Abstract
Study Objectives
Estimate the genetic relationship of cannabis use with sleep deficits and an eveningness chronotype.
Methods
We used linkage disequilibrium score regression (LDSC) to analyze genetic correlations between sleep deficits and cannabis use behaviors. Secondly, we generated sleep deficit polygenic risk score (PRS) and estimated their ability to predict cannabis use behaviors using linear and logistic regression. Summary statistics came from existing genome-wide association studies of European ancestry that were focused on sleep duration, insomnia, chronotype, lifetime cannabis use, and cannabis use disorder (CUD). A target sample for PRS prediction consisted of high-risk participants and participants from twin/family community-based studies (European ancestry; n = 760, male = 64%; mean age = 26.78 years). Target data consisted of self-reported sleep (sleep duration, feeling tired, and taking naps) and cannabis use behaviors (lifetime ever use, number of lifetime uses, past 180-day use, age of first use, and lifetime CUD symptoms).
Results
Significant genetic correlation between lifetime cannabis use and an eveningness chronotype (rG = 0.24, p < 0.001), as well as between CUD and both short sleep duration (<7 h; rG = 0.23, p = 0.017) and insomnia (rG = 0.20, p = 0.020). Insomnia PRS predicted earlier age of first cannabis use (OR = 0.92, p = 0.036) and increased lifetime CUD symptom count (OR = 1.09, p = 0.012).
Conclusion
Cannabis use is genetically associated with both sleep deficits and an eveningness chronotype, suggesting that there are genes that predispose individuals to both cannabis use and sleep deficits.
Keywords: cannabis, cannabis use disorder, sleep duration, insomnia, chronotype, genetics
Statement of Significance.
This study provides the first genomic-based evidence of a genetic relationship between both sleep deficits and an eveningness chronotype with cannabis use behaviors. These results complement prior twin studies and suggest that the relationship between these phenotypes can be partially explained by a common genetic liability, implying that the genetic influences on sleep deficits also have an influence on cannabis use. Furthermore, this is the first study to find a positive genetic relationship between an eveningness chronotype and cannabis use.
Introduction
Cannabis is one of the most widely used psychoactive substances in the world [1] and has a well-documented but unclear relationship with sleep. Cannabis contains cannabinoids, which are the major contributors to psychoactive and medicinal effects [2]. The brain contains cannabinoid receptors, which are affected by exogenous cannabinoids and endogenous cannabinoids (i.e. produced within the brain). Together, these receptors and endogenous cannabinoids comprise the endocannabinoid system. The two most prominent exogenous cannabinoids are tetrahydrocannabinol (THC), the primary psychoactive cannabinoid, and cannabidiol (CBD), which appears to have additional sedating and anxiolytic properties [3]. Evidence suggests that an interplay between THC and CBD may underlie a nuanced relationship with sleep. For example, acute/low-dose THC and high-dose CBD may aide sleep, but low-dose CBD and long-term/high-dose THC may interfere with sleep [4]. While cannabis is often associated with being a sleep aid [5–10], repeated cannabis use may lead to tolerance of its sleep-aid properties and consistent use is linked to negative sleep outcomes via habituation [4].
Increased frequency of cannabis use is associated with an assortment of sleep problems including prolonged latency to sleep onset [11], lower sleep duration [11–16], sleep disturbances [17], sleep quality problems [17–20], later bed times [15], and insomnia-related outcomes [12, 17, 19, 21–23]. These adverse effects might be specific to daily or chronic users, as a recent study found that daily cannabis users endorsed worse sleep quality and increased insomnia symptoms compared with both nonusers and non-daily users, but also found that nonusers and non-daily users demonstrated similar sleep scores [17]. Thus, irregular users might not experience the adverse sleep effects experienced by heavy users. Additionally, sleep disturbances are a primary withdrawal symptom of cannabis use disorders (CUD) and are often a leading risk factor for relapse, suggesting that those with ongoing CUD might suffer from continuous sleep issues stemming from discontinued use or attempts to abstain [4, 24]. Lastly, an eveningness chronotype (a diurnal preference for a sleep–wake pattern of activity and alertness in the evening which is linked to insomnia [25] and sleep complaints [26, 27]) is associated with increased cannabis frequency [28–30] and cannabis addiction [31].
In addition to cross-sectional association, there is evidence of early cannabis exposure and use predicting later sleep outcomes. The fetal brain is densely inhabited with CB1 receptors that spread during gestation [32]. CB1 receptors are thought to be involved in the regulation of sleep processes since they are found in numerous regions of the brain associated with the sleep–wake cycle [33]. THC binds to CB1 receptors, and animal research implies this possibly modifies fetal cortical circuitry in the womb [34]. Several studies have found associations of prenatal cannabis exposure with early sleep factors, such as differences in quiet time, irregular sleep, and sleep-related body movements a few days after birth [35], less efficient sleep and less total sleep time at three years of age [36], and increased endorsement of sleep disorder symptoms at age 9–10 years [37].
The endocannabinoid system also plays a critical role in the development of the adolescent brain [38] and because the brain is changing and developing well throughout early adulthood [39], could be susceptible to the effects of cannabis for a large part of the lifespan. A handful of studies have found that cannabis initiation and early use predict later sleep problems such as tiredness, trouble sleeping [22], short sleep duration [16, 40], and insomnia-related outcomes [23]. Evidence exists for the reverse relationship as well, with premorbid insomnia [21] and generalized sleep problems [40–42] predicting later cannabis use. This effect appears strong in early development, such that early childhood sleep deficits predict cannabis use in later adolescence [22, 43–45] and sleep factors during adolescence predict adult cannabis use [21, 46]. Lastly, endorsements of an adolescent eveningness chronotype are associated with follow-up reports of increased cannabis use controlling for baseline adolescent substance use [41]. With evidence of both cross-sectional associations and a bidirectional relationship between cannabis and sleep deficits/eveningness chronotype, there could be an underlying common liability such as shared or common genetics responsible for this association.
The concept of common genetic liability is that the same genetic influences can act on distinct or separately measured phenotypes. This can be referred to as “shared genetics” or, more formally, genetic pleiotropy. That is, if phenotypes are genetically correlated, the relationship between those phenotypes can be partially explained by a common genetic liability (pleiotropy and shared genetics), implying that the genetic influences on one phenotype also have an influence on another phenotype. There is increasing evidence of a genetic relationship between cannabis use and sleep deficits, which may be biologically centered on the endocannabinoid system’s involvement in the circadian sleep–wake cycle [47–49]. Additionally, disruption of circadian genes might disturb the reward processing system, which can influence substance use [50, 51]. While research has shown evidence of common genetics between both alcohol and tobacco use and disorders with sleep outcomes using both twin studies and genomic methods (e.g. genetic correlations) [52–57], studies specifically focused on the genetic relationship between cannabis and sleep components remain scarce. Two twin studies have found evidence of shared genetics between cannabis use and sleep outcomes, specifically lower adult sleep duration [16] and adult insomnia outcomes [23]. Additionally, several clock gene polymorphisms have been linked to risk for cannabis addiction [58].
Consistent with this literature, there is converging evidence that supports a shared genetic liability hypothesis. Recent large genome-wide association studies (GWAS) on sleep-related and chronotype variables [57, 59–62] have found genes and genetic pathways linked with both cannabis use or cannabinoid activity [63–69]. Likewise, several GWAS of lifetime cannabis use and CUD disorder [66, 67, 70, 71] have found genetic associations that are believed to be involved in circadian rhythm and sleep behaviors [72–75]. These studies imply genetic pleiotropy between cannabis use and sleep deficits, but research is needed to analyze the specific role of the potentially shared genetics in this relationship, especially using modern genomic methods.
