Abstract
Study Objectives
To determine the sociodemographic, behavioral, and clinical risk factors associated with the persistence, remission, and incidence of insomnia symptoms in the transition from childhood to adolescence.
Methods
The Penn State Child Cohort is a random, population-based sample of 700 children (5–12 years at baseline), of whom 421 were followed-up as adolescents (12–23 years at follow-up). Subjects underwent polysomnography, clinical history, physical exam, and parent- and self-reported scales at baseline and follow-up. Insomnia symptoms were defined as a parent- or self-report of difficulty falling and/or staying asleep.
Results
The 421 subjects with baseline (Mage = 8.8 years) and follow-up (Mage = 17 years) data were 53.9% male and 21.9% racial/ethnic minorities. The persistence of childhood insomnia symptoms (CIS) was 56% (95% CI = 46.5–65.4), with only 30.3% (95% CI = 21.5–39.0) fully remitting. The incidence of adolescent insomnia symptoms was 31.1% (95% CI = 25.9–36.3). Female sex, racial/ethnic minority, and low socioeconomic status as well as psychiatric/behavioral or neurological disorders, obesity, smoking, and evening chronotype were associated with a higher persistence or incidence of insomnia symptoms.
Conclusions
CIS are highly persistent, with full remission occurring in only a third of children in the transition to adolescence. Sex-, racial/ethnic-, and socioeconomic-related disparities in insomnia occur as early as childhood, while different mental/physical health and lifestyle/circadian risk factors play a key role in the chronicity of CIS versus their incidence in adolescence. CIS should not be expected to developmentally remit and should become a focus of integrated pediatric/behavioral health strategies.
Keywords: insomnia, pediatrics, epidemiology, longitudinal
Statement of Significance.
The present study is one of the first to follow a large, population-based cohort of children into adolescence to evaluate the natural history of insomnia symptoms and a broad number of relevant clinically and objectively assessed risk factors. Novel long-term, longitudinal results indicate low full remission of childhood insomnia symptoms and high incidence rates of insomnia symptoms in adolescence. The findings also allow for an informed approach to evaluating the risk for persistent and incident insomnia symptoms in youth, and indicate that girls, racial/ethnic minorities, children of low socioeconomic status, those with psychiatric/behavioral, neurological or metabolic disorders, and evening circadian preference should be monitored for chronicity or development of insomnia symptoms during this important developmental period.
Introduction
Difficulties falling and/or staying asleep (DFA/DSA) are the most common parent-reported insomnia symptoms in youth [1], and their prevalence in childhood and adolescence is about 20%–25% [1–3]. Few longitudinal studies have examined the persistence and remission of childhood insomnia symptoms (CIS) as well as the incidence of insomnia symptoms in adolescence and the unique contributions of various risk factors in youth [4–6].
Estimates of persistence rates of insomnia symptoms in youth vary from 0% over a 12-year period to 52% over a 4-year period [7, 8]. Shorter follow-up periods indicate that 46% of adolescents continue exhibiting insomnia symptoms 12 months later [9] and have a relatively high 1-year incidence rate (14%) [10]. Moreover, insomnia symptoms are more persistent in adulthood than childhood, with only 20%–25% of adults with chronic insomnia fully remitting [11–13]. Given the high prevalence but variable persistence rates reported, it is critical to examine individual risk factors that contribute to the persistence and incidence of insomnia symptoms in youth. Cross-sectional studies in population-based child and adolescent samples indicate that a number of demographic, environmental, health, and behavioral factors are associated with a higher prevalence of insomnia symptoms. Demographic factors include female sex in adolescence (but not childhood) [1], low socioeconomic status (SES) [14], racial/ethnic minority status [15], and single-parent household [14]. Environmentally, parental stress, exposure to childhood adversity, and neighborhood- and school-related stress are associated with increased insomnia symptoms [14, 16]. Somatic and psychological problems such as anxiety disorders [3, 17] and behavioral factors such as caffeine intake [18] and media use [14] have also been linked to greater prevalence of insomnia symptoms among youth. In contrast, limited longitudinal studies examining risk factors for insomnia symptoms in youth are available. To date, some studies have found that family conflict, parasomnias, and technology use are associated with the persistence or incidence of insomnia symptoms over the course of 6–12 months [4–6].
Although the state of the field has advanced greatly, there are several notable limitations within the existing longitudinal literature. First, there is a need for large population-based studies that allow estimating naturally occurring persistence, remission, and incidence rates with higher generalizability and replicability. Second, none of the previous studies had available objectively assessed sleep-related problems, such as sleep disordered breathing (SDB). Third, most studies had limited availability of comprehensive clinical assessments of physical health conditions, instead relying on behavioral health measures. Lastly, most previous studies of insomnia symptoms in youth were prospective within either childhood or adolescence, whereas examining changes between, rather than within, developmental periods will allow for better understanding of the natural course of insomnia symptoms.
As such, we sought to examine the natural history of insomnia symptoms in a random, general population sample of young children (5–12 years old) followed up as adolescents (12–23 years old) to establish population-based rates for their persistence, full remission, and incidence. Given the variability in these rates found in previous longitudinal studies of CIS, we hypothesized that identification of their full remission would provide a better understanding of their high persistence in the transition to adolescence; we did not have a priori hypotheses regarding the specific rates to be estimated. Furthermore, we sought to expand the study of individual risk factors associated with insomnia symptoms to include sociodemographic determinants, physical and mental health conditions, as well as sleep problems including SDB, periodic limb movements (PLMS), and objective short sleep duration (OSSD). We hypothesized that demographic factors related to social determinants of health (i.e. sex, race/ethnicity, and SES), clinical factors related to physical health (including, but not limited to, neurological and respiratory disorders), and mental health (particularly mood/anxiety disorders) as well as behavioral health (particularly substance use), will be key predictors of the natural history of insomnia symptoms during this developmental period. Thus, our overarching goal was to address these issues and study the natural history of insomnia symptoms in a population-based sample of 421 children followed up as adolescents to establish population-based rates for their persistence, remission, and incidence and to ascertain the contributions of social determinants and clinical risk factors.
Methods
Sample
The baseline portion of the Penn State Child Cohort was designed as a two-phase study of a random, general population sample; specific recruitment details have been reported in prior studies [19]. In brief, 7,312 questionnaires were sent home with students and 5,740 were returned in phase I (78.5% response rate); thereafter, 1,000 children (5–12 years) were randomly selected from the 5,740 to participate in an in-lab study (phase II) and 700 agreed to participate (70.0% response rate). Out of these subjects, 421 completed a follow-up examination at age 12–23 years (60.1% response rate) [20]. No differences in baseline demographic characteristics were observed in the 279 lost to follow-up [20]. The average length between baseline and follow-up in the 421 subjects was 7.8 (1.4) years, the minimum was 5.8 years and the maximum 13 years (25th percentile = 6.8 years, median = 7.4 years, and 75th percentile = 8.7 years). The Penn State College of Medicine Institutional Review Board approved the study (IRB 98–228). Written informed consent from the parent/legal guardian and assent from those ˂18 years were obtained. Written informed consent was obtained at follow-up from subjects ≥18 years.
Insomnia symptoms
Subjects or their parents completed the Pediatric Behavior Scale (PBS) [21] and the Pediatric Sleep Questionnaire (PSQ) [22]. At baseline, CIS were defined as a parent-report of “often/moderate” or “very often/severe” DFA and/or DSA on the PBS [1, 23–25]; for three cases missing PBS data, a positive response to DFA and/or DSA on the PSQ was used. Whereas parents are considered best reporters of young children’s behavior, youth self-report of behaviors that parents may not witness such as sleep is preferred in adolescents [26]. Therefore, at follow-up, adolescent insomnia symptoms (AIS) were defined as self-report of DFA and/or DSA on the PSQ; for 13 cases missing PSQ data, a parent-report of “often/moderate” or “very often/severe” DFA and/or DSA on the PBS was used [25, 27]. Persistence of CIS was defined by presence of insomnia symptoms at follow-up among those with insomnia symptoms at baseline. Remission of CIS was defined by absence of insomnia symptoms at follow-up among those with insomnia symptoms at baseline. Full remission of CIS was defined by the complete absence of insomnia symptoms at follow-up, based on the PSQ and PBS (i.e. absence of “sometimes/mild” DFA and/or DSA), among those with baseline insomnia symptoms. Incidence of AIS was defined by presence of insomnia symptoms at follow-up among those without baseline insomnia symptoms.
