Abstract
Background
New-onset atrial fibrillation (NOAF) in acute coronary syndrome (ACS) may be associated with a poor prognosis. However, whether restoring sinus rhythm (SR) at discharge in patients with ACS improves outcomes remains unknown.
Methods
A total of 552 patients with ACS at an emergency department during 2011-2016 were enrolled. According to documented electrocardiography at admission and medical records, the patients were classified into without atrial fibrillation (WAF), NOAF, and prior atrial fibrillation (PAF) groups. Major adverse events (MAEs) were defined as cardiac death, recurrent myocardial infarction, heart failure requiring hospitalization, target lesion revascularization, and stroke. The mean follow-up period of MAEs was 25 ± 15 months.
Results
Compared with the NOAF and PAF groups, the WAF group was younger and had a significantly lower heart rate, prior stroke rate, CHA2DS2-VASc score, and lower Global Registry of Acute Coronary Events (GRACE) score in the emergency department (p < 0.001). The patients in the NOAF group had the highest incidence of MAEs (p < 0.001) during follow-up, and those whose SR was restored at discharge had a lower MAE rate than those with AF at discharge (p = 0.001). In multivariable analysis, prior myocardial infarction, GRACE score, use of beta-blockers, and restoring SR at discharge were independent predictors of MAEs in the NOAF group.
Conclusions
The patients with ACS who presented with NOAF had worse outcomes than those with PAF or WAF. The patients with NOAF whose rhythm was restored to SR at discharge were associated with better outcomes than those with AF at discharge.
Keywords: Acute coronary syndrome, Atrial fibrillation, Major adverse events, Myocardial infarction
INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia contributing to adverse cardiovascular events, including worsening of heart failure (HF) and ischemic stroke. AF has been reported to be a complication in 2%-28% of patients with acute coronary syndrome (ACS).1 In addition, the occurrence rates of most in-hospital adverse events has been reported to be significantly higher in patients with any form of AF than in those without AF. Furthermore, in patients with ACS, new-onset AF (NOAF) has been reported to be an independent predictor of in-hospital adverse events.2,3 In the past decade, occurrence rates of AF in patients with ACS have decreased significantly; however, despite improvements in the 1-year survival rates in patients with AF, NOAF in ACS remains an independent predictor of mortality.4 In the present study, we investigated whether restoring sinus rhythm (SR) at discharge in patients with ACS and NOAF could improve long-term outcomes. We examined prevalence rates, clinical characteristics, and major adverse events (MAEs) in patients with different types of heart rhythms and ACS at a single tertiary referral center. We also investigated the association between restoring SR at discharge and MAEs in patients with ACS and NOAF.
METHODS
Study design and population
This study was approved by our institutional review board. All patients diagnosed as having ACS between 2011 and 2016 at the emergency department (ED) of our tertiary medical center were enrolled. Series of electrocardiograms (ECGs) recorded at different periods, including previous visits to the medical center, in the ED, during hospitalization, at discharge, and at the first clinic visit after discharge were independently evaluated by two cardiologists. Data of echocardiographic parameters and biochemical markers were collected during hospitalization. Patients presenting with arrhythmias other than AF, such as ventricular arrhythmia or atrioventricular block, were excluded. Patients without necessary ECG records, clinical data and less than 6 months of follow-up data at out-patient departments were also excluded from the study (Figure 1).
Figure 1.
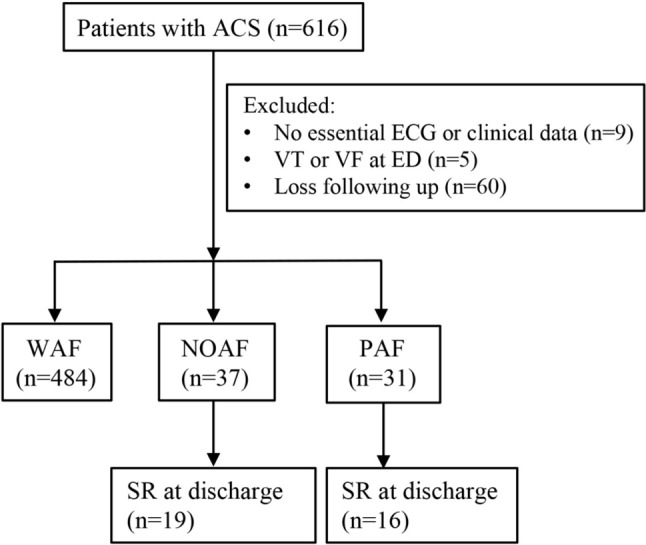
Patients who presented with ACS at the ED were enrolled and categorized into groups based on the occurrence of AF types. ACS, acute coronary syndrome; AF, atrial fibrillation; ECG, electrocardiograms; ED, emergency department; NOAF, new-onset AF; PAF, prior AF; SR, sinus rhythm; VF, ventricular fibrillation; VT, ventricular tachyarrhythmia; WAF, without AF.
Diagnosis and management of different AF types during ACS
The diagnosis of ACS was based on clinical characteristics, electrocardiographic changes, and levels of biochemical markers. Furthermore, the patients were classified as having ST-segment elevation myocardial infarction (STEMI), and non-ST-segment elevation acute coronary syndrome (NSTEACS), which included non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina (UA) according to the guidelines of the American College of Cardiology.5 A diagnosis of AF required absolutely irregular RR intervals without a distinct P wave documented on surface ECG. The patients were divided into three groups depending on the presence or absence of AF. The NOAF group consisted of patients who presented with AF at admission or during hospital stay but did not have a medical history of AF. Patients with prior AF (PAF group) had previously documented AF as either paroxysmal, persistent, or permanent AF, and patients without AF (WAF group) did not have a history of AF or documented AF during hospitalization. ACS and AF management was based on standard international guidelines.6-8 After NOAF was detected, the decision to provide pharmacological treatment or electrical cardioversion or adopt a wait-and-see strategy was made by clinicians depending on the patients’ hemodynamic status and contraindications for antiarrhythmic agents. Failure to restore SR in patients with NOAF was defined as persistent AF 7 days after NOAF; these patients were referred to as the NOAF to AF group, and those with NOAF whose SR was restored were referred to as the NOAF to SR group. Short-term mortality was defined as all-cause mortality within the first 30 days from ACS diagnosis. Long-term mortality was defined as all-cause mortality that occurred more than 30 days after ACS diagnosis. MAEs were defined as a composite of cardiac death, recurrent myocardial infarction (MI), target lesion revascularization, HF requiring hospitalization, and stroke.
