Abstract
Background
Pitch and duration mismatch negativity (pMMN/dMMN) are related to left Heschl’s gyrus gray matter volumes in first-episode schizophrenia (FESz). Previous methods were unable to delineate functional subregions within and outside Heschl’s gyrus. The Human Connectome Project multimodal parcellation (HCP-MMP) atlas overcomes this limitation by parcellating these functional subregions. Further, MMN has generators in inferior frontal cortex, and therefore, may be associated with inferior frontal cortex pathology. With the novel use of the HCP-MMP to precisely parcellate auditory and inferior frontal cortex, we investigated relationships between gray matter and pMMN and dMMN in FESz.
Methods
pMMN and dMMN were measured at Fz from 27 FESz and 27 matched healthy controls. T1-weighted MRI scans were acquired. The HCP-MMP atlas was applied to individuals, and gray matter volumes were calculated for bilateral auditory and inferior frontal cortex parcels and correlated with MMN. FDR correction was used for multiple comparisons.
Results
In FESz only, pMMN was negatively correlated with left medial belt in auditory cortex and area 47L in inferior frontal cortex. Duration MMN negatively correlated with the following auditory parcels: left medial belt, lateral belt, parabelt, TA2, and right A5. Further, dMMN was associated with left area 47L, right area 44, and right area 47L in inferior frontal cortex.
Conclusions
The novel approach revealed overlapping and distinct gray matter associations for pMMN and dMMN in auditory and inferior frontal cortex in FESz. Thus, pMMN and dMMN may serve as biomarkers of underlying pathological deficits in both similar and slightly different cortical areas.
Keywords: gray matter volume, auditory cortex, inferior frontal cortex, mismatch negativity, first-episode schizophrenia
Introduction
The mismatch negativity (MMN) is an event-related potential (ERP) typically measured with electroencephalography (EEG) that has been extensively studied in schizophrenia.1,2 MMN is a response elicited by changes in stimuli and is commonly studied in the auditory domain where frequent standard tones are presented along with infrequent tones that differ in some feature (pitch, duration, loudness, etc…). The MMN is calculated as the difference between the ERP waveform to the standard tones and the ERP waveform to the deviant tones. MMN to both pitch and duration deviant tones is robustly and reliably reduced in chronic schizophrenia.3 MMN is much less reduced at first psychosis.1,4,5 Metanalysis suggests that MMN to duration deviants may be slightly reduced at first episode, while MMN to pitch deviants is not consistently reduced at first-episode.6 When the MMN is followed longitudinally in first-hospitalized schizophrenia patients, their MMN amplitude decreases within the first 2 years of the disorder.7 Therefore, while MMN may not be reduced at first episode, it progressively declines during the early disease course.
Schizophrenia is associated with gray matter deficits that are present early in the disorder and progressively decline in the first few years of the disease course.8,9 Areas that are particularly impacted early are the frontal and temporal lobes.10–13 Gray matter volumes are related to MMN in chronic schizophrenia,14 and during the early course of schizophrenia, the longitudinal decline of pitch MMN is associated with a decline in left Heschl’s gyrus gray matter.7 A recent study found that both pMMN and dMMN are associated with left hemisphere Heschl’s gyrus gray matter in individuals with schizophrenia within 2 months of their first clinical contact for psychosis.15 This suggests that very early in the disorder, the MMNs to pitch and duration deviants are associated with pathological deficits in left auditory cortex. However, Heschl’s gyrus is a strictly anatomical region that contains primary auditory cortex and portions of the medial and lateral belt regions,16 while excluding other important auditory areas, such as parabelt. Thus, it is unable to differentiate between the specific auditory areas’ gray matter contributions to the MMN decline in early schizophrenia.
Pitch MMN and dMMN have slightly different neural generators.17,18 Animal depth recordings, human intracranial recordings, and localization of human-scalp-recorded EEG and magnetoencephalography (MEG) MMN demonstrate that the primary generator for MMN is within primary and secondary auditory cortex.18–26 However, MMN generated by pitch/frequency deviants have a more anterior auditory source compared to the MMN generated by duration deviants.27–30 Similarly, functional MRI reveals that BOLD activity increases to pitch MMN in a more anterior location in auditory cortex, while activity from dMMN is located more posterior and in secondary auditory cortex.31 Further, the generation of the MMN also involves a source in bilateral inferior frontal cortex, with a stronger contribution from the right hemisphere.32–34 This source is difficult to detect with MEG, indicating its source may be oriented radial to the surface of the scalp, such as on a sulcal wall.34 Dynamic causal modeling suggests this inferior frontal source is hierarchically organized with auditory cortex in generating the MMN.35–37 Therefore, pitch and duration MMN are likely generated in slightly different auditory areas with additional contributions from an inferior frontal source.
The current study is a critical improvement on the previous MMN-HG study15 with a new, advanced analysis technique that differentiates gray matter volumes within A1 and surrounding belt regions. We overcame the previous sulcal-gyral limitation by applying the quasi-functional Human Connectome Project Multimodal Parcellation (HCP-MMP) atlas. This parcellation defined cortical regions by both functional and structural information, resulting in a delineation of primary auditory cortex (A1) and surrounding auditory belt regions.38 Due to our previous studies demonstrating that both pMMN and dMMN were associated with gray matter volume in left Heschl’s gyrus in FESz, we used the HCP-MMP atlas to more precisely parcellate Heschl’s gyrus and surrounding auditory cortex into functionally meaningful regions. We assessed correlations between gray matter volume and pMMN/dMMN within these regions to investigate if the pathological association between these biological measures would follow the same anterior-posterior pattern seen in putative auditory generators of pMMN/dMMN. In addition, this study extends the regions of interest to include the inferior frontal cortex to investigate if pMMN and dMMN are also associated with gray matter in the frontal source location of the MMN in FESz.