As mentioned, modern GWAS have been used to identify independent genome-wide significant loci associated with various sleep traits and to estimate genome-wide single nucleotide polymorphism (SNP) heritability for several sleep-related traits such as chronotype (eveningness–morningness; 14%), sleep duration (10%), and insomnia (17%) [57, 59, 76] as well as for cannabis behaviors such as lifetime cannabis use (11%) [67] and CUD (4%) [71]. These results suggest that the combined effects of common SNPs capture a considerable proportion of the heritability of various sleep behaviors and cannabis use behaviors. A polygenic risk score (PRS) is an individual measure of genetic propensity to a trait of risk that is generated by multiplying the number of risk alleles that an individual possesses at a particular SNP by the effect size from a discovery GWAS for that same SNP. By applying summary statistics from a large GWAS to a smaller genotyped target sample, PRS can be generated to estimate if genetic risk for a trait is associated with another trait, implying shared genetics between traits. Additionally, summary statistics from GWAS can be used to analyze genetic correlations by using a technique called linkage disequilibrium score regression (LDSC) which estimates if the direction of the effect of SNPs is correlated between traits. Thus, LDSC analyzes if the direction of effect between the SNPs of two traits are correlated based on the whole genome, while PRS uses the genetic scores assigned to individuals (derived from a GWAS) to determine if the genetic risk attributed to the PRS can predict behaviors observed in a target sample and can readily control for covariates.
In this study, we first conducted genetic correlation analyses using the summary statistics of several large cannabis (lifetime cannabis use and CUD) and sleep (sleep duration, chronotype, and insomnia) GWAS to identify any significant genetic correlations between the sleep deficits and cannabis use behaviors. Secondly, we generated PRS based on summary statistics of various large sleep-related GWAS (chronotype, sleep duration, and insomnia) and analyzed the ability of sleep trait PRS to predict cannabis measures in a target sample consisting of both high-risk participants and participants from twin/family community-based studies.
Methods
Participants
Our target data consisted of subjects who participated in a third wave of data collection from either the Center on Antisocial Drug Dependence (CADD) in Boulder, CO (PI: Hewitt) or the Genetics of Antisocial Drug Dependence (GADD) cohort from Denver, CO and San Diego, CA (PI: Hopfer). To generate accurate and bias-free PRS, the ideal analysis requires that the ancestry of the target data reflect the original GWAS data that the PRS was derived from [77]. With this in mind, we only included subjects in our target sample of European ancestry (n = 868; identified by principal component analysis with the 1,000 Genome phase III European subsample as a reference [78]) in order to match the ethnicity of the discovery GWAS and avoid biased polygenic score estimates [79]. Among those in the sample with a family member (206 total subjects were nested within a family), we kept one member of each family at random to make our final sample 760 subjects. Removing subjects from the same family allowed us to avoid the potential convergent complications of using mixed-effects models as well to avoid the role of the shared environment amongst family members. Of our remaining sample, we had 622 subjects from the CADD and 138 from the GADD. Subjects from the CADD included data from the Colorado Adoption Project (n = 5; [80]), Longitudinal Twin Study (n = 143; [81]), Community Twin Sample (n = 315; [81]), and the Adolescent Substance Abuse Project (n = 115; [82]). In addition to the San Diego and Denver subsamples of the GADD, 44 subjects in this consortium were part of a separate study (PI: Hopfer, DA035804) that were integrated into the third wave of the GADD. The final sample was 64% male (n = 491) with an average age of 26.78 years (SD = 3.09, range = 19–37).
Measures
Cannabis measures in the target sample
Cannabis measures in our target sample were self-reported via a supplement to the Composite International Diagnostic Interview Substance Abuse Module (CIDI-SAM) [83]. Any/lifetime cannabis use was measure with a screening question regarding lifetime cannabis use (“Have you ever used cannabis?”). Subjects who endorsed any lifetime cannabis use (n = 593; no lifetime cannabis use as the reference group) were asked a series of cannabis-related questions, including age of first use (“How old were you the first time you used cannabis?”; mean = 15.28 years, SD = 3.10), and number of lifetimes uses (“How many times in your life have you used cannabis?”). Responses for number of lifetime cannabis uses included: “1–2 times” (n = 36), “3–5 times” (n = 57), “6–9 times” (n = 27), “10–19 times” (n = 41), “20–39 times” (n = 39), and “40 or more times” (n = 393), and were coded as 1–5. Anyone who denied any lifetime cannabis use was assigned a 0 for the number of lifetime cannabis use (n = 167). Previous 180-day cannabis use was measured by asking, “How many days have you used marijuana in the past six months (180 Days)?” (mean = 32.60, SD = 63.31). Past 180-day cannabis use was categorized as 0 days (n = 487), 1–100 days (n = 154), and more than 100 days (n = 119).
We included several measures of DSM-IV CUD [84] taken from the CIDI-SAM to generate a measure that consisted of the sum of the number of both lifetime cannabis dependence and abuse symptoms endorsed (mean = 1.47, SD = 2.47, range = 0–11), with the goal of generating a variable conceptually similar to the unidimensional symptom count of CUD in DSM-5 [85]. Research has determined that the cannabis abuse and dependence criteria of DSM-IV might not distinguish between two separate disorders or constructs [86] and that a unidimensional symptom count classifies CUD more appropriately [87]. Studies with DSM-IV cannabis dependence and abuse symptom data have utilized this summation technique to make a symptom count measure that is comparable to CUD reflecting a single disorder [87, 88].
Sleep measures in target data
Sleep measures in our target sample were assessed using the Jessor Health Questionnaire [89]. Sleep duration was assessed using two questions which asked, “How many hours of sleep do you typically get on a weekend?” and “How many hours of sleep do you typically get on a weekday?” with responses being “5 h or less,” “6 h,” “7 h,” “8 h,” “9 h,” “10 h,” or “11 h or more.” Our measures of short sleep duration were coded to match the GWAS we generated our PRS from [57]. Short sleep duration <7 h was coded as a 1 (n = 225 and n = 158) and the reference group of 7–8 h sleep duration was coded as 0 (n = 471 and n = 395) for both weekday and weekend sleep, respectively. Those who reported 9 or more hours were assigned an NA to match the coding of the sleep duration GWAS. Subjects also were asked: “How often do you feel tired or sleepy when you get up in the morning?,” “How often do you feel tired or low on energy in day?,” and “How often do you take a nap during the daytime?” with possible responses for these three questions being “almost never,” “once a week or so,” “2 or 3 times a week,” “nearly every day,” and “would rather not answer”; coded as 1–4 and NA.
Generating PRS
After removing duplicate SNPs, quality control performed in PLINK [90] included pruning variants based on missingness (>5%), minor allele frequency (<1%), and Hardy–Weinberg equilibrium (p-value < 0.001) followed by pruning variants based on linkage disequilibrium (wherever r2 exceeds 0.20 within a 50 kb window). There were 1,089,148 SNPs available for generating PRS after applying QC. Effect sizes (reported as log odds ratios) from the discovery GWAS of dichotomously measured sleep traits [57, 59, 76] were multiplied by the number of affected alleles at each individual SNP in our target sample to generate a unique PRS for each participant. Each participant in our data was assigned a PRS for each sleep trait by applying the GWAS effect size to their genomic data equating to a summation of log odds ratios weighted genotypes per individual [91]. That is, each participant in our data was assigned a PRS for each sleep trait, by applying GWAS effect sizes to their genomic data. Each PRS comprised of all SNPs that passed quality control steps (all p < 1) and thus explained the highest variance in the sleep phenotypes in our target sample. This is consistent with work suggesting that complex traits display a high amount of polygenicity and that using only genome-wide significant SNPs may exclude many SNPs with small but meaningful additive effects [92]; thus including all possible SNPs captures the highest amount of variance possible for a given trait [93, 94].
Summary statistics from sleep GWAS for PRS analysis and genetic correlations via LDSC
Sleep trait PRS effect sizes were generated from the summary statistics of several large sleep-related GWAS (all performed by the same research team) that utilized data of European ancestry from the UK biobank [57, 59, 76]. The sleep-related traits included self-report chronotype [59], short sleep duration (<7 h) [57], and self-reported insomnia symptoms [76]. The original chronotype GWAS utilized morningness, which was a binary measure of being a morning person or not (if participants endorsed any morningness measures as opposed to any eveningness measure) with morning people coded as 1 and evening people coded as 0. For the purpose of this study and the emphasis on sleep deficits, we reverse coded this measure and interpreted the results as eveningness chronotype (127,622 cases and 120,478 controls). Short sleep was defined as <7 h relative to 7–8 h sleep duration (106,192 cases and 305,742 controls). Severe insomnia (as defined by the GWAS) was classified using a self-report question: “Do you have trouble falling asleep at night or do you wake up in the middle of the night?,” with participants being dichotomized into controls (“never/rarely,” n = 108,357) and frequent insomnia symptoms (“usually,” n = 129,270) and with those reporting “sometimes” excluded. These same summary statistics were used for the genetic correlation analysis.
