Abstract
Study Objective
To prospectively examine the association between sleep quality and incident cancer risk in the elderly.
Methods
A total of 10,036 participants aged ≥50 years free of cancer at baseline from the English Longitudinal Study of Ageing at wave 4 (2008) were included, and followed up until 2016. The primary endpoint was new onset physician-diagnosed cancer. Sleep quality was assessed by four questions regarding the frequency of sleep problems and overall subjective feeling of sleep quality in the last month, with higher score denoting poorer sleep quality. The multivariable Cox regression model was used to calculate hazard ratio (HR) with 95% confidence interval (CI) for incident cancer risk according to sleep quality.
Results
At 8-year follow-up, a total of 745 (7.4%) participants developed cancer. Compared with good sleep quality at baseline, HR (95% CI) for incident cancer risk was 1.328 (1.061, 1.662) for intermediate quality, 1.586 (1.149, 2.189) for poor quality. Similarly, compared with maintaining good sleep quality in the first 4 years, HR (95% CI) for incident cancer risk was 1.615 (1.208, 2.160) for maintaining intermediate quality and 1.608 (1.043, 2.480) for maintaining poor quality. The exclusion of participants with family history of cancer or abnormal sleep duration yielded consistent results.
Conclusions
Poor sleep quality is positively associated with the long-term risk of developing cancer in an elderly cohort. Both medical staffs and the general public should pay more attention to improving sleep hygiene.
Keywords: sleep quality, cancer risk, elderly
Statement of Significance.
Poor sleep quality is a common concern in the elderly but whether it is associated with incident cancer risk remains unclear. By using data from the English Longitudinal Study of Ageing cohort, the current study found that poor sleep quality was positively associated with the long-term risk of developing cancer, highlighting that both medical staffs and the general public should pay more attention to improving sleep hygiene.
Introduction
Poor sleep quality is one of the most concerning public health issue. The prevalence of insomnia, one of the most common sleep disorders, increased from 17.5% in 2002 to 19.2% in 2012 [1] in the United States. This prevalence ranged from 6% to 19% across European countries [2].
Cancer is the leading cause of death globally. One out of six death is estimated to be caused by cancer [3]. Based on GLOBOCAN estimates, there were more than 18 million new cancer cases and 9.5 million cancer deaths in 2018 [4]. There is a close relationship between cancer and sleep. Many studies have demonstrated that cancer survivors suffer from poor sleep quality after a cancer diagnosis, which includes insomnia, sleep disruption, hot flushes, nightmares, etc. [5]. However, much fewer studies have examined whether poor sleep quality was associated with future cancer risk in participants free of cancer. One meta-analysis published in 2020 included 8 cohort studies with more than 500,000 participants, and found a moderate 24% increased risk of cancer for participants with insomnia symptoms compared with those without insomnia [6]. However, all studies focused on specific cancer types, such as breast cancer and prostate cancer, and exclusively included females or males. While a comprehensive investigation of the association between sleep quality and overall cancer risk in both sexes remains lacking.
The objective of the current study is to investigate the association between sleep quality and the risk of overall incident cancer in a representative cohort of participants living in England aged 50 years and over.
Methods
Participants
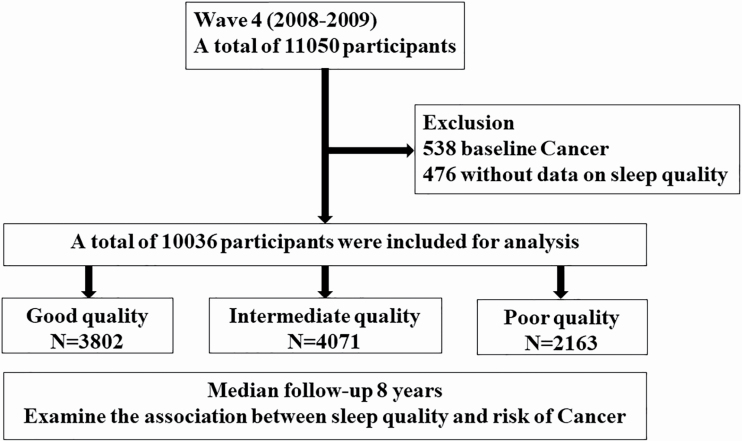
We included participants from the English Longitudinal Study of Ageing (ELSA), a national representative cohort of adults aged greater than 50 years, which was initiated in 2002. Detailed description of the study design has been published [7]. In brief, ELSA included participants aged ≥ 50 years living in England and collected data on demographics, economic and social status, physical and psychological health status, cognitive, etc. ELSA commenced in 2002 (also known as wave 1), and participants were followed up every 2 years. Each follow-up is also known as a wave. Nurse visits were conducted every 4 years to collect biological sample and assess biomarkers. For the current study, we included participants from wave 4 because this is the first wave when data on sleep quality and duration was collected. Those who reported cancer at baseline (N = 538) or with missing data on sleep quality assessment (N = 476) were excluded (Figure 1). We finally included a total of 10,036 participants for analysis. All aspects of this work follow the World Medical Association’s Declaration of Helsinki. All participants were provided informed consent and the project received ethical approval from the National Research Ethics Service.