Methods
Participants
This study included the same participants reported in Salisbury and colleagues15 and as a subset of participants in Murphy and colleagues39, 27 FESz individuals (paranoid: n = 9; undifferentiated: n = 3; residual: n = 2; schizoaffective: n = 5; schizophreniform: n = 2; psychotic disorder NOS: n = 6) and 27 healthy controls. All FESz participated within their first episode of psychosis and had less than 2 months of lifetime antipsychotic medication exposure. Ten FESz were unmedicated. All participants had normal hearing as assessed by audiometry. None of the participants had a history of concussion or head injury with sequelae, history of alcohol or drug addiction, detox in the last 5 years, or neurological comorbidity. Groups were matched for age, gender, parental social economic status, and premorbid IQ, measured by the Wechsler Abbreviated Scale of Intelligence (WASI) (table 1). The work described was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All participants provided informed consent and were paid for participation. All procedures were approved by the University of Pittsburgh IRB.
Table 1.
Demographic, Neuropsychological, and Clinical Information (Previously Reported in Salisbury et al15)
| FESz | HC | t/X 2 | P | Cohen’s d | |
|---|---|---|---|---|---|
| Age | 22.6 (5.1) | 21.1 (3.0) | 1.36 | .18 | 0.36 |
| Gender (M/F) | 19/8 | 18/9 | 0.09 | .77 | |
| SES | 31.9 (13.2) | 33.7 (13.6) | −0.51 | .61 | 0.13 |
| Parental SES | 43.1 (12.6) | 47.5 (13.5) | −1.21 | .23 | 0.34 |
| WASI | 110.4 (14.5) | 108.0 (10.7) | 0.71 | .48 | 0.19 |
| MATRICS | 42.5 (13.3) | 47.2 (8.2) | −1.54 | .13 | 0.43 |
| PANSS Total | 75.2 (14.8) | ||||
| PANSS Positive | 20.0 (5.6) | ||||
| PANSS Negative | 16.9 (4.9) | ||||
| PANSS General | 38.3 (7.5) | ||||
| PANSS TD | 11.2 (3.1) | ||||
| SAPS Global | 5.9 (2.9) | ||||
| SANS Global | 9.7 (3.1) | ||||
| Illness Duration | 73.3 (120.4) | ||||
| DUP (days) | 192.2 (201.1) | ||||
| Medication | 228.8 (147.9) |
Note: SES, socioeconomic status; WASI, Wechsler Abbreviated Scale of Intelligence; MATRICS, composite scaled t score; PANSS, Positive and Negative Syndrome Scale; TD, PANSS Thought Disorder factor; SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms; DUP, duration of untreated psychosis; Medication, chlorpromazine equivalents.
Socioeconomic status (SES) for all participants and their parents was measured with the 4-factor Hollingshead Scale. FESz participants’ diagnoses were based on the Structured Clinical Interview for DSM-IV (SCID-P) and were confirmed 6 months after initial clinical assessment. Symptoms were rated using the Positive and Negative Syndrome Scale (PANSS)40 (table 1). Neuropsychological functioning was assessed with the MATRICS Consensus Cognitive Battery.41 All tests were conducted by an expert diagnostician.
Data Acquisition and Processing
The acquisition and processing methods have previously been described in detail.15 The procedures are briefly described here.
EEG and MMN
EEG was recorded from a custom 72 channel Active2 high impedance system (BioSemi). Auditory stimuli were presented while EEG was recorded, and participants watched a silent video. Stimuli comprised of 1280 standard tones (80%, 1 kHz, 50 ms duration), 160 pitch deviants (10%, 1.2 kHz, 50 ms duration), and 160 duration deviants (10%, 1 kHz, 100 ms). Due to time constraints, 6 FESz and 7 HC participants were only presented a total of 800 tones, including 640 standards (80%), 80 pitch deviants (10%) and 80 duration deviants (10%). Using BESA (BESA GmbH), EEG data were filtered between 0.5 (to remove DC drifts) and 20Hz (to remove muscle and other high frequency artifact), bad channels interpolated, and eye/cardiac artifact removed with independent component analysis. In BrainVision Analyzer2 (brain Products GmbH), data were re-referenced to the nosetip. Epochs of 350ms (including 50ms pre-stimulus baseline) were extracted for deviant tones and standard tones preceding a deviant. Epochs were baseline corrected and a rejection criterion of ±50 μV was applied. Epochs were then averaged for the standard tones, pitch deviants, and duration deviants. The standard average was subtracted from the pitch average to generate the pMMN. To generate the dMMN, the standard average was subtracted from the duration average. MMN was measured by automatic peak detection at Fz for each individual (pMMN range: 90–145 ms, dMMN range: 140–200 ms, verified visually and adjusted if necessary) and averaged over a 100 ms time window (peak ±50 ms). The Fz site was used to quantify the MMNs, where they are typically the largest and to replicate the procedure used in Salisbury and colleagues.7
