Abstract
Head and neck cancers (HNC) represent the seventh most frequent cancer worldwide, with squamous cell carcinomas as the most frequent histologic subtype. Standard treatment for early stage diseases is represented by single modality surgery or radiotherapy, whereas in the locally advanced and recurrent or metastatic settings a more aggressive multi-modal approach is needed with locoregional intervention and/or systemic therapies. Epidermal Growth Factor Receptor (EGFR) plays an important role in HNC biology and has been studied extensively in preclinical and clinical settings. In this scenario, anti-EGFR targeted agent cetuximab, introduced in clinical practice a decade ago, represents the only approved targeted therapy to date, while the development of immune-checkpoint inhibitors has recently changed the available treatment options. In this review, we focus on the current role of anti-EGFR therapies in HNCs, underlying available clinical data and mechanisms of resistance, and highlight future perspectives regarding their role in the era of immunotherapy.
Keywords: cetuximab, EGFR, HNC, HNSCC, immunotherapy
Introduction
Head and neck cancer (HNC) is the seventh most frequent cancer worldwide.1 HNC arises from oral cavity, pharynx, larynx, sinuses, nasal cavity, and salivary gland, and HNC squamous cell carcinoma (HNSCC) is the major histologic subtype (>90%). Alcohol and tobacco abuse are the two most important risk factors for HNSCC. Human papillomavirus (HPV) infection also plays a role in the development of certain HNCs, particularly those in the oropharynx.2 HPV-positive and -negative cancers represent two distinct biologic entities, but these differences have not yet been translated into a different treatment approach in the clinic.3
Unfortunately, the majority of HNSCC patients are diagnosed at later stages. Standard therapies used for treatment of HNSCC achieve only a 40–50% 5-year survival rate in advanced stages.4
Early stage disease (stages I and II) is treated with single modality surgery or radiotherapy (RT) with 5-year survival rates of approximately of 90% and 70%, respectively.
In locally advanced (LA) disease, a more aggressive multimodality treatment combining locoregional intervention and systemic treatment using chemotherapy (CT) and/or anti-Epidermal Growth Factor Receptor (EGFR) targeted therapy is required.5 The anti-EGFR monoclonal antibody catuximab (Cx) was introduced into clinical practice about a decade ago when available treatments for HNSCC were still very limited; to date, however, it remains the only targeted therapy approved for this cancer type.
Approximately 12% of HNSCC cases are diagnosed at the metastatic stage. Furthermore, 10–20% of patients treated for early stage HNSCC disease are expected to experience recurrence during follow up. Prognosis of recurrent or metastatic (R/M) HNSCC is poor, with a median overall survival (mOS) of 1 year.5 In this setting, medical treatment usually consists of doublet platinum-based CT (EXTREME regimen).6
More recently, development of immune-checkpoint inhibitors (ICIs) has greatly changed the treatment of HNSCC. The anti-PD-1 (programmed death 1) antibodies Nivolumab and Pembrolizumab showed survival improvements in platinum-treated patients with R/M disease,7,8 leading to approval of these two drugs by the United States Food and Drug Administration (FDA) in 2016. Finally, given the results of KEYNOTE-048 trial, Pembrolizumab is a candidate to replace, or be associated with, CT in a large portion of patients in front-line treatment.9
In the present article, we review the biological rationale and clinical development of anti-EGFR in the treatment of HNCs, and highlight future perspectives for use of anti-EGFR in the era of immunotherapy.
EGFR in HNSCC: pathogenetic and predictive role
EGFR pathway
EGFR is a transmembrane protein receptor that belongs to the ErbB family of receptor tyrosine kinases (RTKs) (Figure 1). Four different ErbB receptors have been characterized, EGFR (or ErbB 1 or Her-1), Her-2, Her-3, and Her-4, which exert critical physiological functions in epithelial normal cells. EGFR can be activated by soluble ligands (e.g., EGF or transforming growth factor alpha, TGF-α) or by homodimerization or heterodimerization with other HER family receptors (especially ErbB 2); EGFR activation results in stimulation of a proliferative and pro-survival intracellular signaling, through the mitogen-activated protein kinase (MAPKs) cascade, PI3K/AKT/mTOR and JAK/STAT pathway.10,11
Figure 1.