Summary statistics from cannabis GWAS for genetic correlations via LDSC
Summary statistics from several large scale GWAS of cannabis use behaviors were used for LDSC. Summary statistics for lifetime cannabis use were generated from self-report measures of whether a participant had ever used cannabis during their lifetime from a sample comprised of both the UK biobank and the International Cannabis Consortium (n = 162,082 [67]). Summary statistics for CUD were derived via the International Statistical Classification of Diseases and Related Health Problems, 10th revision diagnosis of CUD [95] reflecting a problematic and persistent use of cannabis and were derived from the deCODE cohort based in Iceland (5,501 cases and 301,041 controls) [71].
Covariates
We included known correlates of cannabis use and sleep including age [96, 97], sex [98–100], depression [101–103], and current alcohol and tobacco use [104–107] as covariates in all regression models. Current alcohol and tobacco use were measured using the number of days that tobacco (mean = 33.89, SD = 42.23) and alcohol (mean = 59.61, SD = 80.32) were used in the past 180 days. Past 180-day tobacco use was categorized as 0 days (n = 126), 1–10 days (n = 187), 11–40 days (n = 233), and more than 40 days (n = 214). Past 180-day alcohol use was categorized as 0 days (n = 368), 1–100 days (n = 158), and more than 100 days (n = 234). Depression symptoms were assessed using the Center for Epidemiological Studies-Depression (CES-D) scale [108] with the caveat of the sleep disturbance question being removed due to its direct overlap with our sleep PRS measure (mean = 27.76, SD = 9.29). All regression models also included the first 10 ancestral principal components (PCs 1–10) generated in PLINK as covariates to account for population stratification as is common in genetic and PRS analysis [109, 110].
Statistical analysis
Genetic correlations via LDSC
We used LDSC [111] to calculate genetic correlations between traits. Summary statistics were filtered by INFO > 0.90 and MAF > 0.01. Strand ambiguous SNPs, SNPs with duplicated rs numbers, multi-allelic variants, and insertion/deletions were all removed. SNPs with low Ns (as determined by the LDSC program) were also removed when sample sizes were available. Alleles were merged with the Hap Map 3 [112] reference panel, with the major histone complex removed. The LD scores and beta weights used were pre-computed from 1,000 Genomes European GWAS data included in the LDSC download.
LDSC is a computationally efficient method that regresses chi-square statistics from GWAS on LD scores of the trait of interest [111]. LD scores per SNP are the sum of the variance explained by LD with other SNPs [113]. Genetic correlations were calculated using overlapping SNPs from filtered summary statistic files. Genetic correlations also account for population stratification and are not confounded by overlapping samples.
Polygenic risk regression analysis
All regression analyses were conducted in R version 3.5.1 [114]. Linear and logistic regression models were used to test the association between sleep trait PRS and cannabis use behaviors including the previously mentioned covariates of age, sex, depression symptoms, past 180-day alcohol and tobacco use, and ancestral principal components (PCs 1–10). In terms of the steps of our analyses, we first used LDSC to estimate potential genetic correlations between sleep and cannabis GWAS summary statistics. Second, we ran phenotypic regression models between our sleep measures and cannabis behaviors in our target data to establish associations of sleep deficits and cannabis behaviors amongst our target sample. Third, we ran regression models between our sleep PRS and sleep traits in our discovery sample to confirm that the sleep PRS predicted sleep constructs in our target data. Lastly, we ran regression models to see how sleep PRS predict cannabis behaviors. For all series of regressions models involving PRS, we ran two sets of models: (1) with just age, sex, and PCs 1–10 as covariates and (2) with the prior covariates and the addition of current depression symptoms and past 180-day alcohol and tobacco use. We utilized this two-model approach to (1) look at the associations between sleep PRS and cannabis factors controlling for both basic covariates and more complex covariates and (2) determine if the effects seen in our final models (including all covariates) were driven by the more maladaptive covariates (depression and past 180-day substance use).
Results
Genetic correlations using LDSC
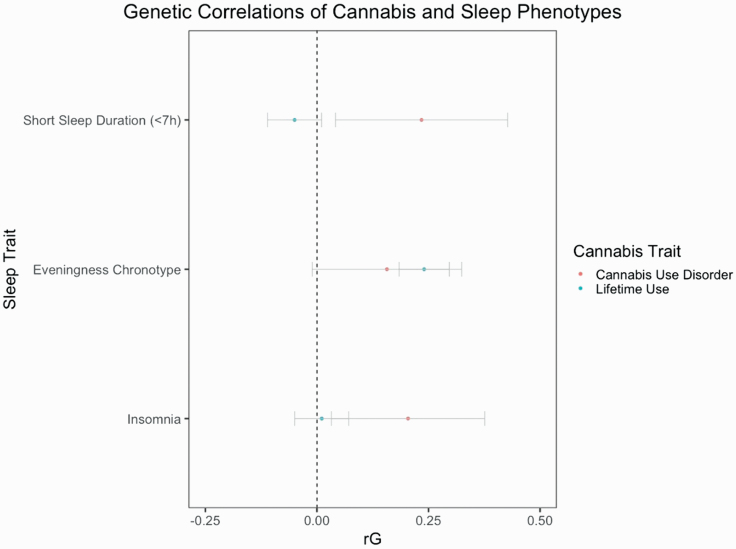
We first used LDSC to look at potential genetic correlations between cannabis and sleep traits using the largest GWAS to date for each trait. Table 1 displays the LDSC analysis between sleep traits and cannabis use traits using large scale GWAS. We found significant positive genetic correlations between any lifetime cannabis use and an eveningness chronotype (rG = 0.24; 95% CI = 0.18,0.30; p = < 0.001). We also found significant genetic correlations between CUD and both short sleep duration (rG = 0.23; 95% CI = 0.03,0.43; p = 0.017) and insomnia (rG = 0.20; 95% CI = 0.02,0.38; p = 0.020). Figure 1 displays the genetic correlations between cannabis and sleep phenotypes using LDSC (error bars are 95% confidence intervals).
Table 1.
Genetic correlations and 95% confidence intervals between sleep and cannabis phenotypes using large scale GWAS
| Lifetime cannabis use (No lifetime cannabis use as the reference group) |
CUD (No CUD as the reference group) |
|
|---|---|---|
| Short sleep duration (<7 h)(7–8 h sleep duration as the reference group) | −0.05 [−0.11,0.01] | 0.23* [0.03,0.43] |
| Eveningness chronotype(Morningness chronotype as the reference group) | 0.24* [0.18,0.30] | 0.16 # [−0.02,0.34] |
| Insomnia (never/rare insomnia symptoms as the reference group) | 0.01 [−0.05,0.07] | 0.20* [0.02,0.38] |
Genetic correlations between cannabis and sleep phenotypes were calculated using LD score regression.
Bold values represent *p < 0.05.
#Italic values represent p = 0.06.
Figure 1.
Genetic correlations between cannabis and sleep phenotypes. Genetic correlations were calculated with LDSC. Error bars are 95% confidence intervals.
Sleep traits predicting cannabis in target data
Table 2 displays regression outputs of our target data sleep traits predicting cannabis use behaviors controlling for sex, age, depression, and past 180-day alcohol and tobacco use. Short sleep duration on the weekday significantly predicted earlier age of first cannabis use (β = −0.06; 95% CI = −0.10,−0.02; p = 0.004). Short sleep duration on the weekend significantly predicted lifetime cannabis use (β = 0.70; 95% CI = 0.12,1.32; p = 0.022), increased number of lifetime cannabis uses (β = 0.24; 95% CI = 0.08,0.40; p = 0.001), and earlier age of first cannabis use (β = −0.06; 95% CI = −0.11,−0.02; p = 0.006). How often one felt tired or low on energy during the day significantly predicted early age of first cannabis use (β = −0.14; 95% CI = −0.22,−0.05; p = 0.001) and increased lifetime CUD symptom count (β = 0.11; 95% CI = 0.02,0.19; p = 0.010). How often one takes a nap during the daytime predicted earlier age of first cannabis use (β = −0.12; 95% CI = −0.20,−0.03; p = 0.001).
