INTRODUCTION
Cardiovascular complications including myocardial injury and myocarditis have been reported in acute COVID-19 infection. Delayed cardiac manifestations with life threatening presentations deserve clinical attention. We report a case of severe stress cardiomyopathy in a patient following recent COVID-19 infection.
CASE
A 34-year-old male with no significant past medical history presented in April 2020 with a 2-day history of fever, cough, vomiting and diarrhea. He also reported a 2-week history of central chest pain, worst in the last 2-days with associated dyspnea. Initial vital signs were: temperature 39.4 °C, heart rate 125 beats/minute, blood pressure 82/45 mm Hg, respiratory rate 30 breaths/minute, oxygen saturation 95% on ambient air.
Laboratory tests revealed: leukocytosis (19.02 × 109/L) with neutrophilia (17.66 × 109/L) and lymphopenia (0.53 × 109/L). He had a raised creatinine (192 μmol) and hyperbilirubinemia (157 μmol) which was predominantly conjugated (98 μmol) and associated with raised aspartate and alanine transaminases. Cardiac troponin-I levels were elevated (Trend: 428-586-395 ng/L). The patient also had raised levels of C-reactive protein (479 mg/L), ferritin (7064 μg/L), and D-Dimer (5.8 μg/ml).
Initial electrocardiogram (ECG) demonstrated sinus rhythm (Figure 1). There were diffuse ST-segment elevations and PR-segment depressions in the inferolateral leads. There was also ST-segment depressions and PR-segment elevations in leads V1, aVR. Chest radiography revealed prominent pulmonary vasculature and left retrocardiac consolidation-collapse.
Figure 1.
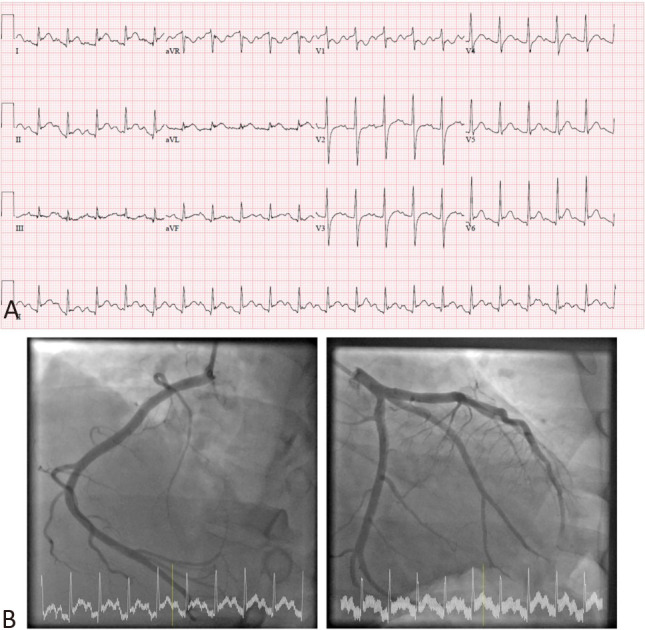
(A) Initial 12-lead electrocardiogram demonstrated diffuse ST-segment elevations with PR-segment depressions in the inferior (II, III, aVF) and lateral (I, aVL, V5, V6) leads. There was also ST-segment depressions and PR-segment elevations in leads V1, aVR. (B) Coronary angiography revealed normal epicardial vessels with slow coronary flow.
The patient was a foreign migrant worker. In Singapore at the time, over 95% of COVID-19 cases had been in or related to the foreign migrant worker community.1 Thus, all care and procedures for this patient were carried out in full personal protective equipment and in airborne isolation.
The patient underwent emergent coronary angiogram due to the antecedent ECG changes. He was found to have normal epicardial vessels with slow coronary flow (Figure 1). Left ventriculography revealed global left ventricular hypokinesia with severe left ventricular systolic dysfunction. Bedside echocardiogram confirmed biventricular systolic dysfunction with a left ventricular ejection fraction (LVEF) of around 30%.
The patient was initially admitted to the intensive care unit (ICU) in a negative pressure room. He rapidly deteriorated and required mechanical ventilation and sedation with paralysis. He was treated with vasopressors (noradrenaline and vasopressin) with limited improvement before finally stabilizing on direct inotropic support with dobutamine and continuous cardiac output monitoring. In view of ongoing shock and fever, he was treated for possible severe community acquired pneumonia with broad spectrum antibiotics (amoxicillin with clavulanic acid, azithromycin, ceftazidime) and oseltamivir for possible influenza myocarditis. He was also maintained on therapeutic anticoagulation with heparin infusion during his ICU stay. Anti-inflammatories for pericarditis were avoided due to renal impairment.
