Abstract
Background
Immediate-release carvedilol requires twice-daily dosing and may have low treatment compliance. We assessed the efficacy of a new formulation of once-daily extended-release carvedilol (carvedilol ER) on systolic blood pressure (SBP) and diastolic blood pressure (DBP) among patients with hypertension in this double-blind, randomized, placebo-controlled trial.
Methods
A total of 134 patients with untreated or uncontrolled hypertension were randomly assigned in a 1:1:1 ratio to receive placebo, low-dose carvedilol ER, or high-dose carvedilol ER for 8 weeks. The primary endpoint was the reduction in office SBP at 8 weeks. Secondary endpoints included the reduction in office DBP and the proportion of patients with blood pressure (BP) < 140/90 mm Hg.
Results
In the intention-to-treat population, placebo-adjusted changes in SBP/DBP were -2.9 mm Hg [95% confidence interval (CI), -9.6 to 3.7]/-1.7 mm Hg (95% CI, -5.6 to 2.3) and -4.9 mm Hg (95% CI, -11.5 to 1.7)/-3.4 mm Hg (95% CI, -7.3 to 0.5) for low-dose carvedilol ER and high-dose carvedilol ER, respectively. In the per-protocol population, high-dose carvedilol ER was associated with a significant DBP reduction [placebo-adjusted difference, -4.7 mm Hg (95% CI, -8.8 to -0.5); adjusted p = 0.026]. There was a gradational improvement in BP control with carvedilol ER (25%, 37%, and 48% for placebo, low-dose carvedilol ER, and high-dose carvedilol ER, respectively; linear-by-linear association p = 0.028). There were no differences in safety among the three groups.
Conclusions
Carvedilol ER, though well tolerated, did not result in a greater reduction in either SBP or DBP compared with placebo.
Keywords: Carvedilol, Extended-release formulation, Hypertension
INTRODUCTION
Elevated blood pressure (BP) is one of the leading causes of cardiovascular and renal diseases. By estimation, at least one fifth of the global adult population is affected by hypertension.1 Controlling BP in patients at risk reduces the occurrence of long-term cardiovascular complications.2,3 Besides diuretics, calcium channel blockers, and blockers of the renin-angiotensin system, beta-blockers are among the commonly used antihypertensive drugs.4,5
Carvedilol is a third-generation beta-blocker. Except for its beta-adrenoceptor blockage effects, it has vasodilatory properties owing to alpha-adrenergic blockage. In addition to decreasing cardiac output, vasodilatory beta-blockers can lower BP further by reducing systemic vascular resistance. Compared with other beta-blockers, carvedilol is more metabolism-friendly as it does not appear to cause carbohydrate and/or lipid disturbances.6,7 Beyond the hypertension treatment, immediate-release carvedilol (carvedilol IR) is approved for the management of heart failure with reduced left ventricular ejection fraction and left ventricular dysfunction after myocardial infarction.8,9 Nevertheless, it requires twice-daily administration. Encapsulated controlled-release carvedilol (carvedilol CR) has been developed to improve the disadvantages of the pharmacokinetics of carvedilol IR and requires only once-daily administration.10 The tolerability of these two formulations has been reported to be very similar.11,12
Although carvedilol CR has been licensed by the U.S. Food and Drug Administration since 2006, it is not yet available in Taiwan. As less frequent dosing regimens result in better compliance to medications,13 the development of extended-release carvedilol (carvedilol ER) given once daily is expected to improve patient adherence to the treatment in Taiwan. The present study was designed to evaluate the efficacy and safety of a new formulation of carvedilol in patients with hypertension and to determine its optimal dose.
METHODS
Study design and oversight
We conducted a three-group, double-blind, randomized, placebo-controlled trial comparing two doses of carvedilol ER with placebo in patients with hypertension at nine centers. The trial was sponsored by TSH Biopharm Corporation and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The protocol and amendments were approved by the health authority of Taiwan and the ethics committee at each participating center. All patients provided written informed consent.