Table 2.
Regression betas and 95% confidence intervals of sleep traits predicting cannabis use behaviors amongst a high-risk and twin/family community-based sample controlling for sex, age, depression, and past 180-day substance using both linear and logistic regression
| Sleep trait | Lifetime cannabis use(No lifetime cannabis use as the reference group) | Number of lifetime uses | Age of first cannabis use | Past 180-day cannabis use | CUD symptom count |
|---|---|---|---|---|---|
| How often do you feel tired or sleepy when you get up in the morning? | 0.02 [−0.19,0.23] |
0.03 [−0.14,0.19] |
−0.03 [−0.12,0.05] |
−0.06# [−0.13,0.01] |
0.06
#
[−0.01,0.14] |
| How often do you feel tired or just low in energy during the day? | 0.14 [−0.09,0.37] |
0.17
#
[−0.01,0.36] |
−0.14*
[−0.22, −0.05] |
0.00 [−0.08,0.08] |
0.11*
[0.02,0.19] |
| How often do you take a nap during the daytime? | 0.15 [−0.11,0.42] |
0.18
#
[−0.03,0.38] |
−0.12*
[−0.20, −0.03] |
0.08
#
[−0.01,0.16] |
0.03 [−0.05,0.12] |
| Short sleep duration on the weekday (<7 h) (7–8 h sleep duration as the reference group) | 0.07 [−0.38,0.54] |
0.10 [−0.16,0.45] |
−0.06**
[−0.10, −0.02] |
−0.03 [−0.18,0.12] |
0.06 [−0.10,0.22] |
| Short sleep duration on the weekend (<7 h) (7–8 h sleep duration as the reference group) |
0.70*
[0.12,1.32] |
0.24**
[0.08,0.40] |
−0.06**
[−0.11, −0.02] |
0.16
#
[−0.02,0.33] |
0.11 [−0.6,0.30] |
Responses for the first three sleep measures included “Almost never,” “Once a week or so,” “2 or 3 times a week,” “Nearly every day,” and “would rather not answer,” while both the weekday and weekend sleep short sleep duration are binary measures.
Bold values represent *p < 0.05; **p < 0.01.
#Italic values represent p = 0.06–0.09.
Sleep PRS predicting sleep traits
Table 3 displays the regression betas between the sleep PRS and our sleep variables in our target data controlling for age, sex, and PCs 1–10. We converted the original coefficients for the PRS regressions from log odds ratios to odds ratios for clearer interpretation, thus the coefficients for the PRS regressions can be interpreted as a summation of odds ratio weighted genotypes of the trait that it represents. A significant association greater than 1 between a PRS and an outcome measure implies a liability such that a genetically determined unit log-odds increase in PRS is positively associated with a liability for the outcome measure. A significant association less than 1 between a PRS and an outcome measure implies a liability such that a genetically determined unit log-odds increase in PRS is negatively associated with a liability for the outcome measure (in the reverse direction or an association with the reference group). We found evidence of significant associations between the PRS for short sleep duration and numerous sleep factors including short weekday sleep duration (OR = 1.37; 95% CI = 1.16,1.64; p < 0.001), short weekend sleep duration (OR = 1.43; 95% CI = 1.15,1.77; p = 0.001), and frequency of taking naps during the day (OR = 1.10; 95% CI = 1.04,0.17; p = 0.002). Insomnia PRS was significantly associated with short sleep duration on the weekday (OR = 1.20; 95% CI = 1.02,1.42; p = 0.031). There were no significant associations between the eveningness chronotype PRS and any of our sleep measures. Table 4 displays regression betas for the sleep PRS predicting sleep outcomes/traits in our target data in our full models controlling for age, sex, PCs 1–10, current depression, and past 180-day alcohol and tobacco use. Short sleep PRS was significantly predicted short sleep on the weekday (OR = 1.38; 95% CI = 1.16,1.66; p < 0.001), short sleep on the weekend (OR = 1.52; 95% CI = 1.22,1.90; p < 0.001) and how often one takes naps during the day (OR = 1.10; 95% CI = 1.04,1.17; p = 0.001). Insomnia PRS significantly predicted how short sleep on the weekday (OR = 1.22; 95% CI = 1.03,1.45; p = 0.024). The eveningness chronotype PRS did not significantly predict any sleep variables in our target data.
Table 3.
Odds ratios and 95% confidence intervals for sleep PRS (p < 1) predicting sleep factors amongst a high-risk and twin/family community-based sample controlling for age, sex, and ancestral principal components (PCs 1–10)
| Sleep trait | Short sleep duration (<7 h) PRS (7–8 h sleep duration as the reference group) |
Eveningness chronotype PRS (Morningness chronotype as the reference group) |
Insomnia PRS (Never/rare insomnia symptoms as the reference group) |
|---|---|---|---|
| How often do you feel tired or sleepy when you get up in the morning? | 1.01 [0.94,1.10] | 0.99 [0.92,1.06] | 1.03 [0.96,1.11] |
| How often do you feel tired or just low in energy during the day? | 1.03 [0.96,1.10] | 1.04 [0.97,1.11] | 1.05 [0.99,1.13] |
| How often do you take a nap during the daytime? | 1.10** [1.04,1.17] | 0.97 [0.92,1.03] | 1.04 [0.98,1.09] |
| Short sleep duration on the weekday (<7 h) (7–8 h sleep duration as the reference group) |
1.37** [1.16,1.64] | 1.02 [0.86,1.19] | 1.20* [1.02,1.42] |
| Short sleep duration on the weekend (<7 h) (7–8 h sleep duration as the reference group) |
1.43** [1.15,1.77] | 1.10 [0.91,1.33] | 1.05 [0.86,1.27] |
Responses for the first three sleep measures included “Almost never,” “Once a week or so,” “2 or 3 times a week,” “Nearly every day,” and “would rather not answer” while both the weekday and weekend sleep short sleep duration are binary measures.
Bold values represent *p < 0.05; **p < 0.01.
Table 4.
Odds ratios and 95% confidence intervals for sleep PRS (p < 1) predicting sleep factors amongst a high-risk and twin/family community-based sample controlling for sex, age, depression, past 180-day substance use, and ancestral principal components (PCs 1–10)
| Sleep trait | Short sleep duration (<7 h) PRS (7–8 h sleep duration as the reference group) |
Eveningness chronotype PRS (Morningness chronotype as the reference group) |
Insomnia PRS (Never/rare insomnia symptoms as the reference group) |
|---|---|---|---|
| How often do you feel tired or sleepy when you get up in the morning? | 1.02 [0.95,1.10] | 0.99 [0.92,1.06] | 1.04 [0.97,1.12] |
| How often do you feel tired or just low in energy during the day? | 1.03 [0.97,1.11] | 1.05 [0.99,1.12] | 1.06 # [0.99,1.13] |
| How often do you take a nap during the daytime? | 1.10** [1.04,1.17] | 0.69 [0.92,1.03] | 1.04 [0.98,1.10] |
| Short sleep on the weekday (<7 h) (7–8 h sleep duration as the reference group) |
1.38** [1.16,1.66] | 1.03 [0.87,1.22] | 1.22* [1.03,1.45] |
| Short sleep on the weekend (<7 h) (7–8 h sleep duration as the reference group) |
1.52** [1.22,1.90] | 1.09 [0.89,1.32] | 1.06 [0.87,1.30] |
Responses for the first three sleep measures included “Almost never,” “Once a week or so,” “2 or 3 times a week,” “Nearly every day,” and “would rather not answer” while both the weekday and weekend sleep short sleep duration are binary measures.
Bold values represent *p < 0.05; **p < 0.01.
#Italic values represent p = 0.07.
