Abstract
Study Objectives
Evaluate efficacy and safety of lower-sodium oxybate (LXB), a novel oxybate medication with 92% less sodium than sodium oxybate (SXB).
Methods
Adults aged 18–70 years with narcolepsy with cataplexy were eligible. The study included a ≤30-day screening period; a 12-week, open-label, optimized treatment and titration period to transition to LXB from previous medications for the treatment of cataplexy; a 2-week stable-dose period (SDP); a 2-week, double-blind, randomized withdrawal period (DBRWP); and a 2-week safety follow-up. During DBRWP, participants were randomized 1:1 to placebo or to continue LXB treatment.
Results
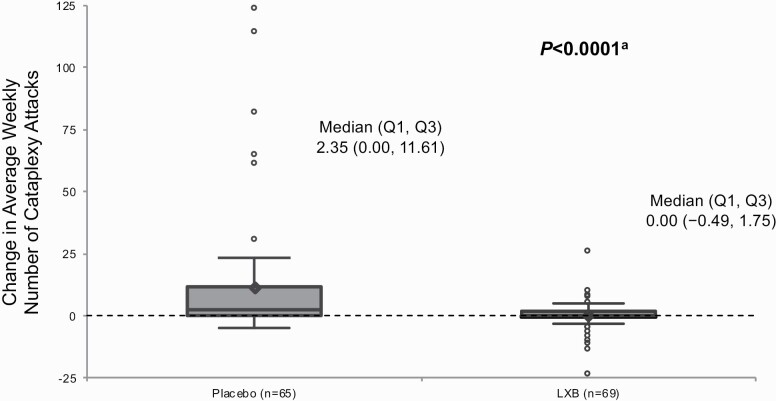
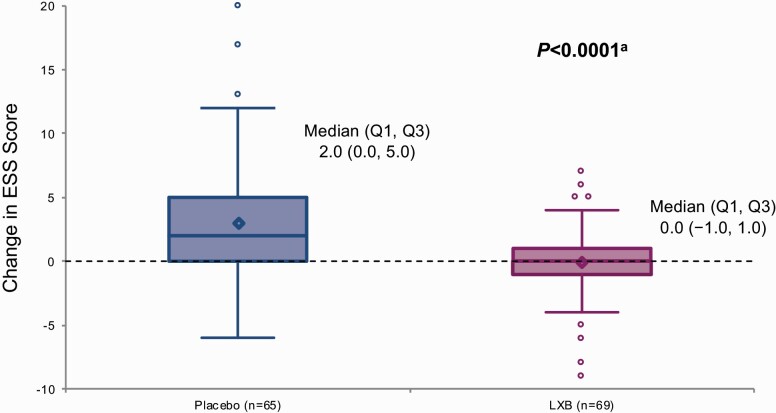
Efficacy was assessed in 134 participants who received randomized treatment, and safety was assessed in all enrolled participants (N = 201). Statistically significant worsening of symptoms was observed in participants randomized to placebo, with median (first quartile [Q1], third quartile [Q3]) change in weekly number of cataplexy attacks from SDP to DBRWP (primary efficacy endpoint) in the placebo group of 2.35 (0.00, 11.61) versus 0.00 (−0.49, 1.75) in the LXB group (p < 0.0001; mean [standard deviation, SD] change: 11.46 [24.751] vs 0.12 [5.772]), and median (Q1, Q3) change in Epworth Sleepiness Scale score (key secondary efficacy endpoint) of 2.0 (0.0, 5.0) in the placebo group versus 0.0 (−1.0, 1.0) in the LXB group (p < 0.0001; mean [SD] change: 3.0 [4.68] vs 0.0 [2.90]). The most common treatment-emergent adverse events with LXB were headache (20.4%), nausea (12.9%), and dizziness (10.4%).
Conclusions
Efficacy of LXB for the treatment of cataplexy and excessive daytime sleepiness was demonstrated. The safety profile of LXB was consistent with SXB.
Clinical trial registration
Keywords: narcolepsy, cataplexy, excessive daytime sleepiness, sodium oxybate, JZP-258
Statement of Significance.
Lower-sodium oxybate (LXB), a medication approved in the United States for the treatment of cataplexy and excessive daytime sleepiness (EDS) in participants with narcolepsy, contains the same active moiety as sodium oxybate (SXB) with 92% less sodium. SXB is recommended as a standard of care for the treatment of narcolepsy symptoms by the American Academy of Sleep Medicine, European Federation of Neurological Societies, and the French consensus group. This double-blind randomized withdrawal study evaluated the efficacy and safety of LXB. Cataplexy attacks and EDS worsened significantly in those assigned to placebo but remained stable in those who continued LXB treatment, demonstrating the efficacy of LXB. The overall safety profile of LXB was as expected based on prior experience with SXB.
Introduction
Narcolepsy is a lifelong neurologic disorder that profoundly impairs quality of life, productivity, and social functioning; requires long-term treatment in many patients; and has a worldwide estimated prevalence of 0.02%–0.067% [1–3]. The cardinal symptom of narcolepsy is excessive daytime sleepiness (EDS); many patients with narcolepsy also experience cataplexy [4]. Cataplexy presentation differs widely among patients, from subtle partial attacks where loss of muscle tone is localized (e.g. facial weakness) to complete attacks leading to postural collapse. The frequency of cataplexy also varies, from sporadic to frequent. EDS and cataplexy are two of the main pentad of symptoms, which also include disrupted nighttime sleep (DNS) [5], sleep-related (hypnagogic/hypnopompic) hallucinations, and sleep paralysis.
Sodium oxybate (SXB; Xyrem) is approved in the United States for the treatment of cataplexy or EDS in patients 7 years of age and older with narcolepsy and in Europe and Canada for the treatment of adult patients with narcolepsy with cataplexy [6–8]. American Academy of Sleep Medicine (AASM) practice parameters designate SXB as a standard of care for the treatment of cataplexy, EDS, and DNS, based on level 1 evidence [9]. European Federation of Neurological Societies (EFNS) guidelines (published prior to marketing approval of SXB in Europe) recommend SXB as a first-line treatment for cataplexy based on class I evidence (level A) and provisionally recommended SXB for EDS and DNS (designated as poor sleep) [10]. French consensus guidelines and other treatment recommendations are similar [11, 12]. Wake-promoting agents (WPAs) such as modafinil and stimulants such as methylphenidate or amphetamine are frequently taken for the treatment of EDS in patients with narcolepsy. Antidepressants are frequently prescribed off-label for the treatment of cataplexy, despite limited evidence from controlled clinical trials of efficacy or safety [9].
Randomized controlled trials conducted in more than 700 adult and pediatric participants with narcolepsy have demonstrated that SXB significantly reduces cataplexy attacks and EDS [13–17]. The most common adverse events (AEs) reported with SXB in adult and pediatric studies were nausea, dizziness, vomiting, somnolence, enuresis, tremor, headache, weight decreased, and decreased appetite [6].
At the recommended dosage range for adults (6–9 g/night), SXB treatment contributes 1,100–1,640 mg to daily sodium intake [6]. Sodium intake increases risk of hypertension and cardiovascular outcomes, particularly stroke, independent of blood pressure [18–20]. Several authoritative organizations have published recommendations to reduce sodium intake to <2,300 mg/day, with a further reduction to <1,500 mg/day recommended as the ideal intake, particularly for individuals with hypertension, diabetes, or chronic kidney disease and for individuals above 50 years of age [18, 21]. In 2019, the National Academy of Sciences established 2,300 mg/day as the chronic disease risk reduction intake (CDRR) for sodium, indicating that reduction of sodium intake at this level is expected to reduce chronic disease risk, not only in older individuals or those with comorbidities, but in an apparently healthy population [22]. Average dietary sodium intake (3,400–5,000 mg/day) far exceeds the recommended limits [23, 24].
Exposure to high sodium–containing medications represents an additional contribution to total daily sodium intake. A systematic review examining the association between high sodium–containing medications and risk of increased blood pressure and cardiovascular events identified three synergistic risk factors: high sodium contribution of medication (>1,500 mg/day), chronic treatment/long-term exposure to medication (≥2 years), and presence of comorbidities, specifically hypertension and diabetes [25]. SXB treatment in patients with narcolepsy may confer all three of these synergistic risk factors. As noted, at the recommended dosage range for adults (6–9 g/night), SXB treatment contributes 1,100–1,640 mg to total daily sodium intake [6]. Furthermore, because narcolepsy is a lifelong condition that requires long-term treatment [1], high sodium intake may confer risk even in younger, apparently healthy individuals with narcolepsy. Finally, multiple studies across geographic regions have identified increased prevalence of cardiovascular and cardiometabolic comorbidities in patients with narcolepsy [3, 26, 27], and multiple lines of evidence implicate narcolepsy pathophysiology in the mechanism for this increased risk of disease [28–34]. Metabolic dysfunction in patients with narcolepsy appears to be independent of body mass index [26, 31, 35]. Additionally, the “non-dipping” phenotype (defined by a blood pressure decrease of <10% during nighttime sleep) is a strong predictor of mortality in the general population, and is significantly more common in patients with narcolepsy than in healthy controls [28, 36]. Thus, patients with narcolepsy are at increased risk of developing cardiovascular and cardiometabolic disease [26, 27, 29, 31, 35].