Study patients
Patients aged between 20 and 90 years with untreated or uncontrolled (despite up to two antihypertensive drugs among which neither was a beta-blocker) mild to moderate essential hypertension [defined as systolic BP (SBP) between 140 mm Hg and 180 mm Hg and/or diastolic BP (DBP) between 90 mm Hg and 110 mm Hg based on the office measurement] were enrolled in the study. Antihypertensive treatments should remain unchanged for at least 4 weeks before screening. Key exclusion criteria included malignant hypertension; hypertension secondary to an identifiable and treatable cause; any other indications or any contraindications for a beta-blocker; clinically significant valvular or arrhythmic diseases; and a heart rate less than 60 beats per minute.
Study procedures
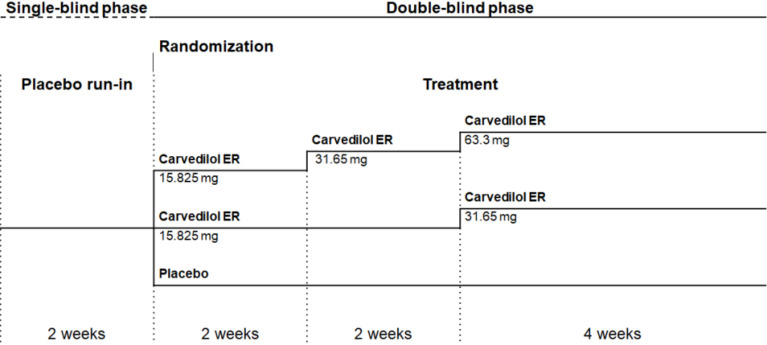
The study scheme is shown in Figure 1. Eligible patients underwent a 2-week, single-blind, placebo run-in period during which background antihypertensive treatments were unchanged. Patients who met the inclusion criteria were then randomly assigned in a 1:1:1 ratio to receive placebo, low-dose (15.825 mg for 4 weeks then 31.65 mg for 4 weeks) or high-dose (15.825 mg for 2 weeks, 31.65 mg for 2 weeks, then 63.3 mg for 4 weeks) carvedilol ER, all given once daily in the morning with food. Randomization with a permuted block (size of 6) was performed with the use of sealed envelopes provided by the sponsor. No stratification was applied.
Figure 1.
Study scheme with dosing schedule. ER, extended-release.
Carvedilol ER 15.825 mg and the matching placebo and carvedilol ER 63.3 mg and the matching placebo were provided as identical-appearing tablets in order to maintain blinding to the treatment. Each patient received two sets of study medications: either carvedilol ER 15.825 mg and the matching placebo or carvedilol ER 63.3 mg and the matching placebo. During the study, up-titration of study medications was stopped if SBP was less than 100 mm Hg or a heart rate was less than 50 beats per minute.
At each visit, office BP was assessed using an oscillometric sphygmomanometer (WatchBP Home; Microlife Corporation, Taipei, Taiwan). At and after randomization, all BP measurements were made in the morning between 8:00 and 12:00 on the same arm, in which higher BP was recorded during screening. The same member of staff in each participating center performed the measurements at the trough drug effect while patients were seated and rested for at least 5 minutes but had not been left unattended. Two BP readings were taken at a two-minute interval and a third measurement was taken if there was a 6 mm Hg or higher difference between the first two SBP readings. Averaged readings were used for all analyses. The first heart rate reading at the cuff pressure measurement was recorded.
Study endpoints
The primary endpoint was the reduction in SBP at 8 weeks. Secondary endpoints included the reduction in DBP at 8 weeks and the proportion of patients with controlled BP, defined as BP less than 140/90 mm Hg, at 8 weeks. Safety was assessed on the basis of vital signs, electrocardiography- and laboratory-associated parameters, and adverse events that occurred during the treatment and were coded according to the Medical Dictionary for Regulatory Activities, version 19.1.
