The level of decrease in TNF-α was not significantly different in the patients with or without comorbidities (all p > 0.05). However, the other biomarkers including IL-6, IL-1ra, galectin-3 and CRP did not show significant differences during the study period.
Figure 4.
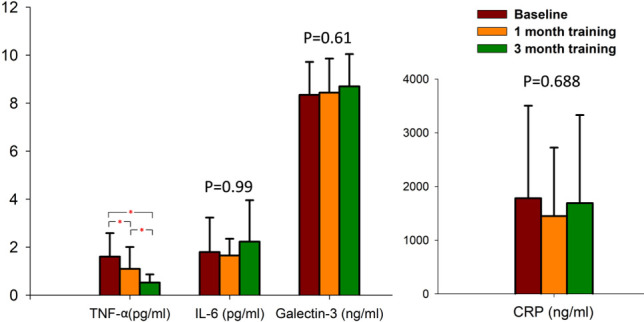
Changes in blood inflammatory biomarkers during the 3-month period of device-guided slow breathing. CRP, C-reaction protein; IL-1ra/IL-1F3, interleukin-1 receptor antagonist; IL-6, interleukins-6; TNF-α, tumor necrosis factor-alpha. * p < 0.05.
Correlation between HRV and blood inflammatory biomarkers
Table 3 shows correlations between HRV parameters and blood inflammatory biomarkers. There were significant positive correlations between the sympathetic indicator, LF/HF ratio, and the two inflammatory biomarkers, TNF-α and galectin-3. The correlations between LF/HF ratio and IL-6, IL-1ra/IL-1F3 and CRP were not significant. There was also a negative correlation between SDNN and CRP (p < 0.05). There were no significant correlations between the other parasympathetic indicators, including pNN50, RMSSD and HFP, and the blood inflammatory biomarkers.
Table 3. Correlation between heart rate variability and blood inflammatory biomarkers. Values presented are correlation coefficients (R-square).
| SDNN | pNN50 | RMSSD | HFP | LF/HF ratio | |
| TNF-α (pg/ml) | -0.014 | 0.013 | 0.045 | -0.002 | 0.294# |
| IL-6 (pg/ml) | -0.021 | 0.097 | 0.114 | 0.063 | 0.046 |
| IL-1ra/IL-1F3 (pg.ml) | 0.000 | -0.032 | 0.002 | 0.034 | 0.012 |
| Galectin-3 (ng/ml) | -0.135 | -0.138 | -0.037 | 0.014 | 0.316# |
| CRP (ng/ml) | -0.184* | -0.02 | -0.042 | -0.079 | 0.165 |
* p < 0.05; # p < 0.001.
CRP, C-reaction protein; HFP, high frequency power (0.15-0.4 Hz) of heartbeat series; IL-1ra/IL-1F3, interleukin-1 receptor antagonist; IL-6, interleukins-6; LF/HF ratio, the ratio between HFP and LFP [low frequency power (0.04-0.15 Hz) of heartbeat series]; pNN50, proportion of heartbeat intervals that are larger than 50 msec; RMSSD, root mean square standard of heart rate intervals; SDNN, standard deviation of heartbeat intervals; TNF-α, tumor necrosis factor-alpha.
DISCUSSION
To the best of our knowledge, this is the first follow-up study to investigate the long-term effects of a real-time DGSB biofeedback intervention on chronic inflammation and DGSB on BP, ANS, arterial stiffness in patients with hypertension in Taiwan. The main findings are as follows. First, DGSB decreased BP, especially DBP, at the first month of the intervention, and this effect was preserved during the intervention at the third month. Second, DGSB significantly decreased TNF-α, but other blood inflammatory biomarkers including IL-6, IL-1ra, galectin-3 and CRP did not show significant differences among the three time period points. Third, an immediate effect of DGSB on BRS could be seen on the low-frequency component of BP and heart rate but not in their respective ratio, which is the BRS index. The effect was independent of the long-term practices in DGSB. Fourth, HRV indexes derived from the wearable ECG device were correlated with blood inflammatory biomarkers. In particular, the sympathetic index (LF/HF ratio) was negatively correlated with galectin-3 and CRP.
