Abstract
Background:
Fetal echocardiography can accurately diagnose critical congenital heart disease prenatally, but relies on referrals from abnormalities identified on routine obstetrical ultrasounds. Critical congenital heart disease that is frequently missed due to inadequate outflow tract imaging includes anomalies such as truncus arteriosus, double outlet right ventricle, transposition of the great arteries, tetralogy of Fallot, pulmonary stenosis, and aortic stenosis.
Objective:
This study evaluated the prenatal detection rate of critical outflow tract anomalies in a single urban pediatric hospital before and after “AIUM Practice Guideline for the Performance of Obstetric Ultrasound Examinations”, which incorporated outflow tract imaging.
Design:
Infants with outflow tract anomalies who required cardiac catheterization and/or surgical procedure(s) in the first 3 months of life were retrospectively identified. This study evaluated two time periods; pre-guidelines from June 2010 - May 2013 and post-guidelines from January 2015 - June 2016. June 2013 - December 2014 was excluded as a theoretical period necessary for obstetrical practices to implement the revised guidelines.
Results:
Overall, prenatal diagnosis occurred in 55% of infants with critical outflow tract anomalies; of the 3 most common defects, prenatal diagnosis occurred in 53% of D-transposition of the great arteries, 63% of tetralogy of Fallot, and 80% of double outlet right ventricle patients. Pre-guidelines, prenatal diagnosis occurred in 52% (52 of 102) infants with critical outflow tract anomalies requiring early cardiac intervention. Post-guidelines, prenatal diagnosis occurred in 61% (33 of 54) infants, not significantly different than the prenatal detection rate pre-guidelines (p=0.31).
Conclusions:
Despite revised obstetrical guidelines highlighting the importance of outflow tract imaging, referrals and prenatal diagnosis of these types of critical congenital heart disease remain low. Education of obstetrical sonographers and practitioners who perform fetal anatomic screening is vital to increase referrals and prenatal detection of critical outflow tract anomalies.
Keywords: Fetal echocardiography, Fetal cardiology, Prenatal diagnosis
Introduction
Congenital heart disease (CHD) is the most common congenital anomaly, accounting for approximately 1% of live births.1 About 1 in 4 infants with CHD have critical CHD requiring timely intervention after birth to prevent significant morbidity or mortality.2 Prenatal diagnosis of critical CHD by fetal echocardiogram (ECHO) leads to appropriate fetal cardiac care, delivery, and postnatal management in a pediatric cardiac center.3 Most referrals for fetal ECHO and subsequent accurate prenatal diagnosis of CHD rely primarily on abnormality detection on screening obstetrical anatomic ultrasound, as many cases of CHD occur in otherwise low risk pregnancies.4,5
Until recently, obstetric anatomic ultrasound guidelines for cardiac evaluation mandated only a four-chamber view, with “views of the outflow tracts attempted as part of the cardiac screening.”6 Critical CHD that can be missed by imaging only the four-chamber view (Figure 1a) include outflow tract anomalies such as D-transposition of the great arteries (TGA) (Figure 1b and 1c), congenitally corrected-TGA (cc-TGA), tetralogy of Fallot (TOF), double outlet right ventricle (DORV), pulmonary atresia with intact ventricular septum (PA/IVS), truncus arteriosus, pulmonary stenosis, and aortic stenosis. Many of these defects require surgical or cardiac catheterization intervention within the first few months of life and usually prior to discharge after delivery. In this study, we defined critical CHD as a cardiac defect requiring cardiac catheterization and/or surgical procedure(s) in the first 3 months of life, with a focus on outflow tract anomalies. Studies have demonstrated the ability of fetal ECHO to predict the need for urgent postnatal intervention,7–9 as well as improved neonatal outcomes with prenatal diagnosis.10–12 With evidence supporting the value of ventricular outflow tract imaging13, in June 2013, the American Institute of Ultrasound in Medicine (in conjunction with the American College of Radiology, American College of Obstetricians and Gynecologists, and the Society of Radiologists in Ultrasound) updated its obstetrical ultrasound guidelines to include evaluation of the ventricular outflow tracts.14
Figure 1.
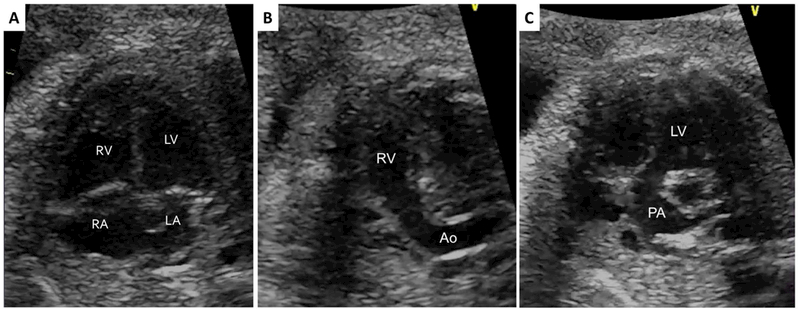
(a) Normal appearing cardiac four-chamber view with abnormal parallel (b) right ventricular and (c) left ventricular outflow tracts in a fetus with D-transposition of the great arteries. RV – right ventricle; LV – left ventricle; RA – right atrium; LA – left atrium; Ao – aorta; PA – pulmonary artery.