Statistical analysis
The study was designed to test the hypothesis that carvedilol ER would be superior to placebo with regard to the SBP reduction. Based on the prior experience of carvedilol CR 40 mg and 80 mg,12 a change from baseline to the end of treatment in SBP between either dose of carvedilol ER and placebo was expected to be around 9 mm Hg. A pooled standard deviation of 12 mm Hg was used. Therefore, it was determined that approximately 38 evaluable patients for each treatment group would provide 80% power at an alpha level of 0.05. Considering a dropout rate of 20%, we originally planned to enroll 143 patients. However, this number was lowered when the review of blinded data suggested a lower-than-expected dropout rate.
The primary efficacy analysis was conducted within the intention-to-treat (ITT) population that included all patients who underwent randomization, received at least one dose of study medications, and had at least one evaluable datum for the efficacy assessment. The per-protocol (PP) population excluded patients who had major protocol violations, compliance to study medications of 80% or less, or no evaluable data at the end of the study. In addition, analyses for safety were performed based on the safety population that included all randomized patients who had taken at least one dose of study medications. We reported compliance to study medications using data for tablets dispensed to, taken by, and returned from each patient.
Changes in BP from baseline within treatment groups were tested by the paired t-test or the Wilcoxon signed rank test if the assumption of normality was violated, and between-group differences were analyzed by the one-way analysis of covariance model using baseline BP as the covariate and the Dunnett’s method for multiple comparisons. Two-sided 95% confidence intervals (CIs) were calculated using the least-squares mean and the root-mean-square error of the analysis of covariance model. For missing BP values, the last observation carried forward method was adopted. For the safety assessment, mean values among treatments and changes from baseline within treatment groups were compared by the analysis of variance model and the paired t-test, respectively. For categorical variables, values were compared with the Fisher’s exact test, the Pearson’s chi-squared test, or the Mantel-Haenszel test as appropriate; multiple comparisons were adjusted using the Bonferroni method where appropriate. Two-tailed p values less than 0.05 were considered to be significant. All analyses were done with SAS version 9.2 (SAS Institute, Cary, North Carolina, USA).
This trial is registered with ClinicalTrials.gov, number NCT02432937.
RESULTS
Patients
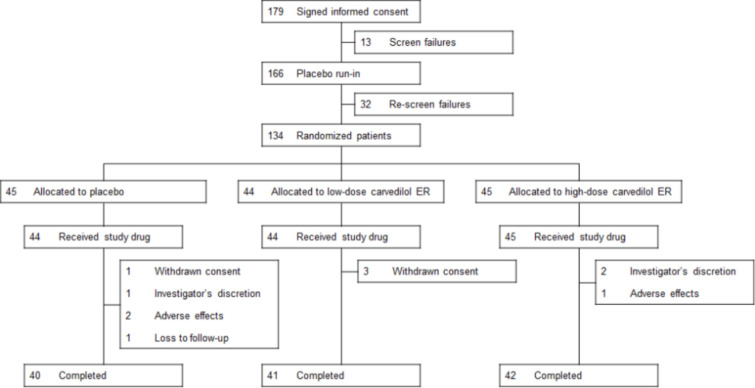
Between January 2015 and July 2016, 179 patients were screened and enrolled, among whom 134 (74.9%) underwent randomization (Figure 2). Of these patients, 133 were treated and included in the primary efficacy analysis. Treatment compliance was greater than 90% in all three groups.
Figure 2.
Flow diagram. ER, extended-release.
At baseline, demographic and clinical characteristics were well balanced between the placebo, low-dose and high-dose carvedilol ER groups (Table 1). Per study design, 52.3%, 62.8%, and 63.6% of the patients in the placebo, low-dose and high-dose carvedilol ER groups, respectively, had taken concomitant antihypertensive treatments during the study (p = 0.482). Of these treatments, renin-angiotensin system antagonists and calcium channel blockers were predominant. One patient was taking a prohibited alpha-blocker (minor protocol violation) and no patient received any additional beta-blockers.