DGSB and BP regulation
DGSB is an US Food and Drug Administration (FDA)-approved adjunctive treatment for hypertension. Although the evidence is conflicting,41 the effect of lowering BP by practicing DGSB for more than 8 weeks is supported by many studies30-32 and has been listed as a Class IIA alternative approach by the American Heart Association.42 In our study, decreases in DBP and MAP in the non-dominant hand were shown after DGSB training for 1 month, and the effect of BP lowering was maintained after 3 months of DGSB.
Interestingly, we did not observe a longitudinal decrease in HRV or PWV, indicating that the decrease in BP was not due to changes in ANS function or vascular stiffness. The possible mechanism of the decreased BP might be an increase in BRS. Previous studies have shown that acute electronic baroreflex stimulation therapy can decrease heart rate, plasma renin concentration, and BP.35,36,43 In this study, an acute enhancement of BRS was observed in the significant increase in the low-frequency power of RRI and BP with DGSB exercise. Although the mechanism of how DGBS enhances BRS is not fully known, most of the present evidence points to an increase in chemical sensitivity or BRS33,34 and the inhibition of an overactive SNS.35,44 In the present study, there was no significant improvement in BRS indexes in the 3-month follow-up period at rest or during DGSB. The hypothetical pathway of the observed effect in lowering BP might be through a decrease in respiratory rate during spontaneous breathing, which further promotes BRS at rest. Future studies monitoring ambulatory respiration during a follow-up period of DGSB exercise are warranted to validate our findings and to elucidate the underlying mechanisms.
DGSB and inflammatory biomarkers
Many studies have revealed the pathway and causality of blood inflammation markers in atherosclerosis. The concentrations of CRP, IL-6, TNF-α and galectin-3 have been associated with the level of atherosclerosis and the risk of cardiovascular disease.45,46 Our findings showed that BRS mainly influence the degree of TNF-α; the decreasing trend was observed at both the first and third months, while the underlying mechanism is unknown. There were negative correlations between LF/HF and TNF-α as well as galectin-3 during the 3 months of DGSB, indicating that the link between ANS and inflammatory system became more prominent. One possible explanation is that the strengthened sensitivity in chemoreceptors or baroreceptors by BRS also enhanced parasympathetic nerve activity and concurrently intensified the modulation of neuro-immune circuits. Therefore, the increase in anti-inflammatory response further decreased TNF-α. Nevertheless, the reason for the lack of differences in the other inflammation markers needs further biochemical investigations.
Application of wearable ECG devices in DGSB, HRV and blood inflammatory biomarkers
The previous contradictory results of the long-term effect of DGSB on BP may be due to different experimental designs, patient background, guiding interface and compliance of the patients.29 The superiority of our DGSB design is the real-time biofeedback interface which integrates instantaneous RRI changes derived from dry-electrode ECG signals during DGSB, and provides immediate evaluation of the synchronization between the user’s respiration and the guiding rhythm. This may have promoted DGSB training efficiency and user compliance. In addition, the other advantage is the HRV data provided by dry-lead ECG, which is both a widely accepted indicator of ANS function and also a promising biomarker for assessing chronic inflammation.
Limitations
The DGSB in this study was carried out by self-practice in a home setting. More than one-third of the patients did not finish five sets of the designed practice protocol every day, even though a reminder was provided every day for each subject in this study. However, > 90% of the subjects still completed at least three sets of DGSB daily. The dosage effect for DGSB can be further investigated in future studies. The small number of subjects and lack of a control group may also limit the generality of our results. Lifestyle changes due to elevated alertness or the placebo effect cannot be ruled out. Differences in DGSB training time and the environment that may have altered the effectiveness of DGSB exercise are also unmanageable variables. Moreover, all the examinations and measurements were performed in the hospital, so the white coat effect may also have biased the derived biomarkers.
CONCLUSION
The slow-breathing exercise guided by an interactive ECG wearable device was able to increase the low-frequency power of heart rate and BP; this might improve the imbalance of ANS function. During the 3-month training period, BP was significantly lowered in the patients with hypertension. One of the blood inflammatory biomarkers, TNF-α, was also decreased during the training period.
Acknowledgments
This work was supported by Taiwan Ministry of Science and Technology under Grants NO.106-2917-I-564-027 and 108-2221-E-008-095-MY2 (to C. L.) and 106-2221-E-008-032-MY2, 107-2622-E-008-024-CC2 and 108-2221-E-008-040-MY3 (to M. T. L.).