Magnetic Resonance Imaging
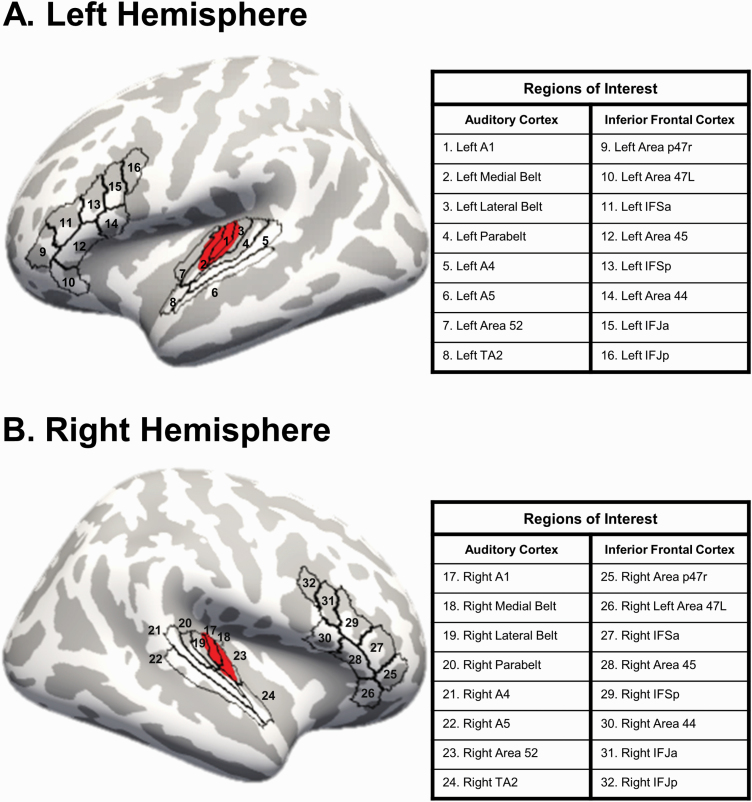
MRI data were acquired using a Siemens Tim Trio on a 32-channel phase array head coil. Sagittal T1-weighted anatomical MR images were obtained with a multi-echo 3D MPRAGE sequence. For quality assurance, the MRI scans were visually inspected for scanner artifacts, motion, and gross neuroanatomical abnormalities. FreeSurfer (stable v6.0) was used for MRI processing.42–44 Briefly, processing involved registration to MNI space, intensity normalization, skull stripping, segmentation of white matter, and the generation of white and pial surfaces. Cortical surfaces were examined to ensure accurate delineation of white matter and pial surfaces. Using the HCP workbench, the HCP-MMP was resampled to fsaverage space. In Freesurfer, the parcellation was applied to individual data (see HCP Users FAQ (https://wiki.humanconnectome.org/display/PublicData/HCP+Users+FAQ#HCPUsersFAQ-9.HowdoImapdatabetweenFreeSurferandHCP?)). Freesurfer was used to calculate gray matter volume in 32 ROIs using the HCP-MMP atlas.38 The regions of interest included the following 8 ROIs in bilateral AC: A1, medial belt (Mbelt), lateral belt (Lbelt), parabelt (Pbelt), A4, A5, Area 52, and area TA2. In the inferior frontal cortex, the following 8 bilateral areas were used as ROIs: Brodmann area 44, Brodmann area 45, lateral subdivision of area 47 (47L), anterior inferior frontal junction (IFJa), posterior inferior frontal junction (IFJp), anterior inferior frontal sulcus (IFSa), posterior inferior frontal sulcus (IFSp), and area posterior 47r (figure 1).
Fig. 1.
Regions of interest. The regions of interest in auditory cortex and inferior frontal cortex defined from the Human Connectome Project multimodal parcellation (HCP-MMP) atlas with Heschl’s gyrus in red. Volumes were estimated from each region.
Analysis
Group demographics were compared using independent samples t-test and chi-square tests where appropriate. Gray matter volume group comparisons were made using analysis of covariance (ANCOVA) with intracranial content (ICC), gender, and age as covariates. The statistical conclusions reported remained the same when we used ANCOVA on the relative volumes ([absolute volume/ICC] × 100) with gender and age as covariates.
Absolute volumes were used for correlations, and correlations were assessed with Spearman’s rho. Benjamini-Hochberg False Discovery Rate (FDR) correction was applied to correct for multiple comparisons for the group comparisons and correlations (q = 0.05). Note that MMN is a negative brainwave, and thus a larger MMN has a more negative amplitude. Therefore, a negative correlation indicates that smaller GM volume is associated with a smaller absolute MMN amplitude.
Results
Mismatch Negativity
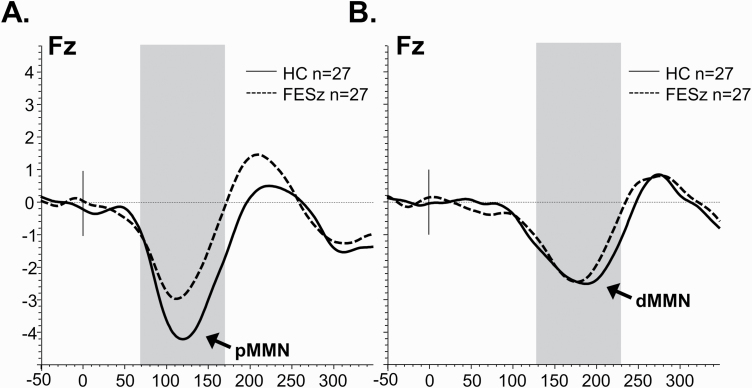
As reported previously in Salisbury and colleagues,15 peak pMMN latency did not differ between FESz (113.1 ± 13.8 ms) and HC (117.8±14.5 ms, t(52) = 1.22, P = .23). Similarly, peak dMMN latency did not differ between groups (FESz: 174.3 ± 14.7 ms; HC: 173.9 ± 20.1 ms, t(52) = 0.08, P = .94). Pitch MMN amplitude was significantly reduced in this sample of FESz (2.0 µV±1.4 µV) compared to HC (3.1 ± 1.7 µV, t(52) = 2.50, P = .016). Duration MMN amplitude did not differ between groups (FESz: 1.8 ±1.5 µV; HC: 2.1 ± 1.6 µV, t(52) = 0.77, P = .44) (figure 2).
Fig. 2.
Group mismatch negativity grand averages information (previously reported in Salisbury et al15). (A) Mismatch negativity subtraction waveforms for pitch deviants in HC and FESz. (B) Mismatch negativity subtraction waveforms for duration deviants in HC and FESz. The averaged time window is highlighted.
Gray Matter Volume Group Comparisons
Groups did not have significantly different intracranial content (FESz: 1619 ± 198 cm3; HC: 1649 ± 178 cm3; t(52) = 0.58, P = .56). There were no regions of interest in which gray matter volume significantly differed between groups (Ps > .05).