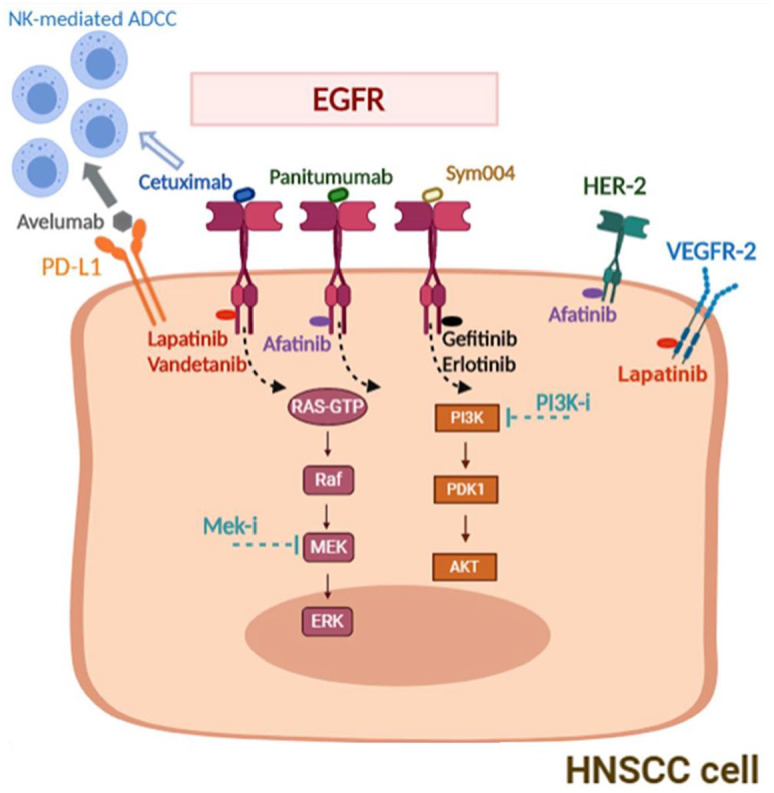
EGFR pathway and targets for combination strategies in HNSCC.
EGFR, epidermal growth factor receptor; HNSCC, head and neck cancer squamous cell carcinoma.
EGFR in HNSCC carcinogenesis
EGFR is overexpressed in 80–90% of HNSCCs, playing a key role in carcinogenesis and tumor evolution. In fact, EGFR expression is higher in HNC patients’ normal mucosa than in healthy people, and EGFR expression gradually increases according to histological malignant transformation, from hyperplasia to invasive carcinoma.12–14
Predictive role of EGFR alterations
Playing a fundamental role in its carcinogenesis, EGFR is an extensively studied biomarker in HNSCC. Although EGFR overexpression and aberrant EGFR gene copy number (GCN) have commonly been associated with poorer prognosis and disease specific survival in HNSCC,15,16 recent reports suggest a controversial prognostic role of EGFR expression in HNSCC, evaluated according different cytogenetic/molecular markers: protein expression levels, protein activation, GCN, polymorphisms, mutation, EGFRvIII expression, and EGFR ligand expression.17
Up to now, results are still conflicting in terms of predictive value of EGFR expression in HNSCC for standard treatments, including RT alone or combined with surgery, CT, and anti-EGFR drugs. Alterations of EGFR, including EGFR mutation frequency and EGFR protein expression/phosphorylation, were not associated with disease free survival (DFS).18 HNSCC with high EGFR expression had poor outcome with RT alone, while no difference was found when using RT+Cx.19
Also, discordant results were obtained by investigating the association between EGFR GCN and clinical outcome after primary CT: in some studies, it was a negative prognostic factor, being significantly associated with shorter progression-free survival (PFS) and OS,15,16 but this was not confirmed by other studies.20
Moreover, an inverse correlation between HPV positivity and EGFR expression has been reported in HPV-positive oropharyngeal squamous cell carcinoma (OSCC).21 This should be taken into account, considering that about 5–20% of HNSCC are HPV positive, with a significantly higher percentage in OSCC(range 40–90%).22 Also, HPV-positive patients are less likely to experience recurrence or disease progression than HPV-negative patients, independent of treatment.23
Biomarkers for anti-EGFR therapy
From the past 10 years, numerous randomized trials have been conducted with the aim to identify patients who can mostly benefit from anti-EGFR therapy [ClinicalTrials.gov identifier: NCT02999087].4–6
In a retrospective analysis on 37 R/M HNSCC patients treated with Cx+CT, high tumor expression of EGFR ligands epiregulin (EREG) and amphiregulin (AREG), correlated with OS and PFS.24
We still lack validated molecular features that can predict clinical outcome to anti-EGFR therapy in HNSCC; however, an important and valid clinical predictive factor is represented by onset of skin toxicity under EGFR treatment. A meta-analysis by Klinghammer et al. showed a positive trend in PFS and OS from the addition of Cx to CT in patients who had experienced a Grade 1 skin rash compared with patients with Grade 0 skin rash.25 A recently published study confirmed a correlation between Grade 3 skin toxicity emerged within 90 days from starting Cx therapy and benefit in OS in R/M HNSCC.26