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Pan HY, Lin HJ, Chen WJ, Wang TD. Prevalence, treatment, control and monitoring of hypertension: a nationwide community-based survey in Taiwan, 2017. Acta Cardiol Sin. 2020;36:375. doi: 10.6515/ACS.202007_36(4).20191220A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang CE, Wang TD, Lin TH, et al. The 2017 focused update of the guidelines of the Taiwan Society of Cardiology (TSOC) and the Taiwan Hypertension Society (THS) for the management of hypertension. Acta Cardiol Sin. 2017;33:213. doi: 10.6515/ACS20170421A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esler M, Rumantir M, Kaye D, et al. Sympathetic nerve biology in essential hypertension. Clin Exp Pharmacol Physiol. 2001;28:986–989. doi: 10.1046/j.1440-1681.2001.03566.x. [DOI] [PubMed] [Google Scholar]
- 4.Carthy ER. Autonomic dysfunction in essential hypertension: a systematic review. Ann Med Surg (Lond) 2013;3:2–7. doi: 10.1016/j.amsu.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein DS. Arterial baroreflex sensitivity, plasma catecholamines, and pressor responsiveness in essential hypertension. Circulation. 1983;68:234–240. doi: 10.1161/01.cir.68.2.234. [DOI] [PubMed] [Google Scholar]
- 6.Bristow JD, Honour AJ, Pickering GW, et al. Diminished baroreflex sensitivity in high blood pressure. Circulation. 1969;39:48–54. doi: 10.1161/01.cir.39.1.48. [DOI] [PubMed] [Google Scholar]
- 7.Huang D, Zhou J, Su D, et al. Variations of perioperative baroreflex sensitivity in hypertensive and normotensive patients. Clin Exp Hypertens. 2017;39:74–79. doi: 10.1080/10641963.2016.1210624. [DOI] [PubMed] [Google Scholar]
- 8.Su DF. Reduction of blood pressure variability: a new strategy for the treatment of hypertension. Trends Pharmacol Sci. 2005;26:388–390. doi: 10.1016/j.tips.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Parati G, Pomidossi G, Albini F, et al. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–98. doi: 10.1097/00004872-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Parati G, Mancia G. Blood pressure variability as a risk factor. Blood Press Monit. 2001;6:341–347. doi: 10.1097/00126097-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Sleight P. The importance of the autonomic nervous system in health and disease. Aust N Z J Med. 1997;27:467–473. doi: 10.1111/j.1445-5994.1997.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 12.Harris KF, Matthews KA. Interactions between autonomic nervous system activity and endothelial function: a model for the development of cardiovascular disease. Psychosom Med. 2004;66:153–164. doi: 10.1097/01.psy.0000116719.95524.e2. [DOI] [PubMed] [Google Scholar]
- 13.Palatini P, Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Hypertens Rep. 2009;11:199–205. doi: 10.1007/s11906-009-0035-4. [DOI] [PubMed] [Google Scholar]
- 14.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 15.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014;114:1815–1826. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 16.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 17.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 18.Bertini R, Garattini S, Delgado R, Ghezzi P. Pharmacological activities of chlorpromazine involved in the inhibition of tumour necrosis factor production in vivo in mice. Immunology. 1993;79:217. [PMC free article] [PubMed] [Google Scholar]
- 19.Spengler RN, Chensue SW, Giacherio DA, et al. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J Immunol. 1994;152:3024–3031. [PubMed] [Google Scholar]
- 20.Nilsson MB, Armaiz-Pena G, Takahashi R, et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem. 2007;282:29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 21.Norris JG, Benveniste EN. Interleukin-6 production by astrocytes: induction by the neurotransmitter norepinephrine. J Neuroimmunol. 1993;45:137–145. doi: 10.1016/0165-5728(93)90174-w. [DOI] [PubMed] [Google Scholar]
- 22.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 23.Dedert EA, Calhoun PS, Watkins LL, et al. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med. 2010;39:61–78. doi: 10.1007/s12160-010-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietri P, Vyssoulis G, Vlachopoulos C, et al. Relationship between low-grade inflammation and arterial stiffness in patients with essential hypertension. J Hypertens. 2006;24:2231–2238. doi: 10.1097/01.hjh.0000249701.49854.21. [DOI] [PubMed] [Google Scholar]