Sleep PRS predicting cannabis traits
Table 5 displays the regression betas between the sleep PRS and cannabis behaviors in our target data controlling for age, sex, and PCs 1–10. The insomnia PRS significantly predicted earlier age of first cannabis use (OR = 0.91; 95% CI = 0.84,0.99; p = 0.023) and increased lifetime CUD symptom count (OR = 1.09; 95% CI = 1.02,1.17; p = 0.019). Eveningness chronotype significantly predicted lifetime cannabis use (OR = 1.21; 95% CI = 1.01,1.45; p = 0.042). Table 6 displays the regression betas for the sleep PRS predicting cannabis use measures in our target data controlling for age, sex, PCS 1–10, current depression, and past 180-day alcohol and tobacco use. The insomnia PRS significantly predicted earlier age of first cannabis use (OR = 0.92; 95% CI = 0.85,0.99; p = 0.036) and increased lifetime CUD symptom count (OR = 1.09; 95% CI = 1.02,1.17; p = 0.012). Both the short sleep duration and eveningness chronotype PRS did not significantly predict any of our cannabis measures.
Table 5.
Odds ratios and 95% confidence intervals for sleep PRS (p < 1) predicting cannabis behaviors amongst a high-risk and twin/family community-based sample controlling for sex, age, and ancestral principal components (PCs 1–10)
| Sleep trait | Short sleep duration (<7 h) PRS (7–8 h sleep duration as the reference group) |
Eveningness chronotype PRS (Morningness Chronotype as the reference group) |
Insomnia PRS (Never/rare insomnia symptoms as the reference group) |
|---|---|---|---|
| Lifetime Cannabis Use (No lifetime cannabis use as the reference group) |
1.04 [0.86,1.26] | 1.21* [1.01,1.45] | 1.09 [0.91,1.32] |
| Number of lifetime cannabis uses | 0.99 [0.93,1.07] | 1.07 # [0.99,1.15] | 1.04 [0.97,1.12] |
| Age of first cannabis use | 0.95 [0.88,1.04] | 0.97 [0.89,1.05] | 0.91** [0.84,0.99] |
| Past 180-day cannabis use | 1.02 [0.95,1.10] | 1.05 [0.98,1.13] | 1.02 [0.96,1.09] |
| CUD symptom count | 1.02 [0.95,1.11] | 1.07 # [0.99,1.14] | 1.09* [1.02,1.17] |
Bold values represent *p < 0.05; **p < 0.01.
#Italic values represent p = 0.07–0.09.
Table 6.
Odds ratios and 95% confidence intervals for sleep PRS (p < 1) predicting cannabis behaviors amongst a high-risk and twin/family community-based sample controlling for sex, age, depression, past 180-day substance use, and ancestral principal components (PCs 1–10)
| Cannabis trait | Short sleep duration (<7 h) PRS (7–8 h sleep duration as the reference group) |
Eveningness chronotype PRS (Morningness chronotype as the reference group) |
Insomnia PRS (Never/rare insomnia symptoms as the reference group) |
|---|---|---|---|
| Lifetime cannabis use (No lifetime cannabis use as the reference group) |
1.02 [−0.19,0.23] | 1.19 # [0.97,1.46] | 1.17 [0.95,1.44] |
| Number of lifetime uses | 0.99 [0.92,1.05] | 1.04 [0.98,1.11] | 1.04 [0.98,1.11] |
| Age of first cannabis use | 0.96 [0.89,1.04] | 0.99 [0.91,1.07] | 0.92* [0.85,0.99] |
| Past 180-day cannabis use | 1.02 [0.96,1.10] | 1.03[0.96,1.10] | 1.12 [0.95,1.09] |
| CUD symptom count | 1.01 [0.94,1.09] | 1.05 [0.98,1.012] | 1.09*[1.02,1.17] |
Bold values represent *p < 0.05.
#Italic values represent p = 0.09.
Discussion
We set out to examine the potential shared genetic liability of sleep deficits and cannabis use behaviors using multiple genomic methods. We found significant positive genetic correlations between lifetime cannabis use and an eveningness chronotype as well as between CUD and both short sleep duration and insomnia. Additionally, we found that an insomnia PRS generated from a large scale sleep GWAS predicted earlier age of first cannabis use and increased number of lifetime CUD symptoms controlling for sex, age, PCs 1–10, current depression, and past 180-day alcohol and tobacco, suggesting that the genetic risk attributed to insomnia can predict several cannabis use behaviors. This study presents the first genomic-based evidence (using PRS and LDSC) of shared genetic influence for cannabis use behaviors with both sleep traits and chronotype. The direction of these correlations and regressions implies a shared genetic relationship between increased cannabis use behaviors and both sleep deficits and an eveningness chronotype.
Our LD score derived genetic correlations are the first such report of a genetic relationship between sleep deficits and cannabis use behaviors, and are similar to prior studies that have shown genetic associations of sleep deficits and other substance use behaviors such as alcohol and tobacco [55–57]. These results and analyses imply that there is a positive genetic correlation between sleep deficits (short sleep duration, insomnia, and an eveningness chronotype) and lifetime cannabis use/CUD, such that the genetic influences on cannabis use also have an influence on sleep deficits or vice versa. While a prior twin study found a genetic correlation between eveningness chronotype and both increased alcohol quantity and binge drinking [52], to our knowledge this is the first report of chronotype being genetically correlated with cannabis use. These results would imply that the genetic influences for being an “evening type” person also have an influence on lifetime cannabis use. Additionally, our PRS analysis findings present the first report of genetic risk for a sleep behavior predicting a substance use behavior, suggesting that the genetic risk for insomnia is associated with cannabis use behaviors such as earlier age of first cannabis use and increased number of lifetime CUD symptoms.
Our sleep duration PRS was validated on phenotypes of sleep behaviors in our target sample. Specifically, our short sleep duration PRS predicted short sleep duration on the weekday/weekend and how often one takes naps during the daytime. The insomnia PRS predicted short sleep duration on the weekday and was trending in its association with how often one feels tired or low in energy during the day. Unfortunately, our target sample did not have a direct measure of insomnia (which was measured in the GWAS as have trouble falling asleep at night or waking up in the middle of the night) nor did our target data sleep trait measures include questions regarding trouble falling asleep or waking up at night. Furthermore, the insomnia GWAS phenotype was defined as severe insomnia, excluding those who had insomnia symptoms “sometimes” and only including those with “usual/always” insomnia symptoms. This exclusion could have led the PRS to predict only extreme cases and this could have influenced the potential variance explained in our regression analysis regarding the sleep outcomes. The eveningness chorotype PRS did not predict any of the sleep measures in our target sample. Similar to our insomnia PRS, our target sample did not have a direct measure of chronotype, and the only chronotype-like measure was restricted to a question regarding how often one feels tired or sleepy in the morning, which differed from the morning person/night person question of the chronotype GWAS. This could explain the lack of significant relationships between the eveningness chronotype PRS and the sleep outcomes in our target data
It is worth noting that we included cannabis measures from differential time points with the goal of not only analyzing the genetic relationship between sleep and various cannabis behaviors across life, but also to look at the phenotypic associations of early cannabis use and later sleep in our target data. We found that several measures of recent sleep characteristics were associated with earlier cannabis use measures in our target data; for example, short sleep duration on the weekend was associated with earlier age of first cannabis use as well as increased number of lifetime cannabis uses, and that short sleep duration on the weekday, how often one feels tired or low in energy during the day, and how often one takes a nap during the daytime were associated with earlier age of first cannabis use. Additionally, we found that increased lifetime CUD symptom count was associated with how often one feels tired or low in energy during the day. These results support prior findings of potentially earlier or preceding cannabis use behaviors being associated with later sleep factors.
While some of the results from our LDSC analysis align with our PRS analysis (e.g. findings of shared genetics between insomnia and CUD) several of our findings were not replicated between the two analyses. For instance, we found a genetic correlation between short sleep duration and CUD, yet our PRS for short sleep duration did not predict any cannabis behaviors. Additionally, we found a significant genetic association between an eveningness chronotype and lifetime cannabis use, yet the eveningness chronotype PRS did not significantly predict any cannabis behaviors in our full models (although there was a significant association between the eveningness chronotype PRS and lifetime cannabis use in our simple model but this association was trending in the full model). Reasons for the lack of convergent results could include population differences between the GWAS and target data in terms of both environmental and genetic differences of the samples. Different locations of the samples will have different environmental influences that can influence phenotype expression and there are both racial and regional differences in terms of common and rare variants, minor allele frequencies, and linkage disequilibrium that can influence results and the variance explained [115]. Additionally, differences in the methodological aspects of the analyses (PRS vs LDSC) could be responsible. While both analyses focused on the effects across all available SNPs, LDSC looks at the overall direction of effect of all SNPs and PRS looks whether the genetic risk for a certain trait predicts a phenotype. Lastly, the difference in sample sizes between the two methods could explain the dissimilarities in results, provided the genetic correlations were between large-sample GWAS and were better powered than the PRS regression which has a much smaller sample size. Overall, our results imply shared genetics between cannabis use and sleep deficits, and the differences seen in the results may be due to population and methodological differences between these analyses.