Sociodemographic factors
Subjects or their parents completed a standard questionnaire with demographic information [19, 20]. At follow-up, subjects also completed a Tanner stage rating scale [28]. Parents reported their own occupational status at baseline and follow-up, which was used to define SES [23]; subjects self-reported their occupational status at follow-up. Commensurate with our previous studies, parent occupation was classified as “professional” if the parent reported a managerial or professional occupation and as “non-professional” if the parent reported a secretarial or non-managerial occupation or was unemployed, disabled, retired, or a student based on US census data [23]. Subjects with at least one parent in the household defined as “professional” were classified as “higher SES,” whereas those with neither parent reporting a professional occupation were classified “low SES.”
Clinical factors
At both baseline and follow-up, height and weight were measured in the lab, and a thorough clinical history and physical examination were conducted to identify physical health and psychiatric/behavioral conditions [19, 20, 23]. Body mass index (BMI) percentile for sex and age was calculated using standard growth curves and the presence of obesity was defined as a BMI percentile ≥ 95. At baseline, a pulmonologist evaluated respiratory functioning, and an ear, nose, and throat (ENT) specialist performed an examination of the nose and throat [19]. Medication use was reported by parents at baseline and/or subjects at follow-up during the clinical history and physical examination and on an evening questionnaire completed before the in-lab polysomnography (PSG) study. Medications were classified into three categories by an experienced registered pediatric nurse (C.K.S.): psychoactive (e.g. stimulants, antidepressants, anxiolytics, and sleep), other (e.g. steroids, asthma/allergy, cardiac, and insulin), and oral contraceptives (in females at follow-up).
During the clinical history, parents at baseline and subjects or their parent at follow-up were asked whether there was a presence of a lifetime history of a psychiatric or behavioral disorder with the question “Has your child/have you ever been treated for a psychiatric/behavioral disorder?,” which was used to classify subjects as having any history of a psychiatric/behavioral disorder (None vs. Yes). This question was followed-up with the option of specifying whether the disorder was attention-deficit/hyperactivity disorder (ADHD), a learning disorder, or another psychiatric/behavioral disorder. For the latter, parents/subjects were asked to specify the psychiatric/behavioral diagnosis with an open-ended question. The vast majority of subjects reporting another psychiatric/behavioral disorder at baseline or follow-up were reported to have mood/anxiety disorders, either alone or comorbid with other conditions. In order to evaluate the natural history rates of insomnia symptoms associated with ADHD, learning disorders, and mood/anxiety disorders, comparisons were made against those without any history of a psychiatric/behavioral disorder (None) as the common reference group in univariate analyses.
Circadian preference was ascertained using the Morningness–Eveningness Questionnaire (MEQ) at follow-up: morning (M-type), intermediate (I-type), and evening types (E-type) were identified using validated scores on the MEQ [29]. To assess substance use, four binary variables of caffeine, tobacco, alcohol, and recreational drug use (yes/no) were created. These data were gathered from: (1) parent- or self-report during the clinical history (i.e. caffeine use, tobacco product use, and alcohol use); (2a) subjects ˂18 years, three parent-reported items on the Child Behavior Checklist (CBCL) [30] assessing the frequency of tobacco (including smokeless tobacco), alcohol, and recreational drug use over the past 6 months or (2b) for subjects ≥18 years, three corresponding self-reported items on the Adult Self Report (ASR) [31], assessing the frequency of tobacco, alcohol, and recreational drug use (i.e. drugs for nonmedical purposes, such as marijuana, cocaine, etc.); and/or (3) three parent-reported items on the PSQ assessing whether their child uses caffeine, tobacco products, and/or recreational drugs. Tobacco, alcohol, and/or recreational drug use were considered to be present if there was a positive response on any of the three sources of information (i.e. clinical history, CBCL/ASR, or PSQ). Female subjects at follow-up self-reported whether they had ever been pregnant and the severity of pre-menstrual symptoms (PMS) on a four-point Likert scale from none to severe; the presence of PMS was defined as moderate-to-severe symptoms.
Polysomnography
At baseline and follow-up, sleep was monitored in all 421 subjects with 9-h PSG [1, 19, 20] and scored according to standard criteria [32]. Based on the apnea/hypopnea index (AHI), SDB was defined by the presence of primary snoring (AHI < 2 events/h and the presence of snoring) or pediatric OSA (AHI ≥ 2) [20]. The presence of abnormal PLMS was defined as a periodic limb movement index ≥ 5 [33, 34]. We defined the presence of OSSD as PSG-measured total sleep time <7.7 h, the median of the entire cohort [24, 25].
Statistical analyses
Descriptive statistics were used to estimate the characteristics (Supplementary Table S1) and persistence, remission, full remission, and incidence rates in the overall sample. Analyses were conducted among the 109 subjects with CIS at baseline for the persistence, remission, and full remission of CIS and among the 312 subjects without CIS at baseline for the incidence of AIS. General linear models for binary data were used to test for statistically significant differences in estimated rates (%) and their 95% confidence intervals (95CI) across sociodemographic and clinical risk factors. Missing data were minimal (<8%, Mmissing = 2.42%) and pairwise deletion was employed. p-values ≤0.05 were considered statistically significant, while p-values >0.05 and ≤0.10 were deemed marginally significant and interpreted cautiously based on sample size and 95CIs. All tables include the effective sample size for each sociodemographic and clinical risk factor. Univariate odds ratios (OR) and their 95CI for persistent CIS, fully remitted CIS, and incident AIS are provided in Supplementary Tables S2–S4. In order to examine the relative association of demographic and clinical risk factors, while simultaneously adjusting for each other, with persistent or fully remitted CIS and incident AIS, multivariable-adjusted ORs were estimated using binary logistic regression models that included each individual childhood or adolescence clinical risk factor (backward conditional elimination, p-entry < 0.05, p-removal > 0.10, classification cutoff = 0.5, 20 maximum iterations), while simultaneously adjusting for sex, age, race/ethnicity, and SES (forced entry). All analyses were conducted using SPSS v24.
Results
Characteristics of the cohort
The characteristics of the cohort at baseline and follow-up have been previously reported [19, 20] and can be found in Supplementary Table S1. Overall, 46.1% of the sample was female and 21.9% identified as a racial/ethnic minority (12.6% Black/African-American and 6.4% Hispanic/Latinx). The mean age was 8.7 years (1.7) at baseline and 16.5 years (2.3) at follow-up, with 39.0% of children from low SES households since baseline.