A transthoracic echocardiogram was performed on day 3 of admission (whilst on dobutamine infusion of 2.5 mcg/kg/min) (Figure 2). In view of the severity of the patient’s condition and isolation status for suspected COVID-19 infection, a targeted acute cardiogenic shock protocol was adopted for echocardiographic evaluation. This demonstrated global left ventricular hypokinesia (Biplane LVEF 32.7% with average left ventricular (LV) global longitudinal strain of -8.7%). There was moderate right ventricular systolic dysfunction with a tricuspid annular plane systolic excursion of 11.7 mm, estimated pulmonary artery systolic pressure of 42 mm Hg, borderline pulmonary artery acceleration time (120 msec) and echocardiographic estimated pulmonary vascular resistance of 3.41 wood units by Abbas et al.’s formula.2 A small pericardial effusion was also found.
Figure 2.
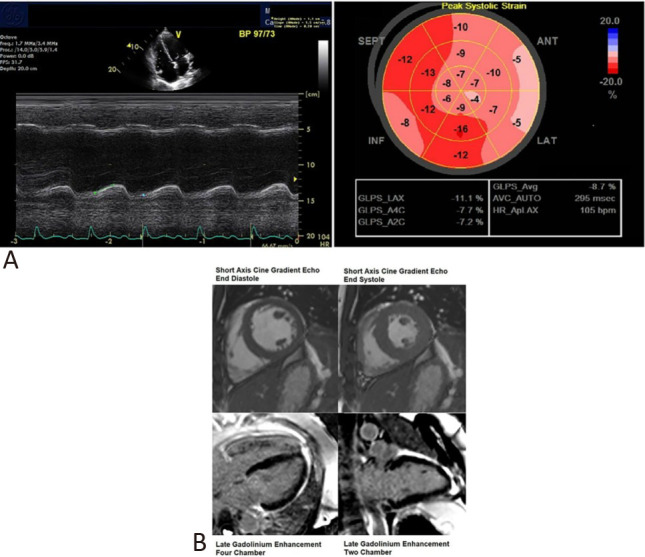
(A) Transthoracic echocardiography was performed on Day 3 of admission whilst the patient was on dobutamine infusion. This demonstrated a tricuspid annular plane systolic excursion of 11.7 mm and an average left ventricular global longitudinal strain of -8.7%. (B) Cardiac magnetic resonance imaging performed on Day 8 of admission after clinical recovery. Short axis cine gradient echo still (end diastole and end systole) images of the heart at mid cavity level showed normal biventricular contraction. Long axis views of the heart demonstrated no late gadolinium enhancement. There was no imaging evidence of myocardial infarction or fibrosis.
In consideration of the local coronavirus outbreak, the patient was tested for COVID-19 infection. SARS-CoV-2 IgG antibodies returned positive, but all subsequent reverse transcriptase polymerase chain reaction (PCR) assays including three nasopharyngeal swabs, two endotracheal tube aspirates, one blood sample, one rectal swab and one stool specimen were negative. Further potential causes of inflammatory cardiomyopathy were also evaluated. Laboratory testing was negative for Influenza A and B, respiratory syncytial virus, Enterovirus, Dengue, human immunodeficiency virus, herpes simplex virus, cytomegalovirus and Hepatitis B, C and E Viruses. Serologies for atypical infections were sent which returned equivocal for Leptospira IgM and negative for spotted fever, typhus and scrub typhus group rickettsia. Blood, respiratory and urine cultures were negative for bacterial organisms.
Over the course of admission, this patient’s respiratory and hemodynamic status, renal and liver function tests gradually improved. He was weaned off inotropes on day 4, extubated to ambient air on day 6 and transferred from the ICU to the general isolation ward on day 8.
The patient underwent cardiac magnetic resonance (CMR) imaging on day 8 of admission (Figure 2) following clinical improvement. CMR showed an improved LVEF of 66%. There was maximal LV wall thickness of 8 mm at the basal anteroseptal segment, normal right ventricular systolic function and indexed volumes and there was no late gadolinium enhancement (LGE) in the myocardium of both ventricles or myocardial edema. Endomyocardial biopsy (EMB) was not pursued given the prompt recovery of the patient.