Objective
As the San Diego region’s only pediatric cardiac and cardiovascular surgery center, all neonates with critical CHD potentially requiring interventional management are cared for at Rady Children’s Hospital. The primary objective of this retrospective study was to evaluate the prenatal detection rate of critical outflow tract anomalies in neonates at Rady Children’s Hospital with critical outflow tract CHD before and after theoretical implementation of the 2013 updated obstetrical imaging guidelines. Ideally, the majority of neonates with critical outflow tract CHD should have been detected by prenatal ventricular outflow tract imaging on obstetrical ultrasound.
Methods
Study Design.
Infants with critical CHD requiring cardiac catheterization and/or surgical procedure(s) in the first 3 months of life were retrospectively identified through Rady Children’s Hospital cardiac and electronic medical record databases. Infants were subdivided into two time frames; the pre-guideline time frame consisted of infants requiring procedures between June 2010 and May 2013, while the post-guideline time frame consisted of infants requiring procedures between January 2015 and June 2016.
The timeframe between June 2013 and December 2014 was specifically excluded to allow for theoretical time necessary for obstetrical providers to implement the recommended guidelines into daily practice. Actual implementation of outflow tract imaging per the new guidelines was at the discretion of individual obstetrical practices; there is currently no universal training of all obstetrical sonographers given the large number of practices in San Diego county.
Infants with the following cardiac outflow tract anomalies identifiable by outflow tract imaging were included: D-TGA, cc-TGA, TOF, DORV, PA/IVS, truncus arteriosus, pulmonary stenosis, and aortic stenosis. Although cc-TGA should also be detectable by the four-chamber view, cc-TGA was included in this study, as it may be unrealistic to expect the general obstetrical screening sonographer the nuance of differentiating the morphologic right versus left ventricle. Those with other forms of CHD potentially diagnosable by the four-chamber view were excluded (i.e. atrioventricular canal defects or single ventricle lesions such as hypoplastic right or left heart syndrome). Isolated aortic coarctation without aortic valve stenosis was also excluded as it is not reliably expected to be diagnosed prenatally. Rady Children’s Hospital also serves as the primary referral center for infants with CHD from Hawaii and the Pacific Islands; out of state patients were excluded. The study was approved by the Institutional Review Boards of Rady Children’s Hospital and University of California, San Diego.
Statistical Analysis.
Interval variables were expressed as mean ± standard deviation or median (range). Categorical variables were counted and expressed as frequencies or percentages. Due to the non-normality of most of our continuous study outcomes, the Mann-Whitney U test was used as a robust rank-based statistic to determine if there was a significant difference between pre-guidelines and post-guidelines or prenatal and postnatal diagnosis groups. Due to some small expected cell counts, Fisher’s Exact test was used to compare categorical variables across groups. A p-value less than 0.05 was considered statistically significant. All analyses were performed using the latest version of R software (version 3.3.2).
Results
All but one of the mothers in each time frame had reportedly received standard prenatal care. There was no association between maternal race or type of health insurance and prenatal versus postnatal diagnosis (p=0.99 and 0.41, respectively) (Table 1).
Table 1.
Maternal Demographics, by Timing of Diagnosis.
| Prenatal Diagnosis n=86 (%) |
Postnatal Diagnosis n=70 (%) |
p-value | |
|---|---|---|---|
| Maternal Race | |||
| White | 34 (40%) | 28 (40%) | 0.99 |
| Hispanic | 32 (37%) | 26 (37%) | |
| Asian | 10 (12%) | 7 (10%) | |
| African-American | 3 (3%) | 3 (4%) | |
| American Indian | 0 | 1 (1%) | |
| Hawaiian | 1 (1%) | 0 | |
| Unknown/Not Reported | 6 (7%) | 5 (7%) | |
| Maternal Insurance Type | |||
| Private | 53 (62%) | 38 (54%) | 0.41 |
| Public | 33 (38%) | 32 (46%) | |
Overall, prenatal diagnosis occurred in 55% of infants with critical outflow tract anomalies. Prenatal diagnosis occurred at a mean age of 26.7 ± 4.8 weeks gestational age. Postnatal diagnosis of a critical outflow tract anomaly was mostly made on day of life 1 or 2 (range 1 – 71 days) (Table 2). The most common clinical findings leading to postnatal diagnosis of CHD were hypoxemia, murmur, or failed pulse oximetry screening. Seven percent of patients (11 of 153 newborns) were discharged prior to diagnosis; none of these patients had ductal dependent lesions. Of the 3 most common defects, prenatal diagnosis occurred in 54% of D-TGA, 63% of TOF, and 80% of DORV patients (Figure 2).
Table 2.