Figure 1.
Study flow chart. A total of 11,050 participants from ELSA cohort in wave 4 (2008–2009) were screened. After exclusion of participants with cancer at baseline (N = 538), with missing data on sleep quality (N = 476), a total of 10,036 participants were included for analysis. Participants were divided into three groups according to sleep quality and followed up over 8 years.
Exposure
Sleep quality was assessed by four questions, compromising a 3-item questionnaire adapted from Jenkins Sleep Problems Scale [8] plus one question regarding the overall sleep quality. Participants were asked the following four questions: (1) How often has difficulty falling asleep; (2) The frequency wake up several times at night; (3) The frequency wake up feeling tired and worn out; and (4) Rating sleep quality overall. Regarding the first three questions, participants were asked to indicate the frequency of each sleep problem that best describes the sleeping situation: (1) Not during the last month (score = 1); (2) Less than once a week (score = 2); (3) Once or twice a week (score = 3); and (4) Three or more times a week (score = 4). Regarding the fourth question, the participant rated the overall sleep quality that best described sleep situation: (1) Very good (score = 1); (2) Good (score = 2); (3) Fairly bad (score = 3); and (4) Very bad (score = 4). Sleep quality score was calculated by summing up the score of each question [9]. Participants were divided into three groups according to sleep quality score: good quality (4 ≤ score < 8), intermediate sleep quality (8 ≤ score < 12), and poor quality (12 ≤ score ≤ 16). Sleep quality change pattern was classified into five groups according to the sleep quality group in wave 4 and wave 6: (1) Maintaining good quality group (good sleep quality in both wave 4 and wave 6); (2) Quality improved group (intermediate quality in wave 4 to good quality in wave 6; poor quality in wave 4 to intermediate quality in wave 6; and poor quality in wave 4 to good quality in wave 6); (3) Maintaining intermediate group (intermediate quality in both wave 4 and wave 6); (4) Quality worsened group (intermediate quality in wave 4 to poor quality in wave 6; good quality in wave 4 to intermediate quality in wave 6; and good quality in wave 4 to poor quality in wave 6); and (5) Maintaining poor quality group (poor sleep quality in both wave 4 and wave 6).
Outcome
Outcome of the current study was incident cancer, defined as new onset physician diagnosed cancer. Participants were shown a list of illnesses during interview, and were asked to report any physician-diagnosed illness they received. Incident cancer was defined as newly report cancer diagnosis from wave 5 to wave 8. Participants who reported cancer diagnosis at wave 4 were excluded. Survival time was calculated as the time interval between wave 4 and the first wave when cancer was reported. If a participant did not develop cancer till wave 8, then survival time was calculated as the time interval between wave 4 and wave 8, which was equal to 8 years. If a participant lost to follow-up but did not report cancer, survival time was calculated as the time interval between wave 4 and the last wave of follow-up.