Statistical analysis
Categorical variables are presented as frequencies (percentages) and compared using the chi-square test or Fisher’s exact test. Continuous variables are presented as mean ± standard deviation and compared using one-way analysis of variance. A Cox proportional-hazards model was used to determine predictors for MAEs using the hazard ratio (HR) with a 95% confidence interval (CI). The variables entered into the multivariate analysis model were those with a p value of < 0.2 in the univariate analysis model. Kaplan-Meier analysis with a log-rank test was performed to analyze MAEs. To evaluate the association between the AF status and MAEs, we constructed three Cox proportional hazard models with time-varying exposure. Model 1 was the unadjusted model; model 2 was the multivariate Cox proportional hazard analysis model adjusted for age, prior stroke, Global Registry of Acute Coronary Events (GRACE) score, and the use of beta-blockers; and model 3 was multivariate Cox proportional hazard analysis adjusted for prior MI, GRACE score, and the use of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), and oral anticoagulants (OACs). A p value of < 0.05 was considered to be statistically significant. All statistical analyses were conducted using SPSS for Microsoft Windows (version 20.0; SPSS, Chicago, IL, USA).
RESULTS
Patient characteristics and AF incidence
In total, 552 patients (aged 63 ± 13 years, 78% men) diagnosed with ACS were enrolled. The mean follow-up duration was 25 ± 15 months. Of these patients, 267 (48.3%), 268 (48.6%), and 17 (3.1%) had a diagnosis of STEMI, NSTEMI, and UA, respectively. All of the patients in the present study received index coronary angiography at their first ED visit. The rate of insignificant coronary artery disease was 2.17% (n = 12). In the first visit to the ED for ACS, the percutaneous coronary intervention (PCI) rate was 91.11% (n = 492) and the coronary artery bypass grafting (CABG) rate was 8.89% (n = 48). Overall, AF was documented in 68 patients (12.3%) with ACS, of whom 37 had NOAF (6.7%) and 31 had PAF (5.6%; Figure 1). Compared to the patients in the PAF and NOAF groups, those in the WAF group were younger (61.5 ± 12.7, 73.35 ± 14.7, and 73.81 ± 12.4 years, respectively, p < 0.001; Table 1), had a lower heart rate (79.0 ± 18.7, 94.2 ± 30.0, and 93.0 ± 28.4 beats per minute, respectively, p < 0.001; Table 1), lower incidence of prior stroke (8.5%, 21.6%, and 19.4%, respectively, p = 0.007; Table 1), lower CHA2DS2-VASc score (2.8 ± 1.6, 4.1 ± 2.2, and 4.7 ± 1.6, respectively, p < 0.001; Table 1), and lower GRACE score (185.5 ± 48.4, 240.4 ± 47.7, and 215.6 ± 47.3, respectively, p < 0.001; Table 1). In addition, they had fewer prescriptions for OACs (7.9%, 21.6%, and 61.3%, p < 0.001; Table 1).
Table 1. Characteristics of 3 groups of patients with acute coronary syndrome.
| Group 1 - WAF (n = 484) | Group 2 - NOAF (n = 37) | Group 3 - PAF (n = 31) | p value | |
| Age | 61.5 ± 12.7 | 73.35 ± 14.7* | 73.81 ± 12.4# | < 0.001 |
| Female | 101 (20.9%) | 9 (24.3%) | 9 (29.0%) | 0.515 |
| HR | 79.0 ± 18.7 | 94.2 ± 30.0* | 93.0 ± 28.4# | < 0.001 |
| SBP | 126.74 ± 25.87 | 118.57 ± 22.18 | 124.35 ± 23.94 | 0.66 |
| DBP | 75.08 ± 16.79 | 68.35 ± 17.54 | 68.06 ± 16.79 | 0.913 |
| Hypertension | 303 (62.6%) | 26 (70.3%) | 22 (71.0%) | 0.439 |
| Diabetes | 151 (31.2%) | 15 (40.5%) | 13 (41.9%) | 0.256 |
| Dyslipidemia | 151 (31.2%) | 8 (21.6%) | 10 (32.3%) | 0.466 |
| Uremia | 37 (7.6%) | 5 (13.5%) | 2 (6.5%) | 0.424 |
| Cirrhosis | 2 (0.4%) | 0 (0.0%) | 0 (0.0%) | 0.868 |
| Prior MI | 78 (16.1%) | 5 (13.5%) | 13 (41.9%)#,† | 0.001 |
| Prior stroke | 41 (8.5%) | 8 (21.6%)* | 6 (19.4%)# | 0.007 |
| Prior CHF | 38 (7.9%) | 6 (16.2%) | 11 (35.5%)# | < 0.001 |
| LVEF | 50.4 ± 15.4 | 46.9 ± 18.4 | 50.9 ± 12.8 | 0.438 |
| eGFR | 73.0 ± 33.0 | 54.8 ± 34.0 | 50.8 ± 23.2 | 0.068 |
| BNP | 713.7 ± 1065.8 | 824.7 ± 1067.0 | 924.8 ± 726.2 | 0.307 |
| STEMI | 233 (48.1%) | 18 (48.6%) | 16 (51.6%) | 0.932 |
| NSTEMI | 235 (48.6%) | 18 (48.6%) | 15 (48.4%) | 1.000 |
| UA | 16 (3.3%) | 1 (2.7%) | 0 (0%) | 0.581 |
| Killip score | 1.7 ± 1.2 | 2.6 ± 1.3* | 2.1 ± 1.3 | < 0.001 |
| CHA2DS2-VASc score | 2.8 ± 1.6 | 4.1 ± 2.2* | 4.7 ± 1.6# | < 0.001 |
| GRACE score | 185.5 ± 48.4 | 240.4 ± 47.7* | 215.6 ± 47.3# | < 0.001 |
| Anti-platelet | 418 (86.4%) | 30 (81.1%) | 27 (87.1%) | 0.661 |
| OACs | 38 (7.9%) | 8 (21.6%)* | 19 (61.3%)#,† | < 0.001 |
| ACEI/ARB | 365 (75.4%) | 15 (40.5%)* | 22 (71%)† | < 0.001 |
| Beta-blockers | 421 (87%) | 27 (73.0%)* | 28 (90.3%) | 0.046 |
| Statin | 403 (83.3%) | 16 (43.2%)* | 19 (61.3%)# | < 0.001 |
| Dual antithrombotic agents | 5 (1%) | 2 (5.4%)* | 3 (9.7%)# | 0.001 |
| Triple antithrombotic agents | 31 (6.4%) | 5 (13.5%) | 16 (51.6%)#,† | < 0.001 |
| Short-term mortality | 23 (4.8%) | 8 (21.6%)* | 5 (16.1%)# | < 0.001 |
| Long-term mortality | 10 (2.1%) | 5 (13.5%)* | 3 (9.7%)# | < 0.001 |
ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blockers; BNP, brain natriurertic peptide (pg/mL); CHF, congestive heart failure; DBP, diastolic pressure (mm Hg); eGFR, estimated glomerular filtration rate; HR, heart rate; GRACE, Global Registry of Acute Coronary Events; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NOAF, new-onset atrial fibrillation; NSTEMI, non-ST-segment elevation myocardial infarction; OACs, oral anticoagulants; PAF, prior AF; SBP, systolic pressure (mm Hg); STEMI, ST-segment elevation myocardial infarction; UA, unstable angina; WAF, without AF.