Patient Characteristics, by Timing of Diagnosis.
| Prenatal Diagnosis n=84 |
Postnatal Diagnosis n=65 |
p-value | |
|---|---|---|---|
| Timing of Diagnosis | Gestational Age 26.7 ± 4.8 weeks |
Day of Life 1 (1–71) |
n/a |
| Gestational Age at birth (weeks) | 37.7 ± 2.4 | 37.9 ± 3.4 | 0.03 |
| Birth Weight (kg) | 2.97 ± 0.69 | 3.18 ± 0.64 | 0.02 |
| Apgar score | |||
| 1 minute of life | 6.9 ± 2.1 | 7.2 ± 1.9 | 0.47 |
| 5 minutes of life | 7.9 ± 1.6 | 8.4 ± 0.9 | 0.01 |
| Genetic syndrome and/or Extracardiac anomaly | 28(33%) | 14 (20%) | 0.10 |
| Ductal Dependent | 63 (73%) | 35 (50%) | 0.004 |
| Highest pre-intervention lactate | 3.07 ± 2.05 | 4.30 ± 4.48 | 0.08 |
| Pre-intervention inotropic support | 21 (26%) | 17 (26%) | 0.99 |
| Timing of 1st Intervention (day of life) | 6 (0–88) | 5 (0–84) | 0.19 |
Continuous data is reported as mean ± SD or median (range), as appropriate, and categorical data is reported as count (percentage).
Figure 2.
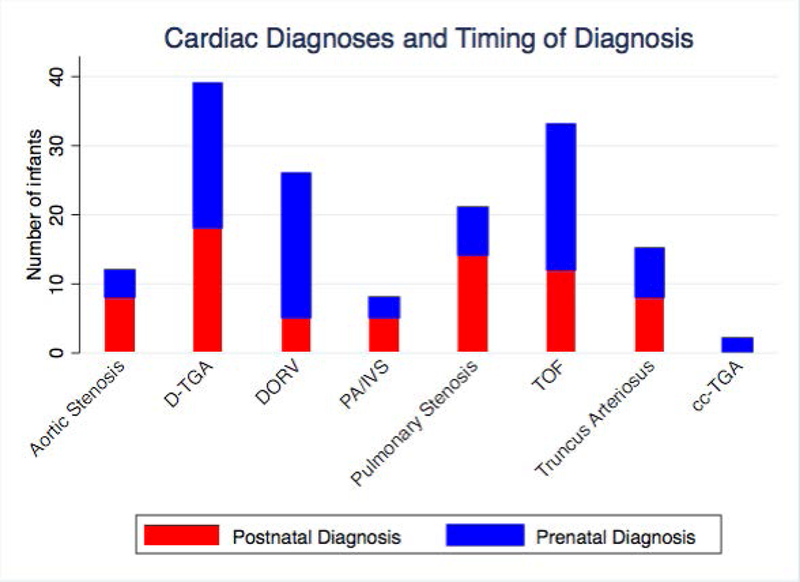
Overall Cardiac Diagnoses and Timing of Diagnosis.
Fifty percent of the infants required transfer from the delivery hospital to our institution; the other infants were born at our adjoining women’s hospital. Sixty-three percent had ductal dependent lesions for systemic or pulmonary blood flow. Most infants (86%) required intervention prior to hospital discharge. Three infants died without procedural intervention due to withdrawal of care. The first cardiac intervention occurred at a median age of 6 days (range 0 – 88 days), with 51 infants undergoing only cardiac catheterization, 69 infants undergoing only surgical palliation/repair, and 33 infants undergoing both catheterization followed by surgical palliation/repair within the first 3 months.
Prenatal versus Postnatal Diagnosis.
Prenatally diagnosed infants had a statistically significant slightly earlier gestational age at birth of 37.7 ± 2.4 weeks (versus 37.9 ± 3.4 weeks in postnatally diagnosed infants, p = 0.03). Prenatally diagnosed infants had statistically lower birth weights (2.97 ± 0.69 kg vs. 3.18 ± 0.64 kg, p=0.02) and 5 minute Apgar scores (7.9 ± 1.6 vs. 8.4 ± 0.9, p=0.01). There was a higher frequency of ductal dependent lesions in those prenatally diagnosed (73% versus 50% postnatally diagnosed, p=0.004). There was no significant difference in degree of pre-intervention lactic acidosis, need for pre-intervention inotropic support, or number of days to first intervention (Table 2).
Pre-Guidelines vs. Post-Guidelines.
Pre-guideline revision, between June 2010 and May 2013, 102 of 424 infants with critical CHD had outflow tract anomalies potentially identifiable by outflow tract imaging which required cardiac catheterization or surgery within the first 3 months of life. Prenatal diagnosis occurred in 52% of the pre-guidelines group. Post-guidelines, between January 2015 – June 2016, 54 of 176 infants with critical CHD had outflow tract anomalies which required cardiac catheterization or surgery within the first 3 months of life. Prenatal diagnosis occurred in 61% of the post-guidelines group, not significantly different than the prenatal detection rate pre-guidelines (p=0.31)(Table 3)(Figure 3). The types of cardiac outflow tract anomalies pre-guidelines versus post-guidelines were similar (Table 3)(Figure 4). The timing of diagnosis was also not significantly different based on type of cardiac defect pre-guidelines vs. post-guidelines (Table 4)(Figure 5).
Table 3.