Calcium, magnesium, potassium, and sodium oxybates (lower-sodium oxybate [LXB]; Xywav; JZP-258) is an oxybate medication recently approved in the United States for the treatment of cataplexy or EDS in patients with narcolepsy 7 years of age and older [37]. LXB contains the same active moiety as SXB but with a unique composition of cations resulting in 92% less sodium (87–131 mg in the dose range of 6–9 g/night) [38]. Lower maximum plasma concentration (Cmax), longer time to Cmax (Tmax), and similar area under the concentration-time curve (AUC) were demonstrated with LXB compared with SXB at equivalent oxybate doses under fasting conditions in healthy volunteers [6, 7, 38]. Although the exact mechanism of action of oxybate in SXB and LXB is not fully understood, therapeutic effects on sleep/wake symptoms are hypothesized to be mediated through modulation of GABAB receptors during sleep [6].
The efficacy and safety of LXB were evaluated in a phase 3, multicenter, placebo-controlled, double-blind, randomized withdrawal study in participants with narcolepsy.
Methods
Study design
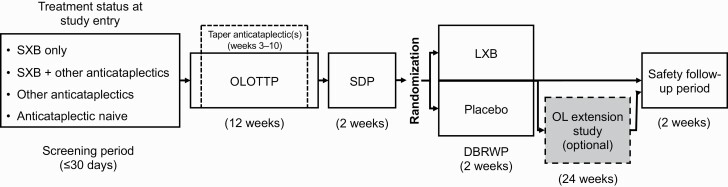
This study (www.clinicaltrials.gov identifier NCT03030599; www.eudract.ema.europa.eu #2016-000426-20) was designed to assess the efficacy and safety of LXB oral solution in adult participants with narcolepsy with cataplexy. The study was conducted in the United States and Europe. The main study consisted of a screening period of up to 30 days; a 12-week, open-label, optimized treatment and titration period (OLOTTP); a 2-week stable-dose period (SDP); a 2-week, double-blind, randomized withdrawal period (DBRWP); and a safety follow-up (Figure 1). The safety follow-up visit occurred 2 weeks after DBRWP or 2 weeks after an optional open-label extension (OLE) period, depending on participation. Participants who completed DBRWP were eligible to enter the 24-week OLE, during which safety was assessed. Participants entered OLE directly from the main study (“rollover”) or after completion of the main study (“re-entry”). Results from the OLE will be reported elsewhere.
Figure 1.
Study design. DBRWP, double-blind randomized withdrawal period; LXB, lower-sodium oxybate; OL, open-label; OLOTTP, open-label optimized treatment and titration period; SDP, stable-dose period; SXB, sodium oxybate.
The study was conducted in accordance with the International Conference on Harmonisation’s Guideline for Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by the independent ethics committee or institutional review board for each site, and each participant provided written informed consent.
Participants
Participants 18–70 years of age with a primary diagnosis of narcolepsy with cataplexy based on criteria from the International Classification of Sleep Disorders, 3rd ed. or Diagnostic and Statistical Manual of Mental Disorders, 5th ed. were eligible to participate [39, 40]. Enrollment criteria included a history of at least 14 cataplexy attacks in a typical 2-week period prior to receiving any narcolepsy treatment.
This study enrolled participants taking various medications for the treatment of narcolepsy symptoms. If participants were taking medication(s) for the treatment of cataplexy at study entry, the medication regimen was to have been stable for at least 2 months prior to study entry; if participants were taking stimulants and/or WPAs (stimulants/WPAs) at study entry, the stimulant/WPA dose and regimen was to remain constant throughout the study. For participants taking SXB at study entry, documentation of prior improvement in cataplexy and EDS with SXB treatment was required.
Participants were categorized into four groups based on medications for the treatment of cataplexy at study entry, as follows: (1) SXB up to 9.0 g/night (SXB only), (2) SXB up to 9.0 g/night and another medication for the treatment of cataplexy (SXB + other anticataplectics), (3) a medication other than SXB for the treatment of cataplexy (other anticataplectics), or (4) no medication for the treatment of cataplexy (anticataplectic naive). Other medications for the treatment of cataplexy were primarily antidepressants, and included selective serotonin-norepinephrine reuptake inhibitors (SSNRIs)/selective norepinephrine reuptake inhibitors (SNRIs; n = 23), tricyclic antidepressants (TCAs; n = 13), selective serotonin reuptake inhibitors (SSRIs; n = 14), pitolisant (n = 6), and antidepressants with other mechanisms of action (n = 2). Other anticataplectics were to be discontinued by week 10 of OLOTTP.
Key exclusion criteria were narcolepsy secondary to another medical condition, restless legs syndrome requiring treatment other than iron supplementation, uncontrolled hypothyroidism, history of seizures (other than early childhood febrile seizures), head trauma associated with loss of consciousness within the past 5 years, clinically significant parasomnias, untreated or inadequately treated sleep-disordered breathing, and succinic semialdehyde dehydrogenase deficiency. Those with major depression or any history of psychotic disorders were excluded, as well as participants treated with an antidepressant for cataplexy that could not be discontinued if considered unsafe due to prior history of depression. Abnormal electrocardiogram or clinically significant laboratory findings disqualified candidates from participation, as did a positive urine screen for benzodiazepines or drugs of abuse, a positive alcohol test, a history of substance abuse, or unwillingness to refrain from consuming alcohol during the study. Participants could not be pregnant or breastfeeding and had to agree to use contraception from at least 2 months prior to the first dose of study medication until 90 days after the study. Central nervous system–sedating agents were prohibited within 2 weeks prior to enrollment and throughout the study.
Treatment and randomization
Enrolled participants entered the 12-week OLOTTP and initiated LXB treatment, with dose titration as needed to optimize efficacy and tolerability. Participants taking SXB only, or SXB + other anticataplectics, at study entry transitioned from SXB to the same dose and regimen of LXB, gram for gram, and remained on that same dose for the first 2 weeks, after which the dose could be adjusted based on efficacy and tolerability as needed through week 10, with a maximum total nightly dose of 9 g/night. Participants not taking SXB at study entry initiated LXB treatment at 4.5 g/night (divided into two doses taken at bedtime and 2.5–4 h later) and titrated to an optimal dose, with a maximal increase of up to 1.5 g/night/week. The maximum total nightly dose maximum was 9 g/night; titration could proceed as rapidly as 1.5 g/night/week, and optimization of the dose for efficacy and tolerability was encouraged. Participants taking other anticataplectics at study entry, with or without SXB, continued taking the other anticataplectic for the first 2 weeks; taper of other anticataplectics could then begin, with discontinuation by week 10 (if necessary, dose adjustments during the last 2 weeks of OLOTTP could occur). The clinical strategy for the taper and discontinuation of other anticataplectics was individualized for each participant at the discretion of the investigator. This transition and titration schema ensured that participants were taking only LXB for the treatment of cataplexy during the last 2 weeks of OLOTTP, at an efficacious and tolerable dose. The OLOTTP was followed by a 2-week SDP during which efficacy assessments were performed, while each participant received a stable dose of LXB.
At the end of SDP, participants were randomized 1:1 to receive placebo or to continue LXB treatment. Randomization was stratified by treatment for cataplexy at study entry. The randomization code was prepared by a sponsor statistician who was not involved in the study, and randomization was assigned using an interactive web response system. Treatment during DBRWP was blinded to participants, investigators, site personnel, and sponsor personnel.
Outcomes
The primary efficacy endpoint was change in weekly number of cataplexy attacks from during the 2 weeks of SDP to during the 2 weeks of DBRWP, as determined from participants’ daily cataplexy diaries. In addition, the number of cataplexy-free days per week was calculated ([# days with 0 cataplexy attacks/# days with diary data] × 7) as a post hoc analysis.