Pitch MMN and GM Volume Correlations
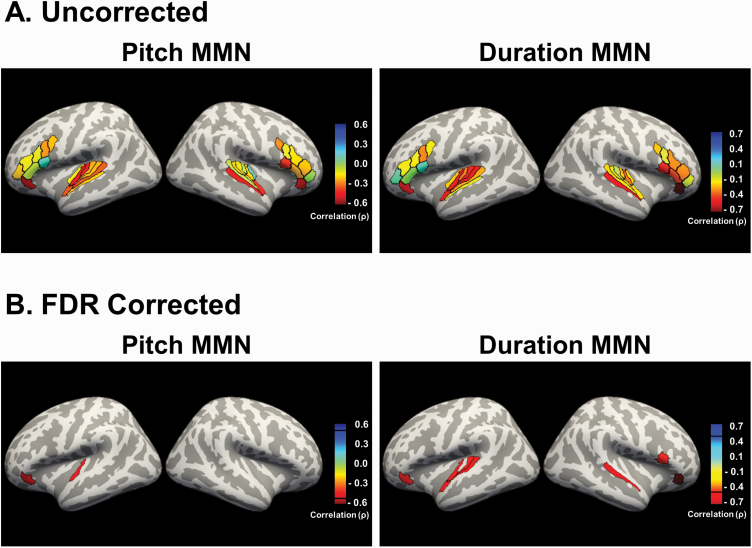
All correlations for FESz are shown in table 2. In FESz, after FDR correction of q = .05, pMMN remained significantly associated with left Mbelt and left 47L (figures 3 and 4). There were no significant correlations in HC (q = .05).
Table 2.
MMN and GM Correlations in FESz
| Pitch MMN | Duration MMN | |||
|---|---|---|---|---|
| Brain Region | ρ | P | ρ | P |
| Auditory Cortex | ||||
| L_A1 | −0.48 | .01 | −0.38 | .05 |
| L_LBelt | −0.30 | .13 | −0.50 | .01a |
| L_MBelt | −0.55 | <.01a | −0.53 | <.01a |
| L_PBelt | −0.30 | .13 | −0.53 | <.01a |
| L_A4 | −0.40 | .04 | −0.34 | .08 |
| L_A5 | −0.27 | .17 | −0.14 | .49 |
| L_52 | −0.16 | .42 | −0.29 | .14 |
| L_TA2 | −0.39 | .05 | −0.52 | .01a |
| R_A1 | −0.07 | .72 | −0.32 | .10 |
| R_LBelt | −0.17 | .40 | 0.05 | .81 |
| R_MBelt | −0.26 | .19 | −0.40 | .04 |
| R_PBelt | −0.26 | .19 | −0.17 | .39 |
| R_A4 | −0.06 | .78 | −0.27 | .17 |
| R_A5 | −0.49 | .01 | −0.53 | <.01a |
| R_52 | 0.10 | .61 | −0.28 | .15 |
| R_TA2 | −0.45 | .02 | −0.26 | .19 |
| Inferior Frontal | ||||
| L_44 | 0.12 | .54 | 0.11 | .57 |
| L_45 | −0.11 | .58 | −0.03 | .89 |
| L_47I | −0.58 | <.01a | −0.48 | .01a |
| L_IFJa | −0.33 | .09 | −0.36 | .06 |
| L_IFJp | −0.29 | .14 | −0.19 | .33 |
| L_IFSa | −0.15 | .45 | −0.19 | .34 |
| L_IFSp | −0.12 | .54 | −0.23 | .26 |
| L_p47r | −0.25 | .21 | 0.08 | .68 |
| R_44 | −0.43 | .03 | −0.48 | .01a |
| R_45 | −0.26 | .19 | −0.41 | .03 |
| R_47I | −0.45 | .02 | −0.70 | <.01a |
| R_IFJa | −0.12 | .55 | −0.10 | .61 |
| R_IFJp | −0.35 | .07 | −0.31 | .12 |
| R_IFSa | −0.28 | .16 | −0.36 | .06 |
| R_IFSp | −0.25 | .21 | −0.37 | .06 |
| R_p47r | −0.04 | .84 | −0.14 | .50 |
Note: Correlation strengths (Spearman’s rho) for the relationships between pitch and duration MMN and regions of interest in auditory and inferior frontal cortex.
adenotes survives FDR correction (q < 0.05).
Fig. 3.
Gray matter volume associations with pitch and duration MMN in FESz. (A) The correlation strengths of auditory and inferior cortex gray matter volumes with pitch and duration MMN in first-episode schizophrenia. (B) Regions that survived FDR correction for multiple comparisons (q < 0.05). In FESz, pitch MMN remained significantly associated with left Mbelt and left 47L. In FESz, duration MMN significantly correlated with left Mbelt, left Lbelt, left Pbelt, left TA2, left 47L, right A5, right area 44, and right 47L.