Table 1. Baseline characteristics of patients.
| Placebo (N = 44) | Low-dose carvedilol ER (N = 43) | High-dose carvedilol ER (N = 44) | p value | |
| Age, yrs | 54.8 ± 13.6 | 55.8 ± 11.8 | 57.2 ± 12.4 | 0.681 |
| Female sex, % | 43.2 | 44.2 | 45.5 | 0.977 |
| Weight, kg | 72.7 ± 11.6 | 72.2 ± 14.8 | 74.1 ± 14.8 | 0.808 |
| Medical history | ||||
| Coronary artery disease, % | 22.7 | 30.2 | 31.8 | 0.600 |
| Kidney disease, %* | 4.5 | 7.0 | 11.4 | 0.513 |
| Diabetes, % | 25.0 | 20.9 | 15.9 | 0.573 |
| Dyslipidemia, % | 56.8 | 48.8 | 63.6 | 0.379 |
| Smoking, % | 15.9 | 11.6 | 18.2 | 0.69 |
| Obesity, %# | 22.7 | 11.6 | 20.5 | 0.369 |
| Physical inactivity, % | 11.4 | 11.6 | 15.9 | 0.777 |
| Newly diagnosed hypertension, %† | 47.7 | 37.2 | 36.4 | 0.482 |
| Prior beta-blocker experience, % | 9.1 | 14.0 | 9.1 | 0.728 |
| Current hypertensive medication | ||||
| ACE inhibitor, ARB, or renin inhibitor, % | 47.7 | 53.5 | 47.7 | 0.826 |
| Alpha-blocker, % | 0.0 | 2.3 | 0.0 | 0.328 |
| Calcium channel blocker, % | 29.5 | 41.9 | 43.2 | 0.349 |
| Diuretic, % | 4.5 | 7.0 | 9.1 | 0.771 |
| SBP, mm Hg | 147.4 ± 8.7 | 148.6 ± 12.3 | 147.2 ± 12.0 | 0.810 |
| DBP, mm Hg | 91.0 ± 7.7 | 91.3 ± 10.2 | 91.3 ± 8.0 | 0.986 |
| Heart rate, beats per minute‡ | 77.1 ± 9.2 | 76.9 ± 9.1 | 74.6 ± 11.2 | 0.411 |
* Kidney disease was defined as microalbuminuria or an estimated glomerular filtration rate < 60 mL/min per 1.73 m2 of body-surface area. # Obesity was defined as a body mass index ≥ 30 kg/m2. † Newly diagnosed hypertension was defined as no concomitant antihypertensive medications at baseline. ‡ Data were based on the safety population.
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; DBP, diastolic blood pressure; ER, extended-release; SBP, systolic blood pressure.
Efficacy
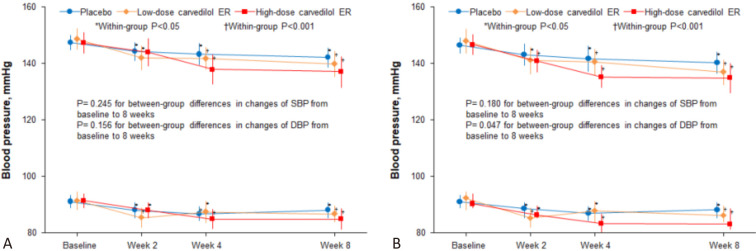
Figure 3A presents the BP changes by treatment group in the ITT population. In all three groups, SBP was significantly reduced by 4 weeks and DBP was reduced at 2 weeks and thereafter. At 4 weeks, the largest SBP reduction occurred in the high-dose carvedilol ER group (9.4 ± 16.6 mm Hg) and sustained at 8 weeks (10.3 ± 17.0 mm Hg). Although reductions in both SBP and DBP were the greatest with high-dose carvedilol ER, the between-group differences in SBP and DBP were not statistically significant. The placebo-adjusted changes in SBP and DBP were -2.9 mm Hg (95% CI, -9.6 to 3.7; adjusted p = 0.509) and -1.7 mm Hg (95% CI, -5.6 to 2.3; adjusted p = 0.532), respectively, in the low-dose carvedilol ER group. In the high-dose carvedilol ER group, the placebo-adjusted changes in SBP and DBP were -4.9 mm Hg (95% CI, -11.5 to 1.7; adjusted p = 0.169) and -3.4 mm Hg (95% CI, -7.3 to 0.5; adjusted p = 0.098), respectively.