The key secondary endpoint was change in the Epworth Sleepiness Scale (ESS) [41] score from the end of SDP to the end of DBRWP. Other secondary efficacy endpoints included Patient Global Impression of Change (PGIc) and Clinical Global Impression of Change (CGIc) in narcolepsy overall scores at the end of DBRWP, change in Physical Component Summary and Mental Component Summary scales from the 36-Item Short Form Health Survey Version 2 (SF-36) [42, 43], and crosswalk index and visual analog scale (VAS) scores from the EuroQoL EQ-5D-5L [44] from the end of SDP to the end of DBRWP. All efficacy assessments were administered during scheduled clinic visits, except for cataplexy diaries, which were completed daily by participants.
Safety assessments included treatment-emergent AEs (TEAEs; defined as any AEs that started or worsened in severity on or after the first dose of open-label study drug in the OLOTTP up to the last dose of study drug during any study period + 30 days), clinical laboratory tests (serum chemistry, hematology, and urinalysis), vital signs (temperature, respiration rate, sitting blood pressure, and heart rate), physical examinations, 12-lead electrocardiograms, the Columbia-Suicide Severity Rating Scale (C-SSRS), and the Patient Health Questionnaire-9 (PHQ-9) [45, 46]. Monitoring for TEAEs was continuous after treatment initiation; vital signs were measured and the C-SSRS was administered at screening and treatment initiation, at weeks 1, 4, and 8 of OLOTTP, start and end of SDP, end of DBRWP or early termination, and safety follow-up; laboratory tests, physical examinations, and 12-lead electrocardiograms were performed at screening and end of DBRWP or early termination. Vital signs were monitored and recorded after the participant had been resting and seated for at least 5 min. The PHQ-9 was administered at screening and treatment initiation, weeks 2, 4, 6, 8, and 10 of OLOTTP, start and end of SDP, end of DBRWP or early termination, and safety follow-up. Safety assessments were performed during scheduled clinic visits, except for TEAEs and the PHQ-9, which also were assessed during scheduled telephone visits.
Statistical analysis
The safety population (N = 201) included all participants who took at least one dose of study medication, and the efficacy population (n = 134) included all randomized participants who received at least one dose of study medication during DBRWP and completed at least one set of post-baseline efficacy assessments.
A hierarchical gate-keeping testing strategy was used to control the family-wise type 1 error rate at the 0.05 significance level for two-sided testing across the primary and key secondary efficacy endpoints. All other efficacy endpoints were tested without multiplicity adjustments and are reported with nominal p values.
The primary and key secondary endpoints were assessed with a rank-based analysis of covariance (ANCOVA) model that evaluated treatment group and included prior treatment (medication for cataplexy at study entry) as a fixed effect and weekly number of cataplexy attacks during SDP as a covariate. Missing post-randomization data for both the primary endpoint and the key secondary endpoint were imputed using values from SDP.
PGIc and CGIc ratings were analyzed as response frequencies using the Cochran–Mantel–Haenszel test for the row mean score difference. Quality-of-life assessment scores were analyzed by ANCOVA.
Results
Population
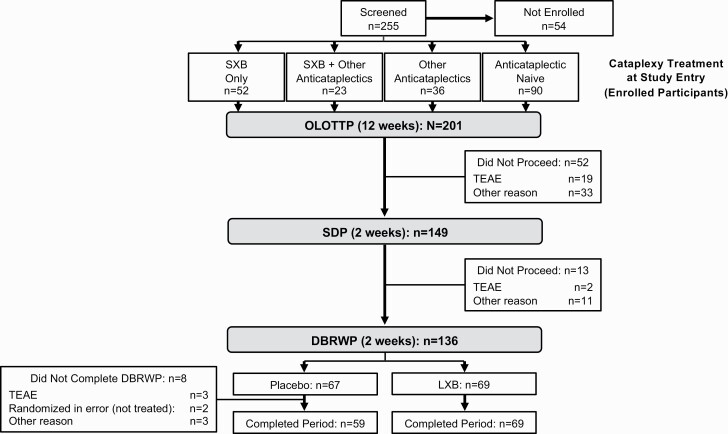
Of 255 candidates who were screened (Figure 2), 201 were enrolled and treated (safety population), 155 completed OLOTTP and 149 entered SDP, 136 were randomized in DBRWP, and 134 were randomized and took at least one dose in DBRWP (efficacy population). All participants randomized to LXB (n = 69) and 59 of 65 participants randomized to take placebo completed DBRWP. Among the 201 participants in the safety population, 74 (36.8%) entered OLE (rollover, n = 47; re-entry, n = 27) and 67 (33.3%) completed OLE.
Figure 2.
Participant disposition. DBRWP, double-blind randomized withdrawal period; LXB, lower-sodium oxybate; OLOTTP, open-label optimized treatment and titration period; SDP, stable-dose period; SXB, sodium oxybate; TEAE, treatment-emergent adverse event.
Discontinuations before SDP (n = 52) were attributed to TEAEs (n = 19), protocol deviation (n = 11, including treatment with disallowed medications/substances [n = 9] and failure to taper anticataplectics by week 10 of OLOTTP [n = 2]), and other reasons (n = 22). Other reasons for discontinuation before SDP (n = 22) included withdrawal by subject (n = 6), noncompliance with study drug (n = 4), investigator decision (n = 3), lost to follow-up (n = 3), lack of efficacy (n = 2), sponsor decision (n = 2), and other (n = 2, without additional information).
The median age of participants in the overall safety population (N = 201; Table 1) was 36.0 years (range, 18–70 years). Most participants were white (88.1%), and the majority were female (60.7%). In addition to cataplexy and EDS, the majority of participants reported having experienced other symptoms of the narcolepsy pentad: disrupted nighttime sleep (63.2%), sleep-related hallucinations (59.7%), and sleep paralysis (59.7%). Common comorbid conditions with possible cardiovascular implications included hypertension (17.4%), obesity (7.0%), hypercholesterolemia (4.5%), diabetes mellitus and type 2 diabetes mellitus (3.0% each), and hyperlipidemia (3.0%). Participant characteristics were well balanced between those randomized to take placebo compared with those randomized to continue taking LXB (Table 1).
Table 1.
Demographic and baseline disease characteristics
| Post-randomization treatment group† | |||
|---|---|---|---|
| Characteristics | Participants (N = 201)* | Placebo (n = 65) | LXB (n = 69) |
| Age (years) | |||
| Mean (SD) | 37.2 (12.21) | 37.8 (12.69) | 37.2 (11.79) |
| Median (range) | 36.0 (18–70) | 35.0 (18–70) | 39.0 (18–68) |
| Sex, n (%) | |||
| Female | 122 (60.7) | 39 (60.0) | 43 (62.3) |
| Male | 79 (39.3) | 26 (40.0) | 26 (37.7) |
| Body mass index (kg/m2)‡ | |||
| Mean (SD) | 28.8 (6.1) | 30.0 (6.0) | 27.3 (5.0) |
| Median (range) | 28.0 (18.7–47.9) | 28.9 (20.7–47.9) | 26.4 (19.1–40.6) |
| Race, n (%) | |||
| White | 177 (88.1) | 59 (90.8) | 64 (92.8) |
| Black or African American | 11 (5.5) | 4 (6.2) | 3 (4.3) |
| Asian | 3 (1.5) | 0 | 0 |
| American Indian or Alaska Native | 0 | 0 | 0 |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 |
| Multiple | 2 (1.0) | 0 | 1 (1.4) |
| Missing | 8 (4.0) | 2 (3.1) | 1 (1.4) |
| Region, n (%) | |||
| Europe | 122 (60.7) | 42 (64.6) | 46 (66.7) |
| North America | 79 (39.3) | 23 (35.4) | 23 (33.3) |
| Past narcolepsy symptoms prior to any narcolepsy treatment, n (%)§ | |||
| Cataplexy | 201 (100) | 65 (100) | 69 (100) |
| Excessive daytime sleepiness | 201 (100) | 65 (100) | 69 (100) |
| Disrupted nighttime sleep | 127 (63.2) | 44 (67.7) | 43 (62.3) |
| Hypnagogic and/or hypnopompic hallucinations | 120 (59.7) | 42 (64.6) | 38 (55.1) |
| Sleep paralysis | 120 (59.7) | 42 (64.6) | 41 (59.4) |
| Cardiovascular/cardiometabolic comorbidities: system organ class, n (%) | |||
| Cardiac disorders | |||
| Atrial fibrillation | 1 (0.5) | 0 | 1 (1.4) |
| Bundle branch block right | 1 (0.5) | 0 | 1 (1.4) |
| Mitral valve incompetence | 1 (0.5) | 0 | 0 |
| Palpitations | 1 (0.5) | 1 (1.5) | 0 |
| Sinus tachycardia | 1 (0.5) | 0 | 0 |
| Supraventricular extrasystoles | 1 (0.5) | 0 | 0 |
| Tricuspid valve incompetence | 1 (0.5) | 0 | 0 |
| Ventricular extrasystoles | 1 (0.5) | 0 | 0 |
| Metabolism and nutrition disorders | |||
| Obesity | 14 (7.0) | 2 (3.1) | 5 (7.2) |
| Hypercholesterolemia | 9 (4.5) | 4 (6.2) | 3 (4.3) |
| Diabetes mellitus|| | 6 (3.0) | 1 (1.5) | 3 (4.3) |
| Type 2 diabetes mellitus | 6 (3.0) | 2 (3.1) | 2 (2.9) |
| Hyperlipidemia | 6 (3.0) | 4 (6.2) | 2 (2.9) |
| Vitamin D deficiency | 4 (2.0) | 1 (1.5) | 2 (2.9) |
| Dyslipidemia | 2 (1.0) | 0 | 0 |
| Glucose tolerance impaired | 2 (1.0) | 1 (1.5) | 0 |
| Hypertriglyceridemia | 2 (1.0) | 1 (1.5) | 1 (1.4) |
| Gluten sensitivity | 1 (0.5) | 0 | 1 (1.4) |
| Gout | 1 (0.5) | 1 (1.5) | 0 |
| Hyperglycemia | 1 (0.5) | 0 | 0 |
| Insulin resistance syndrome | 1 (0.5) | 0 | 1 (1.4) |
| Vascular disorders | |||
| Hypertension | 35 (17.4) | 12 (18.5) | 11 (15.9) |
| Deep vein thrombosis | 1 (0.5) | 0 | 1 (1.4) |
| Raynaud’s phenomenon | 1 (0.5) | 0 | 0 |
| Treatment at study entry, n (%) | |||
| SXB only | 52 (25.9) | 19 (29.2) | 22 (31.9) |
| SXB + other anticataplectics | 23 (11.4) | 8 (12.3) | 6 (8.7) |
| Other anticataplectics | 36 (17.9) | 10 (15.4) | 11 (15.9) |
| Anticataplectic naive | 90 (44.8) | 28 (43.1) | 30 (43.5) |
LXB, lower-sodium oxybate; SD, standard deviation.