Persistence of CIS
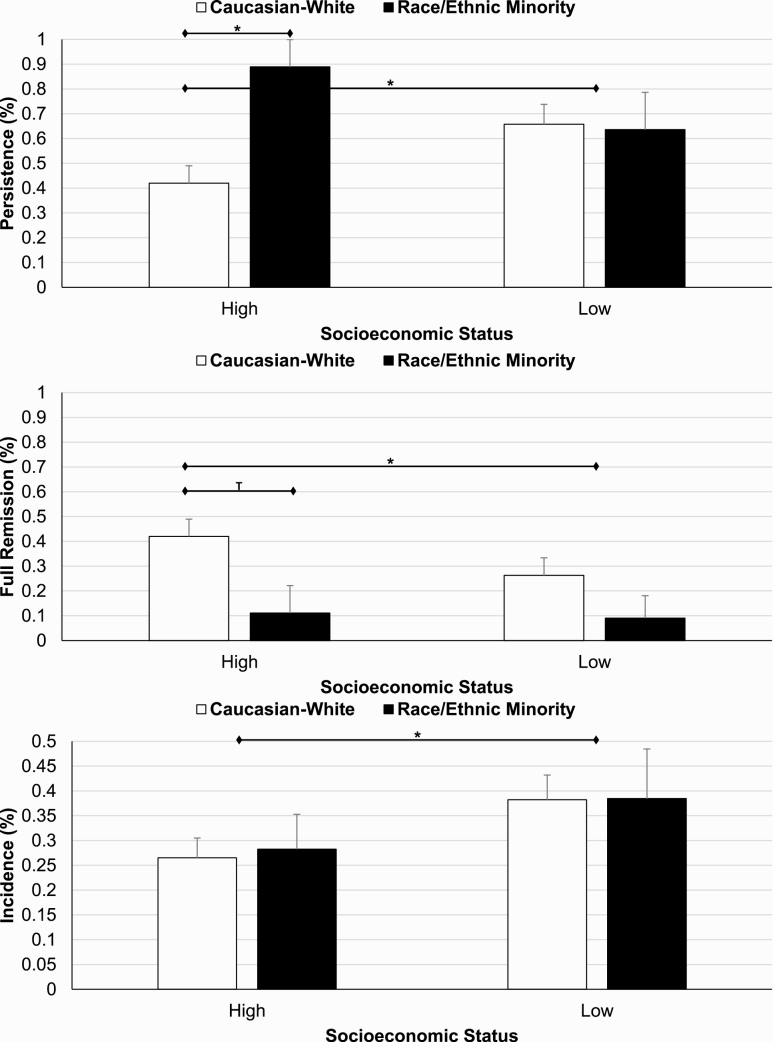
Among subjects with CIS (n = 109), the persistence rate in the transition to adolescence was 56.0% (n = 61). Follow-up length was not significantly or marginally associated with the persistence of CIS (p = 0.742). As shown in Table 1, females and racial/ethnic minorities had a significantly higher persistence rate of CIS compared with males and non-Hispanic whites. SES played a role in the association of race/ethnicity with the persistence of CIS (p-interaction = 0.044). Compared with non-Hispanic whites of higher SES (42.0%), the persistence rate of CIS was significantly higher in non-Hispanic whites of low SES (65.8%) and in racial/ethnic minorities regardless of whether they belonged to higher (88.9%) or low (63.6%) SES households (Figure 1).
Table 1.
Rates for the natural history of insomnia symptoms by sociodemographic factors
| n | Persistence | Remission | Full remission | n | Incidence | |
|---|---|---|---|---|---|---|
| % (95CI) | % (95CI) | % (95CI) | % (95CI) | |||
| Overall | 109 | 56.0 (46.5–65.4) | 44.0 (34.6–53.5) | 30.3 (21.5–39.0) | 312 | 31.1 (25.9–36.3) |
| Sex | ||||||
| Male | 60 | 46.7 (33.7–60.0) | 53.3 (40.3–66.3) | 35.0 (22.6–47.4) | 167 | 24.0 (17.4–30.5) |
| Female | 49 | 67.3 (53.7–81.0)* | 32.7 (19.0–46.3)* | 24.5 (12.0–37.0) | 145 | 39.3 (31.3–47.4)** |
| Age | ||||||
| 5–7 years | 39 | 51.3 (34.9–67.7) | 48.7 (32.3–65.1) | 30.8 (15.6–45.9) | 81 | 28.4 (18.4–38.4) |
| 8–10 years | 55 | 56.4 (42.8–69.9) | 43.6 (30.1–57.2) | 29.1 (16.7–41.5) | 176 | 31.8 (24.9–38.8) |
| 11–12 years | 15 | 66.7 (39.6–93.7) | 33.3 (6.3–60.4) | 33.3 (6.3–60.4) | 55 | 32.7 (19.9–45.5) |
| 12–14 years | 24 | 58.3 (37.1–79.6) | 41.7 (20.4–62.9) | 20.8 (3.3–38.4) | 66 | 30.3 (18.9–41.7) |
| 15–17 years | 50 | 52.0 (37.7–66.3) | 48.0 (33.7–62.3) | 34.0 (20.4–47.6) | 147 | 27.9 (20.6–35.2) |
| 18–23 years | 35 | 60.0 (42.9–77.1) | 40.0 (22.9–57.1) | 31.4 (15.3–47.6) | 99 | 36.4 (26.7–46.0) |
| Tanner stage | ||||||
| Pre-to-mid puberty | 11 | 63.6 (29.7–97.5) | 36.4 (2.5–70.3) | 27.3 (0.0–58.6) | 53 | 28.3 (15.8–40.8) |
| Late puberty-to-adulthood | 94 | 55.3 (45.1–65.6) | 44.7 (34.4–54.9) | 30.8 (21.3–40.4) | 248 | 31.8 (26.0–37.7) |
| Race/ethnicity | ||||||
| Non-Hispanic White | 89 | 51.7 (41.1–62.3) | 48.3 (37.7–58.9) | 34.8 (24.7–44.9) | 240 | 30.8 (24.9–36.7) |
| Racial/Ethnic Minority | 20 | 75.0 (54.2–95.8)* | 25.0 (4.2–45.8)* | 10.0 (0.0–24.4)** | 72 | 31.9 (20.9–43.0) |
| Socioeconomic status | ||||||
| Higher–Higher | 26 | 46.1 (25.6–66.7) | 53.9 (33.3–74.4) | 42.3 (22.0–62.7) | 87 | 24.1 (15.0–33.3) |
| Low–Higher | 4 | 25.0 (0.0–100.0) | 75.0 (0.0–100.0) | 75.0 (0.0–100.0) | 24 | 29.2 (9.6–48.8) |
| Higher–Low | 29 | 55.2 (35.9–74.4) | 44.8 (25.6–64.1) | 27.6 (10.3–44.9) | 86 | 29.1 (19.3–38.9) |
| Low–Low | 49 | 65.3 (51.5–79.1) | 34.7 (20.9–48.5) | 22.4 (10.3–34.6)T | 115 | 38.3 (29.2–47.3)* |
Data are number of total cases (n), rates (%), and their 95% confidence interval (95CI).
T p < 0.10.
* p < 0.05.
** p < 0.01
Figure 1.
Natural history rates of insomnia symptoms as a function of socioeconomic and racial/ethnic status. Racial/ethnic minorities, regardless of their SES, and non-Hispanic whites of low SES had a significantly higher persistence rate of CIS than non-Hispanic whites of higher SES. Children of low SES had a significantly higher incidence rate of AIS than those of higher SES, regardless of their race/ethnicity.
Children and adolescents with a psychiatric/behavioral disorder had significantly higher persistence rates than those without such disorders (see Tables 2 and 3). Adolescents with obesity, those taking psychoactive medications, or using tobacco had significantly higher persistence rates of CIS than adolescents without such disorders/lifestyle factors. Adolescents with a respiratory or neurological disorder had a marginally higher persistence rate of CIS compared with adolescents without such disorders.
As shown in Table 4, multivariable-adjusted logistic regression models showed that females, non-Hispanic whites of low SES, and racial/ethnic minorities remained independently associated with a higher likelihood of persistent CIS, as did clinical factors such as childhood or adolescent psychiatric/behavioral disorders, adolescent obesity, and adolescent tobacco use.
Table 4.