Our findings complement a small collection of research focused on the genetics of cannabis use and sleep behaviors. Two prior studies by our group have used the classical twin design to show that shared genetics played a role in the etiology of the relationship between early cannabis use and shorter adult sleep duration, insomnia, and insomnia with short sleep [16, 23] and a recent study found clock gene polymorphisms that were significant risk factors for cannabis addiction [58]. One possible explanation for this genetic relationship could be that disturbances of circadian rhythm genes might interrupt the reward processing system, which could influence substance use [50, 51]. Another supported explanation could be that the endocannabinoid system is involved in the circadian sleep–wake cycle, such that endocannabinoids influence sleep behaviors and their levels can vary with the time of day and other circadian-related factors [47–49, 116]. Along these lines, several of the genes and genetic pathways found to be significant in sleep-related variable GWAS [57, 59–62] have been associated with cannabis use and cannabinoid activity [63–66, 68, 69, 117–120]. Likewise, GWAS for lifetime cannabis use and CUD [66, 67, 70, 71] have found genes that have been linked to sleep behaviors and circadian rhythm [72–75, 121].
This study demonstrates a genetic relationship between both sleep factors and chronotype with cannabis use behaviors, implying shared genetic liability between these domains, specifically common genetics between short sleep duration, insomnia, and an eveningness chronotype with increased cannabis use behaviors. Future studies should use more novel genetic methods to examine the exact mechanisms for this genetic relationship such as gene set enrichment pathway analysis [122]. While a recent study has made causal inferences between these domains using Mendelian randomization [123], there are mechanisms both genetic and outside of genetics that could be responsible for the associations between these traits and future research should use methods that can make causal inferences like epigenome-wide association studies [124] and experimental-based cannabis administration studies to analyze the relationship between cannabis use and sleep deficits.
Limitation
There are several limitations to this study, which point to important lines of future research. First, our cannabis, sleep, and covariate variables in our target sample were self-report and could be prone to response bias or report error. Second, while both the short sleep duration and insomnia PRS were significantly associated with sleep duration outcomes and other sleep behaviors, the eveningness chronotype PRS was not significantly associated with any of our target data sleep measures. The cohort study lacked a valid insomnia or chronotype measure to validate the usefulness of the respective evening chronotype and insomnia PRS measures. The inclusion of such measures would have ideally been associated with these PRS measures. Third, population differences in environmental factors between target and base data can influence the variance predicted in the models. Our GWAS data for our PRS was gathered from the UK in a cohort known for being older and overtly healthy [125]. Our target sample was from the US, younger, mostly male, and a combination of community-based and high-risk subjects. Fourth, genetic differences due to the regional make-up our samples could influence the variance. While both our GWAS base and target data were from European ancestry, there could still be genetic differences between the samples that could have influenced the predictive ability of the PRS to explain the variance of the outcomes. Fifth, we reported the effects of all SNPs (p < 1) in our results, and while using this threshold method captures the additive effect of additional SNPs often removed by the stringent threshold of genome-wide significance [92–94], it is also susceptible to false positives or noise. Still, studies have shown that the whole-genome approach of using all SNPs captures more signal than it does noise and that this method can outperform PRS generated from using only top hits [126, 127]. Sixth, while LDSC is robust to population stratification/relatedness, there are limitations to consider such as biases in the estimates due to rare copy variants and capturing genetic variation tagged only by common SNPs [111]. Lastly, several of our genetic-based results regarding the relationship between cannabis use and sleep deficits were trending in significance (such as the eveningness chronotype PRS predicting lifetime cannabis use and the genetic correlation between eveningness chronotype and CUD) and it is possible that similar studies with considerably larger samples would yield clearer results.
Summary
Our findings are consistent with the theory that both sleep deficits (such as short sleep duration and insomnia) and an eveningness chronotype share genetic liability with cannabis use behaviors, and that this genetic relationship contributes to the associations between sleep and cannabis. These results extend the current body of research focused on the relationship of sleep and cannabis behaviors to include the first instance of genomic evidence (LDSC and PRS prediction) as well as the first evidence of a genetic relationship between an eveningness chronotype and cannabis use behaviors. Future studies should consider novel genomic methods to examine potential genes as well as specific genetic causal pathways for these relationships.
Acknowledgments
We would like to thank all authors for their insightful comments and suggestions regarding the design, analysis, and wording of the manuscript.
Funding
Funding from the National Institute of Health (NIH) through the National Institute on Drug Abuse (NIDA) via grants DA035804, DA032555, DA042755, DA017637, and DA011015.
Conflict of interest statement
Financial disclosure: This study was not financially supported by industry.
Non-financial disclosure: No potential conflicts of interest.
Ethical approval: Documented consent was obtained from all study participants and all procedures of this study followed the ethical standards of the University of Colorado and the University of California, San Diego Institutional Review Boards.
Preprint repositories
A preprint of this manuscript can be found via BIORXIV/2020/053983
References
- 1. Management of Substance Abuse Cannabis. World Health Organization (WHO). https://www.who.int/substance_abuse/facts/cannabis/en/.