Patient Characteristics, by Time Frame.
| Pre-Guidelines n=102 (%) |
Post-Guidelines n=54 (%) |
p-value | |
|---|---|---|---|
| Timing of Diagnosis | |||
| Prenatal Diagnosis | 53 (52%) | 33 (61%) | 0.31 |
| Postnatal Diagnosis | 49 (48%) | 21 (39%) | |
| Type of Cardiac Defect | |||
| D-TGA | 26 (26%) | 13 (24%) | 0.97 |
| TOF | 19 (18%) | 14 (26%) | |
| DORV | 17 (16%) | 9 (17%) | |
| Pulmonary Stenosis | 15 (15%) | 6 (11%) | |
| Truncus Arteriosus | 10 (10%) | 5 (9%) | |
| Aortic Stenosis | 8 (8%) | 4 (7%) | |
| PA/IVS | 6 (6%) | 2 (4%) | |
| CC-TGA | 1 (1%) | 1 (2%) | |
|
Prenatal Care (if Postnatal Diagnosis) |
|||
| Yes | 48 (98%) | 20 (95%) | 0.52 |
| No | 1 (2%) | 1 (5%) | |
| Gestational Age (weeks) | 38 ± 2.3 | 37.4 ± 3.8 | 0.31 |
| Birth Weight (kg) | 3.1 ± 0.7 | 3 ± 0.7 | 0.33 |
Figure 3.
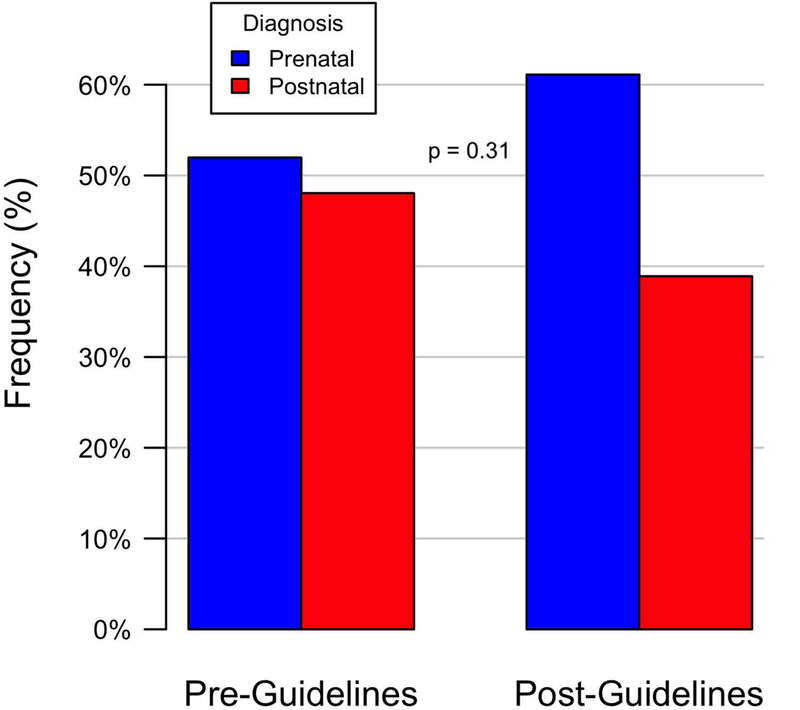
Timing of Diagnosis: Pre-Guidelines versus Post-Guidelines.
Figure 4.
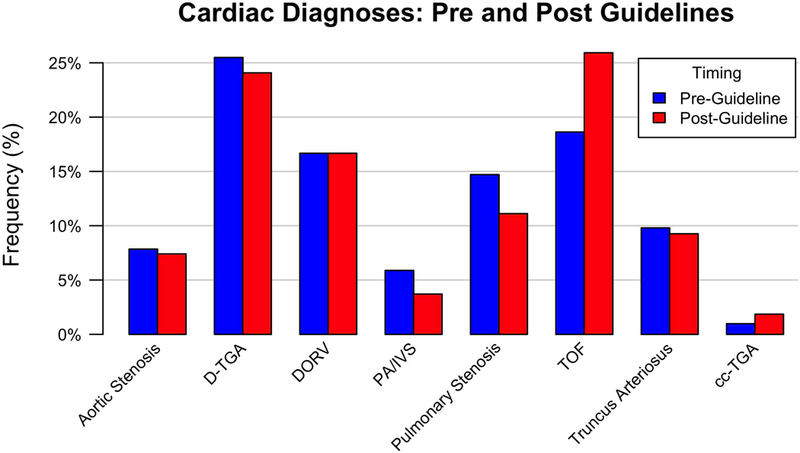
Cardiac Diagnoses: Pre-Guidelines and Post-Guidelines.
Table 4.