Multivariable-adjusted logistic regression models for persistent and fully remitted CIS and for incident AIS
| Persistent CIS | Fully Remitted CIS | Incident AIS | |
|---|---|---|---|
| OR (95CI) | OR (95CI) | OR (95CI) | |
| Demographic factors | |||
| Sex | |||
| Male | 1.00 | 1.00 | 1.00 |
| Female | 2.45 (1.02–5.86)* | 0.70 (0.26–1.87) | 2.25 (1.35–3.76)** |
| Age | |||
| 5–7 years | 1.00 | 1.00 | |
| 8–10 years | 1.38 (0.55–3.46) | 1.17 (0.42–3.24) | |
| 11–12 years | 1.49 (0.37–5.96) | 1.52 (0.35–6.59) | |
| 12–14 years | 1.00 | ||
| 15–17 years | 0.91 (0.46–1.80) | ||
| 18–23 years | 1.13 (0.55–2.31) | ||
| Racial/ethnic minority | |||
| No | 1.00 | ||
| Yes | 1.15 (0.63–2.11) | ||
| Low SES | |||
| No | 1.00 | ||
| Yes | 1.58 (1.01–2.66)* | ||
| Racial/ethnic minority*Low SES | |||
| Non-Hispanic White High SES | 1.00 | 1.00 | |
| Non-Hispanic White Low SES | 3.12 (1.22–7.99)* | 0.39 (0.14–1.04)T | |
| Racial/Ethnic Minority | 3.83 (1.14–12.92)* | 0.14 (0.03–0.73)* | |
| Childhood factors | |||
| Any psychiatric/behavioral disorder | |||
| No | 1.00 | ||
| Yes | 2.04 (0.99–4.19)T | ||
| Mood/anxiety disorder | |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 6.27 (1.19–32.95)* | 0.16 (0.02–1.39)T | 4.74 (1.04–21.56)* |
| Obesity | |||
| No | 1.00 | ||
| Yes | 0.14 (0.03–0.76)* | ||
| Asthma/allergies | |||
| No | 1.00 | ||
| Yes | 3.00 (1.11–8.12)* | ||
| ENT disorder | |||
| No | 1.00 | ||
| Yes | 0.55 (0.33–0.94)* | ||
| Cardiovascular disorder | |||
| No | 1.00 | ||
| Yes | 3.75 (0.95–14.79)T | ||
| Musculoskeletal disorder | |||
| No | 1.00 | ||
| Yes | 0.21 (0.04–1.24)T | ||
| Adolescence factors | |||
| Any psychiatric/behavioral disorder | |||
| No | 1.00 | 1.00 | |
| Yes | 2.86 (1.04–7.92)* | 2.10 (1.14–3.88)* | |
| Obesity | |||
| No | 1.00 | 1.00 | |
| Yes | 7.97 (1.89–33.66)** | 0.07 (0.01–0.55)* | |
| Gastrointestinal disorder | |||
| No | 1.00 | ||
| Yes | 0.36 (0.11–1.17)T | ||
| Neurological disorder | |||
| No | 1.00 | ||
| Yes | 1.62 (0.94–2.79)T | ||
| Tobacco | |||
| No | 1.00 | ||
| Yes | 6.88 (1.19–39.93)* | ||
| Chronotype | |||
| M-type | 0.62 (0.32–1.20) | ||
| I-type | 1.00 | ||
| E-type | 1.94 (1.03–3.67)* |
Data are multivariable-adjusted odds ratios (OR) and their 95% confidence interval (95CI); Multivariable-adjusted binary logistic regression models were ran separately for persistent CIS, fully remitted CIS and incident AIS. Demographic factors (i.e. sex, age, race/ethnicity, and SES) were always entered in the models (forced entry), while childhood or adolescence clinical factors were submitted to stepwise selection (backward conditional elimination). The interaction between race/ethnicity and SES on the persistence of CIS was statistically significant (see text), thus, the resulting three groups were based on the data shown in Figure 1. As the interaction was not statistically significant for the incidence of AIS, race/ethnicity, and SES were entered into the models as separate, individual variables.
T p < 0.10.
*p < 0.05.
**p < 0.01.
Remission of CIS
Among subjects with CIS (n = 109), the remission rate in the transition to adolescence was 44.0% (n = 48), with 30.3% experiencing full remission (n = 33). Follow-up length was not significantly or marginally associated with the full remission of CIS (p = 0.319). Remission rates of CIS were significantly lower in females and racial/ethnic minorities, while full remission rates were not statistically different between females and males. Low SES was marginally associated with a lower full remission rate as compared to high SES (Table 1). Full remission rates were 10% in racial/ethnic minorities regardless of SES and 26.3% in non-Hispanic whites of low SES, while highest (42.0%) in non-Hispanic whites of higher SES (Figure 1).
Children with psychiatric/behavioral disorders, particularly a mood/anxiety disorder, had lower full remission rates than those without such disorders. Similarly, the presence of obesity, musculoskeletal disorders, or primary snoring in childhood was associated with lower full remission rates. In contrast, children with asthma/allergies, cardiovascular disorders or who were on any type of non-psychoactive medication had higher remission rates than children without such conditions (Table 2). Adolescents with obesity, psychiatric/behavioral disorders, or taking psychoactive medications had significantly lower full remission rates of CIS than adolescents without such conditions (Table 3). Furthermore, adolescents with a respiratory disorder had a marginally lower full remission rate of CIS as compared with adolescents without such disorder.
Table 2.
Rates for the natural history of insomnia symptoms by clinical risk factors in childhood
| Childhood risk factor | Persistence | Remission | Full remission | Incidence | ||
|---|---|---|---|---|---|---|
| n | % (95CI) | % (95CI) | % (95CI) | n | % (95CI) | |
| Psychiatric/behavioral disorder | ||||||
| No | 70 | 50.0 (38.0–62.0) | 50.0 (38.0–62.0) | 32.9 (21.6–44.1) | 260 | 27.7 (22.2–33.2) |
| Yes | 39 | 66.7 (51.2–82.1)T | 33.3 (17.9–48.8)T | 25.6 (11.3–40.0) | 52 | 48.1 (34.0–62.1)** |
| ADHD | 28 | 60.7 (41.4–80.0) | 39.3 (20.0–58.6) | 28.6 (10.7–46.4) | 24 | 45.8 (24.3–67.3)T |
| Learning disorder | 15 | 66.7 (39.6–93.7) | 33.3 (6.3–60.4) | 20.0 (0.0–42.9) | 24 | 29.2 (9.6–48.8) |
| Mood/anxiety disorder | 13 | 84.6 (61.9–100.0)** | 15.4 (0.0–38.1)** | 7.7 (0.0–24.4)* | 11 | 72.7 (41.3–100.0)** |
| Psychoactive medication | ||||||
| No | 95 | 54.7 (44.5–64.9) | 45.3 (35.1–55.5) | 30.5 (21.1–40.0) | 294 | 29.9 (24.7–35.2) |
| Yes | 14 | 64.3 (35.6–93.0) | 35.7 (7.0–64.4) | 28.6 (1.5–55.6) | 18 | 50.0 (24.4–75.6)T |
| Obesity | ||||||
| No | 85 | 51.8 (40.9–62.6) | 48.2 (37.4–59.1) | 36.5 (26.0–46.9) | 257 | 30.4 (24.7–36.0) |
| Yes | 24 | 70.8 (51.2–90.4) | 29.2 (9.6–48.8) | 8.3 (0.0–20.3)** | 55 | 34.5 (21.6–47.5) |
| Asthma/allergies | ||||||
| No | 63 | 61.9 (49.6–74.2) | 38.1 (25.8–50.4) | 22.2 (11.7–32.8) | 191 | 29.8 (23.3–36.4) |
| Yes | 46 | 47.8 (32.8–62.8) | 52.2 (37.2–67.2) | 41.3 (26.5–56.1)* | 117 | 34.2 (25.5–42.9) |