Figure 3.
Blood pressure throughout the study in the intention-to-treat population. CI, confidence interval; DBP, diastolic blood pressure; ER, extended-release; SBP, systolic blood pressure.
The patterns and degrees of reductions in both SBP and DBP in the PP population during the study were similar to those observed in the ITT population (Figure 3B). The placebo-adjusted changes in SBP and DBP were -4.0 mm Hg (95% CI, -11.0 to 3.0; adjusted p = 0.338) and -2.8 mm Hg (95% CI, -7.1 to 1.5; adjusted p = 0.254), respectively, in the low-dose carvedilol ER group. In the high-dose carvedilol ER group, the placebo-adjusted changes in SBP and in DBP were -5.5 mm Hg (95% CI, -12.2 to 1.3; adjusted p = 0.128) and -4.7 mm Hg (95% CI, -8.8 to -0.5; adjusted p = 0.026), respectively.
BP goal achievement at 8 weeks
BP goal achievement rates at 8 weeks were 25%, 37%, and 48% for the patients assigned to the placebo, low-dose and high-dose carvedilol ER groups, respectively (Figure 4). There was a gradational improvement in the BP goal achievement (linear-by-linear association p = 0.028). High-dose carvedilol ER appeared to be associated with the highest BP goal achievement rate [odds ratio, 2.74 (95% CI, 1.11-6.76) for high-dose carvedilol ER compared with placebo; unadjusted p = 0.027 and adjusted p = 0.053].
Figure 4.
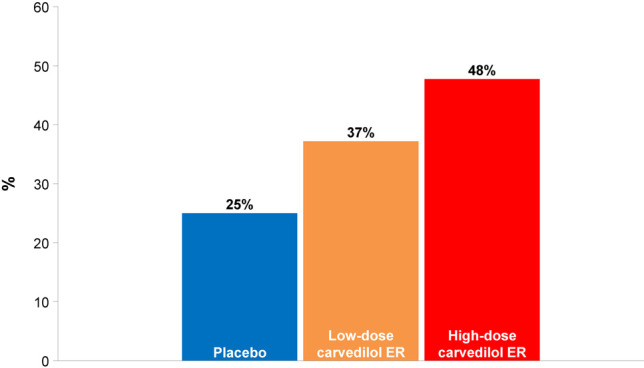
Proportions of patients who achieved systolic and diastolic blood pressure less than 140/90 mm Hg at 8 weeks in the intention-to-treat population. BP, blood pressure; ER, extended-release. Compared with placebo, the higher-dose carvedilol ER was associated with an improvement in BP control (linear-by-linear association p = 0.028; adjusted p = 0.437 comparing low-dose carvedilol ER with placebo; adjusted p = 0.053 comparing high-dose carvedilol ER with placebo).