*Safety population.
†Efficacy population.
‡ N = 199.
§Participant could have been included in >1 category.
||Type of diabetes mellitus not specified.
Concomitant treatment with stimulants and/or wake-promoting agents
A total of 121 of 201 (60.2%) participants in the safety population were taking stimulants/WPAs at study entry (Supplementary Table S1). Stimulant/WPA treatment was similar in participants randomized to take placebo (60.0%) and in those randomized to continue LXB (59.4%) during DBRWP. At study entry, participants were taking amphetamines (10.9%), methylphenidates (13.4%), modafinil/armodafinil (28.9%), or a combination of these (7.0%). Doses of stimulants/WPAs remained stable throughout the study except for dose changes after study entry observed in two participants (efficacy population): in one participant, the dose of amphetamine was decreased during week 7, and in one participant, amphetamine was discontinued and methylphenidate was initiated in week 14.
Optimization of LXB dosing and tapering/discontinuation of other anticataplectics
Among the 75 participants who entered the study taking SXB (safety population), the median (minimum, maximum) dose of SXB at study entry was 7.5 (4.5, 9.0) g/night. Among participants in the efficacy population (n = 134), the median (minimum, maximum) total stable nightly dose during SDP was 7.5 (3.0, 9.0) g/night. The mean (SD) time to reach a stable dose of LXB was 30.1 (24.6) days, and the median (minimum, maximum) time was 29 (1, 84) days.
Among the 59 participants (safety population) who entered the study taking either SXB + other anticataplectics (n = 23) or other anticataplectics (n = 36), all but three participants completed tapering of those medications by week 10 during OLOTTP; as already noted, two of these participants discontinued the study because of this protocol deviation, whereas the other participant continued. The mean (SD) time to taper/discontinue anticataplectic medications was 33.3 (18.1) days, and the median (minimum, maximum) time was 29 (4, 74) days.
Efficacy
Prior to randomization, median (first quartile [Q1], third quartile [Q3]) number of weekly cataplexy attacks did not differ in participants taking LXB who were randomized to placebo (1.08 [0.00, 7.88]) or to continue LXB treatment (1.00 [0.00, 4.45]). During the 2-week DBRWP, there was statistically significant worsening in the weekly number of cataplexy attacks in participants randomized to placebo, and no change in participants randomized to continue LXB treatment. The median (Q1, Q3) change in weekly number of cataplexy attacks was 2.35 (0.00, 11.61) in the placebo group versus 0.00 (−0.49, 1.75) in the LXB group (Figure 3), which was associated with a significant (p < 0.0001) location shift (95% CI) of −3.31 (−6.04, −1.50); mean (SD) change in weekly number of cataplexy attacks was 11.46 (24.751) in the placebo group versus 0.12 (5.772) in the LXB group (Table 2).
Figure 3.
Change in weekly number of cataplexy attacks from the stable-dose period to the double-blind randomized withdrawal period (efficacy population). The bottom and top edges of the box indicate the first and third quartiles, the line inside the box is the median, and the marker inside the box is the mean. Any points that are a distance of more than 1.5 times the interquartile range from the box are considered outliers and are represented by circles; the whiskers extending from the box indicate the minimum and maximum after removing those outliers. LXB, lower-sodium oxybate; Q1, first quartile; Q3, third quartile. aRank-based analysis of covariance.
Table 2.
Primary and key secondary endpoints (efficacy population)
| Endpoint | Placebo (n = 65) | LXB (n = 69) |
| Change in weekly number of cataplexy attacks from SDP to DBRWP (primary efficacy endpoint) | ||
| Mean (SD) | 11.46 (24.751) | 0.12 (5.772) |
| Median | 2.35 | 0.00 |
| Q1, Q3 | 0.00, 11.61 | −0.49, 1.75 |
| Location shift* | −3.308 | |
| 95% CI* | −6.044, −1.500 | |
| p value† | <0.0001 | |
| Change in ESS score from SDP to DBRWP (key secondary efficacy endpoint) | ||
| Mean (SD) | 3.0 (4.68) | 0.0 (2.90) |
| Median | 2.0 | 0.0 |
| Q1, Q3 | 0.0, 5.0 | −1.0, 1.0 |
| Location shift* | −2.00 | |
| 95% CI* | −4.00, −1.00 | |
| p value‡ | <0.0001 |
ANCOVA, analysis of covariance; CI, confidence interval; DBRWP, double-blind randomized withdrawal period; ESS, Epworth Sleepiness Scale; LXB, lower-sodium oxybate; Q1, first quartile; Q3, third quartile; SD, standard deviation; SDP, stable-dose period.
*Location shift between two treatment groups and asymptotic 95% CI from Hodges–Lehmann estimate (LXB–placebo).
†From a rank-based ANCOVA model including the change in average weekly number of cataplexy attacks from the 2 weeks of the SDP to the 2 weeks of the DBRWP as response variable, prior treatment group and study treatment group as fixed effects, and average weekly number of cataplexy attacks during the 2 weeks of the SDP as covariate.
‡From a rank-based ANCOVA model including the change in ESS total score from the end of the SDP to the end of the DBRWP as response variable, prior treatment group and study treatment group as fixed effects, and ESS total score at the end of the SDP as covariate.
At study entry, the median number of cataplexy-free days per week was higher in participants taking SXB (SXB only, 5.8; SXB + other anticataplectics, 6.4) than in participants not taking SXB (other anticataplectics, 4.0; anticataplectic naive, 3.5). Prior to randomization, the median number of cataplexy-free days per week did not differ in participants randomized to placebo or to continue LXB treatment (median, 6.0 days/week for both groups); during the DBRWP, median cataplexy-free days per week decreased to 3.5 in week 1 and week 2 for participants randomized to placebo and remained above 5 days/week for participants randomized to continue LXB treatment (week 1, 5.0; week 2, 5.6).
Prior to randomization, median (Q1, Q3) ESS scores did not differ in participants taking LXB who were randomized to placebo or to continue LXB treatment (13.0 [9.0, 17.0] for placebo and 14.0 [10.0, 19.0] for LXB). As with cataplexy, there was statistically significant worsening of EDS in participants randomized to placebo, and no change in participants randomized to LXB. At the end of DBRWP, the change in median (Q1, Q3) ESS score from SDP was 2.0 (0.0, 5.0) for participants randomized to placebo and 0.0 (−1.0, 1.0) for participants randomized to LXB (Figure 4), which was associated with a significant (p < 0.0001) location shift (95% CI) of −2.0 (−4.0, −1.0); the change in mean (SD) ESS score was 3.0 (4.68) in the placebo group versus 0.0 (2.90) in the LXB group (Table 2).