| ENT disorder | ||||||
| No | 37 | 62.2 (45.8–78.6) | 37.8 (21.4–54.2) | 24.3 (9.8–38.8) | 118 | 37.3 (28.4–46.1) |
| Yes | 72 | 52.8 (41.0–64.6) | 47.2 (35.4–59.4) | 33.3 (22.2–44.5) | 193 | 27.5 (21.1–33.8)T |
| Cardiovascular disorder | ||||||
| No | 93 | 58.1 (47.8–68.3) | 41.9 (31.7–52.2) | 26.9 (17.7–36.1) | 281 | 32.0 (26.5–37.5) |
| Yes | 16 | 43.7 (16.4–71.0) | 56.3 (29.0–83.6) | 50.0 (22.5–77.5)T | 28 | 25.0 (7.9–42.1) |
| Musculoskeletal disorder | ||||||
| No | 94 | 54.3 (44.0–64.5) | 45.7 (35.5–56.0) | 33.0 (23.3–42.7) | 278 | 32.4 (26.8–37.9) |
| Yes | 15 | 66.7 (39.6–93.7) | 33.3 (6.3–60.4) | 13.3 (0.0–32.8)T | 31 | 22.6 (7.0–38.2) |
| Any other medication | ||||||
| No | 65 | 63.1 (51.0–75.1) | 36.9 (24.9–49.0) | 24.6 (13.9–35.4) | 204 | 31.9 (25.4–38.3) |
| Yes | 44 | 45.4 (30.1–60.8)T | 54.6 (39.2–69.9)T | 38.6 (23.7–53.6) | 108 | 29.6 (20.9–38.4) |
| SDB | ||||||
| No | 81 | 53.1 (42.0–64.2) | 46.9 (35.8–58.0) | 33.3 (22.8–43.8) | 200 | 32.0 (25.5–38.5) |
| Yes | 28 | 64.3 (45.4–83.2) | 35.7 (16.8–54.6) | 21.4 (5.2–37.6) | 112 | 29.5 (20.9–38.0) |
| Primary snoring | 17 | 64.7 (39.4–90.0) | 35.3 (10.0–60.6) | 11.8 (0.0–28.8)T | 72 | 31.9 (20.9–43.0) |
| Pediatric OSA | 11 | 63.6 (29.7–97.5) | 36.4 (2.5–70.3) | 36.4 (2.5–70.3) | 40 | 25.0 (11.0–39.0) |
| OSSD | ||||||
| No | 52 | 51.9 (37.9–66.0) | 48.1 (34.0–62.1) | 36.5 (23.0–50.1) | 165 | 32.1 (24.9–39.3) |
| Yes | 57 | 59.6 (46.5–72.8) | 40.4 (27.2–53.5) | 24.6 (13.0–36.1) | 147 | 29.9 (22.4–37.4) |
Data are number of total cases (n), rates (%), and their 95% confidence interval (95CI); only seven subjects had a childhood history of psychoactive medication use other than stimulants (n = 28), thus, the two were merged together (n = 32). ADHD, attention deficit hyperactivity disorder; ENT disorder, ear-nose-throat disorder; OSA, obstructive sleep apnea; OSSD, objective short sleep duration; PLMS, abnormal periodic limb movements; SDB, sleep disordered breathing.
T p < 0.10.
*p < 0.05.
**p < 0.01.
Table 3.
Rates for the natural history of insomnia symptoms by clinical risk factors in adolescence
| Adolescence risk factor | Persistence | Remission | Full remission | Incidence | ||
|---|---|---|---|---|---|---|
| n | % (95CI) | % (95CI) | % (95CI) | n | % (95CI) | |
| Psychiatric/behavioral disorder | ||||||
| No | 49 | 44.9 (30.5–59.3) | 55.1 (40.7–69.5) | 36.7 (22.7–50.7) | 227 | 26.9 (21.1–32.7) |
| Yes | 60 | 65.0 (52.6–77.4)* | 35.0 (22.6–47.4)* | 25.0 (13.7–36.3) | 85 | 42.4 (31.6–53.1)* |
| ADHD | 42 | 59.5 (44.0–75.0) | 40.5 (25.0–56.0) | 28.6 (14.3–42.8) | 56 | 39.3 (26.1–52.5)T |
| Learning disorder | 17 | 52.9 (26.5–79.4) | 47.1 (20.6–73.5) | 35.3 (10.0–60.6) | 22 | 40.9 (18.6–63.2) |
| Mood/anxiety disorder | 19 | 79.0 (58.8–99.0)** | 21.0 (1.0–41.2)** | 10.5 (0.0–25.7)* | 31 | 38.7 (20.5–56.9) |
| Psychoactive medication | ||||||
| No | 91 | 50.5 (40.1–61.0) | 49.6 (39.0–59.9) | 34.1 (24.1–44.0) | 290 | 30.3 (25.0–35.7) |
| Yes | 18 | 83.3 (64.3–100.0)** | 16.7 (0.0–35.7)** | 11.1 (0.0–27.2)* | 22 | 40.9 (18.6–63.2) |
| Obesity | ||||||
| No | 87 | 48.3 (37.6–59.0) | 51.7 (41.0–62.4) | 36.8 (26.4–47.1) | 270 | 31.5 (25.9–37.1) |
| Yes | 22 | 86.4 (70.8–100.0)** | 13.6 (0.0–29.2)** | 4.5 (0.0–14.0)** | 42 | 28.6 (14.3–42.8) |
| Respiratory disorder | ||||||
| No | 73 | 50.7 (38.9–62.4) | 49.3 (37.6–61.1) | 35.6 (24.4–46.9) | 247 | 28.3 (22.7–34.0) |
| Yes | 35 | 68.6 (52.4–84.7)T | 31.4 (15.3–47.6)T | 20.0 (6.1–33.9)T | 65 | 41.5 (29.2–53.8)T |
| Gastrointestinal disorder | ||||||
| No | 78 | 53.8 (42.5–65.2) | 46.2 (34.8–57.5) | 33.3 (22.6–44.0) | 241 | 28.2 (22.5–33.9) |
| Yes | 30 | 63.3 (45.0–81.6) | 36.7 (18.4–55.0) | 23.3 (7.3–39.4) | 71 | 40.8 (29.1–52.6)T |
| Neurological disorder | ||||||
| No | 54 | 48.1 (34.4–61.9) | 51.9 (38.1–65.6) | 37.0 (23.7–50.3) | 197 | 25.9 (19.7–32.1) |
| Yes | 54 | 64.8 (51.7–78.0)T | 35.2 (22.0–48.3)T | 24.1 (12.3–35.8) | 115 | 40.0 (30.9–49.1)* |
| Any other medication | ||||||
| No | 62 | 53.2 (40.4–66.0) | 46.8 (34.0–59.5) | 32.3 (20.3–44.2) | 186 | 30.1 (23.4–36.8) |
| Yes | 47 | 59.6 (45.0–74.1) | 40.4 (25.9–55.0) | 27.7 (14.4–40.9) | 126 | 32.5 (24.2–40.8) |
| SDB | ||||||
| No | 40 | 55.0 (38.9–71.1) | 45.0 (28.9–61.1) | 25.0 (11.0–39.0) | 115 | 28.7 (20.3–37.1) |
| Yes | 69 | 56.5 (44.5–68.5) | 43.5 (31.5–55.5) | 33.3 (21.9–44.7) | 197 | 32.5 (25.9–39.1) |
| Primary snoring | 27 | 48.1 (28.0–68.3) | 51.8 (31.7–72.0) | 33.3 (14.3–52.3) | 80 | 33.7 (23.2–44.3) |
| Pediatric OSA | 42 | 61.9 (46.6–77.2) | 38.1 (22.8–53.4) | 33.3 (18.5–48.2) | 117 | 31.6 (23.1–40.2) |
| PLMS | ||||||
| No | 80 | 60.0 (49.0–71.0) | 40.0 (29.0–51.0) | 30.0 (19.7–40.3) | 238 | 32.8 (26.8–38.8) |
| Yes | 29 | 44.8 (25.6–64.1) | 55.2 (35.9–74.4) | 31.0 (13.1–48.9) | 74 | 25.7 (15.5–35.9) |
| OSSD | ||||||
| No | 56 | 57.1 (43.8–70.5) | 42.9 (29.5–56.2) | 25.0 (13.3–36.7) | 152 | 32.2 (24.7–39.7) |
| Yes | 53 | 54.7 (40.9–68.6) | 45.3 (31.4–59.1) | 35.8 (22.5–49.2) | 160 | 30.0 (22.8–37.2) |
| Tobacco | ||||||
| No | 94 | 51.1 (40.8–61.4) | 48.9 (38.6–59.2) | 35.1 (25.3–44.9) | 282 | 28.4 (23.1–33.7) |
| Yes | 15 | 86.7 (67.2–100.0)** | 13.3 (0.0–32.8)** | 0.0 | 30 | 56.7 (37.8–75.5)** |
| Alcohol | ||||||
| No | 80 | 58.7 (47.7–69.8) | 41.2 (30.2–52.3) | 30.0 (19.7–40.3) | 225 | 27.6 (21.7–33.4) |
| Yes | 25 | 56.0 (35.1–76.9) | 44.0 (23.1–64.9) | 28.0 (9.1–46.9) | 81 | 43.2 (32.2–54.2)* |
| Chronotype | ||||||
| M-type | 26 | 42.3 (22.0–62.7)T | 57.7 (37.3–78.0)T | 34.6 (15.0–54.2) | 109 | 21.1 (13.3–28.9) |
| I-type | 41 | 63.4 (48.0–78.8) | 36.6 (21.2–52.0) | 29.3 (14.7–43.8) | 113 | 27.4 (19.1–35.8) |
| E-type | 42 | 57.1 (41.5–72.7) | 42.9 (27.2–58.5) | 28.6 (14.3–42.8) | 89 | 48.3 (37.7–58.9)** |
Data are number of total cases (n), rates (%), and their 95% confidence interval (95CI). ADHD, attention deficit hyperactivity disorder; BMI, body mass index; E-type, evening chronotype; I-type, intermediate chronotype; M-type, morning chronotype; OSA, obstructive sleep apnea; OSSD, objective short sleep duration; PLMS, abnormal periodic limb movements; SDB, sleep disordered breathing.