Prenatal Detection Rate of Cardiac Diagnoses, by Timeframe.
| Pre-Guideline % (# prenatal diagnosis / total) |
Post-Guideline % (# prenatal diagnosis / total) |
p-value | |
|---|---|---|---|
| D-TGA | 50% (13/26) | 61% (8/13) | 0.73 |
| TOF | 58% (11/19) | 71% (10/14) | 0.48 |
| DORV | 76% (13/17) | 88% (8/9) | 0.62 |
| Pulmonary Stenosis | 27% (4/15) | 50% (3/6) | 0.35 |
| Truncus Arteriosus | 50% (5/10) | 40% (2/5) | 0.99 |
| Aortic Stenosis | 50% (4/8) | 0% (0/4) | 0.21 |
| PA/IVS | 33% (2/6) | 50% (1/2) | 0.99 |
Figure 5.
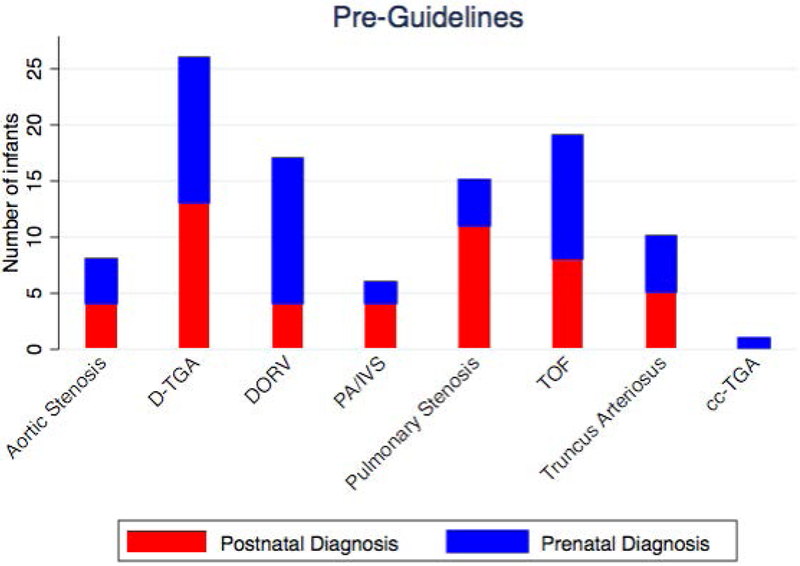
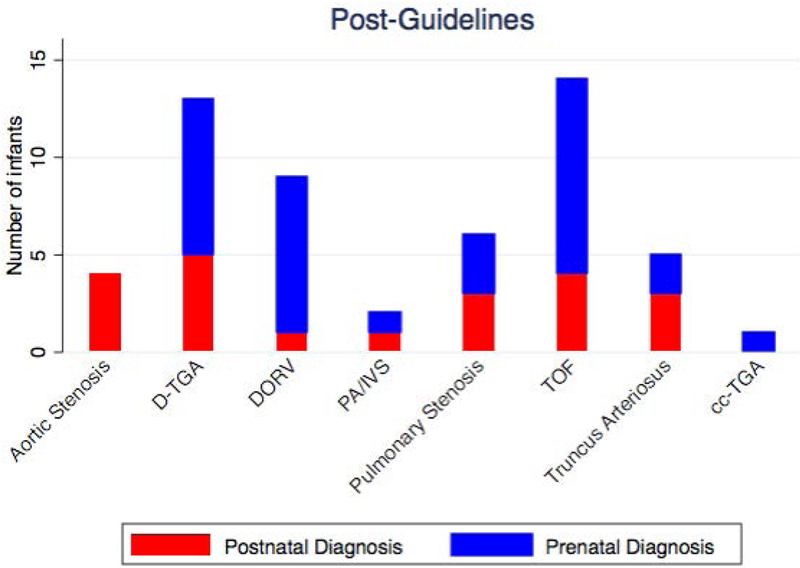
Timing of Diagnosis by Cardiac Diagnoses: (a) Pre-Guidelines and (b) Post-Guidelines.
Discussion
Screening for congenital anomalies should occur during routine anatomic obstetrical ultrasound examination at approximately 18–20 weeks gestational age in all pregnancies.14 The most common congenital anomaly is CHD, affecting approximately 1% of live infants.1 Of these, approximately 25% have critical CHD requiring timely intervention to prevent significant infant morbidity or mortality.2
Although a multitude of maternal and/or fetal factors can prompt referrals for fetal ECHO to evaluate for the presence (or absence) of prenatal CHD4, most CHD occurs in pregnancies without maternal or fetal risk factors.5 Referrals for fetal ECHO in these particular cases rely solely on the identification of cardiac abnormalities on screening obstetrical ultrasound. Studies have shown that less experienced obstetric sonographers have lower detection rates of cardiac abnormalities on screening ultrasound.15 In the mid-1990’s, a single major referral center in New South Wales showed that obstetrics screening detected only 6.7% of ventricular outflow tract anomalies prenatally.16 In 1992, a study at a single obstetrics center in the U.S. also demonstrated significant limitations to the four-chamber view, with only 63% of all CHD detected, while the detection rate increased to 83% when ventricular outflow tract imaging was included.13 Outflow tract imaging is practically feasible when incorporated into a standard screening protocol.17
Despite increased awareness and education, prenatal detection of various forms of CHD remains low,18,19 with outflow tract anomalies historically lower than for other types of critical CHD (such as single ventricle lesions).20 Contemporary studies have shown a wide range of prenatal diagnosis rates for conotruncal anomalies (Table 5).21–28 Many outflow tract anomalies can appear normal when visualizing only the four-chamber view (Figure 1a). The ability of obstetric anatomic ultrasound to identify outflow tract anomalies increases substantially when ventricular outflow tract imaging supplements the more traditional four-chamber view.18,19,29 In June 2013, the American Institute of Ultrasound in Medicine officially recognized the importance of imaging ventricular outflow tracts by updating its guidelines on obstetric anatomic ultrasound to include this critical outflow tract view into its basic cardiac examination.14 These guidelines (developed in conjunction with the American College of Radiology, American College of Obstetricians and Gynecologists, and the Society of Radiologists in Ultrasound) are the standard of care reference for obstetric practices in the United States. The International Society of Ultrasound in Obstetrics and Gynecology also published similar guidelines to include outflow tract views.30
Table 5.