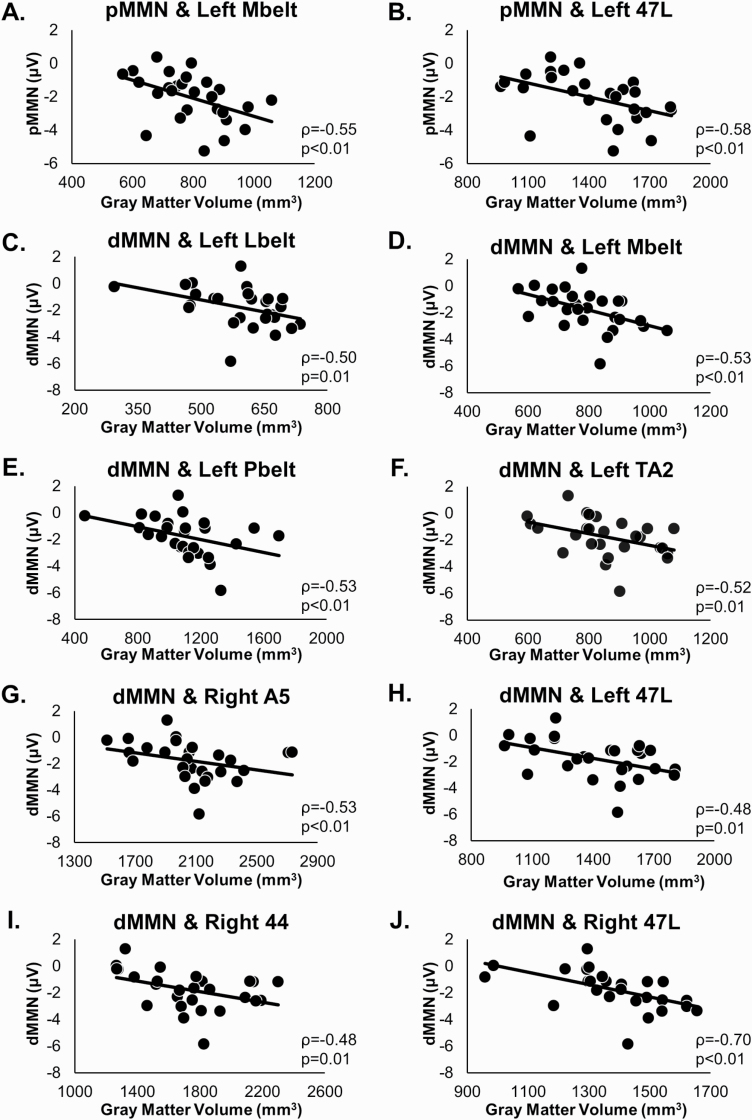
Fig. 4.
Scatterplots of significant MMN and gray matter correlations. In FESz, pitch MMN was significantly (q < 0.05) associated with gray matter in left Mbelt (A) and left 47L (B). Duration MMN was significantly (q < 0.05) associated with gray matter in left Mbelt (C), left Lbelt (D), left Pbelt (E), left TA2 (F), right A5 (G), left 47L (H), right area 44 (I), and right 47L (J).
Duration MMN and GM Volume Correlations
All correlations for FESz are shown in table 2. In FESz, after FDR correction (q=.05), dMMN significantly correlated with left Mbelt, left Lbelt, left Pbelt, left TA2, right A5, left 47L, right area 44, and right 47L in FESz (figures 3 and 4). There were no significant correlations in HC (q = .05).
Discussion
With the novel use of the specific and functionally relevant HCP-MMP atlas, this study provides a notably distinct and more precise understanding of the underlying pathology associated with impaired MMN in FESz. The previous analysis on this dataset was restricted to a strictly anatomically defined region, Heschl’s gyrus, which contains portions of primary auditory cortex and auditory belt areas.15 The method utilized in this study provided an exquisite functional parcellation of cortex, allowing for the study of relationships with temporal and frontal MMN generators with high specificity to functional areas. The novel use of the HCP-MMP atlas combined with the extension to investigate the inferior frontal MMN generator, provided a new understanding of the relationships between MMN and gray matter, revealing both overlapping and distinct associations for pMMN and dMMN in FESz. After correction for multiple comparisons, both pMMN and dMMN were associated with gray matter volume of left Mbelt and left 47L. Duration MMN was uniquely related to volume in left Lbelt, left Pbelt, left TA2, right A5, right area 44, and right 47L. The significant correlations were all in the negative direction and only within the FESz group, suggesting that less gray matter was associated with a smaller MMN in FESz. In light of the evidence of progressive gray matter decline in the early course of schizophrenia that begins as early as the prodrome,8,9,12,45,46 it suggests that within a subset of FESz there is enough underlying gray matter loss in these regions to impair functioning, represented by a reduced pMMN or dMMN. These findings provide important insights into the relationship of MMN and gray matter within MMN generators in the very early course of schizophrenia.
The shared and unique auditory cortex subregions associated with pMMN and dMMN in FESz align with previous reports regarding the underlying neural generators of the MMN. EEG and MEG source localization and dynamic causal modeling suggest MMN generators in the supratemporal plane (Heschl’s gyrus and STG) and inferior frontal cortex.26,34,36 Both pMMN and dMMN were related to gray matter within each of these generators: left Heschl’s gyrus (Mbelt) and left inferior frontal cortex (47L). Further, pitch MMN has a more anterior auditory cortex generator, while the dMMN has a generator more posterior in secondary auditory cortex.27–31 In FESz, pitch MMN was associated with gray matter volume in Mbelt, while dMMN was associated with Mbelt with the addition of other secondary auditory cortex parcels, some slightly more posterior (Lbelt and Pbelt). The additional posterior parcels related to dMMN may provide evidence that (similar to the neural generator) dMMN is associated with underlying pathology in more posterior secondary auditory areas.
The strong correlations of MMN and gray matter early in schizophrenia is consistent with the progressive gray matter loss in the disorder.8,9 Gray matter loss in schizophrenia is due to a reduction in cortical neuropil,47 and in auditory cortex specifically, there is a reduction in layer III pyramidal soma size and a decreased number and density of dendritic spines.48–51 It is hypothesized that the consequence of these reductions is reduced synaptic connectivity, and thus reduced MMN.7,50,52 Because there were no volume group differences and these associations were only present in the FESz individuals, it suggests that within the HC there is enough neuropil to support healthy functioning and the production of a healthy MMN to deviant stimuli. In FESz, some individuals have experienced more gray matter loss in certain auditory and inferior frontal cortex regions beyond healthy limits, which is associated with either a deficient pMMN and/or dMMN response.