Safety and adverse events
Both low-dose and high-dose carvedilol ER were well tolerated, as the proportions of patients who had adverse events were nominally lower in the low-dose and high-dose carvedilol ER groups than in the placebo group (Table 2). Serious adverse events were uncommon and were not related to study procedures or medications. The mean heart rate was lower in both the low-dose and high-dose carvedilol ER groups than in the placebo group at 4 weeks (Table 3). Changes in the heart rate from baseline to 8 weeks were -8.4 ± 9.0 beats per minute (p < 0.001) for low-dose carvedilol ER and -8.6 ± 11.0 beats per minute (p < 0.001) for high-dose carvedilol ER. Although the heart rate decreased with both doses of carvedilol ER, only two patients in the high-dose carvedilol ER group had a heart rate less than 60 beats per minute. Only one patient each in the low-dose and high-dose carvedilol ER groups who had no clinically significant abnormal electrocardiographic finding at baseline reported clinically significant abnormal changes during the study. There were no within- nor between-group differences in low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and fasting plasma glucose with either treatment between randomization and the end of the study.
Table 2. Adverse events.
| Placebo (N = 44) | Low-dose carvedilol ER (N = 44) | High-dose carvedilol ER (N = 45) | |
| Patients with any AEs† | 18 (40.9) | 8 (18.2) | 14 (31.1) |
| Palpitation | 0 (0.0) | 0 (0.0) | 2 (4.4) |
| Sinus bradycardia | 0 (0.0) | 0 (0.0) | 1 (2.2) |
| Decreased heart rate | 0 (0.0) | 0 (0.0) | 1 (2.2) |
| Prolonged QT interval by electrocardiogram | 0 (0.0) | 1 (2.3) | 0 (0.0) |
| Hypertension | 1 (2.3) | 0 (0.0) | 1 (2.2) |
| Fatigue or malaise | 2 (4.5) | 3 (6.8) | 0 (0.0) |
| Dizziness | 1 (2.3) | 0 (0.0) | 1 (2.2) |
| Postural dizziness | 0 (0.0) | 0 (0.0) | 1 (2.2) |
| Headache | 0 (0.0) | 1 (2.3) | 1 (2.2) |
| Depressed mood | 1 (2.3) | 0 (0.0) | 0 (0.0) |
| Insomnia | 2 (4.5) | 1 (2.3) | 1 (2.2) |
| Dyslipidemia | 0 (0.0) | 0 (0.0) | 1 (2.2) |
| Eye disorders | 1 (2.3) | 1 (2.3) | 0 (0.0) |
| Musculoskeletal and connective tissue disorders | 2 (4.5) | 3 (6.8) | 2 (4.4) |
| Respiratory, thoracic, and mediastinal disorders | 2 (4.5) | 1 (2.3) | 2 (4.4) |
| Gastrointestinal disorders | 3 (6.8) | 0 (0.0) | 1 (2.2) |
| Hepatobiliary disorders | 2 (4.5) | 0 (0.0) | 0 (0.0) |
| Infections | 3 (6.8) | 2 (4.5) | 1 (2.2) |
| AEs leading to study medication discontinuation | 2 (4.5) | 0 (0.0) | 1 (2.2) |
| Patients with any SAEs | 1 (2.3) | 1 (2.3) | 0 (0.0) |
* Data were based on the safety population. # Definitions were based on the Medical Dictionary for Regulatory Activities, version 19.1. † p value for the Pearson’s chi-squared test was 0.066. ‡ One patient in the placebo group was hospitalized for abdominal wound treatment and one patient in the low-dose carvedilol ER group developed urinary tract infection that required inpatient treatment. None of SAEs were judged by investigators to be study related.
AE, adverse event; ER, extended-release; SAE, serious adverse event.
Table 3. Changes in the heart rate from baseline.
| Placebo | Low-dose carvedilol ER | High-dose carvedilol ER | p value# | |
| Week 2 | 0.7 ± 7.6 | -3.6 ± 8.3 | -1.9 ± 10.8 | 0.092 |
| p = 0.565 | p = 0.007 | p = 0.240 | ||
| Week 4 | 0.5 ± 8.3 | -4.0 ± 7.5 | -6.5 ± 11.1 | 0.002 |
| p = 0.680 | p = 0.001 | p < 0.001 | ||
| Week 8 | -1.2 ± 9.9 | -8.4 ± 9.0 | -8.6 ± 11.0 | < 0.001 |
| p = 0.449 | p < 0.001 | p < 0.001 |
* Data were based on the safety population. # Between-group differences were compared by the analysis of variance model.