Covariates
Covariates were selected based on their association with sleep and cancer risk, as well as previous publications [10]. Covariates included age, sex, wealth, education, social economic classification, marital status, current smoking, alcohol consumption, BMI, physical activity, family history of cancer, chronic pulmonary lung disease, coronary heart disease (CHD), diabetes, hypertension, high blood cholesterol, depression, and sleep duration. Wealth was defined as total non-pension wealth and categorized into fifths [11], which included net financial, physical wealth, and net owner occupied housing wealth, but did not include pension wealth. Education level was classified into seven groups according to the highest educational qualification in wave 4: national vocational qualification (NVQ)4/NVQ5/Degree or equivalent; higher education below degree; NVQ3/General Certificate of Education Advanced (GCE A) level equivalent; NVQ2/GCE O level equivalent; NVQ1/Certificate of Secondary Education (CSE) other grade equivalent; and Foreign/other; no qualification. Socioeconomic status was classified into three groups according to National Statistics-Socio Economic Classification (NS-SEC): managerial and professional occupations; intermediate occupations; and routine and manual occupations. Marital status was categorized into seven groups: single; married first and only marriage; remarried; legally separated; divorced; widowed; and civil partner/others. Alcohol consumption was modeled as a binary variable according to whether respondent reported alcoholic drink once or more per week. BMI was calculated as weight in kilograms divided by height in meters square. Regular physical activity was defined as moderate/vigorous physical activity once or more per week. Family history of cancer was defined as participants’ father or mother died of cancer. Chronic pulmonary lung disease, CHD (angina or heart attack), diabetes, hypertension, and high blood cholesterol was defined as a medical history of or newly reported corresponding illnesses at wave 4. Depression symptom was assessed by the 8-item Centre for Epidemiological Studies Depression scale (CES-D), with a score ≥4 indicating depression symptoms [12]. Sleep duration was defined as self-reported average number of sleep hours on week night.
Statistical analysis
Categorical variables were presented as count (frequency) and compared by using the chi-square test. Continuous variables were presented as mean ± standard deviation (SD), and compared across groups by using the analysis of variance (ANOVA) test. The age-adjusted and multivariable Cox regression model was used to calculate the hazard ratio (HR) and 95% CI for incident cancer risk according to baseline sleep quality. We used three models with various levels of adjustment. Model 1 adjusted for basic demographic and socioeconomic variables, which included age, sex, wealth, education, NS-SEC, and marital status. Model 2 additionally adjusted for lifestyle factors including current smoking, alcohol consumption, BMI, and regular physical activity. Model 3 adjusted for all the covariates in model 2 plus a family history of cancer, chronic obstructive pulmonary disease (COPD), CHD, diabetes, hypertension, high blood cholesterol, depression, and sleep duration. Subgroup analyses were performed to investigate whether the association differed according to age (<60, [60–70), [70–80), ≥80), sex (male or female), depression symptoms (with or without depression), or socioeconomic status (managerial and professional occupations, intermediate occupations, or routine and manual occupations). Three sensitivity analyses were performed to validate the robustness of our findings. Firstly, participants with a family history of cancer were excluded, and the same multivariate Cox regression model was performed to examine the association between sleep quality and incident cancer risk. The second sensitivity analysis was performed by excluding those with abnormal sleep duration (less than 6 h or greater than 9 h). The definition of abnormal sleep duration was chosen in accordance with the previous reports [13], and we would like to enroll enough participants as many as possible to increase the extrapolation of our findings. Finally, we excluded those who reported cancer within 2 years after baseline to address possible reverse casualty since poor sleep quality is prevalent among individuals with prodromal cancer symptoms have poor quality sleep.
Results
Baseline characteristics
Of the 10,036 participants included for analysis, 3,802 (37.9%) had good sleep quality, 4,071 (40.6%) had intermediate sleep quality, and 2,163 (21.6%) had poor sleep quality. Compared with participants with good sleep quality, those who reported poor quality were more likely to be female and current smokers, and less likely to be physically active (Table 1). The proportion of COPD, CHD, diabetes, hypertension, and depression increased progressively as sleep quality decreased. Sleep duration decreased progressively as sleep quality decreased. No significant difference in the socioeconomic status, education, marital status, and family history of cancer was observed between the groups.
Table 1.