The regions that were strongly related to MMN are functionally important for auditory and attention processing. In the auditory cortex, the medial belt, located on the medial surface of Heschl’s gyrus, is a relatively early auditory processing stage with local connectivity to surrounding auditory areas.53,54 The lateral belt, located on the lateral surface of Heschl’s gyrus, is also a relatively early auditory processing stage with connections to local auditory areas and inferior frontal gyrus.53 The parabelt resides between Heschl’s gyrus and the inferior portion of the supramarginal gyrus. Compared to the medial and lateral belts, the parabelt is considered higher in the regional auditory hierarchy. It has connectivity with local auditory areas and with motor, premotor, and inferior frontal cortices. The parabelt integrates across different frequency bands and is involved in more complex behaviors such as figure-ground segregation.55 Area TA2, residing in the anterior portion of the superior temporal gyrus, is important for auditory stimuli perception. Left TA2 is more involved in temporal patterns of speech, while right TA2 appears to be more involved with tone of speech.53,56 This supports our finding that left TA2 gray matter was significantly related to duration MMN in FESz. Right A5, located on the superior lateral surface of the posterior superior temporal gyrus, has connectivity with inferior frontal gyrus and other local temporal lobe regions. Right A5 may be important for tone-matching performance in schizophrenia, as schizophrenia patients with tone-matching deficits have decreased connectivity between the medial geniculate nucleus and A5.57 In the inferior frontal cortex, area 47L is functionally connected to several cortical regions, including dorsolateral frontal, medial frontal, premotor, parietal, and temporal cortex. In the left hemisphere, commonly referred to as part of Broca’s area, it is important for language production and semantic processing.58,59 In the right hemisphere, it is important for response inhibition and attention.58,60–62 Finally, area 44, located in posterior inferior frontal gyrus, also has connectivity with a number of frontal, parietal, and temporal cortical regions and is involved in response inhibition and attention processing.58,60–62 While we have identified gray matter within these regions to be related to MMN in FESz, it is unclear if they should be considered individually or as part of larger networks. For example, the auditory regions are part of an auditory somatosensory network, and resting fMRI activity within this entire network is related to responses to frequency and duration deviant tones.63 In addition, the inferior frontal regions are commonly included in the attention network, and resting fMRI activity within this network is related to responses to duration deviant tones.63 The areas 47L and 44 may be part of this attention network, which may be particularly relevant to the automatic attention switching process of MMN.33,34 Future work can be done to further dissociate between the regional and network-level contributions to MMN deficits in FESz.
There are some caveats to consider. This study may have been underpowered to detect some significant relationships, as it included a modest sample size and many regions of interest. Due to the many comparisons, a conservative FDR correction was used, and as a result, several parcels with moderate effect sizes did not survive FDR correction. In particular, the correlation between dMMN and right Mbelt did not survive FDR correction. In the previous analysis of this data, dMMN was associated at a trend level with right Heschl’s gyrus.15 The current analysis with the HCP-MMP atlas suggests that the association between dMMN and right HG is likely reflected primarily in right Mbelt. Future studies are needed to investigate these other relationships and investigate directly whether dMMN is associated with right Mbelt volume. In addition, a subset of individuals had 800 tones for their MMNs, and although 800 trials did not previously change MMN statistics,39 it is possible the individuals with fewer trials had poorer signal to noise. While this study focused on the known cortical generators of the MMN, deviance detection deficits in schizophrenia may be present in subcortical structures.64 While beyond the scope of this study, future analyses examining relationships between deviance detection and volumes may extend the analysis to subcortical structures. Further, the analysis was correlational, and therefore, we cannot conclude that gray matter loss directly causes MMN deficits. Finally, the current study was limited to MMN measured with EEG, making source localization difficult. Future analyses with both EEG and MEG combined with high-resolution MRI will resolve MMN activity to the cortical surface and investigate how it relates to gray matter within these structures.
This study was also limited to data at one time-point in the very early course of psychosis. It will be important for future analyses to investigate these relationships longitudinally during the first year of psychosis to better understand the nature of these relationships between MMN and gray matter. It has been proposed that pMMN and dMMN may be indicators of different processes in schizophrenia. Pitch MMN appears to be more related to the degeneration experienced over the course of schizophrenia,3,6,7 while dMMN may be more closely associated with the conversion to schizophrenia.6,52,65–67 Our previous study suggested that pMMN and dMMN may track Heschl’s gyrus neuropil, but this improved, precise analysis suggests that pMMN and dMMN may indicate neuropil loss in slightly different areas, and thus may be related to different underlying processes associated with schizophrenia. Alternatively, there could be a common underlying dendrotoxic process associated with schizophrenia, but the differences may be attributed to a difference in specialization of the affected cortical areas. For example, duration deviant detection may be more sensitive to neuropil loss in areas important for precise timing mechanisms such as right prefrontal cortex.68–71 Nonetheless, the results suggest that pitch and duration MMN may be tools to identify distinct subgroups of individuals with neuropil loss in slightly different areas, potentially improving specificity for treatments, such as non-invasive brain stimulation. In addition, identifying that deficits are in both sensory and higher-order cognitive areas can inform individualized cognitive enhancement therapy, which can be neuroprotective in early schizophrenia.72 Future longitudinal and clinical studies in the early course of schizophrenia can investigate this speculation further.
To summarize, pMMN and dMMN are impaired in some individuals that are early in the disease course of schizophrenia. Pitch and duration MMN have slightly different neural generators in auditory cortex with the addition of an inferior frontal generator. With the novel, precise parcellation of these regions into functionally meaningful parcels, it appears that pathological associations between MMN and gray matter volume align with these generators. Both pMMN and dMMN were associated with gray matter in the more anterior medial belt, while only dMMN was also associated with gray matter in more secondary auditory areas and right inferior frontal. As the early course of schizophrenia is associated with loss of neuropil, MMN may serve as biomarker of underlying pathological deficits, with pMMN and dMMN reflecting gray matter in both similar and slightly different cortical areas.
Acknowledgments
We thank the faculty and staff of the WPH Psychosis Recruitment and Assessment Core, the Conte Center for Translational Mental Health Research (P50 MH103204, David Lewis, MD, Director), and the University of Pittsburgh Clinical Translational Science Institute (UL1 RR024153, Steven E. Reis, MD) for their assistance in recruitment, diagnostic and psychopathological assessments, and neuropsychological evaluations. We thank Timothy Murphy, Justin Leiter, Vanessa Fishel, Natasha Torrence, Julia Lekht, William Foran, Jusmita Saifullan, Maria Jalbrzikowski, Ph.D., and Beatriz Luna, Ph.D., for their assistance in data collection. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This work was supported by National Institute of Mental Health (R01 MH94328, R01 MH113533, and P50 MH103204 to D.F.S.).