- 25.Llaurado G, Ceperuelo-Mallafre V, Vilardell C, et al. Arterial stiffness is increased in patients with type 1 diabetes without cardiovascular disease: a potential role of low-grade inflammation. Diabetes Care. 2012;35:1083–1089. doi: 10.2337/dc11-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 27.Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- 28.Harrison DG, Guzik TJ, Lob HE, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gavish B. Device-guided breathing in the home setting: technology, performance and clinical outcomes. Biol Psychol. 2010;84:150–156. doi: 10.1016/j.biopsycho.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Meles E, Giannattasio C, Failla M, et al. Nonpharmacologic treatment of hypertension by respiratory exercise in the home setting. Am J Hypertens. 2004;17:370–374. doi: 10.1016/j.amjhyper.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Grossman E, Grossman A, Schein M, et al. Breathing-control lowers blood pressure. J Hum Hypertens. 2001;15:263–269. doi: 10.1038/sj.jhh.1001147. [DOI] [PubMed] [Google Scholar]
- 32.Logtenberg SJ, Kleefstra N, Houweling ST, et al. Effect of device-guided breathing exercises on blood pressure in hypertensive patients with type 2 diabetes mellitus: a randomized controlled trial. J Hypertens. 2007;25:241–246. doi: 10.1097/HJH.0b013e32801040d5. [DOI] [PubMed] [Google Scholar]
- 33.Joseph CN, Porta C, Casucci G, et al. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46:714–718. doi: 10.1161/01.HYP.0000179581.68566.7d. [DOI] [PubMed] [Google Scholar]
- 34.Bernardi L, Gabutti A, Porta C, Spicuzza L. Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. J Hypertens. 2001;19:2221–2229. doi: 10.1097/00004872-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Oneda B, Ortega KC, Gusmao JL, et al. Sympathetic nerve activity is decreased during device-guided slow breathing. Hypertens Res. 2010;33:708–712. doi: 10.1038/hr.2010.74. [DOI] [PubMed] [Google Scholar]
- 36.Fonkoue IT, Marvar PJ, Norrholm SD, et al. Acute effects of device-guided slow breathing on sympathetic nerve activity and baroreflex sensitivity in posttraumatic stress disorder. Am J Physiol Heart Circ Physiol. 2018;315:H141–H149. doi: 10.1152/ajpheart.00098.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolan RP, Reid GJ, Seidelin PH, Lau HK. C-reactive protein modulates vagal heart rate control in patients with coronary artery disease. Clin Sci (Lond) 2007;112:449–456. doi: 10.1042/CS20060132. [DOI] [PubMed] [Google Scholar]
- 38.Thayer JF, Fischer JE. Heart rate variability, overnight urinary norepinephrine and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J Intern Med. 2009;265:439–447. doi: 10.1111/j.1365-2796.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- 39.Gunterberg V, Simren M, Ohman L, et al. Autonomic nervous system function predicts the inflammatory response over three years in newly diagnosed ulcerative colitis patients. Neurogastroenterol Motil. 2016;28:1655–1662. doi: 10.1111/nmo.12865. [DOI] [PubMed] [Google Scholar]
- 40.Liu YC, Hung CS, Wu YW, et al. Influence of non-alcoholic fatty liver disease on autonomic changes evaluated by the time domain, frequency domain, and symbolic dynamics of heart rate variability. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahtani KR, Beinortas T, Bauza K, Nunan D. Device-guided breathing for hypertension: a summary evidence review. Curr Hypertens Rep. 2016;18:33. doi: 10.1007/s11906-016-0631-z. [DOI] [PubMed] [Google Scholar]
- 42.Brook RD, Appel LJ, Rubenfire M, et al. American Heart Association Professional Education Committee of the Council for High Blood Pressure Research CoC; Stroke Nursing CoE; Prevention and Council on Nutrition PA. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the american heart association. Hypertension. 2013;61:1360–1383. doi: 10.1161/HYP.0b013e318293645f. [DOI] [PubMed] [Google Scholar]
- 43.Heusser K, Tank J, Engeli S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–626. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 44.Hering D, Kucharska W, Kara T, et al. Effects of acute and long-term slow breathing exercise on muscle sympathetic nerve activity in untreated male patients with hypertension. J Hypertens. 2013;31:739–746. doi: 10.1097/HJH.0b013e32835eb2cf. [DOI] [PubMed] [Google Scholar]
- 45.Paffen E, DeMaat MP. C-reactive protein in atherosclerosis: a causal factor? Cardiovasc Res. 2006;71:30–39. doi: 10.1016/j.cardiores.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]