Prenatal Diagnosis Rates of Conotruncal Anomalies From Recent Studies.
| Location | Author | Years | Number of patients | Prenatal diagnosis rate (%) | |
|---|---|---|---|---|---|
| D-TGA | Texas | Lara | 1999–2007 | 468 | 10 |
| Czech Republic | Marek | 2000–2006 | 223 | 25 | |
| Netherlands | van Velzen | 2002–2012 | 172 | 36 | |
| Paris | Khoshnood | 2005–2008 | 85 | 70 | |
| Belgium | De Groote | 2006–2014 | 79 | 24 | |
| TOF | Czech Republic | Marek | 2000–2006 | 142 | 37 |
| Netherlands | van Velzen | 2002–2012 | 111 | 30 | |
| Paris | Khoshnood | 2005–2008 | 60 | 68 | |
| Belgium | De Groote | 2006–2014 | 117 | 23 | |
| Truncus Arteriosus | Boston | Swanson | 1992–2007 | 136 | 32 |
| Czech Republic | Marek | 2000–2006 | 47 | 80 | |
| Netherlands | van Velzen | 2002–2012 | 46 | 62 | |
| PA/IVS | Italy | Tuo | 1993–2009 | 60 | 60 |
| Czech Republic | Marek | 2000–2006 | 88 | 68 | |
| cc-TGA | Toronto | Wan | 1999–2006 | 54 | 29 |
Fetal ECHO performed by fetal/pediatric cardiac sonographers with interpretation by fetal/pediatric cardiologists provide a more accurate prenatal cardiac diagnosis31,32 as compared to obstetrical ultrasound. Fetal ECHO accurately detects simple and complex CHD,3 such as outflow tract anomalies,33–35 and allows for directed prenatal parental counseling as well as delivery management in an appropriate setting.36 The most common outflow tract anomalies, such as TOF, d-TGA, DORV with pulmonary stenosis, or DORV with malposed great arteries, can cause significant cyanosis and hypoxia after birth leading to poor neonatal outcomes if not appropriately treated in a timely manner; the long-term survival rates are high if appropriately treated. Prenatal detection with timely post-delivery access to cardiac medical and surgical management improves mortality rates and outcomes.12,35,37,38
The overall prenatal detection rate of 55% in this study remains low in the modern era of specialty medicine capable of prenatally diagnosing and managing critical cardiac outflow tract anomalies. Despite the official revised obstetrical ultrasound guidelines, this study shows no significant difference in the prenatal diagnosis rate of these critical outflow tract anomalies following guideline revision. The limitation stems primarily from lack of obstetrical identification of abnormalities – presumably from inadequate imaging of the ventricular outflow tracts on routine anatomic screening ultrasounds. This study supports the need for more resources dedicated to educating obstetrical sonographers and practitioners in optimizing imaging of ventricular outflow tracts and/or encouraging referrals for fetal echocardiograms if unable to confidently confirm normal outflow tracts.
In this study, prenatally diagnosed infants had a statistically significant earlier gestational age at birth, although the majority were still born at or near term. We speculate that prenatally detected CHD placed these pregnancies into a higher risk category and mayhave prompted increased pregnancy and fetal monitoring and a lower threshold for induction or delivery. Prenatally diagnosed infants also had slightly lower birth weights and 5 minute Apgar scores, which may have been related to their earlier gestational age at birth. The majority of infants had ductal dependent lesions and/or required intervention prior to hospital discharge, indicating high risk for hemodynamic compromise if not diagnosed and treated in an expeditious manner.
No differences were seen in pre-operative lactic acidosis, need for inotropic support, or number of days to first procedure between the prenatal and postnatal diagnosis groups. The heterogeneous nature of cardiac diagnoses in the two groups limited the power to detect statistical differences and draw conclusions between pre-intervention course between the prenatal and postnatal diagnosis groups. Prenatal diagnosis of critical CHD has been shown to decrease pre-operative mortality in other studies.12
Limitations
Given the large number of hospitals delivering infants in San Diego county, infants with postnatal diagnosis of critical CHD that did not survive to admission to our hospital were not able to be identified, as it was outside the scope of our study. Theoretically, the addition of these infants would further decrease the prenatal detection rate in both timeframes.