T p < 0.10.
*p < 0.05.
**p < 0.01.
As shown in Table 4, multivariable-adjusted logistic regression models showed that racial/ethnic minorities remained independently associated with a lower likelihood of full remission of CIS, as did clinical risk factors such as childhood and adolescent obesity, whereas non-Hispanic whites of low SES, childhood psychiatric/behavioral disorders, and musculoskeletal disorders were only marginally associated with a lower likelihood of full remission of CIS. These models also showed that childhood asthma/allergies and cardiovascular disorders remained independently associated with a higher likelihood of full remission of CIS.
Incidence of insomnia symptoms in adolescence
Among subjects without CIS (n = 312), the incidence rate of AIS was 31.1% (n = 97). Follow-up length was not significantly or marginally associated with the incidence of AIS (p = 0.929). Females and children of low SES had significantly higher incidence rates of AIS compared with males and children of higher SES, respectively (Table 1). The higher incidence rate in females was significant at the age of 15–17 years old. There were no significant differences in terms of race/ethnicity, except Hispanic/Latinx showing a significantly lower incidence rate (n = 20, 10.0% [0.0–24.4]) than non-Hispanic whites (n = 240, 30.8% [24.9–36.7]). As shown in Figure 1, the incidence rate was significantly higher in those of low SES, regardless of whether they identified as non-Hispanic white (38.2%) or as a racial/ethnic minority (38.5%), when compared with those of higher SES (26.5% in non-Hispanic whites and 28.3% in racial/ethnic minorities).
Children with psychiatric/behavioral disorders had a significantly higher incidence rate of AIS than those without such disorders, whereas children with ADHD had a marginally higher incidence rate of insomnia symptoms (Table 2). Children who were on psychoactive medications had marginally higher incidence rates of AIS than those not taking such medications. Interestingly, those with a childhood history of an ENT disorder had a marginally lower incidence rate of AIS than those without. Adolescents with psychiatric/behavioral or neurological disorders, who used tobacco or drank alcohol had significantly higher incidence rates of AIS than those without such disorders/lifestyle behaviors (Table 3). Adolescents classified as E-types had a significantly higher incidence rate of AIS than those identified as I-types or M-types. Adolescents with respiratory or gastrointestinal disorders had a marginally higher incidence rate of AIS as compared to those without such disorders.
As shown in Table 4, multivariable-adjusted logistic regression models showed that females and those of low SES remained independently associated with a higher likelihood of incident AIS as did clinical factors such as childhood or adolescent psychiatric/behavioral disorders, E-types, and, to a lesser extent, neurological disorders. In contrast, childhood ENT disorders and, to a lesser extent, adolescent gastrointestinal disorders remained associated with a lower likelihood of incident AIS.
Clinical risk factors not reported above were not associated with the natural course of insomnia symptoms or had a frequency in childhood or adolescence too low (n < 10) to be reliably analyzed and interpreted (e.g. adolescent full-time employment, childhood endocrine disorder, childhood PLMS, adolescent contraceptive medication, and adolescent pregnancy) and all can be found in Supplementary Tables S5 and S6.
Discussion
This study examined the natural history of insomnia symptoms in 421 children followed up after a median of about 7.5 years in the transitional period from childhood (median 9 years) to adolescence (median 16 years), with detailed assessments of clinically relevant risk factors. The persistence of CIS in the present study was 56%, with a full remission rate of only 30%, consistent with some studies in youth and adults [11–13, 35, 36]. While previous studies with different follow-up lengths have reported varying persistence rates, this study did not find a statistically or marginally significant impact of length of follow-up, despite the overall persistence rate decreasing from 64% among subjects followed-up 6 years later to 50% among those who were followed-up 9 or more years later. Nevertheless, these data suggest that insomnia symptoms should not be regarded as a transient phenomenon in a significant proportion of children. The incidence of AIS in the present study was 31%, which is higher than studies that relied on shorter follow-ups of about a year [10, 35] but comparable to rates reported in adults [12, 37]. These data also suggest that insomnia symptoms should be thoroughly screened for in adolescents regardless of whether they had insomnia symptoms earlier during childhood.
Our novel data indicate that sex-related, racial/ethnic, and socioeconomic disparities in insomnia symptoms occur early in development, and these social determinants differentially impact their natural course over time. Girls with CIS had higher persistence rates in the transition to adolescence than boys. Furthermore, girls without CIS had a 15% higher incidence rate of AIS than boys. In a previous cross-sectional study, girls entering puberty had a significantly higher prevalence of insomnia symptoms, while the prevalence in boys remained similar at ages 5–12 [1]. The present study expands upon these findings, revealing the incidence of AIS was significantly higher in girls age 15–17 years and suggesting that mid-adolescence is a second developmental period for the onset of insomnia symptoms in girls that should be a target of preventative efforts. This finding is also clinically important considering the limited evidence for the effectiveness of cognitive behavioral therapies for insomnia in adolescents, as compared with adults.
Children belonging to a racial/ethnic minority showed higher persistence and lower remission rates of CIS than non-Hispanic white children; in fact, the full remission rate was as low as 10% in racial/ethnic minorities. Furthermore, among non-Hispanic white children, belonging to a low SES household was associated with a higher persistence of CIS when compared to those of higher SES. Also, the incidence rate of AIS was significantly higher in subjects belonging to a low SES household regardless of their racial/ethnic identification. These data have important public health and clinical implications. First, they suggest that social determinants of health in disadvantaged groups (i.e. low SES and racial/ethnic minorities) puts them at greater risk of persistent CIS throughout adolescence. Potential factors explaining these findings, particularly such low full remission rate in racial/ethnic minorities, include experienced discrimination, which has a negative impact on sleep [38], structural racism, and implicit biases toward race, ethnicity, and poverty in the healthcare system, which may lead clinicians to not consider insomnia symptoms clinically relevant in these children [14], lack of access to adequate healthcare, which may preclude early parental education, prevention, and intervention [39], among many other potential factors. Second, these data reinforce the need to implement community-based and clinical interventions in children and families of racial/ethnic minority and/or low SES backgrounds in order to prevent the chronicity and development of insomnia symptoms over time, rather than expecting these symptoms to resolve with normal development. From a social policy standpoint, these health disparities reinforce the need to target the underlying inequalities that our current cultural and economic systems perpetuate since early childhood, particularly in light of their effects on downstream health consequences [38, 40].