Figure 4.
Change in the ESS score from the end of the stable-dose period to the end of the double-blind randomized withdrawal period (efficacy population). The bottom and top edges of the box indicate the first and third quartiles, the line inside the box is the median, and the marker inside the box is the mean. Any points that are a distance of more than 1.5 times the interquartile range from the box are considered outliers and are represented by circles; the whiskers extending from the box indicate the minimum and maximum after removing those outliers. ESS, Epworth Sleepiness Scale; LXB, lower-sodium oxybate; Q1, first quartile; Q3, third quartile. aRank-based analysis of covariance.
The distribution of PGIc ratings for narcolepsy overall demonstrated that more participants randomized to placebo experienced worsening of symptoms compared with those randomized to continue LXB treatment (nominal p < 0.0001; Table 3), with a greater percentage of participants randomized to placebo rating their narcolepsy as “much worse” or “very much worse” compared with participants randomized to continue LXB treatment (44.6% vs 4.3%; nominal p < 0.0001). Similarly, the distribution of CGIc ratings for narcolepsy overall demonstrated worsening in more participants randomized to placebo (nominal p < 0.0001), with a greater percentage of participants randomized to placebo rated as “much worse” or “very much worse” compared with the percentage of participants randomized to continue LXB treatment (60.0% vs 5.9%; nominal p < 0.0001).
Table 3.
Patient and clinical global impression of change ratings for narcolepsy overall from the end of the stable-dose period to the end of the double-blind randomized withdrawal period
| Ratings | Placebo, n (%) | LXB, n (%) |
|---|---|---|
| PGIc* | 65 | 69 |
| Very much better | 1 (1.5) | 1 (1.4) |
| Much better | 1 (1.5) | 12 (17.4) |
| A little better | 3 (4.6) | 6 (8.7) |
| No change | 10 (15.4) | 39 (56.5) |
| A little worse | 21 (32.3) | 8 (11.6) |
| Much worse | 20 (30.8) | 2 (2.9) |
| Very much worse | 9 (13.8) | 1 (1.4) |
| p value† | <0.0001 | |
| CGIc‡ | 65 | 68 |
| Very much improved | 0 (0.0) | 1 (1.5) |
| Much improved | 0 (0.0) | 12 (17.6) |
| Minimally improved | 3 (4.6) | 9 (13.2) |
| No change | 11 (16.9) | 34 (50.0) |
| Minimally worse | 12 (18.5) | 8 (11.8) |
| Much worse | 28 (43.1) | 4 (5.9) |
| Very much worse | 11 (16.9) | 0 (0.0) |
| p value† | <0.0001 |
CGIc, Clinical Global Impression of Change; LXB, lower-sodium oxybate; PGIc, Patient Global Impression of Change.
*Results are shown for participants with data for ≥1 PGIc survey.
†Cochran–Mantel–Haenszel row means score test; due to no adjustments for multiplicity (or multiple comparisons), the p values presented are nominal.
‡Results are shown for participants with data for ≥1 CGIc survey.
In participants randomized to placebo, a deterioration in the quality of life was observed from the end of SDP to the end of DBRWP compared with those randomized to continue LXB treatment. Declines in the median Physical Component Summary and least-squares mean Mental Component Summary scales of the SF-36 were greater (nominal p = 0.0174 and nominal p = 0.0331, respectively) in participants randomized to placebo compared with participants randomized to continue LXB treatment (Table 4). Several SF-36 subscales worsened more in the placebo group relative to the LXB group (Supplementary Figure S1): vitality (nominal p < 0.0001), role-physical (nominal p = 0.0092), and general health (nominal p = 0.0277). On the EQ-5D-5L, although there were minimal changes in the crosswalk index score, the VAS score (a global self-assessment of current health status) decreased in participants randomized to placebo and remained stable in those randomized to continue LXB treatment (nominal p = 0.0056; Table 4).
Table 4.
Change in quality-of-life scores from the end of SDP to the end of DBRWP (efficacy population)
| Score | Placebo (n = 65) | LXB (n = 69) |
|---|---|---|
| SF-36 physical component summary | ||
| End of SDP | ||
| Median (Q1, Q3) | 51.5 (44.0, 57.4) | 53.9 (46.9, 58.3)* |
| End of DBRWP | ||
| Median (Q1, Q3) | 50.7 (43.2, 55.8) | 53.7 (47.2, 58.6) |
| Change from SDP to DBRWP | ||
| Median (Q1, Q3) | −1.9 (−3.5, 1.7) | 0.0 (−2.1, 2.4)* |
| Location shift (95% CI)† | 1.4 (−0.1, 2.8) | |
| p value‡ | 0.0174 | |
| SF-36 Mental Component Summary | ||
| End of SDP | ||
| Mean (SD) | 48.1 (9.9) | 45.2 (12.2)* |
| End of DBRWP | ||
| Mean (SD) | 46.0 (9.9) | 46.8 (11.3) |
| Change from SDP to DBRWP | ||
| Mean (SD) | −2.0 (7.8) | 1.3 (6.6) |
| LS mean (SE)‡ | −2.2 (0.9) | 0.4 (0.9) |
| LS mean difference (SE; 95% CI)‡ | 2.5 (1.2; 0.2, 4.8) | |
| p value‡ | 0.0331 | |
| EQ-5D-5L Crosswalk Index | ||
| End of SDP | ||
| Median (Q1, Q3) | 0.89 (0.80, 1.00) | 0.87 (0.80, 1.00)§ |
| End of DBRWP | ||
| Median (Q1, Q3) | 0.88 (0.81, 1.00) | 0.91 (0.83, 1.00) |
| Change from SDP to DBRWP | ||
| Median (Q1, Q3) | 0.00 (−0.05, 0.03) | 0.00 (−0.01, 0.03)§ |
| Location shift (95% CI)† | 0.000 (0.000, 0.014) | |
| p value|| | 0.3918 | |
| EQ-5D-5L Visual Analog Scale | ||
| End of SDP | ||
| Median (Q1, Q3) | 75.0 (70.0, 90.0) | 80.0 (70.0, 90.0) |
| End of DBRWP | ||
| Median (Q1, Q3) | 75.0 (60.0, 85.0) | 80.0 (70.0, 90.0) |
| Change from SDP to DBRWP | ||
| Median (Q1, Q3) | −5.0 (−10.0, 5.0) | 0.0 (0.0, 5.0) |
| Location shift (95% CI)† | 5.0 (0.0, 7.0) | |
| p value|| | 0.0056 |
ANCOVA, analysis of covariance; CI, confidence interval; DBRWP, double-blind randomized withdrawal period; EQ-5D-5L, EuroQoL EQ-5D-5L; LS, least squares; LXB, lower-sodium oxybate; Q1, first quartile; Q3, third quartile; SD, standard deviation; SDP, stable-dose period; SE, standard error; SF-36, 36-Item Short Form Health Survey Version 2.
*n = 67.
†The location shift between 2 treatment groups and 95% asymptotic CI are from Hodges–Lehmann estimate.
‡The LS means, SEs, LS mean difference, 95% CI, and p value were obtained from an ANCOVA model including the change in the scale-normalized score/overall component score from the end of SDP to the end of DBRWP as response variable; prior treatment group and study treatment group as fixed effects; and scale-normalized score/overall component score at the end of SDP as covariate.
§ n = 68.
||The p value was obtained from a rank-based ANCOVA model including the change in the crosswalk index score/visual analog scale value from the end of SDP to the end of DBRWP as response variable; prior treatment group and study treatment group as fixed effects; and crosswalk index score/visual analog scale value at the end of SDP as covariate.