This study did not aim to encompass all critical CHD, only those detectable by outflow tract imaging. Other critical CHD, such as single ventricle lesions and isolated aortic arch anomalies, were specifically excluded as the authors aimed to highlight the importance of obstetric screening ultrasounds incorporating adequate outflow tract imaging.
Conclusions
Despite revised obstetrical guidelines highlighting the importance of outflow tract imaging, referrals and prenatal diagnosis of these types of critical CHD remain suboptimal. Education of obstetrical sonographers and practitioners who perform fetal anatomic screening is vital to increase referrals for fetal cardiac evaluation and optimize prenatal detection of critical outflow tract anomalies.
Acknowledgements
The project was partially supported by the National Institutes of Health, Grant UL1TR001442 of CTSA funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: This study was partially funded by the National Institutes of Health, Grant UL1TR001442 of CTSA.
Footnotes
Disclosure Statement: The authors report no conflicts of interest.
Author Contributions
All authors have read and approved the final version of the manuscript.
Concept/Design, Data collection/interpretation, Drafting/revision of article: Sun
Statistics: Proudfoot
Critical revision of article: McCandless
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890–900. [DOI] [PubMed] [Google Scholar]
- 2.Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics 2013;131:e1502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkley EM, Goens MB, Karr S, Rappaport V. Utility of fetal echocardiography in postnatal management of infants with prenatally diagnosed congenital heart disease. Prenat Diagn 2009;29:654–8. [DOI] [PubMed] [Google Scholar]
- 4.Donofrio MT, Moon-Grady AJ, Hornberger LK et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 2014;129:2183–242. [DOI] [PubMed] [Google Scholar]
- 5.Stumpflen I, Stumpflen A, Wimmer M, Bernaschek G. Effect of detailed fetal echocardiography as part of routine prenatal ultrasonographic screening on detection of congenital heart disease. Lancet 1996;348:854–7. [DOI] [PubMed] [Google Scholar]
- 6.International Society of Ultrasound in O, Gynecology. Cardiac screening examination of the fetus: guidelines for performing the ‘basic’ and ‘extended basic’ cardiac scan. Ultrasound Obstet Gynecol 2006;27:107–13. [DOI] [PubMed] [Google Scholar]
- 7.Punn R, Silverman NH. Fetal predictors of urgent balloon atrial septostomy in neonates with complete transposition. J Am Soc Echocardiogr 2011;24:425–30. [DOI] [PubMed] [Google Scholar]
- 8.Maeno YV, Kamenir SA, Sinclair B, van der Velde ME, Smallhorn JF, Hornberger LK. Prenatal features of ductus arteriosus constriction and restrictive foramen ovale in d-transposition of the great arteries. Circulation 1999;99:1209–14. [DOI] [PubMed] [Google Scholar]
- 9.Arya B, Levasseur SM, Woldu K, Glickstein JS, Andrews HF, Williams IA. Fetal echocardiographic measurements and the need for neonatal surgical intervention in Tetralogy of Fallot. Pediatr Cardiol 2014;35:810–6. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet D, Coltri A, Butera G et al. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation 1999;99:916–8. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs IB, Muller H, Abdul-Khaliq H, Harder T, Dudenhausen JW, Henrich W. Immediate and long-term outcomes in children with prenatal diagnosis of selected isolated congenital heart defects. Ultrasound Obstet Gynecol 2007;29:38–43. [DOI] [PubMed] [Google Scholar]
- 12.Holland BJ, Myers JA, Woods CR, Jr. Prenatal diagnosis of critical congenital heart disease reduces risk of death from cardiovascular compromise prior to planned neonatal cardiac surgery: a meta-analysis. Ultrasound Obstet Gynecol 2015;45:631–8. [DOI] [PubMed] [Google Scholar]
- 13.Bromley B, Estroff JA, Sanders SP et al. Fetal echocardiography: accuracy and limitations in a population at high and low risk for heart defects. Am J Obstet Gynecol 1992;166:1473–81. [DOI] [PubMed] [Google Scholar]
- 14.American Institute of Ultrasound in M. AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med 2013;32:1083–101. [DOI] [PubMed] [Google Scholar]
- 15.Tegnander E, Eik-Nes SH. The examiner’s ultrasound experience has a significant impact on the detection rate of congenital heart defects at the second-trimester fetal examination. Ultrasound Obstet Gynecol 2006;28:8–14. [DOI] [PubMed] [Google Scholar]
- 16.Jaeggi ET, Sholler GF, Jones OD, Cooper SG. Comparative analysis of pattern, management and outcome of pre- versus postnatally diagnosed major congenital heart disease: a population-based study. Ultrasound Obstet Gynecol 2001;17:380–5. [DOI] [PubMed] [Google Scholar]