References
- 1. Erickson MA, Ruffle A, Gold JM. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biol Psychiatry. 2016;79(12):980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Light GA, Näätänen R. Mismatch negativity is a breakthrough biomarker for understanding and treating psychotic disorders. Proc Natl Acad Sci U S A. 2013;110(38):15175–15176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76(1):1–23. [DOI] [PubMed] [Google Scholar]
- 4. Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59(8):686–694. [DOI] [PubMed] [Google Scholar]
- 5. Salisbury DF, Polizzotto NR, Nestor PG, Haigh SM, Koehler J, McCarley RW. Pitch and duration mismatch negativity and premorbid intellect in the first hospitalized schizophrenia spectrum. Schizophr Bull. 2017;43(2):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haigh SM, Coffman BA, Salisbury DF. Mismatch negativity in first-episode schizophrenia: a meta-analysis. Clin EEG Neurosci. 2017;48(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1-2):1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60(8):766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160(1):156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cropley VL, Klauser P, Lenroot RK, et al. Accelerated gray and white matter deterioration with age in schizophrenia. Am J Psychiatry. 2017;174(3):286–295. [DOI] [PubMed] [Google Scholar]
- 13. Keshavan MS, Haas GL, Kahn CE, et al. Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res. 1998;32(3-4):161–167. [DOI] [PubMed] [Google Scholar]
- 14. Rasser PE, Schall U, Todd J, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull. 2011;37(1):131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salisbury DF, Shafer AR, Murphy TK, Haigh SM, Coffman BA. Pitch and duration mismatch negativity and Heschl’s gyrus volume in first-episode schizophrenia-spectrum individuals. Clin EEG Neurosci. 2020;51(6):359–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sweet RA, Dorph-Petersen KA, Lewis DA. Mapping auditory core, lateral belt, and parabelt cortices in the human superior temporal gyrus. J Comp Neurol. 2005;491(3):270–289. [DOI] [PubMed] [Google Scholar]
- 17. Paavilainen P, Alho K, Reinikainen K, Sams M, Näätänen R. Right hemisphere dominance of different mismatch negativities. Electroencephalogr Clin Neurophysiol. 1991;78(6):466–479. [DOI] [PubMed] [Google Scholar]
- 18. Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 1995;16(1):38–51. [DOI] [PubMed] [Google Scholar]
- 19. Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG Jr, Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res. 1994;667(2):192–200. [DOI] [PubMed] [Google Scholar]
- 20. Csépe V. On the origin and development of the mismatch negativity. Ear Hear. 1995;16(1):91–104. [DOI] [PubMed] [Google Scholar]
- 21. Rosburg T, Trautner P, Dietl T, et al. Subdural recordings of the mismatch negativity (MMN) in patients with focal epilepsy. Brain. 2005;128(Pt 4):819–828. [DOI] [PubMed] [Google Scholar]
- 22. Kropotov JD, Näätnen R, Sevostianov AV, Alho K, Reinikainen K, Kropotova OV. Mismatch negativity to auditory stimulus change recorded directly from the human temporal cortex. Psychophysiology. 1995;32(4):418–422. [DOI] [PubMed] [Google Scholar]
- 23. Hari R, Rif J, Tiihonen J, Sams M. Neuromagnetic mismatch fields to single and paired tones. Electroencephalogr Clin Neurophysiol. 1992;82(2):152–154. [DOI] [PubMed] [Google Scholar]
- 24. Hari R, Hämäläinen M, Ilmoniemi R, et al. Responses of the primary auditory cortex to pitch changes in a sequence of tone pips: neuromagnetic recordings in man. Neurosci Lett. 1984;50(1-3):127–132. [DOI] [PubMed] [Google Scholar]
- 25. Kasai K, Nakagome K, Itoh K, et al. Multiple generators in the auditory automatic discrimination process in humans. Neuroreport. 1999;10(11):2267–2271. [DOI] [PubMed] [Google Scholar]
- 26. Näätänen R, Alho K. Generators of electrical and magnetic mismatch responses in humans. Brain Topogr. 1995;7(4):315–320. [DOI] [PubMed] [Google Scholar]
- 27. Frodl-Bauch T, Kathmann N, Möller HJ, Hegerl U. Dipole localization and test-retest reliability of frequency and duration mismatch negativity generator processes. Brain Topogr. 1997;10(1):3–8. [DOI] [PubMed] [Google Scholar]
- 28. Rosburg T. Left hemispheric dipole locations of the neuromagnetic mismatch negativity to frequency, intensity and duration deviants. Brain Res Cogn Brain Res. 2003;16(1):83–90. [DOI] [PubMed] [Google Scholar]
- 29. Giard MH, Lavikahen J, Reinikainen K, et al. Separate representation of stimulus frequency, intensity, and duration in auditory sensory memory: an event-related potential and dipole-model analysis. J Cogn Neurosci. 1995;7(2):133–143. [DOI] [PubMed] [Google Scholar]
- 30. Levänen S, Ahonen A, Hari R, McEvoy L, Sams M. Deviant auditory stimuli activate human left and right auditory cortex differently. Cereb Cortex. 1996;6(2):288–296. [DOI] [PubMed] [Google Scholar]
- 31. Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ. The neural circuitry of pre-attentive auditory change-detection: an fMRI study of pitch and duration mismatch negativity generators. Cereb Cortex. 2005;15(5):545–551. [DOI] [PubMed] [Google Scholar]
- 32. Park HJ, Kwon JS, Youn T, et al. Statistical parametric mapping of LORETA using high density EEG and individual MRI: application to mismatch negativities in schizophrenia. Hum Brain Mapp. 2002;17(3):168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27(6):627–640. [DOI] [PubMed] [Google Scholar]
- 34. Rinne T, Alho K, Ilmoniemi RJ, Virtanen J, Näätänen R. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage. 2000;12(1):14–19. [DOI] [PubMed] [Google Scholar]