Safety
At least one TEAE was reported by 76.1% of participants while receiving LXB (Table 5). TEAEs were reported less frequently by participants taking SXB only at study entry compared with other prior-treatment groups. The most common TEAEs reported were headache (20.4%), nausea (12.9%), dizziness (10.4%), worsening cataplexy (10.0%), decreased appetite (7.5%), nasopharyngitis (7.5%), influenza (7.0%), diarrhea (5.5%), and vomiting (5.0%). Nausea and dizziness occurred more frequently in participants who were not taking SXB at study entry. Incidences of enuresis and somnambulism were 3.5% and 2.0% of participants, respectively. Worsening cataplexy was reported as a TEAE by 20 (10.0%) participants; 17 of the 20 were tapering other anticataplectics, and 3 were cataplexy treatment naive at study entry; most occurred during OLOTTP. The most common TEAEs leading to discontinuation of LXB during the main study were worsening cataplexy (7/201; 3.5%), nausea (3/201; 1.5%), and anxiety, depressed mood, depression, headache, and irritability (each 2/201; 1.0%). During DBRWP, TEAEs were reported by 33.8% of participants in the placebo group (22/65) and 18.8% of participants in the LXB group (13/69) (Supplementary Table S2). Specific TEAEs in >1 participant in the placebo or LXB groups during DBRWP were, respectively, somnolence (9.2% and 0%), worsening cataplexy (7.7% and 1.4%), nasopharyngitis (3.1% and 2.9%), sleep disorder (4.6% and 0%), influenza (3.1% and 0%), insomnia (3.1% and 0%), nightmare (3.1% and 0%), poor quality sleep (3.1% and 0%), and urinary tract infection (0% and 2.9%). During DBRWP, three participants (all taking placebo) discontinued because of TEAEs (somnolence and sleep disorder [unspecified], n = 1; influenza, n = 1; muscle enzyme increased, n = 1 [a serious TEAE, noted below]). Most TEAEs during the main study were mild or moderate in severity: 10 participants (5.0%) reported severe TEAEs.
Table 5.
TEAEs in the main study (≥5% of total participants) by treatment at study entry, excluding placebo data (safety population)
| TEAE, n (%) | SXB only (n = 52) | SXB + other anticataplectics (n = 23) | Other anticataplectics (n = 36) | Anticataplectic naive (n = 90) | Total (N = 201) |
|---|---|---|---|---|---|
| Participants with ≥1 TEAE | 31 (59.6) | 20 (87.0) | 30 (83.3) | 72 (80.0) | 153 (76.1) |
| Preferred term in ≥5% of total participants | |||||
| Headache | 7 (13.5) | 3 (13.0) | 7 (19.4) | 24 (26.7) | 41 (20.4) |
| Nausea | 2 (3.8) | 1 (4.3) | 7 (19.4) | 16 (17.8) | 26 (12.9) |
| Dizziness | 1 (1.9) | 1 (4.3) | 6 (16.7) | 13 (14.4) | 21 (10.4) |
| Cataplexy* | 0 | 11 (47.8) | 6 (16.7) | 3 (3.3) | 20 (10.0) |
| Decreased appetite | 0 | 1 (4.3) | 2 (5.6) | 12 (13.3) | 15 (7.5) |
| Nasopharyngitis | 2 (3.8) | 1 (4.3) | 5 (13.9) | 7 (7.8) | 15 (7.5) |
| Influenza | 5 (9.6) | 3 (13.0) | 3 (8.3) | 3 (3.3) | 14 (7.0) |
| Diarrhea | 4 (7.7) | 0 | 0 | 7 (7.8) | 11 (5.5) |
| Vomiting | 1 (1.9) | 0 | 4 (11.1) | 5 (5.6) | 10 (5.0) |
SXB, sodium oxybate; TEAE, treatment-emergent adverse event.
*TEAEs of cataplexy were reported when cataplexy worsened when compared with the study baseline.
Serious TEAEs were reported by six participants during the main study, including three during OLOTTP, one during SDP, and two reported the day after 2 weeks of placebo treatment in DBRWP. Serious TEAEs were considered study medication related in two participants. One participant experienced confusion and hallucinations after inadvertently taking the second dose of 4.5 g of LXB shortly after the first dose of 4.5 g (during OLOTTP). The participant was hospitalized and discharged after resolution of symptoms. The other participant experienced muscle enzyme increase (the day after 2 weeks of placebo treatment in DBRWP) in the context of intense physical exertion. The participant was hospitalized and discharged after resolution. No deaths were reported.
No participants reported TEAEs of suicidal ideation or suicidal behavior. However, two participants endorsed items on the C-SSRS. One participant who endorsed an item on the C-SSRS had reported a history of suicidal ideation at screening; the participant was discontinued from study participation when the protocol deviation was identified. The second participant had a history of depression; this participant reported a TEAE of depression during OLOTTP but did not endorse any items on the C-SSRS at that time. After study medication was discontinued in response to the TEAE of depression, this participant endorsed two items on the C-SSRS.
Mean PHQ-9 scores at beginning of OLOTTP were higher, indicating more severe depressive symptoms, in participants not taking SXB at study entry (SXB only, 5.1; SXB + other anticataplectics, 5.3; other anticataplectics, 8.3; anticataplectic naive, 7.7). By the end of SDP, mean PHQ-9 scores ranged from 4.8 to 5.9 across groups by prior treatment. The improved scores were generally maintained through the end of DBRWP.
At study entry, observed mean (SD) systolic blood pressure values were higher in participants taking SXB (126.2 [15.05] mmHg and 129.5 [17.55] mmHg in the SXB only and SXB + other anticataplectics groups, respectively) than in participants not taking SXB at study entry (123.3 [14.44] mmHg and 122.3 [13.54] mmHg in the other anticataplectics and anticataplectic naive groups, respectively). Mean (SD) diastolic blood pressure values at study entry were 80.6 (9.20), 82.4 (10.88), 80.1 (9.76), and 78.8 (9.13) in the SXB only, SXB + other anticataplectics, other anticataplectics, and anticataplectic naive groups, respectively.
Discussion
This study was a placebo-controlled, double-blind, randomized withdrawal trial in adult participants with narcolepsy with cataplexy that demonstrated the efficacy of LXB for the treatment of cataplexy and EDS in narcolepsy with an overall safety profile generally consistent with the established safety profile of SXB [6, 13, 14, 16, 47]. The study design allowed enrollment of participants currently treated with SXB and participants currently treated with anticataplectics other than SXB (primarily antidepressants), and the taper and discontinuation of those anticataplectic agents was incorporated into the study and not performed prior to study entry. Thus, this study is representative of potential treatment strategies that would occur in clinical practice: transition from SXB to LXB, initiation of LXB, and cross-titration with taper and discontinuation of other medications that are prescribed off-label for the treatment of cataplexy. A majority (60.2%) of participants in the safety population were taking stimulants/WPAs at study entry, including amphetamines, methylphenidates, and modafinil/armodafinil. Percentages taking these agents were similar in the post-randomization (placebo and LXB) groups, and stimulant/WPA treatment remained consistent throughout the study in all but two participants. The results of this study demonstrate the efficacy and safety of LXB in participants with narcolepsy across prior treatment histories. In addition, the results demonstrate the feasibility of transitioning to LXB from other anticataplectics with less well-established efficacy [9] and known potential for rebound even with gradual taper and discontinuation [48], and the feasibility of initiating treatment with LXB in cataplexy treatment–naive individuals.
Participants in the present study who were randomized to placebo experienced a significant increase in the average weekly number of cataplexy attacks and significant worsening of EDS during DBRWP versus those randomized to continue treatment with LXB. The poorer outcomes for those randomized to placebo were also reflected in significantly poorer participant and investigator ratings (PGIc and CGIc) of narcolepsy overall. Worsening of quality of life measures in participants taking placebo during DBRWP was also observed. Worsening in SF-36 subscales of role-physical, general health, and vitality is consistent with SF-36 impairments reported previously in patients with narcolepsy [49, 50]. Although differences were not identified by the EQ-5D-5L crosswalk index, there were differences in VAS scores, which is consistent with previous findings indicating that the EQ-5D-5L may not be as sensitive as other measures of quality of life in patients with sleep disorders [51].
The overall safety profile of LXB in this trial was as expected based on prior experience with SXB [6]. Prior SXB exposure likely contributed to the tolerability of LXB, as the most common TEAEs (headache, nausea, and dizziness) were reported less frequently in participants who were taking SXB at study entry. In contrast to the anticipated benefits of continuing LXB therapy, randomized withdrawal to placebo treatment was associated with higher incidences of somnolence, worsening cataplexy, and sleep problems. Under fasting conditions in two single-dose studies in healthy volunteers, the incidences of nausea and vomiting were lower with LXB than with SXB; with that exception, the overall safety profiles of LXB and SXB in healthy volunteers were similar [38]. In a pooled analyses of the two studies, incidences of nausea and vomiting were positively related with Cmax, suggesting that lower peak plasma concentration may have been the reason for better gastrointestinal tolerability in the studies in healthy individuals. The design of the present study precluded comparison of gastrointestinal tolerability of LXB and SXB.