Baseline characteristics according to sleep quality
| Variable | Good quality N = 3,802 | Intermediate quality N = 4,071 | Poor quality N = 2,163 | P value |
|---|---|---|---|---|
| Age | 64.9 ± 10.5 | 65.5 ± 10.2 | 64.0 ± 10.1 | <0.001 |
| Sex | 1,835 (48.3) | 2,268 (55.7) | 1,492 (69.0) | <0.001 |
| Wealth quintile | 0.129 | |||
| 1 (lowest) | 562 (17.1) | 660 (18.6) | 353 (18.8) | |
| 2 | 658 (20.0) | 675 (19.0) | 389 (20.7) | |
| 3 | 670 (20.3) | 703 (19.8) | 380 (20.3) | |
| 4 | 665 (20.2) | 777 (21.9) | 381 (20.3) | |
| 5 (highest) | 742 (20.5) | 735 (20.7) | 374 (19.9) | |
| Education* | ||||
| ≥NVQ3/GCE A level | 1,574 (41.4) | 1,660 (40.8) | 845 (39.07) | 0.206 |
| NS-SEC classification | 0.577 | |||
| 1 | 1,065 (32.0) | 1,077 (30.2) | 594 (31.9) | |
| 2 | 818 (24.6) | 892 (25.04) | 456 (24.5) | |
| 3 | 1,448 (43.5) | 1,593 (44.7) | 813 (43.6) | |
| Marital status | ||||
| First and only marriage | 2,128 (56.0) | 2,214 (54.4) | 1,171 (54.1) | 0.259 |
| Current smoking | 523 (15.3) | 494 (13.7) | 426 (22.0) | <0.001 |
| ≥1 alcoholic drink/week | 2,167 (66.2) | 2,235 (63.1) | 932 (51.9) | <0.001 |
| BMI | 27.3±7.1 | 27.4±7.3 | 27.0±7.7 | 0.258 |
| Regular physical activity (%) | 3,098 (81.5) | 3,214 (79.0) | 1,389 (64.2) | <0.001 |
| Family history of cancer (%) | 117 (3.1) | 137 (3.4) | 69 (3.2) | 0.767 |
| COPD (%) | 106 (2.8) | 196 (4.8) | 168 (7.8) | <0.001 |
| CHD (%) | 267 (7.0) | 405 (10.0) | 266 (12.3) | <0.001 |
| Diabetes (%) | 277 (7.3) | 397 (9.8) | 250 (11.6) | <0.001 |
| Hypertension (%) | 1,803 (47.4) | 2,089 (51.3) | 1,132 (52.3) | <0.001 |
| High blood cholesterol (%) | 1,242 (32.7) | 1,347 (33.1) | 670 (31.0) | 0.226 |
| Depression symptom (%) | 36 (1.0) | 107 (3.2) | 128 (10.1) | <0.001 |
| Sleep duration (h) | 7.3±1.0 | 7.0±1.2 | 5.8±1.5 | <0.001 |
NVQ, national vocational qualification; GCE A, General Certificate of Education Advanced; NS-SEC, national statistics social-economic classification; 1, managerial and professional occupations; 2, intermediate occupations; 3, routine and manual occupations; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
*Education: NVQ3/GCE A level is equivalent to senior high school.
Baseline characteristics between participants lost or did not lost to follow-up are shown in Supplementary Table S1. Compared with those who did not lose to follow-up, participants lost to follow-up were older, more likely to be current smoker and have comorbidities including COPD, CHD, diabetes, and hypertension. No significant difference in sleep duration and sleep quality score was found (Supplementary Table S1).
Baseline sleep quality, sleep quality change, and incident cancer
A total of 745 (7.4%) incident cancer occurred during a median 8-year follow-up. Compared with good sleep quality, intermediate and poor sleep quality was associated with higher risk of cancer in the age-adjusted and all three multivariate models (Table 2). In multivariate model 3 adjusted for demographics, socioeconomic characteristics, life style factors, and comorbidities, the HR (95% CI) for incident cancer risk was 1.328 (1.061, 1.662) for intermediated sleep quality and 1.586 (1.149, 2.189) for poor sleep quality.
Table 2.
Association between baseline sleep quality and risk of incident cancer
| Good quality, N = 3,802 | Intermediate quality, N = 4,071 | Poor quality, N = 2,163 | |
|---|---|---|---|
| No. of cases/person-years | 250/22,810 | 338/24,904 | 157/12,538 |
| Age-adjusted model | 1 (reference) | 1.255 (1.004, 1.570) | 1.432 (1.067, 1.923) |
| P value | 0.047 | 0.017 | |
| Model 1 | 1 (reference) | 1.287 (1.038, 1.597) | 1.520 (1.143, 2.020) |
| P value | 0.022 | 0.004 | |
| Model 2 | 1 (reference) | 1.317 (1.055, 1.643) | 1.541 (1.149, 2.067) |
| P value | 0.015 | 0.004 | |
| Model 3 | 1 (reference) | 1.328 (1.061, 1.662) | 1.586 (1.149, 2.189) |
| P value | 0.013 | 0.005 |
Model 1: adjusted for age, sex, wealth, education, social economic classification, marital status; Model 2: model 1+ current smoking, alcohol consumption, BMI, physical activity; Model 3: model 2+ family history of cancer, chronic pulmonary lung disease, CHD, diabetes, hypertension, high blood cholesterol, depression and sleep duration.
We also investigated the association between sleep quality change and incident cancer risk (Table 3). A detailed description of the number of participants in each change group, the mean sleep quality score in wave 4 and wave 6, and mean score change are shown in Supplementary Table S2.
