Abstract
Epithelial-mesenchymal transition (EMT) is a reversible process of epithelial cell transdifferentiation into a mesenchymal cell, that enables initiation of cell migration. EMT plays an important role in embryonic development, tissue repair and cancer metastasis. Better understanding of cellular and molecular events during EMT will not only provide novel insights on how mammalian organism develops and how epithelial tissues regenerate, but also can identify novel therapeutic targets for cancer therapy. Here we aim to provide a detailed protocol on how to induce EMT in Madin-Darby Canine Kidney (MDCK) II epithelial cell line and perform immunofluorescent staining on EMT-induced cells.
Keywords: Epithelial-mesenchymal transition, MDCK cells, Hepatocyte growth factor, Epithelial plasticity, EMT, Mesenchymal cell differentiation from epithelial cell
Background
Epithelial cells are characterized by cell plasticity, that is the ability to adopt different cellular phenotypes ( Carter et al., 2019 ; Yuan et al., 2019 ). Epithelial-mesenchymal transition (EMT) is a form of epithelial cell plasticity. During EMT epithelial cells disrupt cell-cell junctions, lose their polarity and change their shape from squamous, cuboidal, or columnar into spindle-like and become migratory, thus gaining the properties of mesenchymal cells (Kalluri and Weinberg, 2009). EMT can be evaluated by immunostainings and measurement of expression levels of markers such as E-cadherin, ZO-1, vimentin, fibronectin and N-cadherin (Kalluri and Weinberg, 2009). The studies on EMT during the last decade centered mostly on the role of transcription factors e.g., Snail1/2, ZEB1/2, Twist and microRNAs (Gonzalez and Medici, 2014), cytokines like tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) ( Yadav et al., 2011 ; Li et al., 2012 ), and signaling mechanisms such as transforming growth factor beta (TGF-β) signaling and NF-κB signaling (Gonzalez and Medici, 2014; Song et al., 2014 ; Dongre and Weinberg, 2019). However, regulatory mechanisms of early stages EMT and the precise sequence of molecular events during EMT remain largely unknown.
Madin-Darby Canine Kidney (MDCK) epithelial cell line is a cellular model to study epithelial cell polarity and junctions ( Dukes et al., 2011 ; Yonemura, 2014; Vidal- Quadras et al., 2017 ). MDCK II cell line is a strain, that originates from higher passage of parental MDCK cells ( Dukes et al., 2011 ). MDCK II cells differ from parental MDCK cells in that MDCK II cells have leaky cell junctions. Importantly, MDCK cells can be cultured in a standard 2D cell culture system, as well as in a more physiologically relevant 3D cell culture system (Yonemura, 2014; Vidal- Quadras et al., 2017 ).
EMT can be easily stimulated in 2D cell cultures of MDCK II cells by treatment with hepatocyte growth factor (HGF), that decreases cell roundness, upregulates the expression of vimentin, and increases the cell distance to the first and the second neighbor cell ( Farrell et al., 2014 ). Here we provide a detailed description of the procedure on how to stimulate EMT in 2D cell cultures of MDCK II cells. Additionally, we describe the procedure for immunostaining of EMT-induced cells for ZO-1, a tight junction protein, and vimentin, the classical EMT marker.
Materials and Reagents
Sterile Cell Culture Dish, 100 × 20 mm (Sarstedt, catalog number: 83.3902)
Serological pipettes: 5 ml (Sarstedt, catalog number: 86.1253.001), 10 ml (Sarstedt, catalog number: 86.1254.001), 50 ml (Sarstedt, catalog number: 86.1256.001)
Tube 15 ml (Sarstedt, catalog number: 62.554.502)
Tube 50 ml (Sarstedt, catalog number: 62.547.254)
1.5 ml microtubes (Sarstedt, catalog number: 72.690.301)
-
Culture chambers: Lumox 8-well specimen slide detachable (Sarstedt, catalog number: 94.6150.801)
Note: The procedure also works with FalconTM Chambered Cell Culture Slides, 8 wells, (Corning, catalog number: 354118).
Laboratory bottle, borosilicate 3.3 glass, 50 ml (VWR, catalog number: 215-3261)
Laboratory bottle, borosilicate 3.3 glass, 1,000 ml (VWR, catalog number: 215-1595)
Aluminium foil
ARTTM Barrier Specialty Pipette Tips: 10 µl (Thermo Scientific, catalog number: 2140), 200 µl (Thermo Scientific, catalog number: 2770), 1,000 µl (Thermo Scientific, catalog number: 2279)
Microscope slide holder
Cover slips Menzel Glaser 24 × 60 mm (ThermoFisher Scientific, catalog number: E-4137)
Parafilm
MDCK II cell line (Merck/Sigma-Aldrich, catalog number: ECACC 00062107)
Eagle's Minimum Essential Medium (EMEM), 500 ml (ATCC, catalog number: 30-2003)
-
Fetal bovine serum (FBS), 500 ml (Gibco, catalog number: 10270-106)
Note: FBS does not require heat inactivation.