Discontinuation of other anticataplectics during OLOTTP was a potential contributor to the incidence of TEAEs. Adverse effects associated with discontinuation of TCAs [48], SNRIs [52], and SSRIs [53], including nausea, light-headedness, chills, aches, paresthesia, insomnia, and electric shock–like experiences, are well known, and were recently codified as antidepressant discontinuation syndrome (ADDS) in the Diagnostic and Statistical Manual of Mental Disorders, 5th ed. [54]. The overall incidence of TEAEs during the main study was higher in participants taking other anticataplectics at study entry compared with participants who were not (SXB only, 59.6%; SXB + other anticataplectics, 87.0%; other anticataplectics, 83.3%; anticataplectic naive, 80.0%). However, TEAEs of nausea and dizziness, which were among those reported by ≥5% of participants during the main study, did not occur more frequently in those taking other anticataplectics at study entry compared with those who were not. TEAEs of worsening cataplexy in this study occurred mainly in participants taking other anticataplectics at study entry (17/20), and occurred during tapering and discontinuation of these other anticataplectics. In this study, serious TEAEs were uncommon, usually not considered related to LXB, and resolved with appropriate medical care.
Several limitations of this study should be noted. The study did not include a direct comparison of LXB and SXB; however, participants previously taking SXB only were able to successfully transition to LXB during OLOTTP. In participants transitioning to LXB from SXB, treatment was initiated at the same dose gram-for-gram, with the instruction to titrate if needed to optimize efficacy and tolerability. Despite the opportunity to titrate, the same dose was maintained in the majority of participants.
Approximately one-quarter of the participants (52/201) discontinued before SDP, including 19 who discontinued due to TEAEs. The remaining participants discontinued due to protocol deviations, withdrawal by participant, or other reasons. The discontinuations due to TEAEs were most common in participants who were taking anticataplectics other than SXB at study entry. In those participants, treatment with other anticataplectics was tapered and stopped during OLOTTP, which could have led to rebound effects that might explain the TEAEs of worsening cataplexy and the higher incidence of discontinuations because of TEAEs. The effects of discontinuation of anticataplectics on worsening cataplexy can be protracted; in a study of SXB, starting during a 3-week tapering and discontinuation period, participants experienced elevated rates of cataplexy that peaked 40–60 days (5.7–8.6 weeks) later and did not return to previous levels until another 28 days (4 weeks) had passed [48].
The randomized withdrawal design of the current study inherently enriches for responders to the drug under evaluation, and is intended to increase study feasibility [55]. This responder population was further enriched by requiring participants taking SXB at study entry to have a documented history of clinical improvement. To minimize potential bias arising from prior treatments, randomization was stratified by treatment for cataplexy at study entry. In order to minimize the duration of placebo exposure, the DBRWP was 2 weeks in duration, potentially limiting the magnitude of worsening observed in participants taking placebo. As already noted, participants entering the study taking other off-label anticataplectics experienced increased cataplexy during taper and discontinuation of these medications; this increase may have extended into the DBRWP, thereby blunting differences between LXB and placebo. It is unknown how quickly cataplexy returns after abrupt cessation of long-term treatment. Because of robust and compelling evidence that SXB improves DNS in patients with narcolepsy [56–61], neither symptoms of DNS nor polysomnography data were collected as outcomes in this study.
Rigorous evaluation of changes in blood pressure with LXB in patients previously treated with SXB was not performed, as this evaluation was outside the scope of this study. Blood pressure measurements were recorded during vital signs assessments at study clinic visits as part of the evaluation of the overall safety profile of LXB, but without ambulatory blood pressure monitoring as would occur in a study intended to examine changes in blood pressure. Additional research under well-controlled conditions could provide more definitive data; future studies designed to examine changes in blood pressure are needed.
Participants in this study exhibited cardiovascular and cardiometabolic comorbidities that were observed in prior research [3, 26, 27]. Given these cardiovascular risk factors, the 92% reduction of sodium intake with LXB relative to SXB treatment may be beneficial for mitigating the risk of cardiovascular events in a population with narcolepsy. The absolute magnitude of sodium reduction at the recommended doses of LXB relative to SXB is 1,013–1,509 mg/day. American College of Cardiology/American Heart Association guidelines for the general population suggests that a reduction of 1,000 mg/day in sodium intake may decrease systolic blood pressure by 2–3 mmHg in normotensive individuals and 5–6 mmHg in those with hypertension [62]. It has been estimated from a model based on the US general population ≥35 years of age that a reduction of 1,200 mg/day in sodium intake would decrease incident coronary heart disease, new and recurring myocardial infarction, and incident stroke by as much as 9.6%, 12.0%, and 7.8%, respectively [63]. However, the impact on cardiovascular disease risk of the reduction in sodium content of LXB in patients previously treated with SXB could not be assessed due to the relatively short duration of the study.
In conclusion, the efficacy of LXB for the treatment of cataplexy and EDS in adults with narcolepsy was demonstrated in this placebo-controlled, double-blind, randomized withdrawal study. Statistically significant worsening in cataplexy and EDS was observed in patients randomized to placebo compared with participants randomized to continue LXB treatment. Participant and clinician ratings of change in narcolepsy overall indicated worsening in participants randomized to placebo compared with those randomized to LXB. The overall safety profile of LXB was consistent with SXB. LXB represents a novel oxybate treatment option for patients with narcolepsy, with the benefit of 92% less sodium.
Supplementary Material
Acknowledgments
The authors would like to thank the study participants, study investigators, and study staff for their contributions to this research. This study was sponsored by Jazz Pharmaceuticals. Under the direction of the authors, Michael J. Theisen, Ph.D., an employee of Peloton Advantage, LLC, an OPEN Health company, provided medical writing and editorial support. Jazz Pharmaceuticals provided funding to Peloton Advantage for medical writing and editorial support.
Clinical trial: A Study of the Efficacy and Safety of JZP-258 in Subjects With Narcolepsy With Cataplexy; https://clinicaltrials.gov/ct2/show/NCT03030599, ClinicalTrials.gov identifier: NCT03030599
Disclosure statement
Financial disclosure: Richard K. Bogan has served on the speakers’ bureau and participated in advisory boards for Jazz Pharmaceuticals and Harmony Biosciences and has received research/grant support from Jazz Pharmaceuticals, Harmony Biosciences, Balance Therapeutics, Axsome Therapeutics, Merck, and Avadel Pharmaceuticals. Michael J. Thorpy has received research/grant support and consultancy fees from Jazz Pharmaceuticals, Harmony Biosciences, Balance Therapeutics, Axsome Therapeutics, and Avadel Pharmaceuticals. Yves Dauvilliers is a consultant for and has participated in advisory boards for Jazz Pharmaceuticals, UCB Pharma, Flamel Technologies, Idorsia, Takeda, Theranexus, and Bioprojet. Markku Partinen has participated in advisory boards of AOP Orphan, Bioprojet, UCB, and Umecrine. He has participated in clinical trials for Bioprojet, Jazz Pharmaceuticals, MSD, and Umecrine. Rafael Del Rio Villegas has participated in advisory boards for Bioprojet and trials for Jazz Pharmaceuticals and Bioprojet. Nancy Foldvary-Schaefer has served on an advisory committee for Jazz Pharmaceuticals and participated in clinical trials for Suven and Takeda. Roman Skowronski is an employee of Jazz Pharmaceuticals who, in the course of his employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Lihua Tang is a former consultant with Jazz Pharmaceuticals. Franck Skobieranda is an employee of Jazz Pharmaceuticals who, in the course of his employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Karel Šonka has served on the speakers’ bureau for Sanofi, Angelini, and Stada and participated in advisory boards for UCB and in clinical trials for Jazz Pharmaceuticals, Flamel-Avadel, and Luitpold Pharmaceuticals.
Nonfinancial disclosure: None.
Data availability
All relevant data are provided within the manuscript and supporting files.