* NOAF vs. WAF, p < 0.05. # PAF vs. WAF, p < 0.05. † PAF vs. NOAF, p < 0.05.
Clinical outcomes in patients in different groups
The total MAE rate was 15.4%. Compared to the WAF group, the NOAF and PAF groups had higher short-term (21.6% vs. 4.8%, p < 0.001; 16.1% vs. 4.8%, p < 0.001, respectively; Table 1) and long-term (13.5% vs. 2.1%, p < 0.001 and 9.7% vs. 2.1%, p < 0.001, respectively; Table 1) mortality rates. The NOAF group had the highest MAE rate among the three groups (48.6%, p < 0.001; Table 2). Kaplan-Meier survival analysis of the three groups for MAEs demonstrated that the NOAF group had a significant higher MAE rate than the PAF and WAF groups (p = 0.029; p < 0.001, respectively; Figure 2). The NOAF group also had the highest cardiac death rate (29.7%, p < 0.001; Table 2). Regarding the association between different AF status and MAEs, model 1 showed that NOAF was significantly associated with a higher MAE rate compared to WAF and PAF (HR = 5.075, 95% CI = 2.991-8.611, p < 0.001; HR = 2.558, 95% CI = 1.065-6.146, p = 0.036, respectively; Supplementary Table 1). In model 2, after adjusting for confounding factors including age, prior stroke, GRACE score, and the use of beta-blockers, the multivariable Cox proportional hazard analysis showed that the NOAF group had a higher MAE rate than the WAF group (HR = 1.820, 95% CI = 1.008-3.286, p = 0.047; Supplementary Table 1). In model 3, after adjusting for confounding factors including prior MI, GRACE score, and the use of ACEI/ARB and OACs, the NOAF group had a higher MAE rate than the WAF and PAF groups (HR = 1.810, 95% CI = 1.011-3.240, p = 0.046; HR = 2.785, 95% CI = 1.017-7.628, p = 0.046, respectively; Supplementary Table 1).
Table 2. Major adverse event during follow-up.
| Group 1 – WAF (n = 484) | Group 2 – NOAF (n = 37) | Group 3 – PAF (n = 31) | p value | |
| Cardiac death | 22 (4.5%) | 11 (29.7%)*,# | 3 (9.7%) | < 0.001 |
| Recurrent MI | 13 (2.7%) | 2 (5.4%) | 0 (0.0%) | 0.425 |
| Target lesion revascularization | 4 (0.8%) | 0 (0.0%) | 1 (3.2%) | 0.328 |
| HF requiring hospitalization | 16 (3.3%) | 5 (13.5%)* | 2 (6.5%) | 0.009 |
| Stroke | 5 (1.0%) | 0 (0.0%) | 1 (3.2%) | 0.419 |
| Total | 60 (12.4%) | 18 (48.6%)*,# | 7 (22.6%) | < 0.001 |
HF, heart failure; MI, myocardial infarction.
* NOAF vs. WAF, p < 0.001. # NOAF vs. PAF, p < 0.05.
Figure 2.
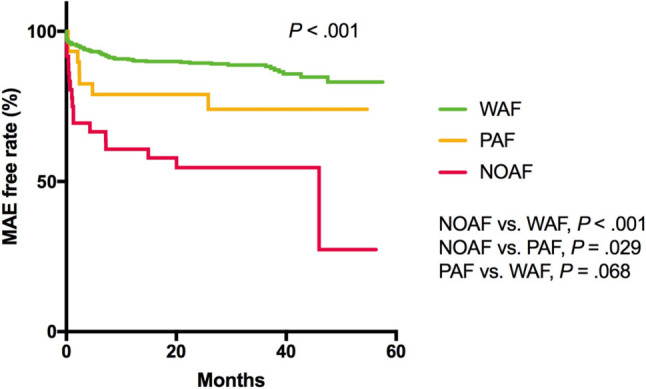
Kaplan-Meier curves during follow-up exhibited differences in MAEs among the 3 groups, and patients with ACS and NOAF had significantly worse outcomes (p < .001). ACS, acute coronary syndrome; AF, atrial fibrillation; MAE, major adverse event; NOAF, new-onset AF; PAF, prior AF; WAF, without AF.
Supplementary Table 1. Association between different AF status and MAEs.
| Model | HR | 95% CI of HR | p value |
| Model 1* | |||
| NOAF vs. WAF | 5.075 | 2.991-8.611 | 0.000 |
| PAF vs. WAF | 2.038 | 0.931-4.460 | 0.075 |
| NOAF vs. PAF | 2.558 | 1.065-6.146 | 0.036 |
| Model 2# | |||
| NOAF vs. WAF | 1.820 | 1.008-3.286 | 0.047 |
| PAF vs. WAF | 1.093 | 0.484-2.471 | 0.830 |
| NOAF vs. PAF | 1.988 | 0.811-4.871 | 0.133 |
| Model 3† | |||
| NOAF vs. WAF | 1.810 | 1.011-3.240 | 0.046 |
| PAF vs. WAF | 0.860 | 0.365-2.029 | 0.731 |
| NOAF vs. PAF | 2.785 | 1.017-7.628 | 0.046 |
* Unadjusted model. # Adjusted for age, prior stroke, GRACE score, beta-blockers. † Adjusted for prior MI, GRACE score, ACEI/ARB, and OACs.
ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CI, confidence interval; HR, hazard ratio; MAE, major adverse event; MI, myocardial infarction; NOAF, new-onset atrial fibrillation; OAC, oral anticoagulant; PAF, prior AF; WAF, without AF.
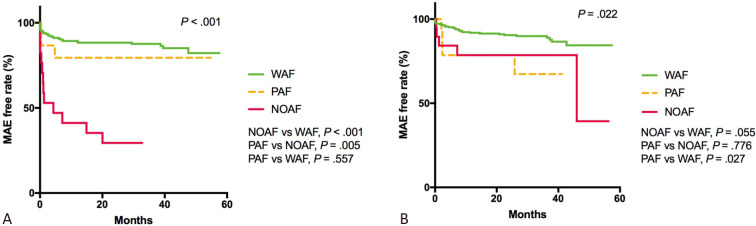
Kaplan-Meier survival analysis of the three groups for MAEs in patients with STEMI demonstrated that those with NOAF had a higher MAE rate than those with PAF and WAF (72.2% vs. 18.8%, p = 0.005; 72.2% vs. 13.7%, p < 0.001, respectively; Figure 3(A)). In the patients with NSTEACS, those with PAF had a higher MAE rate than those WAF (26.3% vs. 11.2%, p = 0.027; Figure 3(B)).
Figure 3.
(A) Kaplan-Meier survival analysis of three groups for MAEs in patients with STEMI demonstrated that patients with NOAF had higher MAE rate than PAF and WAF (72.2% vs. 18.8%, p = .005; 72.2% vs. 13.7%, p < .001, respectively). (B) In patients with NSTEACS, patients with PAF had higher MAE rate than those in WAF (26.3% vs. 11.2%, p = .027). MAE, major adverse event; NOAF, new-onset AF; NSTEACS, non-ST-segment elevation acute coronary syndrome; PAF, prior AF; STEMI, ST-segment elevation myocardial infarction; WAF, without AF.
Management of ACS and NOAF
In the NOAF group, SR was restored successfully in 19 patients at discharge (NOAF to SR group), but SR could not be restored in 18 patients (NOAF to AF group). As shown in Table 3, amiodarone (78.4%) was the most used drug in the NOAF group, followed by beta-blockers (73.0%), non-dihydropyridine calcium channel blockers (37.8%), and digoxin glycoside (24.3%). The prescription of amiodarone was higher in the NOAF to AF group than in the NOAF to SR group (58.6% vs. 41.4%, p = 0.042). Only one patient with hemodynamic compromise received electrical cardioversion despite receiving pharmacological therapies.
Table 3. Therapeutic interventions in patients with ACS and NOAF.
| Overall (n = 37) | NOAF to SR at discharge (n = 19) | NOAF to AF at discharge (n = 18) | p value | |
| Amiodarone | 29 (78.4%) | 12 (41.4%) | 17 (58.6%) | 0.042 |
| Beta-blockers | 27 (73.0%) | 16 (59.3%) | 11 (40.7%) | 0.151 |
| Non-DHP CCBs | 14 (37.8%) | 6 (42.6%) | 8 (57.1%) | 0.508 |
| Digoxin glycoside | 9 (24.3%) | 3 (33.3%) | 6 (66.7%) | 0.269 |
| Electrical cardioversion | 1 (2.7%) | 1 (5.3%) | 0 (0.0%) | 1.000 |
ACS, acute coronary syndrome; NOAF, new-onset AF; Non-DHP CCB, non-dihydropyridine calcium channel blocker; SR, sinus rhythm.
Prognosis of restoring SR in NOAF
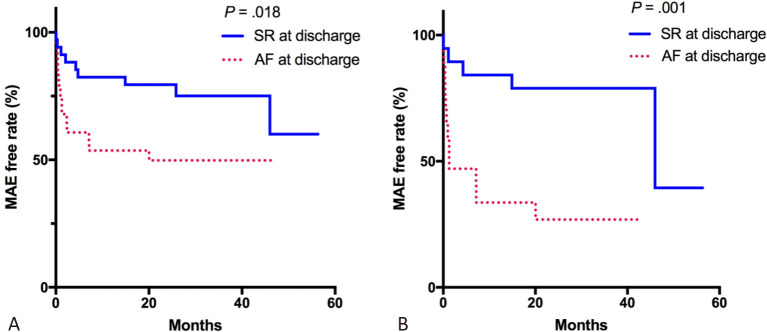
Patients with AF at discharge had significantly worse outcomes (p = 0.018; Figure 4(A)) than those whose SR was restored at discharge. In the NOAF group, the patients whose SR was restored successfully at discharge had a lower MAE rate than those with AF at discharge (p = 0.001; Figure 4(B)). In the multivariable analysis, prior MI, GRACE score, the use of beta-blockers and restoring SR at discharge were independent predictors of MAEs in the NOAF group (HR 5.582, 95% CI 1.331-23.418, p = 0.019; HR 1.014, 95% CI 1.002-1.026, p = 0.026; HR 0.129, 95% CI 0.037-0.449, p = 0.001; HR 0.219, 95% CI 0.061-0.789, p = 0.020, respectively; Table 4).
Figure 4.
(A) Kaplan-Meier curves for MAEs during follow-up showing differences in long-term outcomes in patients with ACS and AF. Patients with AF at discharge have significantly worse outcomes (p = .018). (B) Kaplan-Meier curves for MAEs in the NOAF group during follow-up also showed that patients with AF at discharge have higher MAE rate than patients with SR at discharge (p = .001). ACS, acute coronary syndrome; AF, atrial fibrillation; MAE, major adverse event; SR, sinus rhythm.