Dulbecco's phosphate-buffered saline (DPBS) 1×, no calcium, no magnesium, 500 ml (Gibco, catalog number: 14190-094)
Trypsin-EDTA (0.25%), phenol red, 100 ml (Gibco, catalog number: 25200056)
Recombinant Human HGF (HEK293 derived), 25 μg (Peprotech, catalog number: 100-39H)
Goat normal serum, 10 ml (Agrisera, catalog number: AS10 1548)
Saponin, 10 g (Sigma-Aldrich, catalog number: S4521)
Paraformaldehyde (PFA), 1 kg (Sigma-Aldrich, catalog number: 16005)
Sodium chloride (NaCl), 1 kg (VWR Chemicals, catalog number: 27810.295)
Potassium chloride (KCl), reagent grade, Reag. Ph Eur, 1 kg (Scharlau, catalog number: PO02001000)
di-Sodium hydrogen phosphate dihydrate (Na2HPO4·2H2O), 1 kg (Scharlau, catalog number: SO03391000)
Potassium dihydrogen phosphate (KH2PO4), 1 kg (Merck, catalog number: 1048731000)
VECTASHIELD® Antifade Mounting Medium with DAPI, 10 ml (Vector Laboratories, catalog number: H-1200)
Nail polish
Sodium hydroxide (NaOH), 1 kg (Merck, catalog number: 1064691000)
Primary antibodies (Table 1)
Secondary antibodies (Table 2)
Complete medium for MDCK II cells (see Recipes)
PBS 10× (see Recipes)
PBS 1× (see Recipes)
Blocking buffer (see Recipes)
Washing buffer (see Recipes)
3% PFA (see Recipes)
5% saponin (see Recipes)
Table 1. Primary antibodies.
| Antibody name | Host | Company | Catalog number | |
|---|---|---|---|---|
| 1 | Vimentin monoclonal antibody (V9) | Mouse | eBioscience | 14-9897-82 |
| 2 | ZO-1 polyconal antibody | Rabbit | Proteintech | 21773-1-AP |
Table 2. Secondary antibodies.
| Species reactivity | Fluorescent dye | Company | Catalog number | |
|---|---|---|---|---|
| 1 | Anti-mouse | Alexa Fluor® 568 | Life Technologies | A11031 |
| 2 | Anti-rabbit | Alexa Fluor® 488 | Life Technologies | A11034 |
Equipment
Counting chamber, type Burker (Hirschmann, catalog number: 8100201)
Fume hood
Eppendorf® Research® plus pipette, 3-pack: 0.5-10 µl, 10-100 µl, 100-1,000 µl (Sigma-Aldrich, catalog number: Z683884)
Integra Pipetboy 2 (VWR, catalog number: 612-0927)
Heating and magnetic stirrer RH basic 2 (Dulova, catalog number: QSA337)
Centrifuge VWR Compact Star CS4
37 °C, 5% CO2 cell culture incubator
Standard phase contrast microscope
Confocal microscope Zeiss 710
Software
Procedure
-
Starting MDCK II cell culture
Prepare the complete medium for MDCK II cells (see Recipes).
Thaw a vial with frozen MDCK II cells.
Transfer the cells into a 15 ml Falcon tube containing the complete medium.
Centrifuge the cells for 5 min at 1,500 rpm (214 × g).
Discard the supernatant.
Add 10 ml of complete medium. Pipette up and down.
Transfer the cells into a cell culture dish.
Culture cells at 37 °C, 5% CO2 in cell culture incubator.
-
MDCK II cell passage
Pre-warm complete medium and trypsin.
Remove medium from the cells.
Wash with 10 ml of DPBS.
Add 2 ml of trypsin.
Incubate cells for 5 min at 37 °C.
-
Check under the microscope if cells are detached.
Note: If cells are not detached, continue incubation for 2-5 min at 37 °C.
Add 10 ml of complete medium.
Pipette up and down.
Transfer the cell suspension into a 15 ml tube.
Centrifuge the cells for 5 min at 1,500 rpm (214 × g).
Discard the supernatant.
Seed the cells in a new cell culture dish. Use split ratio of 1:5.
Culture cells at 37 °C.
-
Induction of EMT
Day 0
-
Seed 16,000 cells/well in an 8-well chamber slide.
Note: For the induction of EMT in MDCK II cells, the cells were passaged less than 10 times.
Incubate cells overnight.
Day 1
-
Prepare HGF stock 100 μg/ml and make aliquots:
Spin down the vial with lyophilized HGF.