Table 3.
Association between sleep quality change and risk of incident cancer
| Model 1 | Model 2 | Model 3 | ||
|---|---|---|---|---|
| Cases/person-year | HR (95% CI) | |||
| Maintaining good quality | 133/13,314 | 1 (reference) | 1 (reference) | 1 (reference) |
| Quality improved | 109/10,636 | 1.190 (0.850, 1.665) | 1.187 (0.843, 1.672) | 1.196 (0.845, 1.692) |
| Maintaining intermediate quality | 204/13,286 | 1.591 (1.198, 2.111) | 1.599 (1.200, 2.133) | 1.615 (1.208, 2.160) |
| Quality worsened | 138/11,868 | 1.104 (0.804, 1.516) | 1.041 (0.749, 1.447) | 1.044 (0.750, 1.454) |
| Maintaining poor quality | 87/6,810 | 1.559 (1.054, 2.306) | 1.554 (1.040, 2.322) | 1.608 (1.043, 2.480) |
Model 1: adjusted for age, sex, wealth, education, social economic classification, marital status; Model 2: model 1+ current smoking, alcohol consumption, BMI, physical activity; Model 3: model 2+family history of cancer, chronic pulmonary lung disease, CHD, diabetes, hypertension, high blood cholesterol, depression, and sleep duration.
Compared with those maintaining good quality in wave 4 and wave 6, those who maintained intermediate quality (HR: 1.615, 95% CI: 1.208, 2.160) and maintained poor quality (HR: 1.608, 95% CI: 1.043, 2.480) had increased cancer risk in the fully adjusted multivariate model. No significant association between incident cancer risk and sleep quality improved/worsened was observed (Table 3).
Subgroup and sensitivity analysis
Subgroup analysis according to age, sex, depression symptoms, and socioeconomic status are shown in Table 4. No significant interaction between sleep quality and age was found (p = 0.465). In each age subgroup, the intermediate and poor quality was associated with a trend toward higher, or higher cancer risk compared with good sleep quality. Similar results were found in sex, depression, and socioeconomic subgroup. The sensitivity analyses yielded consistent results as the entire cohort (Supplementary Tables S3–S7). For participants without family history of cancer, baseline intermediate and poor sleep quality was associated with increased cancer risk (Supplementary Table S3). Similarly, compared with maintaining good quality, maintaining intermediate quality, or poor quality was associated with increased cancer risk (Supplementary Table S4). Consistent results were found in participants with normal sleep duration (Supplementary Tables S5 and S6) and those who did not report cancer within 2 years after baseline (Supplementary Table S7).
Table 4.
Association between baseline sleep quality and risk of incident cancer according to age, sex, depression, and socio-economic status
| Good quality | Intermediate quality | Poor quality | P interaction | |
|---|---|---|---|---|
| Age | 0.465 | |||
| <60 (N = 3,439) | 1 (reference) | 1.910 (1.088, 3.354) | 2.458 (1.267, 4.769) | |
| [60–70) (N = 3,408) | 1 (reference) | 1.223 (0.855, 1.750) | 1.045 (0.612, 1.784) | |
| [70–80) (N = 2,230) | 1 (reference) | 1.268 (0.850, 1.891) | 1.837 (1.053, 3.206) | |
| ≥80 (N = 959) | 1 (reference) | 1.022 (0.432, 2.416) | 1.522 (0.470, 4.922) | |
| Sex | 0.576 | |||
| Male (N = 4,441) | 1 (reference) | 1.389 (1.004, 1.921) | 1.295 (0.736, 2.275) | |
| Female (N = 5,595) | 1 (reference) | 1.257 (0.902,1.752) | 1.654 (1.095, 2.498) | |
| Depression symptom | 0.409 | |||
| Yes (N = 271) | 1 (reference) | 3.502 (0.435, 28.206) | 2.308 (0.281, 18.958) | |
| No (N = 7,826) | 1 (reference) | 1.292 (1.020, 1.636) | 1.590 (1.125, 2.245) | |
| NS-SEC | 0.875 | |||
| 1 (N = 2,736) | 1 (reference) | 1.290 (0.868, 1.919) | 1.151 (0.665, 1.995) | |
| 2 (N = 1,996) | 1 (reference) | 1.375 (0.866, 2.184) | 1.707 (0.924, 3.151) | |
| 3 (N = 3,573) | 1 (reference) | 1.206 (0.841, 1.730) | 1.431 (0.892, 2.297) |
NS-SEC, national statistics social-economic classification; 1, managerial and professional occupations; 2, intermediate occupations; 3, routine and manual occupations; adjusted for age, sex, wealth, education, social economic classification, marital status, current smoking, alcohol consumption, BMI, physical activity, family history of cancer, chronic pulmonary lung disease, CHD, diabetes, hypertension, high blood cholesterol, depression, and sleep duration.