Generally, targeted therapy holds great promise to improve patients’ outcome while limiting toxicity as compared with CT. Thus, identifying predictive biomarkers for anti-EGFR treatment is an important challenge to guide HNSCC patients’ selection.
Anti-EGFR drugs in treatment of HNSCC
Two different anti-EGFR therapeutic strategies have been developed (Figure 1): the first is to target the extracellular domain of the receptor with monoclonal antibodies as Cx and Panitumumab,27 and the second is to target the intracellular domain of the receptor with low-molecular-weight tyrosine kinase inhibitors (TKIs) such as Gefitinib, Erlotinib or Afatinib.28
Cx is a chimeric mouse-human monoclonal IgG1 antibody that binds EGFR at its extracellular domain and blocks EGF-induced autophosphorylation of EGFR.11 It has preclinical activity in vitro and in vivo both as a single agent and in combination with cytotoxic compounds and RT in different human cancer models, including HNC.29–31 Anti-EGFR antibody competes with EGFR ligands, resulting in internalization and degradation of the antibody-receptor complex and leading to the death of tumor cells also through the indirect mechanism of NK-dependent antibody mediated cytotoxicity [antibody dependent cell-mediated cytotoxicity (ADCC)].32,33 It also induces the dimerization and downregulation of EGFR, perturbs cell cycle progression,31 and inhibits tumor-induced angiogenesis.34 Beyond Cx, other anti-EGFR antibodies have been developed in HNSCC.35 Zalutumumab is a human monoclonal antibody against EGFR that has shown activity in preclinical models by blocking the EGFR signaling pathway and, as Cx, by stimulating ADCC.36 Panitumumab is a fully human anti-EGFR monoclonal antibody that effectively inhibits EGFR signaling similarly to Cx. It diverges from Cx due to its IgG2-based structure, which does not allow an enhanced NK-dependent ADCC activity.37 The other class of drugs is represented by TKIs, which inhibit EGFR signaling through preventing the intracellular phosphorylation cascade.38 First-generation TKI, gefitinib and erlotinib, are anilinoquinazolines that bind reversibly to the K745 site in the ATP binding pocket,39 with anti-tumor activity in vitro mediated by inhibition of AKT and MAPK.40 Also, erlotinib is able to radio-sensitize HPV-negative HNSCC cells by inhibiting DNA double-strand-break (DSB) repair via MAPK and PARP1,41 and inducing arrest of the cells in the G2 cell cycle phase.42 Afatinib is a second-generation pan-EGFR-TKI that irreversibly binds to EGFR1, HER2, and HER4,43 performing a sustained receptor inhibition compared with first-generation TKI inhibitors. Macha et al. demonstrated that afatinib is more potent than erlotinib in EGFR inhibition in HNSCC in vitro models, and is able to inhibit the expression of cancer stem cells (CSCs) markers, including CD44 and Oct3/4, and CSCs growth. Of interest, they showed also that afatinib significantly radio-sensitizes preclinical model of HNSCC through eradication of CSCs.44 These results encourage clinical testing of afatinib in the setting of heterogenous HNSCC.45
Anti-EGFR antibodies
Cetuximab
Cx remains to date the only targeted drug approved for the treatment of LA and R/M HNSCC (Table 1).