Table 4. Univariate and multivariable analysis for MAE in patients with NOAF.
| Variables | Univariate p value | Hazard ratio | 95% confidence interval | Multivariable p value | Hazard ratio | 95% confidence interval |
| Age | 0.131 | 1.029 | 0.992-1.067 | 0.941 | 1.002 | 0.957-1.049 |
| HR | 0.772 | 0.998 | 0.982-1.013 | |||
| Prior MI | 0.113 | 2.364 | 0.815-6.860 | 0.019 | 5.582 | 1.331-23.418 |
| Prior stroke | 0.784 | 1.170 | 0.381-3.598 | |||
| Prior CHF | 0.300 | 1.814 | 0.588-5.591 | |||
| Killip score | 0.716 | 0.937 | 0.659-1.331 | |||
| CHA2DS2-VASc score | 0.200 | 1.147 | 0.930-1.414 | |||
| GRACE score | 0.091 | 1.008 | 0.999-1.018 | 0.026 | 1.014 | 1.002-1.026 |
| OACs | 0.164 | 2.030 | 0.749-5.498 | 0.492 | 1.496 | 0.474-4.723 |
| ACEI/ARB | 0.026 | 0.278 | 0.090-0.860 | |||
| Beta-blockers | 0.002 | 0.223 | 0.085-0.584 | 0.001 | 0.129 | 0.037-0.449 |
| Statin | 0.562 | 0.754 | 0.290-1.960 | |||
| Amiodarone | 0.234 | 2.452 | 0.559-10.745 | |||
| Returned to SR at discharge | 0.003 | 0.181 | 0.058-0.563 | 0.020 | 0.219 | 0.061-0.789 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CHF, congestive heart failure; HR, heart rate; MAE, major adverse event; MI, myocardial infarction; NOAF, new-onset AF; OAC, oral anticoagulant; SR, sinus rhythm.
DISCUSSION
Main findings
In the present ACS cohort study, we observed that patients with AF during the index hospitalization were not uncommon, and that they had a higher MAE rate than those without AF. Compared to the patients in the WAF group, those in the NOAF group had a higher incidence of HF requiring hospitalization. The patients in the NOAF had the highest MAE rate among the three groups. The cardiac death rate was also the highest in the NOAF group. In addition, the patients with ACS and AF in both the PAF and NOAF groups whose SR was restored at discharge had better clinical outcomes than those whose SR was not restored. In our multivariate analysis, prior MI, GRACE score, the use of beta-blockers, and restoring SR at discharge were independent predictors for MAEs in the patients with ACS and NOAF.
Incidence and possible mechanism underlying NOAF in ACS
Varying prevalence rates of NOAF have been reported in patients with ACS, and these variations have been associated with the severity of ACS. A study involving a total 21,785 patients with ACS enrolled in the multinational GRACE study reported that 6.2% of the patients had NOAF. Furthermore, the incidence of STEMI in patients in the NOAF group was higher than that in the WAF group (43% vs. 34%, p < 0.001).2 Another study reported that 4.4% of their patients with ACS had NOAF, and that the incidence of NOAF in the STEMI group was higher than that in the NSTEACS group.9 A recent study reported that the prevalence of NOAF in ACS was 9.2%, and patients in the NOAF group had a significantly higher incidence of STEMI and higher GRACE score than those in the WAF or PAF group.10 In the present ACS cohort study, approximately 1 in every 8 patients presented with NOAF or PAF. The incidence of NOAF in ACS was 6.7%, and nearly half of the patients with NOAF had STEMI (48.6%), although the incidence of STEMI in the patients with NOAF was not significantly higher than that in the other patients. Patients in the NOAF group had higher Killip score, higher CHA2DS2-VASc score and higher GRACE score than the WAF group. These findings indicate that the incidence of NOAF may be associated with the severity of ACS and underlying diseases.
The pathogenesis of AF in ACS can be multifactorial. A previous study showed a significantly higher incidence rate of AF in patients with hydropericardium during the first 3 days after Q wave anterior MI. In addition, the author stated that increased atrial pressure related to atrial distention, which resulted from hemodynamic changes due to extensive myocardial damage, and not inflammatory infiltration, was a crucial factor in the patients who developed AF after acute Q wave anterior infarction.11 Nagahama et al. reported that patients with infarction-associated pericarditis had significantly higher pulmonary capillary wedge pressure than those without pericarditis; moreover, pericarditis was a crucial sign of an increased incidence of AF.12 Nishida et al. reported that chronic atrial infarction in dogs promoted both AF triggers and substrate for AF maintenance by creating spontaneous ectopic beats and re-entrant circuits.13 In addition, Shino et al. showed that an experimental atrial-arterial occlusion, which slowed the atrial conduction within the ischemic zone and created a substrate for AF maintenance, promoted AF genesis.14 These findings suggest that AF could be triggered by factors involved in atrial ischemia during ACS. In addition, inflammation has been implicated in AF and the risk of coronary artery disease. Marcus et al. reported that while myocardial inflammation levels were highest in the early stage during acute MI, the serum level of interleukin 6, an inflammatory marker, was independently associated with the development of AF.15 Several previous studies have established models using patient characteristics to predict NOAF in patients with ACS, including advanced age, female sex, and unstable hemodynamics.2,16-18 Dritan et al. demonstrated that after ACS, patients who developed NOAF were less likely to have previous MI, CABG, PCI, or HF, but they had the highest rates of STEMI and reinfarction.19 Héctor et al. demonstrated that patients with NOAF had higher requirements of inotropes, vasopressors, ventilation, and intraaortic balloon pumps, which reflected the high severity of the acute illness.3 As shown in Table 1, age, heart rate, history of prior stroke, Killip score, CHA2DS2-VASc score, and GRACE score were higher in NOAF than WAF. These findings also suggest that the development of NOAF may be due to advanced age, multiple comorbidities, high severity of ACS, and deteriorating hemodynamic status.