Add 250 μl of sterile BSA 1 mg/ml (in PBS) to the vial with HGF.
Mix by pipetting.
Spin down.
Make aliquots. Use sterile 1.5 ml tubes.
Store the aliquots at -80 °C.
Dilute the HGF stock with complete medium to final concentration 20 ng/ml. Mix by vortexing.
Remove medium from the cells, that were seeded in the 8-well chamber slide.
Add 0.5 ml of DPBS per well.
Remove DPBS.
-
Add complete medium containing HGF, use 0.5 ml medium per well.
Note: For the control, treat cells with complete medium without HGF.
Incubate the cells for 24 h at 37 °C.
-
-
Immunofluorescent staining and imaging
Day 2
-
Thaw:
3% PFA (see Recipes)
Goat serum
5% saponin (see Recipes)
-
Prepare:
Washing Buffer (see Recipes)
Blocking Buffer (see Recipes)
Remove culture medium.
-
Fix the cultures in 3% PFA for 10-15 min. Use 0.5 ml of PFA/well.
Note: All immunostaining steps are performed at room temperature, except where otherwise provided.
Wash the cultures with PBS 1× (see Recipes). Use 0.5 ml of PBS 1×/well.
Add 0.5 ml of Blocking Buffer/well. Incubate for 1 h at room temperature.
-
Incubate the cultures with primary antibodies (Table 1), overnight, at 4 °C. Cover the cultures with parafilm.
Dilute anti-ZO-1 antibody at a ratio 1:200.
Dilute anti-vimentin antibody at a ratio 1:500.
Use Washing Buffer for dilution of antibodies.
Use minimum 200 μl antibody solution/well.
Day 3
Remove antibody solution.
Wash with PBS 1×. Use 0.5 ml of PBS 1×/well.
-
Incubate the cultures with fluorescent secondary antibodies (Table 2) for 1 h.
Dilute secondary antibodies at a ratio 1:500.
Use Washing Buffer for dilution of antibodies.
Use minimum 200 μl antibody solution/well.
Cover the cultures with aluminium foil to protect from the light.
Wash with PBS 1×. Use 0.5 ml of PBS 1x/well.
Discard PBS 1×.
Remove media chamber. Use removing device.
-
Mounting
Put few drops of mounting medium on a coverslip slide.
Put the coverslip slide on the top of the slide containing the cultures. Avoid bubbles.
Seal the mounted slide with nail polish.
Store the slides in a microscope slide folder at 4 °C. Protect from light.
Day 4
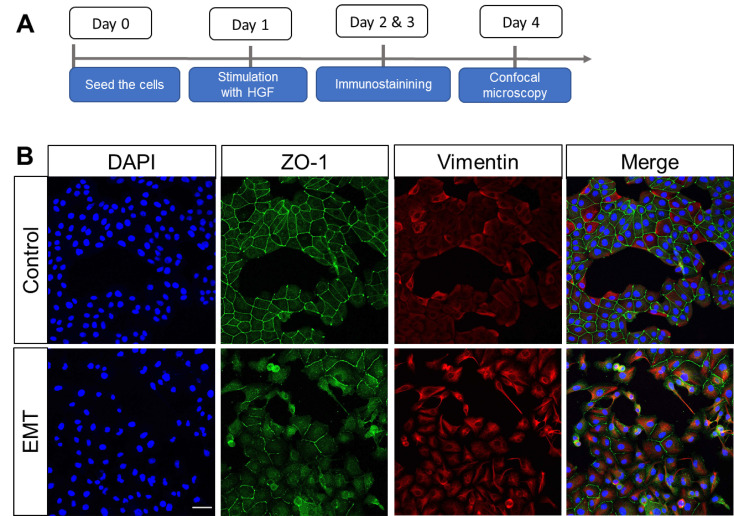
Take images of the stained cells with a confocal microscope (Figure 1).
-
Figure 1. Phenotypical characterization of EMT in MDCK II cells.
A. Experimental schedule: MDCK II cells were treated with hepatocyte growth factor (HGF) for 24 h, fixed and then stained for EMT markers. B. Immunostaining for vimentin and tight junction protein-1 (zonula occludens-1, ZO-1). DAPI was used for nuclear counterstaining. Objective 20×. Scale bar, 50 μm.
Data analysis
Confocal microscope images are analyzed with ImageJ software. In addition to phenotypical characterization by immunofluorescence, EMT-induced MDCK cells can be analyzed by western blotting, evaluation of cell roundness as well as measurement of the cell distance to the first and the second neighbor cell, as described by Farrell et al. (2014) .
Notes
For successful and reproducible results, we recommend:
To strictly follow the manufacturer’s guidelines on HGF storage and to avoid repeated HGF freeze-thaw cycles.