- 2. Groce E. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. J Med Regul. 2018;104:32. doi: 10.30770/2572-1852-104.4.32 [DOI] [Google Scholar]
- 3. Batalla A, et al. Neuroimaging studies of acute effects of THC and CBD in humans and animals: a systematic review. Curr Pharm Des. 2014;20(13):2168–2185. [DOI] [PubMed] [Google Scholar]
- 4. Babson KA, et al. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4):23. [DOI] [PubMed] [Google Scholar]
- 5. Bachhuber M, et al. Use of cannabis to relieve pain and promote sleep by customers at an adult use dispensary. J Psychoactive Drugs. 2019;51(5):400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belendiuk KA, et al. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goodhines PA, et al. Self-medication for sleep in college students: concurrent and prospective associations with sleep and alcohol behavior. Behav Sleep Med. 2019;17(3):327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jensen B, et al. Medical marijuana and chronic pain: a review of basic science and clinical evidence. Curr Pain Headache Rep. 2015;19(10):50. doi: 10.1007/s11916-015-0524-x [DOI] [PubMed] [Google Scholar]
- 9. Ogborne AC, et al. Self-reported medical use of marijuana: a survey of the general population. CMAJ. 2000. [PMC free article] [PubMed] [Google Scholar]
- 10. Pedersen W, Sandberg S. The medicalisation of revolt: A sociological analysis of medical cannabis users. Sociol Heal Illn. 2013. doi: 10.1111/j.1467-9566.2012.01476.x [DOI] [PubMed] [Google Scholar]
- 11. Bolla KI, et al. Sleep disturbance in heavy marijuana users. Sleep. 2008;31(6):901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freeman D, et al. Persecutory ideation and insomnia: findings from the second British National Survey of Psychiatric Morbidity. J Psychiatr Res. 2010;44(15):1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKnight-Eily LR, et al. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med. 2011;53(4–5):271–273. [DOI] [PubMed] [Google Scholar]
- 14. Mednick SC, et al. The spread of sleep loss influences drug use in adolescent social networks. PLoS One. 2010;5(3):e9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Troxel WM, et al. Examining racial/ethnic disparities in the association between adolescent sleep and alcohol or marijuana use. Sleep Health. 2015;1(2):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Winiger E, et al. The relationship between early regular cannabis use & adult sleep duration: genetic variation and the implications of a predictive relationship. Drug Alcohol Depend. 2019;204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conroy DA, et al. Marijuana use patterns and sleep among community-based young adults. J Addict Dis. 2016;35(2):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fakier N, et al. Associations among sleep problems, learning difficulties and substance use in adolescence. J Adolesc. 2011;34(4):717–726. [DOI] [PubMed] [Google Scholar]
- 19. Johnson EO, Breslau N. Sleep problems and substance use in adolescence. Drug Alcohol Depend. 2001;64:1–7. doi: 10.1016/S0376-8716(00)00222-2 [DOI] [PubMed] [Google Scholar]
- 20. Ogeil RP, et al. Risky drug use and effects on sleep quality and daytime sleepiness. Hum Psychopharmacol. 2015;30(5):356–363. [DOI] [PubMed] [Google Scholar]
- 21. Roane BM, et al. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2008;31(10):1351–1356. [PMC free article] [PubMed] [Google Scholar]
- 22. Wong MM, et al. Childhood sleep problems, early onset of substance use and behavioral problems in adolescence. Sleep Med. 2009;10(7):787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winiger EA, et al. Onset of regular cannabis use and young adult insomnia: an analysis of shared genetic liability. Sleep. 2019;43(5):zsz293. doi: 10.1093/sleep/zsz293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Babson KA, Bonn-Miller MO. Sleep disturbances: implications for cannabis use, cannabis use cessation, and cannabis use treatment. Curr Addict Reports. 2014;1:109–114. doi: 10.1007/s40429-014-0016-9 [DOI] [Google Scholar]
- 25. Li SX, et al. Eveningness chronotype, insomnia symptoms, and emotional and behavioural problems in adolescents. Sleep Med. 2018;47:93–99. [DOI] [PubMed] [Google Scholar]
- 26. Merikanto I, et al. Relation of chronotype to sleep complaints in the general Finnish population. Chronobiol Int. 2012;29(3):311–317. [DOI] [PubMed] [Google Scholar]
- 27. Susman EJ, et al. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Dev Psychol. 2007;43(4):811–822. [DOI] [PubMed] [Google Scholar]
- 28. Fernández-Mendoza J, et al. Circadian preference, nighttime sleep and daytime functioning in young adulthood. Sleep Biol Rhythms. 2010;8:52–62. doi: 10.1111/j.1479-8425.2010.00430.x [DOI] [Google Scholar]
- 29. Prat G, et al. Influence of circadian typology on drug consumption, hazardous alcohol use, and hangover symptoms. Chronobiol Int. 2011;28(3):248–257. [DOI] [PubMed] [Google Scholar]
- 30. Hasler BP, et al. Eveningness and later sleep timing are associated with greater risk for alcohol and marijuana use in adolescence: initial findings from the National Consortium on Alcohol and Neurodevelopment in Adolescence Study. Alcohol Clin Exp Res. 2017;41(6):1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kervran C, et al. Association between morningness/eveningness, addiction severity and psychiatric disorders among individuals with addictions. Psychiatry Res. 2015;229(3):1024–1030. [DOI] [PubMed] [Google Scholar]
- 32. Harkany T, et al. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28(2):83–92. [DOI] [PubMed] [Google Scholar]
- 33. Bowles NP, et al. Recent legalization of cannabis use: effects on sleep, health, and workplace safety. Nat Sci Sleep. 2017;9:249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tortoriello G, et al. Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 2014;33(7):668–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scher MS, et al. The effects of prenatal alcohol and marijuana exposure: disturbances in neonatal sleep cycling and arousal. Pediatr Res. 1988;24(1):101–105. [DOI] [PubMed] [Google Scholar]
- 36. Dahl RE, et al. A longitudinal study of prenatal marijuana use: effects on sleep and arousal at age 3 years. Arch Pediatr Adolesc Med. 1995;149(2):145–150. doi: 10.1001/archpedi.1995.02170140027004 [DOI] [PubMed] [Google Scholar]
- 37. Winiger EA, Hewitt JK. Prenatal cannabis exposure and sleep outcomes in children 9–10 years of age in the adolescent brain cognitive development study. Sleep Heal. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meyer HC, et al. The role of the endocannabinoid system and genetic variation in adolescent brain development. Neuropsychopharmacology. 2018;43(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mills KL, et al. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. Neuroimage. 2016;141:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pasch KE, et al. Longitudinal bi-directional relationships between sleep and youth substance use. J Youth Adolesc. 2012;41(9):1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hasler BP, et al. Eveningness and later sleep timing are associated with greater risk for alcohol and marijuana use in adolescence: initial findings from the National Consortium on Alcohol and Neurodevelopment in Adolescence Study. Alcohol Clin Exp Res. 2017;41(6):1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nguyen-Louie TT, et al. Effects of sleep on substance use in adolescents: a longitudinal perspective. Addict Biol. 2018;23(2):750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong MM, et al. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol Clin Exp Res. 2004;28(4):578–587. [DOI] [PubMed] [Google Scholar]
- 44. Wong MM, et al. Childhood sleep problems, response inhibition, and alcohol and drug outcomes in adolescence and young adulthood. Alcohol Clin Exp Res. 2010;34:1033–1044. doi: 10.1111/j.1530-0277.2010.01178.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller MB, et al. The prospective association between sleep and initiation of substance use in young adolescents. J Adolesc Heal. 2017;60:154–160. doi: 10.1016/j.jadohealth.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hasler BP, et al. Restless sleep and variable sleep timing during late childhood accelerate the onset of alcohol and other drug involvement. J Stud Alcohol Drugs. 2016;77(4):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vaughn LK, et al. Endocannabinoid signalling: has it got rhythm? Br J Pharmacol. 2010;160(3):530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murillo-Rodriguez E, et al. The emerging role of the endocannabinoid system in the sleep-wake cycle modulation. Cent Nerv Syst Agents Med Chem. 2011;11(3):189–196. [DOI] [PubMed] [Google Scholar]
- 49. Prospéro-García O, et al. Endocannabinoids and sleep. Neurosci Biobehav Rev. 2016;71:671–679. doi: 10.1016/j.neubiorev.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 50. Hasler BP, et al. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. J Sleep Res. 2012;21(5):515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hasler BP, et al. Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol. 2015;49(4):377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Watson NF, et al. A twin study of genetic influences on diurnal preference and risk for alcohol use outcomes. J Clin Sleep Med. 2013;9(12):1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kranzler HR, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 2019;10(1):1–11. doi: 10.1038/s41467-019-09480-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walters RK, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21(12):1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hammerschlag AR, et al. Genome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Nat Genet. 2017;49(11):1584–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gibson M, et al. Evidence for genetic correlations and bidirectional, causal effects between smoking and sleep behaviors. Nicotine Tob Res. 2018;21:731–738. doi: 10.1093/ntr/nty230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dashti HS, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. 2019;10:1–12. doi: 10.1038/s41467-019-08917-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saffroy R, et al. Several clock genes polymorphisms are meaningful risk factors in the development and severity of cannabis addiction. Chronobiol Int. 2019;36(1):122–134. [DOI] [PubMed] [Google Scholar]
- 59. Jones SE, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 2019;10:1–11. doi: 10.1038/s41467-018-08259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Doherty A, et al. GWAS identifies 14 loci for device-measured physical activity and sleep duration. Nat Commun. 2018;9(1):5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jansen PR, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51:394–403. doi: 10.1038/s41588-018-0333-3 [DOI] [PubMed] [Google Scholar]