Discussion
Major findings
In this prospective cohort study of elderly participants living in England, even a moderately impaired sleep quality was associated with a 33% increased risk of incident cancer at the 8-year follow-up, and a greater increase of 59% was observed for severely impaired quality. Consistent findings were observed in the sensitivity analysis in which participants with a family history of cancer, abnormal sleep duration, and who reported cancer diagnosis within 2 years after baseline were excluded. Our findings suggested poor sleep quality as a novel modifiable risk factor for developing cancer. Physicians, health-care givers, and the general public should increase the awareness of this issue in order to promote health and well-being.
Comparison with previous studies
Most previous studies in this field exclusively enrolled men or women, and investigated specific cancer types. Studies of both sexes and overall cancer incidence remain lacking. In female population, two studies used data from Women Health Initiative, a prospective cohort study that included post-menopausal women aged 50–79 years, and investigated the association between sleep quality and the risk of breast [14] and thyroid cancer [15]. Another prospective cohort study included female participants from HUNT study with a mean age 55 years [16], and looked into breast cancer risk. Fewer studies have been conducted in the male population. Only one large-scale prospective cohort study included male participants from the Cancer Prevention Study-II cohort with mean age of 55 years, and investigated the risk of fatal prostate cancer [17]. Current available epidemiological studies did not yield consistent conclusion, while some observed a higher cancer risk in participants with insomnia than those without insomnia, and other studies found no significant relationship between sleep quality and cancer risk. To our knowledge, this is the first large-scale prospective study of both sexes, and investigated overall incident cancer risk in the elderly.
Erran et al. performed a meta-analysis which included 23 publications with over 1,500,000 study individuals in 13 countries [18]. Of these studies, six studies provided data on sleep quality and was combined to calculate incident cancer risk for “poor quality” group relative to “good quality” group. The combined HR (95% CI) for breast cancer in women was 1.03 (0.94–1.13), while the estimates for colorectal cancer for both sexes was 1.23 (0.78–1.92). Compared with this meta-analysis, the association in our study was stronger. We proposed the following two reasons: first, we studied overall cancer risk and did not distinguish specific types of cancers. Second, all the studies in the meta-analysis divided all participants into two groups (poor or good), while our study participants were divided into three groups. Differences in sleep quality between two groups may be smaller compared with three groups.
The second difference between our study and previous studies is that we investigated not only baseline sleep quality, but also changes between two sleep quality assessments. Most previous studies assessed sleep quality only once at baseline, and examined its association with incident cancer risk. We also firstly examined baseline sleep quality and found that cancer risk increased progressively as sleep quality decreased. We went on to further classify participants into five groups according to their sleep quality change pattern, and found that compared with maintaining good sleep quality, maintaining intermediate/poor sleep quality was associated with approximately 60% increased cancer risk. No significant association between sleep improved or worsened was found. Explanations may involve the high heterogeneity of sleep quality in quality improved or worsened group. For instance, participants in the sleep quality improved group had the following three change patterns: poor to intermediate, poor to good, and intermediate to good. Similarly, the quality worsened group also had three change patterns. In summary, our findings highlight the importance of maintaining good sleep.
Biological mechanisms
Our findings are biological plausible. In general, poor sleep quality may cause cancer via reduction in the production of melatonin, sleep disruption, and lifestyle disturbance [19]. The mechanisms also vary according to specific cancer type. Melatonin is a natural hormone produced by pineal gland with multiple functions including regulation of the circadian rhythm. Previous studies suggested that shift-workers who were more likely to be exposed to light at night had a trend toward lower level of melatonin or its metabolites [20]. A growing body of evidence supported melatonin as an anti-cancer agent at the initiation, progression, and metastasis phases [21], and played a key role in interfering various cancer hallmarks, including sustained proliferation, metastasis, angiogenesis, resisting cell death, etc. [22]. Second, sleep disruption and circadian disruption are closely related, which may contribute to oncogenesis via affecting both innate and acquired immune function, increasing reactive oxygen species (ROS) leading to DNA damage and disrupting metabolic function [23]. Finally, some well-established risk factors for cancer, including tobacco smoking, less physical activity, overweight, and obesity [24], are more common in participants with poor sleep quality, which may explain the higher risk of cancer incidence.