ER, extended-release.
DISCUSSION
In this double-blind, randomized, placebo-controlled trial in patients with untreated or uncontrolled hypertension, changes in BP at 8 weeks did not differ significantly between both doses of carvedilol ER and placebo despite that a dose-dependent effect on the BP goal achievement was observed with carvedilol ER compared with placebo. There were no major safety concerns for either dose of carvedilol ER.
The absence of a significant difference in changes in SBP from baseline to the end of the study between carvedilol ER and placebo in our study did not support the hypothesis that either dose of carvedilol ER would further reduce SBP by at least 9 mm Hg after correcting for the placebo effect. The lack of the between-group difference was probably attributed to several factors. First, we observed greater BP reductions with placebo in our study than those in patients with similar baseline BP in other studies with nebivolol.14 The BP changes with placebo in our study were also larger compared with patients enrolled in a prior study of carvedilol CR using ambulatory BP monitoring as one of the inclusion criteria (-5.4/-3.0 mm Hg versus -1.5/-1.9 mm Hg). In fact, the magnitude of BP reductions in the placebo group is often nontrivial in studies with no ambulatory BP monitoring.15-17 Second, the sample size of only a little over 40 patients per treatment group in our study was based on a prior study of carvedilol CR showing a 9 mm Hg reduction, on average, in SBP with 40 mg and 80 mg carvedilol CR at 6 weeks.12 However, the patients enrolled in the prior study had nominally higher BP than those in our study. The regression to the mean phenomenon,18 that is the higher the baseline BP, the bigger the fall after intervention,19 might be more apparent in the referenced study, in which more than 80 patients per treatment group were included.12 Furthermore, compared to office BP measurements, ambulatory BP monitoring is more sensitive when assessing BP changes. Using office measurements only is more susceptible to unexpected BP changes in the placebo group in a limited sample size, leveraging the outcome to the null.20 Practice guidelines endorse ambulatory BP monitoring to evaluate BP in clinical trials,21 nevertheless, patients may not tolerate wearing the equipment which could result in missing data. Considering conducting this clinical trial in nine centers, we opted to use office BP measurements for pragmatic reasons.
Although the reductions in SBP and DBP with either dose of carvedilol ER were not significant when compared with placebo in the ITT population, a considerably greater proportion of the patients who received carvedilol ER achieved a BP less than 140/90 mm Hg compared to those who received placebo. In the PP population, we observed a greater reduction in DBP with high-dose carvedilol ER than with placebo [between-group difference, -4.7 mm Hg (95% CI, -8.8 to -0.5)]. Also in the PP population, SBP/DBP was reduced by approximately 6.6/4.2 mm Hg, 11.2/6.6 mm Hg, and 11.8/7.2 mm Hg with 15.825 mg, 31.65 mg, and 63.3 mg carvedilol ER, respectively. Comparable reductions were observed in the prior study which assessed 20 mg, 40 mg, and 80 mg carvedilol CR.12 We further observed a dose-dependent achievement in BP control with higher dose carvedilol ER [odds ratio, 2.74 (95% CI, 1.11-6.76) for high-dose carvedilol ER compared with placebo]. In addition to BP reductions, beta-blockers reduce the heart rate. In our study, both 31.65 mg and 63.3 mg carvedilol ER were associated with reductions in the heart rate by approximately 8 beats per minute at 8 weeks. Similar reductions in the mean heart rate over 24 hours were observed in the prior study with 40 mg and 80 mg carvedilol CR.12
CONCLUSION
Neither dose of carvedilol ER, given once daily after 8 weeks, resulted in significant reductions in BP compared with placebo. Both doses were well tolerated.