- 62. Wang H, et al. Genome-wide association analysis of self-reported daytime sleepiness identifies 42 loci that suggest biological subtypes. Nat Commun. 2019;10(1):3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rubino T, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19(8):763–772. [DOI] [PubMed] [Google Scholar]
- 64. Colizzi M, et al. Interaction between functional genetic variation of DRD2 and cannabis use on risk of psychosis. Schizophr Bull. 2015;41(5):1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cardinal P, et al. Cannabinoid type 1 (CB1) receptors on Sim1-expressing neurons regulate energy expenditure in male mice. Endocrinology. 2015;156(2):411–418. [DOI] [PubMed] [Google Scholar]
- 66. Gage SH, et al. Assessing causality in associations between cannabis use and schizophrenia risk: a two-sample Mendelian randomization study. Psychol Med. 2017;47(5):971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pasman JA, et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat Neurosci. 2018;21(9):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Philibert RA, et al. Role of GABRA2 on risk for alcohol, nicotine, and cannabis dependence in the Iowa Adoption Studies. Psychiatr Genet. 2009;19(2):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Alpár A, et al. Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling. Nat Commun. 2014;5:4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stringer S, et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32,330 subjects from the International Cannabis Consortium. Transl Psychiatry. 2016;6:e769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Demontis D, et al. Genome-wide association study implicates CHRNA2 in cannabis use disorder. Nat Neurosci. 2019;22(7):1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yang SY, et al. Effects of genetic variants of ST8SIA2 and NCAM1 genes on seasonal mood changes and circadian preference in the general population. Chronobiol Int. 2018;35:405–415. doi: 10.1080/07420528.2017.1410827 [DOI] [PubMed] [Google Scholar]
- 73. Tabuchi S, et al. Influence of inhibitory serotonergic inputs to orexin/hypocretin neurons on the diurnal rhythm of sleep and wakefulness. Sleep. 2013;36(9):1391–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yan J, et al. Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput Biol. 2008;4. doi: 10.1371/journal.pcbi.1000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stadler F, et al. Lack of calbindin-D28k alters response of the murine circadian clock to light. Chronobiol Int. 2010;27(1):68–82. [DOI] [PubMed] [Google Scholar]
- 76. Lane JM, et al. Biological and clinical insights from genetics of insomnia symptoms. Nat Genet. 2019;51(3):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Martin AR, et al. Human demographic history impacts genetic risk prediction across diverse populations. Am J Hum Genet. 2017;100(4):635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Auton A, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cardon LR, et al. Population stratification and spurious allelic association. Lancet. 2003;361(9357):598–604. [DOI] [PubMed] [Google Scholar]
- 80. Rhea SA, et al. The Colorado Adoption Project. Twin Res Hum Genet. 2013;16(1):358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rhea SA, et al. Colorado twin registry: An update. Twin Res Hum Genet. 2013;9:351–357. doi: 10.1017/thg.2012.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stallings MC, et al. A genome-wide search for quantitative trait Loci that influence antisocial drug dependence in adolescence. Arch Gen Psychiatry. 2005;62(9):1042–1051. [DOI] [PubMed] [Google Scholar]
- 83. Cottler LB, et al. The reliability of the CIDI‐SAM: a comprehensive substance abuse interview. Br J Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x [DOI] [PubMed] [Google Scholar]
- 84. Association AP Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Barber, J P, Connolly, M B, Crits-Christoph, P, Gladis, L, Siqueland, L. Washington, DC: Author; 1994. [Google Scholar]
- 85. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 1. New York, NY: American Psychiatric Association; 2013. 10.1176/appi.books.9780890425596.893619 [DOI] [Google Scholar]
- 86. Hartman CA, et al. Item response theory analysis of DSM-IV cannabis abuse and dependence criteria in adolescents. J Am Acad Child Adolesc Psychiatry. 2008;47(2):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hasin DS, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170(8):834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hasin DS, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991–1992 to 2012–2013. JAMA Psychiatry. 2017;74:579–588. doi: 10.1001/jamapsychiatry.2017.0724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jessor R, Jessor SL Problem Behavior and Psychosocial Development: A Longitudinal Study of Youth. Washington, DC: Academic Press; 1977. [Google Scholar]
- 90. Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Choi SW, et al. Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc. 2020:1–14. doi: 10.1038/s41596-020-0353-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Visscher PM, et al. Five years of GWAS discovery. Am J Hum Genet. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Belsky DW, et al. Integrating genetics and social science: genetic risk scores. Biodemography Soc Biol. 2014;60(2):137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ware EB, et al. Heterogeneity in polygenic scores for common human traits. bioRxiv. 2017. doi: 10.1101/106062 [DOI] [Google Scholar]
- 95. ICD-10. Practical Predictive Analytics and Decisioning Systems for Medicine: Informatics Accuracy and Cost-Effectiveness for Healthcare Administration and Delivery Including Medical Research. US and UK: Academic Press; 2014. doi: 10.1177/1071100715600286 [DOI] [Google Scholar]
- 96. Mauro PM, et al. Age differences in daily and nondaily cannabis use in the United States, 2002–2014. J Stud Alcohol Drugs. 2018:423–431. doi: 10.15288/jsad.2018.79.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Silva A, et al. Gender and age differences in polysomnography findings and sleep complaints of patients referred to a sleep laboratory. Braz J Med Biol Res. 2008;41(12):1067–1075. [DOI] [PubMed] [Google Scholar]
- 98. Cuttler C, et al. Sex differences in cannabis use and effects: a cross-sectional survey of cannabis users. Cannabis Cannabinoid Res. 2016;1(1):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhu H, et al. Sex differences in cannabis use disorder diagnosis involved hospitalizations in the United States. J Addict Med. 2017;11(5):357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Krishnan V, et al. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12(6):383–389. [DOI] [PubMed] [Google Scholar]
- 101. Chen CY, et al. Marijuana use and the risk of major depressive episode. Epidemiological evidence from the United States National Comorbidity Survey. Soc Psychiatry Psychiatr Epidemiol. 2002;37(5):199–206. [DOI] [PubMed] [Google Scholar]
- 102. Tsuno N, et al. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254–1269. [DOI] [PubMed] [Google Scholar]
- 103. Lev-Ran S, et al. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. 2014;44(4):797–810. [DOI] [PubMed] [Google Scholar]
- 104. Degenhardt L, et al. The relationship between cannabis use and other substance use in the general population. Drug Alcohol Depend. 2001. doi: 10.1016/S0376-8716(01)00130-2 [DOI] [PubMed] [Google Scholar]
- 105. Fergusson DM, et al. Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction. 2006;101(4):556–569. [DOI] [PubMed] [Google Scholar]
- 106. Boakye D, et al. Tobacco exposure and sleep disturbance in 498 208 UK Biobank participants. J Public Health (Oxf). 2018;40(3):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Roehrs T, et al. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5(4):287–297. [DOI] [PubMed] [Google Scholar]
- 108. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 109. Salvatore JE, et al. Sibling comparisons elucidate the associations between educational attainment polygenic scores and alcohol, nicotine and cannabis. Addiction. 2020;115:337–346. doi: 10.1111/add.14815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Johnson EC, et al. Exploring the relationship between polygenic risk for cannabis use, peer cannabis use and the longitudinal course of cannabis involvement. Addiction. 2019;114(4):687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bulik-Sullivan B, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–295. doi: 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Duan S, et al. FstSNP-HapMap3: a database of SNPs with high population differentiation for HapMap3. Bioinformation. 2008;3(3):139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bulik-Sullivan B, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. R Foundation for Statistical Computing. Development Core Team: R: A Language and Environment for Statistical Computing. ISBN 3-900051-07-0, http//wwwR-project.org. 2015. [Google Scholar]
- 115. Martin AR, et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019;51:584–591. doi: 10.1038/s41588-019-0379-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hanlon EC. Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide). Psychoneuroendocrinology. 2020;111:104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Szutorisz H, et al. Cross-generational THC exposure alters the developmental sensitivity of ventral and dorsal striatal gene expression in male and female offspring. Neurotoxicol Teratol. 2016;58:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pagotto U, et al. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27(1):73–100. [DOI] [PubMed] [Google Scholar]
- 119. Flores A, et al. Cannabinoid-hypocretin cross-talk in the central nervous system: what we know so far. Front Neurosci. 2013;7:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kozela E, et al. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J Neuroinflammation. 2016;13(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jiang P, et al. A systems approach identifies networks and genes linking sleep and stress: implications for neuropsychiatric disorders. Cell Rep. 2015;11(5):835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Luo W, et al. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics. 2009;10:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pasman JA, et al. Causal relationships between substance use and insomnia. Drug Alcohol Depend. 2020;214:108151. [DOI] [PubMed] [Google Scholar]
- 124. Wahl S, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541(7635):81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fry A, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9(3):e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Evans DM, et al. Harnessing the information contained within genome-wide association studies to improve individual prediction of complex disease risk. Hum Mol Genet. 2009;18(18):3525–3531. [DOI] [PubMed] [Google Scholar]