Clinical risk factors such as psychiatric/behavioral or neurological disorders, obesity, smoking, alcohol use, or an evening chronotype were associated with the persistence of CIS or the incidence of AIS. Interestingly, other clinical risk factors, such as pediatric OSA or specific physical health problems, did not contribute to the persistence or incidence of insomnia symptoms in youth. The presence of a childhood mood/anxiety disorder was associated with very high persistence and low full remission rates of CIS as well as a very high incidence rate of AIS. The presence of ADHD was only marginally associated with the course of insomnia symptoms. Given this marginal association, the finding must be interpreted with caution and replication is needed before strong conclusions are drawn. Nevertheless, these data are consistent with the established association of internalizing disorders with insomnia and suggest that the course of insomnia symptoms in youth with ADHD may depend upon the presence of comorbid mood/anxiety disorders, which occur more frequently in ADHD [41]. Commensurate with these findings, psychoactive medication use in adolescence, was associated with higher persistence and lower full remission rates; however, its use did not remain as independently associated in multivariable models. This suggests that CIS may remain persistent despite pharmacological treatment of the psychiatric/behavioral disorder, indicating that behavioral sleep treatments in youth should be a clinical priority.
Obesity and, to a lesser extent, a musculoskeletal disorder or childhood primary snoring, were also associated with lower full remission of CIS, whereas obesity was not associated with higher incidence rates of AIS. Interestingly, primary snoring was no longer associated with lower full remission of CIS in multivariable models. These findings suggest that childhood obesity is a key risk factor for the persistence of CIS and strategies should be implemented early to treat this modifiable risk factor in order to prevent the chronicity of both childhood SDB [42] and insomnia symptoms. Similarly, the presence of a respiratory or neurological disorder in adolescence was associated with marginally higher persistence rates of CIS, but these associations did not remain in multivariable models. Neurological and gastrointestinal disorders were associated with a significantly higher incidence of AIS but these associations became marginally significant in multivariable models. Together, these data suggest that the comorbidity or clustering of these physical health conditions may better predict the persistence of CIS in the transition to adolescence than each individual clinical condition alone. In contrast, other physical health problems in childhood (i.e. asthma/allergies and cardiovascular disorders) were associated with higher full remission of CIS, even in multivariable models. It is likely that these children may have received treatment for these medical disorders, which suggests that their insomnia symptoms were at least partially driven by the disorder itself [23]. In support of this hypothesis, our data showed that childhood use of medications for these and other physical health conditions was associated with a higher remission of CIS. These findings suggest the need to not only clinically assess for insomnia symptoms among children with these health conditions, but to also actively treat insomnia symptoms in youth with obesity, musculoskeletal, respiratory and neurological disorders, or their clustering, in order to prevent their chronicity over time.
Importantly, SDB or PLMS were not associated with higher persistence or incidence rates of insomnia symptoms in the transition to adolescence, consistent with the fact that these disorders do not necessarily manifest with prolonged periods of wakefulness at the beginning or in the middle of the night [43, 44]. However, the low frequency of cases with childhood PLMS that contributed to the analyses precluded any reliable estimates of the persistence or full remission of CIS (Supplementary Table S5). In addition, OSSD, as measured by PSG total sleep time, was also not associated with the course of insomnia symptoms. Differences in biomarker level and behavioral profiles in youth with insomnia symptoms and OSSD compared to those with normal sleep duration have been reported in previous studies [24, 25, 45]. The present study suggests that the persistence of CIS cannot be attributed solely to increased biological severity, indexed by short sleep, and that behavioral factors may play a stronger role during this developmental period [24, 27]. Alternatively, OSSD in young children may not yet be associated with the expression of biological vulnerability due to the strong homeostatic sleep drive in this age group. Future studies should examine whether fine-grained neurophysiologic measures of sleep drive and arousal (e.g., sleep EEG) may better predict the natural history of insomnia symptoms during this developmental period [3, 46–48].
As it pertains to lifestyle factors, tobacco and alcohol use in adolescence were significantly associated with the course of insomnia symptoms; however, only tobacco use remained significantly associated in multivariable models. Given alcohol and nicotine’s known effects on sleep [49], it appears that these substances also have deleterious effects on the development of AIS and that the high co-occurrence of tobacco and alcohol use in adolescence [50] may explain the independent association found for smoking in multivariable models. Adolescent health and drug education programs should include information about the adverse effects of tobacco and alcohol use on both the chronicity and the new-onset of insomnia symptoms. Furthermore, an evening circadian preference was associated with a significantly higher incidence of AIS, in line with existing evidence that this chronotype is a risk factor not only for a circadian misalignment [48] but also insomnia [51, 52]. Given the known shift in circadian phase occurring in the transition to adolescence [53], targeted circadian interventions in these youth are warranted to mitigate the development of insomnia symptoms.
Despite the relatively large population-based cohort, data collected longitudinally over a developmental transition, and examination of numerous subjectively and objectively measured clinical factors, the present study has several limitations. First, the study was underpowered to examine changes in insomnia symptoms among youth belong to specific groups or with certain risk factors. Specifically, some demographic groups (e.g. Hispanic/Latinx) or clinical factors (e.g. childhood PLMS and adolescent pregnancy) had a low number of cases after stratifying by the presence or absence of CIS. As such, a much larger cohort with objective sleep measures and thorough clinical history is still needed to provide a large number of cases with and without CIS and capture low-prevalence risk factors. Nevertheless, this study provides supportive evidence on the persistence of CIS and incidence of AIS helpful to clinical studies on the demographic groups and clinical conditions examined. Second, most clinical factors were collected by parent- or self-report and diagnoses could not be confirmed via medical record review, which would have enhanced the study’s validity. However, data collection relied on structured clinical history and physical examination procedures typically used in routine clinical practice, which reduces the likelihood of differences across evaluators and misreporting by study subjects (e.g., subjects were requested to report on previous diagnosis or treatment of a clinical condition and not symptoms or complaints of a given condition). Third, the PSG measures of SDB, PLMS, and OSSD should be considered within the context of the potential impact of a first-night effect. Future population-based studies should examine the natural course of insomnia symptoms using multiple nights of objective (e.g. PSG, actigraphy) and subjective (i.e. sleep diary) sleep measures. Fourth, the focus was on insomnia symptoms, while the presence of an insomnia disorder could not be ascertained. Lastly, trajectories of the clinical risk factors were not considered, which precluded examining whether increased remission rates of insomnia symptoms in specific physical health conditions were associated with a reduction in disease severity over time. Nevertheless, the findings of the present study have important public health and clinical implications as they identify specific pediatric populations at risk for insomnia symptoms chronicity and development.
In summary, this study highlights the unique contribution of individual sociodemographic and clinical risk factors to the persistence, remission, and incidence of insomnia symptoms in childhood and adolescence, including the identification of health disparities and critical developmental periods for the prevention of insomnia in girls. These findings allow healthcare providers to make informed decisions regarding the need for assessment and treatment of insomnia symptoms and reinforce the need of community-based interventions in racial/ethnic and/or underprivileged minorities to prevent the development and chronicity of insomnia. Early screening and treatment for insomnia symptoms in youth with specific clinical risk factors (e.g. mood/anxiety disorders and obesity) should also be a priority, while appropriate management of some medical conditions (e.g. asthma/allergies) may reduce the risk for persistent or incident insomnia symptoms.
Supplementary Material
Acknowledgments
This study was performed at the Sleep Research & Treatment Center and Clinical Research Center of Penn State College of Medicine and all their staff are highly commended by their efforts.