Acknowledgments
The authors acknowledge the assistance of Tristan Tang for the statistical analyses, which were performed by a contract organization (Formosa Biomedical Technology Corporation) funded by the sponsor.
FUNDING SOURCES
This study was supported, in part, by the grant from the Ministry of Health and Welfare (MOHW109-TDU-B-211-114001), was funded by TSH Biopharm Corporation.
DISCLOSURE
Kang-Ling Wang reports honoraria from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, and Pfizer.
Chih-Yuan Fang, Wen-Ter Lai, Tzung-Dau Wang, Kwo-Chang Ueng, Kuo-Yang Wang, Ji-Hung Wang, and Kou-Gi Shyu, all have none to disclose.
Chern-En Chiang has been on the speaker bureau for AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi, and Servier.
REFERENCES
- 1.NCD Risk Factor Collaboration. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet. 2017;389:37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–598. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 3.Chiang CE, Wang TD, Lin TH, et al. The 2017 focused update of the guidelines of the Taiwan Society of Cardiology (TSOC) and the Taiwan Hypertension Society (THS) for the management of hypertension. Acta Cardiol Sin. 2017;33:213–225. doi: 10.6515/ACS20170421A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc. 2015;78:1–47. doi: 10.1016/j.jcma.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 6.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 7.Bakris GL, Fonseca V, Katholi RE, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292:2227–2236. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 8.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 9.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 10.Henderson LS, Tenero DM, Baidoo CA, et al. Pharmacokinetic and pharmacodynamic comparison of controlled-release carvedilol and immediate-release carvedilol at steady state in patients with hypertension. Am J Cardiol. 2006;98:17L–26L. doi: 10.1016/j.amjcard.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Kitakaze M, Sarai N, Ando H, et al. Safety and tolerability of once-daily controlled-release carvedilol 10-80 mg in Japanese patients with chronic heart failure. Circ J. 2012;76:668–674. doi: 10.1253/circj.cj-11-0210. [DOI] [PubMed] [Google Scholar]
- 12.Weber MA, Bakris GL, Tarka EA, et al. Efficacy of a once-daily formulation of carvedilol for the treatment of hypertension. J Clin Hypertens (Greenwich) 2006;8:840–849. doi: 10.1111/j.1524-6175.2006.05696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 14.Germino FW. Efficacy and tolerability of nebivolol monotherapy by baseline systolic blood pressure: a retrospective analysis of pooled data from two multicenter, 12-week, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging studies in patients with mild to moderate essential hypertension. Clin Ther. 2009;31:1946–1956. doi: 10.1016/j.clinthera.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Howard JP, Nowbar AN, Francis DP. Size of blood pressure reduction from renal denervation: insights from meta-analysis of antihypertensive drug trials of 4,121 patients with focus on trial design: the CONVERGE report. Heart. 2013;99:1579–1587. doi: 10.1136/heartjnl-2013-304238. [DOI] [PubMed] [Google Scholar]
- 16.Patel HC, Hayward C, Ozdemir BA, et al. Magnitude of blood pressure reduction in the placebo arms of modern hypertension trials: implications for trials of renal denervation. Hypertension. 2015;65:401–406. doi: 10.1161/HYPERTENSIONAHA.114.04640. [DOI] [PubMed] [Google Scholar]
- 17.Wang TD. From real-world evidence to consensus of renal denervation in Taiwan: a call for the incorporation of ambulatory blood pressure monitoring after witnessed intake of medications. Acta Cardiol Sin. 2019;35:553–556. doi: 10.6515/ACS.201911_35(6).20191103A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pocock SJ, Bakris G, Bhatt DL, et al. Regression to the mean in SYMPLICITY HTN-3: implications for design and reporting of future trials. J Am Coll Cardiol. 2016;68:2016–2025. doi: 10.1016/j.jacc.2016.07.775. [DOI] [PubMed] [Google Scholar]
- 19.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–220. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt DL, Kandzari DE, O’Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 21.Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]