That MDCK II cells should be weekly observed under the microscope to monitor the stability of phenotype.
That MDCK II cultures should never be overgrown (avoid > 80% confluency).
That cell culture media should be exchanged regularly (avoid acidic medium, that can be identified by color change from pink into yellow, as it can induce EMT).
Recipes
-
Complete medium for MDCK II cells
450 ml Eagle's Minimum Essential Medium (EMEM)
50 ml fetal bovine serum (FBS)
-
PBS 10×
80 g NaCl
2.2 g KCl
14.2 g Na2HPO4·2H2O
2 g KH2PO4
Adjust final volume to 1 L with distilled water
-
PBS 1×
100 ml PBS 10×
900 ml distilled water
-
Blocking buffer
500 μl goat serum
100 μl 5% saponin
Adjust final volume to 10 ml with PBS 1×
Store short-term at 4 °C
-
Washing buffer
500 μl goat serum
500 μl 5% saponin
Adjust final volume to 50 ml with PBS 1×
Store short-term at 4 °C
-
3% PFA
1.5 g PFA
12.5 μl NaOH 6 M
Adjust final volume to 50 ml with PBS 1×
Combine all components in a glass bottle in a fume hood
Add magnetic stirring bar
Dissolve the PFA by stirring the solution on a magnetic stirrer at 56 °C in the fume hood
Store aliquots at -20 °C
Avoid freeze-thaw cycles
-
5% saponin
2.5 g saponin
Adjust final volume to 50 ml with distilled water
Make aliquots and store at -20 °C
Acknowledgments
The protocol was adapted from Farrell et al. (2014). This work was supported by the Swedish Cancer Society (Cancerfonden, CAN2017/735). We acknowledge the Biochemical Imaging Center at Umeå University. We thank Wai-Lok Yau for critical reading of the manuscript.
Competing interests
The authors declare no conflicts of interest.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Carter L. E., Cook D. P. and Vanderhyden B. C.(2019). Phenotypic plasticity and the origins and progression of ovarian cancer. In: The Ovary(Third Edition). Leung, P. C. K. and Adashi, E. Y.(Eds.). Academic Press: 529-545. [Google Scholar]
- 2. Dongre A. and Weinberg R. A.(2019). New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20(2): 69-84. [DOI] [PubMed] [Google Scholar]
- 3. Dukes J. D., Whitley P. and Chalmers A. D.(2011). The MDCK variety pack: choosing the right strain. BMC Cell Biol 12(1): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farrell J., Kelly C., Rauch J., Kida K., Garcia-Munoz A., Monsefi N., Turriziani B., Doherty C., Mehta J. P. and Matallanas D.(2014). HGF induces epithelial-to-mesenchymal transition by modulating the mammalian hippo/MST2 and ISG15 pathways. J Proteome Res 13(6): 2874-2886. [DOI] [PubMed] [Google Scholar]
- 5. Gonzalez D. M. and Medici D.(2014). Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 7(344): re8-re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalluri R. and Weinberg R. A.(2009). The basics of epithelial-mesenchymal transition. The J Clin Invest 119(6): 1420-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li C. W., Xia W., Huo L., Lim S. O., Wu Y., Hsu J. L., Chao C. H., Yamaguchi H., Yang N. K. and Ding Q.(2012). Epithelial–mesenchymal transition induced by TNF-α requires NF-κB–mediated transcriptional upregulation of Twist1. Cancer Res 72(5): 1290-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song F. N., Duan M., Liu L. Z., Wang Z. C., Shi J. Y., Yang L. X., Zhou J., Fan J., Gao Q. and Wang X. Y.(2014). RANKL promotes migration and invasion of hepatocellular carcinoma cells via NF-κB-mediated epithelial-mesenchymal transition. PLoS One 9(9): e108507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vidal-Quadras M., Holst M. R., Francis M. K., Larsson E., Hachimi M., Yau W.-L., Peränen J., Martín-Belmonte F. and Lundmark R.(2017). Endocytic turnover of Rab8 controls cell polarization. J Cell Sci 130(6): 1147-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yadav A., Kumar B., Datta J., Teknos T. N. and Kumar P.(2011). IL-6 Promotes Head and Neck Tumor Metastasis by Inducing Epithelial–Mesenchymal Transition via the JAK-STAT3-SNAIL Signaling Pathway. Mol Cancer Res 9(12): 1658-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yonemura S.(2014). Differential sensitivity of epithelial cells to extracellular matrix in polarity establishment. PLOS One 9(11): e112922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yuan S., Norgard R. J. and Stanger B. Z.(2019). Cellular plasticity in cancer. Cancer Discov. DOI: 10.1158/2159-8290.CD-19-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]