Disclosure statement
Financial disclosure: Research reported in this publication was supported in part by the National Heart, Lung, and Blood Institute, National Institute of Mental Health, and the National Center for Advancing Translational Sciences of the National Institutes of Health under Awards Number R01HL136587 (Fernandez-Mendoza), R01MH118308 (Fernandez-Mendoza), R01HL097165 (Bixler/Liao), R01HL063772 (Bixler), and UL1TR000127 (Sinoway). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no other financial interests.
Non-financial disclosure: none.
References
- 1. Calhoun SL, et al. Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: gender effects. Sleep Med. 2014;15(1):91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ohayon MM, et al. Prevalence and patterns of problematic sleep among older adolescents. J Am Acad Child Adolesc Psychiatry. 2000;39(12):1549–1556. [DOI] [PubMed] [Google Scholar]
- 3. de Zambotti M, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alvaro PK, et al. The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. J Affect Disord. 2017;207:167–174. [DOI] [PubMed] [Google Scholar]
- 5. Gregory AM, et al. Family conflict in childhood: a predictor of later insomnia. Sleep. 2006;29(8):1063–1067. [DOI] [PubMed] [Google Scholar]
- 6. Tokiya M, et al. Predictors of insomnia onset in adolescents in Japan. Sleep Med. 2017;38:37–43. [DOI] [PubMed] [Google Scholar]
- 7. Morrison DN, et al. Sleep problems in adolescence. J Am Acad Child Adolesc Psychiatry. 1992;31(1):94–99. [DOI] [PubMed] [Google Scholar]
- 8. Patten CA, et al. Depressive symptoms and cigarette smoking predict development and persistence of sleep problems in US adolescents. Pediatrics. 2000;106(2):E23. [DOI] [PubMed] [Google Scholar]
- 9. Roberts RE, et al. Chronic insomnia and its negative consequences for health and functioning of adolescents: a 12-month prospective study. J Adolesc Health. 2008;42(3):294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts RE, et al. Persistence and change in symptoms of insomnia among adolescents. Sleep. 2008;31(2):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morin CM, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169(5):447–453. [DOI] [PubMed] [Google Scholar]
- 12. Fernandez-Mendoza J, et al. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012;35(5):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vgontzas AN, et al. Persistent insomnia: the role of objective short sleep duration and mental health. Sleep. 2012;35(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hale L, et al. Recent updates in the social and environmental determinants of sleep health. Curr Sleep Med Rep. 2015;1(4):212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matthews KA, et al. Sleep in healthy black and white adolescents. Pediatrics. 2014;133(5):e1189–e1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, et al. Childhood adversity and insomnia in adolescence. Sleep Med. 2016;21:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson EO, et al. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700–708. [DOI] [PubMed] [Google Scholar]
- 18. Wikoff D, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxicol. 2017;109(Pt 1):585–648. [DOI] [PubMed] [Google Scholar]
- 19. Bixler EO, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bixler EO, et al. Natural history of sleep disordered breathing in prepubertal children transitioning to adolescence. Eur Respir J. 2016;47(5):1402–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindgren SD, Koeppl GK. Assessing child behavior problems in a medical setting: development of the Pediatric Behavior Scale. In: Prinz RJ, ed. Advances in Behavioral Assessment of Children and Families. Greenwich, CT: JAI; 1987:57–90. [Google Scholar]
- 22. Chervin RD, et al. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32. [DOI] [PubMed] [Google Scholar]
- 23. Singareddy R, et al. Medical complaints are more common in young school-aged children with parent reported insomnia symptoms. J Clin Sleep Med. 2009;5(6):549–553. [PMC free article] [PubMed] [Google Scholar]
- 24. Calhoun SL, et al. Behavioral profiles associated with objective sleep duration in young children with insomnia symptoms. J Abnorm Child Psychol. 2017;45(2):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernandez-Mendoza J, et al. Insomnia symptoms, objective sleep duration and hypothalamic-pituitary-adrenal activity in children. Eur J Clin Invest. 2014;44(5):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McLeod BD, eds et al. Overview of Diagnostic and Behavioral Assessment. New York, NY: The Guilford Press; 2013. [Google Scholar]
- 27. Fernandez-Mendoza J, et al. Insomnia phenotypes based on objective sleep duration in adolescents: depression risk and differential behavioral profiles. Brain Sci. 2016;6(4):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carskadon MA, et al. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14(3):190–195. [DOI] [PubMed] [Google Scholar]
- 29. Carskadon MA, et al. Association between puberty and delayed phase preference. Sleep. 1993;16(3):258–262. [DOI] [PubMed] [Google Scholar]
- 30. Achenbach TM, Rescorla LA Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 31. Achenbach TM, Rescorla LA Manual for the ASEBA Adult Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2003. [Google Scholar]
- 32. Rechtschaffen A, Kales AA. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Clin Neurophysiol. 1969;26:644. [DOI] [PubMed] [Google Scholar]
- 33. American Academy of Science Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frye SS, et al. Neurocognitive and behavioral significance of periodic limb movements during sleep in adolescents with attention-deficit/hyperactivity disorder. Sleep. 2018;41(10). doi:10.1093/sleep/zsy129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo C, et al. One-year course and effects of insomnia in rural Chinese adolescents. Sleep. 2013;36(3):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roberts RE, et al. One-year incidence of psychiatric disorders and associated risk factors among adolescents in the community. J Child Psychol Psychiatry. 2009;50(4):405–415. [DOI] [PubMed] [Google Scholar]
- 37. LeBlanc M, et al. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32(8):1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slopen N, et al. Discrimination and sleep: a systematic review. Sleep Med. 2016;18:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi L, et al. Access to medical care, dental care, and prescription drugs: the roles of race/ethnicity, health insurance, and income. South Med J. 2010;103(6):509–516. [DOI] [PubMed] [Google Scholar]
- 40. Johnson DA, et al. Are sleep patterns influenced by race/ethnicity—a marker of relative advantage or disadvantage? Evidence to date. Nat Sci Sleep. 2019;11:79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daviss WB. A review of co-morbid depression in pediatric ADHD: etiology, phenomenology, and treatment. J Child Adolesc Psychopharmacol. 2008;18(6):565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frye SS, et al. Childhood obesity, weight loss and developmental trajectories predict the persistence and remission of childhood sleep-disordered breathing. Pediatr Obes. 2019;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hornyak M, et al. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10(3):169–177. [DOI] [PubMed] [Google Scholar]
- 44. Redline S, et al. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164(4):406–418. [DOI] [PubMed] [Google Scholar]
- 45. Fernandez-Mendoza J, et al. Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain Behav Immun. 2017;61:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fernandez-Mendoza J, et al. Insomnia is associated with cortical hyperarousal as early as adolescence. Sleep. 2016;39(5):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernandez-Mendoza J, et al. Childhood high-frequency EEG activity during sleep is associated with incident insomnia symptoms in adolescence. J Child Psychol Psychiatry. 2019;60(7):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Colrain IM. Sleep and the brain. Neuropsychol Rev. 2011;21(1):1–4. [DOI] [PubMed] [Google Scholar]
- 49. Htoo A, et al. Smoking and sleep disorders. Med Clin North Am. 2004;88(6):1575–91, xii. [DOI] [PubMed] [Google Scholar]
- 50. Pohjanpää AKJ, et al. Is the strong positive correlation between smoking and use of alcohol consistent over time? A study of Finnish adolescents from 1977 to 1993. Health Educ Res. 1997;12(1):25–36. [Google Scholar]
- 51. Fernandez-Mendoza J, et al. Circadian preference, nighttime sleep and daytime functioning in young adulthood. Sleep Biol Rhythms. 2010;8(1):52–62. [Google Scholar]
- 52. Ong JC, et al. Characteristics of insomniacs with self-reported morning and evening chronotypes. J Clin Sleep Med. 2007;3(3):289–294. [PMC free article] [PubMed] [Google Scholar]
- 53. Crowley SJ, et al. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602–612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.