- 35. Garrido MI, Kilner JM, Kiebel SJ, Friston KJ. Dynamic causal modeling of the response to frequency deviants. J Neurophysiol. 2009;101(5):2620–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garrido MI, Friston KJ, Kiebel SJ, Stephan KE, Baldeweg T, Kilner JM. The functional anatomy of the MMN: a DCM study of the roving paradigm. Neuroimage. 2008;42(2):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lieder F, Stephan KE, Daunizeau J, Garrido MI, Friston KJ. A neurocomputational model of the mismatch negativity. PLoS Comput Biol. 2013;9(11):e1003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murphy TK, Haigh SM, Coffman BA, Salisbury DF. Mismatch Negativity and Impaired social functioning in long-term and in first episode schizophrenia spectrum psychosis. Front Psychiatry. 2020;11:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 41. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. [DOI] [PubMed] [Google Scholar]
- 42. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 44. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 45. Chung Y, Allswede D, Addington J, et al. ; North American Prodrome Longitudinal Study (NAPLS) Consortium . Cortical abnormalities in youth at clinical high-risk for psychosis: findings from the NAPLS2 cohort. Neuroimage Clin. 2019;23:101862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cannon TD, Chung Y, He G, et al. ; North American Prodrome Longitudinal Study Consortium . Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77(2):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45(1):17–25. [DOI] [PubMed] [Google Scholar]
- 48. Parker EM, Sweet RA. Stereological Assessments of Neuronal Pathology in Auditory Cortex in Schizophrenia. Front Neuroanat. 2017;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34(2):374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119(4):706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sweet RA, Bergen SE, Sun Z, Sampson AR, Pierri JN, Lewis DA. Pyramidal cell size reduction in schizophrenia: evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004;55(12):1128–1137. [DOI] [PubMed] [Google Scholar]
- 52. Todd J, Harms L, Schall U, Michie PT. Mismatch negativity: translating the potential. Front Psychiatry. 2013;4:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baker CM, Burks JD, Briggs RG, et al. A Connectomic Atlas of the Human Cerebrum-Chapter 5: The Insula and Opercular Cortex. Oper Neurosurg (Hagerstown). 2018;15(suppl_1):S175–S244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kusmierek P, Rauschecker JP. Functional specialization of medial auditory belt cortex in the alert rhesus monkey. J Neurophysiol. 2009;102(3):1606–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schneider F, Dheerendra P, Balezeau F, et al. Auditory figure-ground analysis in rostral belt and parabelt of the macaque monkey. Sci Rep. 2018;8(1):17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Burton H, Firszt JB, Holden T, Agato A, Uchanski RM. Activation lateralization in human core, belt, and parabelt auditory fields with unilateral deafness compared to normal hearing. Brain Res. 2012;1454:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dondé C, Martínez A, Kantrowitz JT, et al. Bimodal distribution of tone-matching deficits indicates discrete pathophysiological entities within the syndrome of schizophrenia. Transl Psychiatry. 2019;9(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baker CM, Burks JD, Briggs RG, et al. A connectomic atlas of the human cerebrum-chapter 2: the lateral frontal lobe. Oper Neurosurg (Hagerstown). 2018;15(suppl_1):S10–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ardila A, Bernal B, Rosselli M. How localized are language brain areas? A review of Brodmann areas involvement in oral language. Arch Clin Neuropsychol. 2016;31(1):112–122. [DOI] [PubMed] [Google Scholar]
- 60. Neef NE, Bütfering C, Anwander A, Friederici AD, Paulus W, Sommer M. Left posterior-dorsal area 44 couples with parietal areas to promote speech fluency, while right area 44 activity promotes the stopping of motor responses. Neuroimage. 2016;142:628–644. [DOI] [PubMed] [Google Scholar]
- 61. Hartwigsen G, Neef NE, Camilleri JA, Margulies DS, Eickhoff SB. Functional segregation of the right inferior frontal gyrus: Evidence from coactivation-based parcellation. Cereb Cortex. 2019;29(4):1532–1546. [DOI] [PubMed] [Google Scholar]
- 62. Lai VT, van Dam W, Conant LL, Binder JR, Desai RH. Familiarity differentially affects right hemisphere contributions to processing metaphors and literals. Front Hum Neurosci. 2015;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee M, Sehatpour P, Hoptman MJ, et al. Neural mechanisms of mismatch negativity dysfunction in schizophrenia. Mol Psychiatry. 2017;22(11):1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gaebler AJ, Zweerings J, Koten JW, et al. Impaired subcortical detection of auditory changes in schizophrenia but not in major depression. Schizophr Bull. 2020;46(1):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Michie PT, Innes-Brown H, Todd J, Jablensky AV. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol Psychiatry. 2002;52(7):749–758. [DOI] [PubMed] [Google Scholar]
- 66. Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol. 2001;42(2):177–194. [DOI] [PubMed] [Google Scholar]
- 67. Näätänen R, Kähkönen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: a review. Int J Neuropsychopharmacol. 2009;12(1):125–135. [DOI] [PubMed] [Google Scholar]
- 68. Gooch CM, Wiener M, Hamilton AC, Coslett HB. Temporal discrimination of sub- and suprasecond time intervals: a voxel-based lesion mapping analysis. Front Integr Neurosci. 2011;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Merchant H, Harrington DL, Meck WH. Neural basis of the perception and estimation of time. Annu Rev Neurosci. 2013;36:313–336. [DOI] [PubMed] [Google Scholar]
- 70. Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. J Neurosci. 1997;17(14):5528–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Harrington DL, Haaland KY, Knight RT. Cortical networks underlying mechanisms of time perception. J Neurosci. 1998;18(3):1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Eack SM, Hogarty GE, Cho RY, et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch Gen Psychiatry. 2010;67(7):674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]