In addition to general mechanisms, poor sleep quality may also contribute to specific type of cancer development via different mechanisms. For instance, sleep disturbance increased the level of thyroid-stimulating hormone, which could lead to increased risk of thyroid cancer [15]. Repeated sleep disruption may cause chronically elevated estrogen levels, which contributed to breast cancer risk across life [23]. However, the causal relationship between sleep and overall or specific cancer risk and underlying molecular mechanisms are largely unclear and require further investigation.
Clinical significance
Our findings highlighted the clinical significance of good sleep quality, and found that poor sleep quality was associated with increased cancer risk. Both medical staff and the general public should increase the awareness of this issue, and commence early screening when necessary, which is helpful in promoting early diagnosis. Early detection of cancer can significantly increase the chances of successful treatment and improve survival rate. This is particularly relevant in source-poor settings, where cancer diagnosed at a later stage often results in higher treatment costs, greater comorbidity, and lower survival.
Many effective treatment methods have been proposed for sleep disorders such as insomnia in cancer survivors. In comparison with pharmacological therapy, cumulating evidence have supported non-pharmacological therapy as first-line treatment method due to substantial side effects of sedative medications [25]. One of the most studied methods is cognitive behavior therapy for Insomnia (CBT-I) [26]. A meta-analysis included 8 studies with 752 cancer survivors demonstrated the efficiency of CBT-I in alleviate insomnia compared with control group [26]. In addition, one randomized clinical trial demonstrated that CBT-I was more effective than acupuncture on improving insomnia, although both methods produced clinical meaningful improvements [25]. Exercise intervention is also an effective method. One meta-analysis included 22 RCT with 1,833 patients and demonstrated exercise intervention, particular regular aerobic exercises, benefits sleep quality in cancer survivors [27], despite various exercise duration and intensity. Future studies are required to investigate whether improvement in sleep quality results in a reduction in cancer risk.
Strengths and limitations
The strengths of the current study included prospective study design and high-quality data. The majority of important covariates were available, which allowed us to investigate the independent association between sleep quality and incident cancer risk, after adjustment of a wide range of factors including demographics, socioeconomic factors, medical history, multiple common comorbidities, and sleep duration. Second, we not only investigated baseline sleep quality, but also used data from two sleep assessments, which provided a more accurate and comprehensive assessment of participants’ sleep status.
There are several limitations that need to be addressed. First, sleep quality and cancer were self-reported, which may subject to reporting bias. However, we found that even subjective perception of sleep quality, though not measured objectively, was associated with increased cancer risk, suggesting the importance of subjective feeling of good sleep. Cancer diagnosis was established by physicians, although reported by participants. Second, the specific type of cancer was available in only a small proportion of patients, and we were unable to analyze the association between sleep quality and site-specific cancer risk. However, the development of cancer, regardless of specific type, causes impaired quality of life and life expectancy. Therefore, our findings highlighted the importance of maintaining good sleep quality. Third, data are not available regarding the stages of cancer, and whether sleep quality have adverse impact on cancer severity remains unclear. Fourth, some important covariates which may be associated with cancer risk were not be adjusted because such data were not collected in ELSA, including hormone treatment, family history of specific cancer, medical history of breast/thyroid disease, polycystic ovaries, etc. Fifth, a total of 3,326 participants lost-to follow-up. Those who lost to follow-up were older and more likely to have comorbidities. We currently do not have data access to survival status or cause or death. Therefore we were unable to investigate sleep quality and fatal cancer risk. Sixth, individuals with prodromal cancer symptoms have poor quality sleep. Although we performed sensitivity analysis by excluding these participants, the potential of reverse casualty cannot be eliminated. Finally, our findings are not generalizable to younger age-groups and other ethnicities. However, the main objective of the current study is to investigate the sleep quality-CHD risk association in the elderly.
Conclusions
Even moderately impaired sleep quality is associated with increased incident cancer, with a greater increase for severely impaired sleep quality, independent of socioeconomic characteristics, many common comorbidities, and a family history of cancer and sleep duration. Our findings suggested poor sleep quality as a novel risk factor for incident cancer risk, which deserves the attention of both the medical staff and the general public.
Supplementary Material
Acknowledgments
We acknowledged ETIM OKON INIEKUNG for prJiaqiang Liao for his help in statistical analysis. We acknowledged UK data service officers and all the staff for their kind help in accessing and using data from English Longitudinal study of ageing.