References
- 1. Ohayon MM. Epidemiology of narcolepsy. In: Bassetti C, Mignot E, Billard M, eds. Narcolepsy and Hypersomnia. Milton Park, UK: Taylor and Francis; 2007:125–132. [Google Scholar]
- 2. Ruoff CM, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry. 2017;78(2):171–176. [DOI] [PubMed] [Google Scholar]
- 3. Black J, et al. Medical comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study. Sleep Med. 2017;33:13–18. [DOI] [PubMed] [Google Scholar]
- 4. American Academy of Sleep Medicine. Narcolepsy type 1. The International Classification of Sleep Disorders. 3rd ed. 2014. Available at: https://learn.aasm.org/Public/Catalog/Details.aspx?id=%2FgqQVDMQIT%2FEDy86PWgqgQ%3D%3D&returnurl=%2fUsers%2fUserOnlineCourse.aspx%3fLearningActivityID%3d%252fgqQVDMQIT%252fEDy86PWgqgQ%253d%253d. Accessed November 12, 2019. [Google Scholar]
- 5. Roth T, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med. 2013;9(9):955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xyrem® (Sodium Oxybate) Oral Solution Prescribing Information. Palo Alto, CA: Jazz Pharmaceuticals; 2018. [Google Scholar]
- 7. Xyrem Summary of Product Characteristics. Berkshire: UCB Pharma; 2019. [Google Scholar]
- 8. Xyrem Product Monograph Including Patient Medication Information. Dublin: Jazz Pharmaceuticals Ireland Limited; 2018. [Google Scholar]
- 9. Morgenthaler TI, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Billiard M, et al. EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13(10):1035–1048. [DOI] [PubMed] [Google Scholar]
- 11. Barateau L, et al. Treatment options for narcolepsy. CNS Drugs. 2016;30(5):369–379. [DOI] [PubMed] [Google Scholar]
- 12. Lopez R, et al. French consensus. management of patients with hypersomnia: which strategy? Rev Neurol (Paris). 2017;173(1–2):8–18. [DOI] [PubMed] [Google Scholar]
- 13. U.S. Xyrem Multicenter Study Group. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25(1):42–49. [PubMed] [Google Scholar]
- 14. U.S. Xyrem Multicenter Study Group. Sodium oxybate demonstrates long-term efficacy for the treatment of cataplexy in patients with narcolepsy. Sleep Med. 2004;5(2):119–123. [DOI] [PubMed] [Google Scholar]
- 15. Xyrem International Study Group. Further evidence supporting the use of sodium oxybate for the treatment of cataplexy: a double-blind, placebo-controlled study in 228 patients. Sleep Med. 2005;6(5):415–421. [DOI] [PubMed] [Google Scholar]
- 16. Black J, et al. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep. 2006;29(7):939–946. [DOI] [PubMed] [Google Scholar]
- 17. Plazzi G, et al. Treatment of paediatric narcolepsy with sodium oxybate: a double-blind, placebo-controlled, randomised-withdrawal multicentre study and open-label investigation. Lancet Child Adolesc Health. 2018;2(7):483–494. [DOI] [PubMed] [Google Scholar]
- 18. Institute of Medicine. Sodium Intake in Populations: Assessment of Evidence. Washington, DC: The National Academies Press; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gardener H, et al. Dietary sodium and risk of stroke in the northern Manhattan study. Stroke. 2012;43(5):1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. George J, et al. Association between cardiovascular events and sodium-containing effervescent, dispersible, and soluble drugs: nested case-control study. BMJ. 2013;347:f6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Heart Association. Why should I limit sodium?2017. Available at: https://www.heart.org/-/media/data-import/downloadables/pe-abh-why-should-i-limit-sodium-ucm_300625.pdf. Accessed July 18, 2019.
- 22. National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Sodium and Potassium. Washington, DC: The National Academies Press; 2019. [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention. Get the facts: sodium and the dietary guidelines. 2017. Available at: https://www.cdc.gov/salt/pdfs/sodium_dietary_guidelines.pdf. Accessed October 4, 2019.
- 24. European Commission. Survey on members states’ implementation of the EU salt reduction framework. 2012. Available at: https://ec.europa.eu/health/sites/health/files/nutrition_physical_activity/docs/salt_report1_en.pdf. Accessed November 12, 2019.
- 25. Perrin G, et al. Cardiovascular risk associated with high sodium-containing drugs: a systematic review. PLoS One. 2017;12(7):e0180634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14(6):488–492. [DOI] [PubMed] [Google Scholar]
- 27. Cohen A, et al. Comorbidities in a community sample of narcolepsy. Sleep Med. 2018;43:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dauvilliers Y, et al. Non-dipping blood pressure profile in narcolepsy with cataplexy. PLoS One. 2012;7(6):e38977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McAlpine CS, et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566(7744):383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Medic G, et al. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poli F, et al. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep. 2009;32(11):1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grimaldi D, et al. Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: effects of hypocretin deficiency in humans. Sleep. 2012;35(4):519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Domínguez F, et al. Association of sleep duration and quality with subclinical atherosclerosis. J Am Coll Cardiol. 2019;73(2):134–144. [DOI] [PubMed] [Google Scholar]
- 34. Thurston RC, et al. Sleep characteristics and carotid atherosclerosis among midlife women. Sleep. 2017;40(2). doi: 10.1093/sleep/zsw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Honda Y, et al. Increased frequency of non-insulin-dependent diabetes mellitus among narcoleptic patients. Sleep. 1986;9(1 Pt 2):254–259. [DOI] [PubMed] [Google Scholar]
- 36. Ben-Dov IZ, et al. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension. 2007;49(6):1235–1241. [DOI] [PubMed] [Google Scholar]
- 37. XywavTM (calcium, magnesium, potassium, and sodium oxybates) oral solution, CIII Prescribing Information. Palo Alto, CA: Jazz Pharmaceuticals; 2020. [Google Scholar]
- 38. Chen C, et al. Pharmacokinetics, relative bioavailability, and food effect of JZP-258 and sodium oxybate: results of two phase 1, open-label, randomised crossover studies in healthy volunteers [poster]. Presented at: Biennial World Sleep Congress; September 20–25, 2019; Vancouver, Canada. [Google Scholar]
- 39. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 40. American Psychiatric Association. Narcolepsy. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:372–378. [Google Scholar]
- 41. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 42. Hays RD, et al. Construct validity of MOS health measures. In: Stewart AL, Ware JE, eds. Measuring Functioning and Well-being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992:325–342. [Google Scholar]
- 43. Ware JE Jr, et al. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 44. van Reenen M, et al. EQ-5D-5L User Guide. Rotterdam: EuroQol Research Foundation; 2015. [Google Scholar]
- 45. Posner K, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kroenke K, et al. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xyrem International Study Group. A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med. 2005;1(4):391–397. [PubMed] [Google Scholar]
- 48. Ristanovic RK, et al. Exacerbation of cataplexy following gradual withdrawal of antidepressants: manifestation of probable protracted rebound cataplexy. Sleep Med. 2009;10(4):416–421. [DOI] [PubMed] [Google Scholar]
- 49. Daniels E, et al. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10(1):75–81. [DOI] [PubMed] [Google Scholar]
- 50. Bogan R, et al. Evaluation of quality of life in patients with narcolepsy treated with sodium oxybate: use of the 36-item short-form health survey in a clinical trial. Neurol Ther. 2016;5(2):203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jenkinson C, et al. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6(3):199–204. [DOI] [PubMed] [Google Scholar]
- 52. Fava GA, et al. Withdrawal symptoms after serotonin-noradrenaline reuptake inhibitor discontinuation: systematic review. Psychother Psychosom. 2018;87(4):195–203. [DOI] [PubMed] [Google Scholar]
- 53. Renoir T. Selective serotonin reuptake inhibitor antidepressant treatment discontinuation syndrome: a review of the clinical evidence and the possible mechanisms involved. Front Pharmacol. 2013;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. American Psychiatric Association. Medication-Induced Movement Disorders and Other Adverse Effects of Medication. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 55. US Food and Drug Administration. Guidance for Industry: Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products. 2019. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enrichment-strategies-clinical-trials-support-approval-human-drugs-and-biological-products. Accessed March 27, 2020.
- 56. Black J, et al. The nightly administration of sodium oxybate results in significant reduction in the nocturnal sleep disruption of patients with narcolepsy. Sleep Med. 2009;10(8):829–835. [DOI] [PubMed] [Google Scholar]
- 57. Black J, et al. The nightly use of sodium oxybate is associated with a reduction in nocturnal sleep disruption: a double-blind, placebo-controlled study in patients with narcolepsy. J Clin Sleep Med. 2010;6(6):596–602. [PMC free article] [PubMed] [Google Scholar]
- 58. Dauvilliers Y, et al. Effect of sodium oxybate, modafinil, and their combination on disrupted nighttime sleep in narcolepsy. Sleep Med. 2017;40:53–57. [DOI] [PubMed] [Google Scholar]
- 59. Roth T, et al. Effect of sodium oxybate on disrupted nighttime sleep in patients with narcolepsy. J Sleep Res. 2017;26(4):407–414. [DOI] [PubMed] [Google Scholar]
- 60. Filardi M, et al.. In-field assessment of sodium oxybate effect in pediatric type 1 narcolepsy: an actigraphic study. Sleep. 2018;41(6). doi: 10.1093/sleep/zxy050. [DOI] [PubMed] [Google Scholar]
- 61. Thorpy MJ, et al. The Nexus narcolepsy registry: assessment of sodium oxybate (SXB) on functioning, productivity, and health-related quality of life in participants with narcolepsy [abstract]. Neurology. 2018;90(15 Suppl):P1.105. [Google Scholar]
- 62. Whelton PK, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13–e115. [DOI] [PubMed] [Google Scholar]
- 63. Bibbins-Domingo K, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362(7):590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are provided within the manuscript and supporting files.