Prognostic effect of AF types in patients with ACS
Over the past decades, the incidence of AF among patients with ACS has decreased significantly and the 1-year survival of ACS patients has improved. However, the development of AF in patients with ACS remains an independent predictor of mortality.4 Studies have demonstrated that AF has detrimental effects on cardiovascular disease. Patients with previous AF have also been shown to have higher prevalence rates of stroke and diabetes mellitus, which may increase their cardiovascular risk during ACS. In addition, patients with previous AF have also been reported to have a high incidence of HF and increased mortality.20,21 Giglioli et al. reported that the mortality rate was higher in NSTEACS patients who developed AF that lasted more than 6 hours than those without AF.22 Several investigators have noted that AF facilitated the induction of ventricular arrhythmia, which may increase the risk of sudden cardiovascular death (SCD). A study revealed that AF was an independent predictor of in-hospital ventricular fibrillation (VF) after MI.23 Another study showed that patients with AF after acute MI had a significant increase in SCD and non-SCD.24 These findings highlight the importance of AF in mortality following ACS. AF in patients with ACS would cause an ischemic burden, resulting in worse in-hospital adverse outcomes. Some studies have reported the detrimental effects of AF on coronary circulation. During AF, the coronary flow cannot adequately support the increased myocardial demand, and diastolic coronary circulation is influenced during AF, which leads to impaired subendocardial perfusion. In addition, irregular ventricular rhythm during AF contributes to a reduction in coronary flow reserve. These mechanisms result in a vicious circle comprising AF and myocardial ischemia, in which each condition aggravates the other.9,25-27
Several studies have also reported that NOAF should be differentiated from prior AF because both conditions have different clinical presentations and outcomes.1,19,28 In addition, previous studies have demonstrated different prognostic implications between previous AF and NOAF in patients with ACS. In a large meta-analysis that included more than 275,000 patients across 43 studies, Jabre et al. reported that NOAF was associated with higher mortality in patients with MI regardless of the timing of AF.29 Another study also demonstrated that patients in the NSTEACS group with NOAF that developed during intensive care stay had a 4.4-fold higher risk of in-hospital mortality than other patients.3 Moreover, even in the absence of structural heart disease, the vicious electromechanical cycle of NOAF may induce the development of left ventricular dysfunction and HF,30 and patients with NOAF after acute MI have been reported to be twice as likely to develop HF than other patients.31 AF also predisposes patients to thrombus formation and is associated with higher risks of stroke and mortality. Zusman et al. reported an annual incidence of ischemic stroke of 4.4% in NOAF vs. 0.2% in the non-AF group after acute MI; furthermore, they reported that NOAF in ACS patients was an independent predictor of stroke.32 Kundu et al. observed a 2.5 fold higher risk of in-hospital stroke in patients developing NOAF following MI compared with patients without AF.31 Asanin et al. further reported several predictors of stroke in ACS with NOAF, including the absence of anticoagulation at discharge, recurrence of AF and HF during follow-up.33
In our study, patients with NOAF and PAF had higher short-term and long-term mortality rates compared to those with WAF (Table 1). Compared to the WAF group, the NOAF group had a higher incidence of HF requiring hospitalization. Moreover, the patients in the NOAF group had the highest MAE rate among three groups (Table 2 and Figure 2). Kaplan-Meier survival analysis of the three groups for MAEs demonstrated that the NOAF group had a significantly higher MAE rate than the PAF and WAF groups (p = 0.029; p < 0.001, respectively; Figure 2). The cardiac death rate was highest in the NOAF group (29.7%, p < 0.001; Table 2). The univariate and multivariate Cox proportional hazard analyses showed that NOAF was significantly associated with a higher MAE rate compared to WAF and PAF (Supplementary Table 1). Approximately 1 in every 5 patients with ACS who developed NOAF died within the first 30 days of ACS diagnosis. In the present study, the MAE rate of the NOAF group was 48.6%, and the cardiac death rate was 29.7%. Previous studies have reported a total mortality rate ranging from 14% to 20% in patients with NOAF.3,9,10,34,35 Patients in the NOAF group in the present study had a higher prior stroke rate (21.6% vs. 2.5%-10.6%3,35) and GRACE score [240.4 ± 47.7 vs. 152 (125-183)10-172 ± 4235] than in the previous studies. Our analysis also demonstrated that the incidence rates of cardiac death and hospital admission due to HF were higher in the patients in the NOAF group than in those in the WAF group (p < 0.001; p = 0.009, respectively; Table 2). These findings are consistent with previous studies and support that NOAF increases cardiovascular adverse outcomes in patients with ACS.29,36-38 However, our analysis did not show a significant differences in thromboembolic events between the patients with and without AF. This might be explained by the small numbers of patients in the NOAF and PAF groups in this ACS registry and the lower survival rate in the NOAF group during follow-up.
Predictors of MAE in ACS patients with AF
Previous studies have demonstrated that prior MI history, higher GRACE score, and less use of beta-blockers were associated with poor outcomes in patients with ACS and NOAF,4,9,19,37-39 and the multivariate analysis in the present study also suggested the same findings. Whether a rhythm-control strategy or rate-control strategy can provide survival or cardiovascular benefits in patients with paroxysmal or persistent AF remains controversial. The Atrial Fibrillation Follow-up Investigation of Rhythm Management Investigators trial showed that a rhythm-control strategy provided no survival advantage over a rate-control strategy in patients with AF.40 In a group analysis of the VALIANT trial, patients with AF after MI who were treated with antiarrhythmic drugs had a higher risk of death than patients treated with a rate-control strategy.41 Wong et al. also noted a strong trend associated with the use of antiarrhythmic agents in managing patients with AF after acute MI.42 Therefore, some investigators have suggested that the poor outcomes in the rhythm-control group may partially be due to the adverse effects of antiarrhythmic agents. In our study, the patients with ACS and NOAF whose SR could not be restored had more prescriptions for amiodarone. However, the difference in amiodarone use did not predict outcomes in our multivariable analysis.