DISCUSSION
Cardiovascular complications have been reported with COVID-19 infection although the exact pathophysiology remains unclear. Myocardial injury affects a significant number of patients with active COVID-19 infection in the form of raised cardiac biomarkers, myocarditis, acute myocardial infarction and arrhythmias.3,4 The temporal association of rising cardiac biomarkers with inflammatory markers (e.g. D-dimer, ferritin, C-reactive protein) suggests a role for the systemic inflammatory response in acute COVID-19 infection in causing myocardial injury.4 There have also been reports suggesting COVID-19 associated myopericarditis,3 with the angiotensin converting enzyme II receptor proposed as an entry point for the virus into cardiac myocytes.4 Recent reports have detected the presence of SARS-CoV-2 genome in EMB of patients with clinical or suspected myocarditis, though this has not been a universal finding.5
The findings in our case are consistent with stress cardiomyopathy with transient biventricular dysfunction. This is evidenced by the rapid recovery of the LV systolic function and the lack of myocardial edema and LGE on CMR. However, neither the classic nor even the reverse Takotsubo pattern on echocardiography were present in our patient, which has been reported in other patients with acute COVID-19 infection.6,7 Potential mechanisms for the development of stress cardiomyopathy include catecholamine surge, microvascular dysfunction and LV outflow tract obstruction.4 It is possible that the heightened inflammatory response with resultant cytokine storm in COVID-19 infection could contribute to the development of stress cardiomyopathy.
What makes this case unique is the manifestation of severe stress cardiomyopathy in a patient who had recovered from COVID-19. At the time this patient presented in April 2020, aggressive testing and contact tracing was being performed in the foreign migrant worker community in Singapore due to over 95% of COVID-19 cases being from or related to this population group.1 Thus, recent infection with COVID-19 can be assumed given this patient’s epidemiological high risk, positive serology with negative PCRs and ongoing fever with raised inflammatory markers driving acute inflammation and myocardial injury. The SARS-CoV-2 IgG antibody was run on the Abbott Architect i400SR platform. This assay is a chemiluminescent microparticle immunoassay for detecting human serum IgG targeting the SARS-CoV-2 nucleocapsid with a high sensitivity of 100% and specificity of 99.9%.8 Furthermore, extensive testing for alternative bacterial and viral etiologies returned negative. Of note, we did not pursue an endomyocardial biopsy and are unable to confirm any direct role of SARS-COV-2 virus in the heart.
There is increasing recognition in the literature that there may be delayed cardiac complications from COVID-19. Huang et al. followed 26 patients who recovered from COVID-19 infection but reported cardiac complaints subsequently and found that fifteen patients (58%) continued to have abnormal findings on CMR including myocardial edema, fibrosis, and impaired RV function.9 Similar to this case, Wenzel et al. reported two patients with LV dysfunction with negative COVID-19 PCR by nasopharyngeal swab who subsequently demonstrated seroconversion with SARS-CoV-2 IgG.5 These patients had CMR features of myocarditis including LGE and had EMB pursued which demonstrated PCR positivity for the SARS-CoV-2 mRNA and inflammatory infiltrate without myocardial necrosis (consistent with borderline myocarditis by the Dallas criteria). In the pediatric setting, Grimaud et al. reported a case series of 20 children who presented with acute severe myocarditis of whom 6 had positive serologies with negative nasopharyngeal or fecal PCR.10
This is a poignant reminder that our understanding of COVID-19 infection and its complications remains limited. Cardiovascular complications, especially delayed ramifications, of COVID-19 can be severe and warrant clinical consideration and further research.
LEARNING POINTS
• Diagnostic modalities including transthoracic echocardiogram, cardiac magnetic resonance imaging and endomyocardial biopsy with histological examination are important tools for demonstrating COVID-19 associated cardiomyopathy or myocarditis.
• Patients can present with delayed cardiac complications of COVID-19 infection, including in the form of severe stress cardiomyopathy.
Acknowledgments
None.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Ministry of Health, Singapore. Updates on COVID-19 (coronavirus disease 2019) Accessed June 17, 2020;Available at: https://www.moh.gov.sg/covid-19 [Google Scholar]
- 2.Abbas AE, Franey LM, Marwick T, et al. Noninvasive assessment of pulmonary vascular resistance by Doppler echocardiography. J Am Soc Echocardiogr. 2013;26:1170–1177. doi: 10.1016/j.echo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babapoor-Farrokhran S, Gill D, Walker J, et al. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenzel P, Kopp S, Göbel S, et al. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res. 2020;116:1661–1663. doi: 10.1093/cvr/cvaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giustino G, Croft LB, Oates CP, et al. Takotsubo cardiomyopathy in COVID-19. J Am Coll Cardiol. 2020;76:628–629. doi: 10.1016/j.jacc.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho McAdam AJ, editor. J Clin Microbiol. 2020;58:e00941-20. doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L, Zhao P, Tang D, et al. Cardiac involvement in recovered COVID-19 patients identified by magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimaud M, Starck J, Levy M, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