- 17.Vettraino IM, Lee W, Bronsteen RA, Comstock CH. Sonographic evaluation of the ventricular cardiac outflow tracts. J Ultrasound Med 2005;24:566. [DOI] [PubMed] [Google Scholar]
- 18.Levy DJ, Pretorius DH, Rothman A et al. Improved prenatal detection of congenital heart disease in an integrated health care system. Pediatr Cardiol 2013;34:670–9. [DOI] [PubMed] [Google Scholar]
- 19.Sklansky MS, Berman DP, Pruetz JD, Chang RK. Prenatal screening for major congenital heart disease: superiority of outflow tracts over the 4-chamber view. J Ultrasound Med 2009;28:889–99. [DOI] [PubMed] [Google Scholar]
- 20.Friedberg MK, Silverman NH, Moon-Grady AJ et al. Prenatal detection of congenital heart disease. J Pediatr 2009;155:26–31, 31 e1. [DOI] [PubMed] [Google Scholar]
- 21.Wan AW, Jevremovic A, Selamet Tierney ES et al. Comparison of impact of prenatal versus postnatal diagnosis of congenitally corrected transposition of the great arteries. Am J Cardiol 2009;104:1276–9. [DOI] [PubMed] [Google Scholar]
- 22.Swanson TM, Selamet Tierney ES, Tworetzky W, Pigula F, McElhinney DB. Truncus arteriosus: diagnostic accuracy, outcomes, and impact of prenatal diagnosis. Pediatr Cardiol 2009;30:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marek J, Tomek V, Skovranek J, Povysilova V, Samanek M. Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21-year experience. Heart 2011;97:124–30. [DOI] [PubMed] [Google Scholar]
- 24.Khoshnood B, Lelong N, Houyel L et al. Impact of prenatal diagnosis on survival of newborns with four congenital heart defects: a prospective, population-based cohort study in France (the EPICARD Study). BMJ Open 2017;7:e018285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Velzen CL, Clur SA, Rijlaarsdam ME et al. Prenatal detection of congenital heart disease--results of a national screening programme. BJOG 2016;123:400–7. [DOI] [PubMed] [Google Scholar]
- 26.De Groote K, Vanhie E, Roets E et al. Outcome after prenatal and postnatal diagnosis of complex congenital heart defects and the influence of genetic anomalies. Prenat Diagn 2017;37:983–991. [DOI] [PubMed] [Google Scholar]
- 27.Lara DA, Fixler DE, Ethen MK, Canfield MA, Nembhard WN, Morris SA. Prenatal diagnosis, hospital characteristics, and mortality in transposition of the great arteries. Birth Defects Res A Clin Mol Teratol 2016;106:739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuo G, Volpe P, Bondanza S et al. Impact of prenatal diagnosis on outcome of pulmonary atresia and intact ventricular septum. J Matern Fetal Neonatal Med 2012;25:669–74. [DOI] [PubMed] [Google Scholar]
- 29.Zhang YF, Zeng XL, Zhao EF, Lu HW. Diagnostic Value of Fetal Echocardiography for Congenital Heart Disease: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015;94:e1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho JS, Allan LD, Chaoui R et al. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol 2013;41:348–59. [DOI] [PubMed] [Google Scholar]
- 31.Meyer-Wittkopf M, Cooper S, Sholler G. Correlation between fetal cardiac diagnosis by obstetric and pediatric cardiologist sonographers and comparison with postnatal findings. Ultrasound Obstet Gynecol 2001;17:392–7. [DOI] [PubMed] [Google Scholar]
- 32.Lai CW, Chau AK, Lee CP. Comparing the accuracy of obstetric sonography and fetal echocardiography during pediatric cardiology consultation in the prenatal diagnosis of congenital heart disease. J Obstet Gynaecol Res 2016;42:166–71. [DOI] [PubMed] [Google Scholar]
- 33.Gelehrter S, Owens ST, Russell MW, van der Velde ME, Gomez-Fifer C. Accuracy of the fetal echocardiogram in double-outlet right ventricle. Congenit Heart Dis 2007;2:32–7. [DOI] [PubMed] [Google Scholar]
- 34.Galindo A, Mendoza A, Arbues J, Graneras A, Escribano D, Nieto O. Conotruncal anomalies in fetal life: accuracy of diagnosis, associated defects and outcome. Eur J Obstet Gynecol Reprod Biol 2009;146:55–60. [DOI] [PubMed] [Google Scholar]
- 35.Dominguez-Manzano P, Mendoza A, Herraiz I et al. Transposition of the Great Arteries in Fetal Life: Accuracy of Diagnosis and Short-Term Outcome. Fetal Diagn Ther 2016;40:268–276. [DOI] [PubMed] [Google Scholar]
- 36.Donofrio MT, Levy RJ, Schuette JJ et al. Specialized delivery room planning for fetuses with critical congenital heart disease. Am J Cardiol 2013;111:737–47. [DOI] [PubMed] [Google Scholar]
- 37.Dominguez-Manzano P, Herraiz I, Mendoza A et al. Impact of prenatal diagnosis of transposition of the great arteries on postnatal outcome. J Matern Fetal Neonatal Med 2017;30:2858–2863. [DOI] [PubMed] [Google Scholar]
- 38.. Pruetz JD, Carroll C, Trento LU et al. Outcomes of critical congenital heart disease requiring emergent neonatal cardiac intervention. Prenat Diagn 2014;34:1127–32. [DOI] [PubMed] [Google Scholar]