In contrast, several investigations have reported associations between rhythm control and improvements in left ventricular function, AF symptoms, exercise tolerance, the ability to perform activities of daily living, and quality of life; furthermore, restoration of SR has been reported to reduce AF-associated morbidity and mortality.43 Choi et al. reported that a rhythm-control strategy was associated with fewer cardiovascular events in the short term than a rate-control strategy.44 Maintenance of SR has also been associated with a low risk of composite of cardiovascular death or hospitalization for HF in patients with HF with preserved ejection fraction and AF.45 AF might result in alterations in atrial structure and architecture that make the atrial myocardium more susceptible to arrhythmia, and AF has been associated with impaired endothelial dysfunction.46 Therefore, restoring SR may prevent further atrial remodeling that promotes AF progression, preserve endothelial dysfunction, and reduce the occurrence of negative sequelae.47,48 In our study, the patients with ACS and NOAF whose SR was restored at discharge had significantly better outcomes than those with AF at discharge (Figure 3). Restoring heart rhythm at discharge was also shown to be an independent predictor of MAEs (Table 4). This novel finding highlights the importance of restoring SR in ACS patients who develop NOAF. Because interrupting AF can stop the vicious circle of arrhythmia and myocardial ischemia, an aggressive rhythm-control strategy in ACS patients may provide better benefits.
This is a retrospective, observational and non-randomized study, and there are several limitations. First, the decision of pharmacological treatment or electrical cardioversion to treat AF was made at the discretion of the clinicians, and bias existed regarding the therapeutic strategy towards restoring SR. Second, the patients may not have received the same frequency and duration of ECG monitoring during follow-up. Third, the patients who returned to SR after discharged may have had undetected AF. Fourth, the burdens and duration of AF cannot be exactly calculated without aggressive monitoring such as an insertable cardiac monitoring system.
CONCLUSIONS
Among the patients with ACS in this study, those who presented with AF during the index hospitalization had a higher MAE rate than those without AF; furthermore, those who presented with NOAF had worse outcomes compared to those with PAF and WAF. In the NOAF group, the patients whose rhythm was restored to SR at discharge were associated with better outcomes than those with AF at discharge.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Schmitt J, Duray G, Gersh BJ, et al. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30:1038–1045. doi: 10.1093/eurheartj/ehn579. [DOI] [PubMed] [Google Scholar]
- 2.Mehta RH, Dabbous OH, Granger CB, et al. Comparison of outcomes of patients with acute coronary syndromes with and without atrial fibrillation. Am J Cardiol. 2003;92:1031–1036. doi: 10.1016/j.amjcard.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Pacheco H, Marquez MF, Arias-Mendoza A, et al. Clinical features and in-hospital mortality associated with different types of atrial fibrillation in patients with acute coronary syndrome with and without ST elevation. J Cardiol. 2015;66:148–154. doi: 10.1016/j.jjcc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Erez A, Goldenberg I, Sabbag A, et al. Temporal trends and outcomes associated with atrial fibrillation observed during acute coronary syndrome: real-world data from the Acute Coronary Syndrome Israeli Survey (ACSIS), 2000-2013. Clin Cardiol. 2017;40:275–280. doi: 10.1002/clc.22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon CP, Battler A, Brindis RG, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol. 2001;38:2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 6.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 8.Sousa-Uva M, Neumann FJ, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 2019;55:4–90. doi: 10.1093/ejcts/ezy289. [DOI] [PubMed] [Google Scholar]
- 9.Lau DH, Huynh LT, Chew DP, et al. Prognostic impact of types of atrial fibrillation in acute coronary syndromes. Am J Cardiol. 2009;104:1317–1323. doi: 10.1016/j.amjcard.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 10.Worme MD, Tan MK, Armstrong DWJ, et al. Previous and new onset atrial fibrillation and associated outcomes in acute coronary syndromes (from the Global Registry of Acute Coronary Events). Am J Cardiol. 2018;122:944–951. doi: 10.1016/j.amjcard.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura T, Iwasaka T, Takahashi N, et al. Factors associated with atrial fibrillation in Q wave anterior myocardial infarction. Am Heart J. 1991;121:1409–1412. doi: 10.1016/0002-8703(91)90146-9. [DOI] [PubMed] [Google Scholar]
- 12.Nagahama Y, Sugiura T, Takehana K, et al. The role of infarction-associated pericarditis on the occurrence of atrial fibrillation. Eur Heart J. 1998;19:287–292. doi: 10.1053/euhj.1997.0744. [DOI] [PubMed] [Google Scholar]
- 13.Nishida K, Qi XY, Wakili R, et al. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation. 2011;123:137–146. doi: 10.1161/CIRCULATIONAHA.110.972778. [DOI] [PubMed] [Google Scholar]
- 14.Sinno H, Derakhchan K, Libersan D, et al. Atrial ischemia promotes atrial fibrillation in dogs. Circulation. 2003;107:1930–1936. doi: 10.1161/01.CIR.0000058743.15215.03. [DOI] [PubMed] [Google Scholar]
- 15.Marcus GM, Whooley MA, Glidden DV, et al. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155:303–309. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathore SS, Berger AK, Weinfurt KP, et al. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. 2000;101:969–974. doi: 10.1161/01.cir.101.9.969. [DOI] [PubMed] [Google Scholar]
- 17.Eldar M, Canetti M, Rotstein Z, et al. Significance of paroxysmal atrial fibrillation complicating acute myocardial infarction in the thrombolytic era. SPRINT and Thrombolytic Survey Groups. Circulation. 1998;97:965–970. doi: 10.1161/01.cir.97.10.965. [DOI] [PubMed] [Google Scholar]
- 18.Crenshaw BS, Ward SR, Granger CB, et al. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO-I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30:406–413. doi: 10.1016/s0735-1097(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 19.Poci D, Hartford M, Karlsson T, et al. Effect of new versus known versus no atrial fibrillation on 30-day and 10-year mortality in patients with acute coronary syndrome. Am J Cardiol. 2012;110:217–221. doi: 10.1016/j.amjcard.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 21.Camm AJ, Savelieva I. Atrial fibrillation: the rate versus rhythm management controversy. J R Coll Physicians Edinb. 2012;42 Suppl 18:23–34. doi: 10.4997/JRCPE.2012.S03. [DOI] [PubMed] [Google Scholar]