Table 1.
Summary of clinical data investigating anti-EGFR therapy in HNSCC.
| Drug | Study | Phase | Treatment | Setting | Results |
|---|---|---|---|---|---|
| Cetuximab | EXTREME | III | Cisplatin and 5-FU ± cetuximab (PFEx) | R/M | OS (10.1 versus 7.4) and PFS (5.6 versus 3.3) for triplet arm |
| RTOG 1016 | III | RT plus cetuximab or cisplatin in HPV + oropharyngeal cancer | LA | Outcomes at 5 years of treatment: cetuximab + RT inferiority in terms of OS (78% versus 85%), PFS (67% versus 78%), locoregional failure (17% versus 10%), distant metastasis (12% versus 9%) | |
| De-ESCALaTE | III | RT plus cetuximab or cisplatin in HPV + oropharyngeal cancer | LA | ORR at 12 weeks: 44.4%, PFS 6.2 months, OS 14.0 months. TPEx regimen is effective and might be substitute for PFEx | |
| GORTEC | II | Cetuximab, docetaxel and cisplatin combination (TPEx) | R/M | ORR at 12 weeks: 44.4%, PFS 6.2 months, OS 14.0 months. TPEx regimen is effective and might be substitute for PFEx | |
| Panitumumab | PRISM | II | Panitumumab in monotherapy | R/M | Limited activity in previously treated patients |
| SPECTRUM | III | Cisplatin and 5-FU ± panitumumab | R/M | No improvement in OS (11 versus 9 months) | |
| Afatinib | LUX- Head & Neck 1 | III | Afatinib versus Metotrexate | R/M | Afatinib improved PFS (2.6 versus 1.7) with a manageable safety profile |
| LUX- Head & Neck 2 | III | Afatinib versus placebo | Adjuvant after CRT | Afatinib after CRT did not improve DFS versus placebo | |
| LUX- Head & Neck 3 | III | Afatinib versus Metotrexate | R/M | Result are consistent with Trial 1 | |
| Gefitinib | IMEX | III | Gefitinib versus Methotrexate | R/M | No OS improvement compared with methotrexate |
CRT, chemoradiation; EGFR, epidermal growth factor receptor; 5-FU, 5-fluorouracil; HNSCC, head and neck cancer squamous cell carcinoma; HPV, human papillomavirus; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; R/M, recurrent or metastatic; RT, radiotherapy.