Funding
ELSA cohort is funded by the National Institute on Aging and by a consortium of UK government departments. Funding has also been provided by the Economic and Social Research Council (ESRC) (https://www.elsa-project.ac.uk/funders).
Disclosure statement
Financial disclosure: All authors declared no conflict of interest.
Non-financial disclosure: None declared.
References
- 1. Ford ES, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16(3):372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riemann D, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. [DOI] [PubMed] [Google Scholar]
- 3. American Cancer Society. Global Cancer Facts & Figures. 4th ed. Atlanta, GA: American Cancer Society; 2018. [Google Scholar]
- 4. Global Cancer Observatory. Cancer incidence and mortality databases. https://gco.iarc.fr/databases.php.
- 5. Walker WH, 2nd, Borniger JC. Molecular mechanisms of cancer-induced sleep disruption. Int J Mol Sci. 2019;20(11):2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi T, et al. Does insomnia predict a high risk of cancer? A systematic review and meta-analysis of cohort studies. J Sleep Res. 2020;29(1):e12876. [DOI] [PubMed] [Google Scholar]
- 7. Steptoe A, et al. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol. 2013;42(6):1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jenkins CD, et al. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol. 1988;41(4):313–321. [DOI] [PubMed] [Google Scholar]
- 9. Campanini MZ, et al. Duration and quality of sleep and risk of physical function impairment and disability in older adults: results from the ENRICA and ELSA cohorts. Aging Dis. 2019;10(3):557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Y, et al. Sleep duration and the risk of cancer: a systematic review and meta-analysis including dose-response relationship. BMC Cancer. 2018;18(1):1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fancourt D, et al. The art of life and death: 14 year follow-up analyses of associations between arts engagement and mortality in the English Longitudinal Study of Ageing. BMJ. 2019;367:l6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie W, et al. Cognitive decline before and after incident coronary events. J Am Coll Cardiol. 2019;73(24):3041–3050. [DOI] [PubMed] [Google Scholar]
- 13. Daghlas I, et al. Sleep duration and myocardial infarction. J Am Coll Cardiol. 2019;74(10):1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vogtmann E, et al. Association between sleep and breast cancer incidence among postmenopausal women in the Women’s Health Initiative. Sleep. 2013;36(10):1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo J, et al. Sleep disturbance and incidence of thyroid cancer in postmenopausal women the Women’s Health Initiative. Am J Epidemiol. 2013;177(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sen A, et al. Insomnia and the risk of breast cancer: the HUNT study. Psychosom Med. 2017;79(4):461–468. [DOI] [PubMed] [Google Scholar]
- 17. Gapstur SM, et al. Work schedule, sleep duration, insomnia, and risk of fatal prostate cancer. Am J Prev Med. 2014;46(3 Suppl 1):S26–S33. [DOI] [PubMed] [Google Scholar]
- 18. Erren TC, et al. Sleep and cancer: synthesis of experimental data and meta-analyses of cancer incidence among some 1,500,000 study individuals in 13 countries. Chronobiol Int. 2016;33(4):325–350. [DOI] [PubMed] [Google Scholar]
- 19. Fritschi L, et al. Hypotheses for mechanisms linking shiftwork and cancer. Med Hypotheses. 2011;77(3):430–436. [DOI] [PubMed] [Google Scholar]
- 20. Hunter CM, et al. Measuring light at night and melatonin levels in shift workers: a review of the literature. Biol Res Nurs. 2017;19(4):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reiter RJ, et al. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci. 2017;18(4):843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Talib WH. Melatonin and cancer hallmarks. Molecules (Basel, Switzerland). 2018;23(3):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samuelsson LB, et al. Sleep and circadian disruption and incident breast cancer risk: an evidence-based and theoretical review. Neurosci Biobehav Rev. 2018;84:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boniol M, et al. Prevalence of main cancer lifestyle risk factors in Europe in 2000. Eur J Cancer. 2010;46(14):2534–2544. [DOI] [PubMed] [Google Scholar]
- 25. Garland SN, et al. Acupuncture versus cognitive behavioral therapy for insomnia in cancer survivors: a randomized clinical trial. J Natl Cancer Inst. 2019;111(12):1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson JA, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. [DOI] [PubMed] [Google Scholar]
- 27. Fang YY, et al. Meta-analysis: exercise intervention for sleep problems in cancer patients. Eur J Cancer Care (Engl). 2019;28(5):e13131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.