- 22.Giglioli C, Minelli M, Chiostri M, et al. Prognostic impact of atrial fibrillation occurrence in patients with non-ST-elevation acute coronary syndromes: is dysrhythmia duration a parameter to focus on? Intern Emerg Med. 2014;9:521–528. doi: 10.1007/s11739-013-0959-1. [DOI] [PubMed] [Google Scholar]
- 23.Rubenstein JC, Cinquegrani MP, Wright J. Atrial fibrillation in acute coronary syndrome. J Atr Fibrillation. 2012;5:551. doi: 10.4022/jafib.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen OD, Abildstrom SZ, Ottesen MM, et al. Increased risk of sudden and non-sudden cardiovascular death in patients with atrial fibrillation/flutter following acute myocardial infarction. Eur Heart J. 2006;27:290–295. doi: 10.1093/eurheartj/ehi629. [DOI] [PubMed] [Google Scholar]
- 25.White CW, Holida MD, Marcus ML. Effects of acute atrial fibrillation on the vasodilator reserve of the canine atrium. Cardiovasc Res. 1986;20:683–689. doi: 10.1093/cvr/20.9.683. [DOI] [PubMed] [Google Scholar]
- 26.Wichmann J, Ertl G, Rudolph G, et al. Effect of experimentally induced atrial fibrillation on coronary circulation in dogs. Basic Res Cardiol. 1983;78:473–491. doi: 10.1007/BF01906459. [DOI] [PubMed] [Google Scholar]
- 27.Kochiadakis GE, Skalidis EI, Kalebubas MD, et al. Effect of acute atrial fibrillation on phasic coronary blood flow pattern and flow reserve in humans. Eur Heart J. 2002;23:734–741. doi: 10.1053/euhj.2001.2894. [DOI] [PubMed] [Google Scholar]
- 28.Kober L, Swedberg K, McMurray JJ, et al. Previously known and newly diagnosed atrial fibrillation: a major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. Eur J Heart Fail. 2006;8:591–598. doi: 10.1016/j.ejheart.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Jabre P, Roger VL, Murad MH, et al. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. 2011;123:1587–1593. doi: 10.1161/CIRCULATIONAHA.110.986661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cha YM, Redfield MM, Shen WK, et al. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation. 2004;109:2839–2843. doi: 10.1161/01.CIR.0000132470.78896.A8. [DOI] [PubMed] [Google Scholar]
- 31.Kundu A, O'Day K, Shaikh AY, et al. Relation of atrial fibrillation in acute myocardial infarction to in-hospital complications and early hospital readmission. Am J Cardiol. 2016;117:1213–1218. doi: 10.1016/j.amjcard.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zusman O, Amit G, Gilutz H, et al. The significance of new onset atrial fibrillation complicating acute myocardial infarction. Clin Res Cardiol. 2012;101:17–22. doi: 10.1007/s00392-011-0357-5. [DOI] [PubMed] [Google Scholar]
- 33.Asanin MR, Vasiljevic ZM, Matic MD, et al. The long-term risk of stroke in patients with acute myocardial infarction complicated with new-onset atrial fibrillation. Clin Cardiol. 2009;32:467–470. doi: 10.1002/clc.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta RH, Dabbous OH, Granger CB, et al. Comparison of outcomes of patients with acute coronary syndromes with and without atrial fibrillation. Am J Cardiol. 2003;92:1031–1036. doi: 10.1016/j.amjcard.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Braga CG, Ramos V, Martins J, et al. Impact of atrial fibrillation type during acute coronary syndromes: clinical features and prognosis. Rev Port Cardiol. 2015;34:403–410. doi: 10.1016/j.repc.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 36.McManus DD, Huang W, Domakonda KV, et al. Trends in atrial fibrillation in patients hospitalized with an acute coronary syndrome. Am J Med. 2012;125:1076–1084. doi: 10.1016/j.amjmed.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bang CN, Gislason GH, Greve AM, et al. New-onset atrial fibrillation is associated with cardiovascular events leading to death in a first time myocardial infarction population of 89,703 patients with long-term follow-up: a nationwide study. J Am Heart Assoc. 2014;3:e000382. doi: 10.1161/JAHA.113.000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almendro-Delia M, Valle-Caballero MJ, Garcia-Rubira JC, et al. Prognostic impact of atrial fibrillation in acute coronary syndromes: results from the ARIAM registry. Eur Heart J Acute Cardiovasc Care. 2014;3:141–148. doi: 10.1177/2048872613517370. [DOI] [PubMed] [Google Scholar]
- 39.Pokorney SD, Rao M, Nilsson KR, et al. Atrial fibrillation complicating acute coronary syndromes. J Atr Fibrillation. 2012;5:611. doi: 10.4022/jafib.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson KR, Jr., Al-Khatib SM, Zhou Y, et al. Atrial fibrillation management strategies and early mortality after myocardial infarction: results from the Valsartan in Acute Myocardial Infarction (VALIANT) Trial. Heart. 2010;96:838–842. doi: 10.1136/hrt.2009.180182. [DOI] [PubMed] [Google Scholar]
- 42.Wong CK, White HD, Wilcox RG, et al. Management and outcome of patients with atrial fibrillation during acute myocardial infarction: the GUSTO-III experience. Global use of strategies to open occluded coronary arteries. Heart. 2002;88:357–362. doi: 10.1136/heart.88.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saliba W, Wazni OM. Sinus rhythm restoration and treatment success: insight from recent clinical trials. Clin Cardiol. 2011;34:12–22. doi: 10.1002/clc.20826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi YJ, Kang KW, Kim TH, et al. Comparison of rhythm and rate control strategies for stroke occurrence in a prospective cohort of atrial fibrillation patients. Yonsei Med J. 2018;59:258–264. doi: 10.3349/ymj.2018.59.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machino-Ohtsuka T, Seo Y, Ishizu T, et al. Relationships between maintenance of sinus rhythm and clinical outcomes in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Cardiol. 2019;74:235–244. doi: 10.1016/j.jjcc.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Yoshino S, Yoshikawa A, Hamasaki S, et al. Atrial fibrillation-induced endothelial dysfunction improves after restoration of sinus rhythm. Int J Cardiol. 2013;168:1280–1285. doi: 10.1016/j.ijcard.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Bunch TJ, Gersh BJ. Rhythm control strategies and the role of antiarrhythmic drugs in the management of atrial fibrillation: focus on clinical outcomes. J Gen Intern Med. 2011;26:531–537. doi: 10.1007/s11606-010-1574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kallergis EM, Goudis CA, Kanoupakis EM, et al. Sinus rhythm restoration affects collagen turnover in patients with persistent atrial fibrillation. Europace. 2014;16:1726–1730. doi: 10.1093/europace/eut401. [DOI] [PubMed] [Google Scholar]