Exclusive treatment with concomitant RT
In a pivotal randomized study reported by Bonner et al., 424 patients with LA-HNSCC were randomized to RT alone or combined with Cx (RT-Cx).46 The mOS was 49 months after combined therapy compared with 29 months after RT alone (p = 0.03). The 5-year OS (46 versus 36%) and 3-year loco-regional control (47 versus 34%) were prolonged with the use of Cx in all clinical subgroups.46 Interestingly, Cx-induced skin rash (grade 2 or above) and p16-positivity predicted better outcomes in terms of OS (HR 0.38 versus 0.93, respectively).47
Based on these data, RT-Cx is incorporated in guidelines as an alternative to standard chemoradiation (CRT) in this setting for patients considered unfit for cisplatin, even given the lack of a direct comparison with standard concurrent CRT with cisplatin in a phase III randomized clinical trial and toxicity profile. A randomized phase II trial evaluating CRT versus RT-Cx was stopped prematurely for slow accrual, resulting in being underpowered for efficacy outcomes. However, a higher rate of acute toxicity (severe cutaneous toxicity and need for nutritional support) was found for RT-Cx, with 11% of toxic death and 13% of discontinuation rate of RT versus 0% of CRT group (p = 0.05).48
In a meta-analysis of 15 trials (3 of which were perspective), including 1088 patients, conducted by Petrelli et al., CRT was associated with better PFS (RR 0.68, p = 0.02) and OS (RR 0.66, p = 0.02) at 2 years compared with RT-Cx in treatment of LA-HNSCC.49 Conversely, a meta-analysis of 31 studies by Huang et al., revealed no significant difference in 3 years OS and PFS (p > 0.05), and confirmed a better outcome in HPV + and primary OSCC patients.50
Because CRT and RT-Cx were demonstrated as superior to RT alone for LA-HNSCC, a randomized trial was performed to determine whether adding Cx to CRT could enhance its effects – the RTOG 0522 study. The intensification regimen did not result in improved OS; in the EGFR high subgroup, increased toxicity (grade 3–4 mucositis 43.2% versus 33.3%, p = 0.002) and higher discontinuation rate of RT (26.9% versus 15.1%) were detected.51
Recently, two randomized phase III trials, RTOG 1016 and De-ESCALaTE, investigated the substitution of cisplatin with Cx in patients with advanced HPV + OSCC. Historically, it has been considered a more chemo- and radiosensitive disease, but, since it arises in younger patients without classical risk factors for HNSCC, the long-term impact on quality of life of traditional therapeutic interventions led to investigation of chemo-sparing regimens. However, both trials showed that substitution of cisplatin with Cx had no significant impact on toxicity and did not improve survival: no difference in OS was found in RTOG study (HR 1.45, p = 0.5056)52 and worse OS (97.5% versus 89.4%, p = 0.0012) in De-ESCALaTE trial in Cx group.53 Collectively, these data indicate that cisplatin should be used as first-choice radiosensitizer in all eligible patients with HPV + OPC. A study comparing CRT and RT-Cx in overall HNSCC population is currently ongoing and will provide further results (ARTSCAN III).
In an induction setting, the addition of Cx to CT appears to improve overall response rate (ORR), especially with taxane-based treatment, but it is still not a standard of care for higher toxicity in the absence of survival benefit.54 Sequential RT-Cx after induction CT appears, at the moment, to be the most promising and feasible option as part of an organ preservation strategy.55
Adjuvant treatment
To date, no evidence supports the use of Cx, awaiting the result of the phase III study ACCRA-HN comparing RT-Cx plus Cisplatin-5FU and RT-Cx.
Metastatic setting
Approval of Cx in the metastatic setting was based on the EXTREME trial. A total of 442 patients with R/M HNSCC were randomized to cisplatin/carboplatin and 5-fluorouracil (5-FU) with or without Cx for six cycles, followed by maintenance Cx. No crossover was allowed. On triplet arms, both mOS (10.1 versus 7.4 months, p = 0.04) and PFS (5.6 versus 3.3, p < 0.01) were improved, with an increase of 16% in ORR in the arm with Cx. These data led to the introduction of the EXTREME protocol in clinical practice for the treatment of HNCs in the forefront of recurrent or metastatic setting.
Although no differences in quality of life outcomes were reported, more sepsis and skin reactions were observed in the experimental arm. EGFR expression was not predictive of treatment benefit.56 Several attempts have been made to replace 5-FU in the EXTREME scheme. In the GORTEC phase II study, the combination named TPEx (docetaxel, cisplatin and weekly Cx for four cycles followed by Cx maintenance) obtained an ORR at 12 weeks of 44.4% with a manageable safety profile; median PFS and OS were 6.2 and 14.0 months, respectively.57 Preclinical evidence suggested a mechanism for the synergistic activity of Cx with taxanes, represented by prevention of taxane-induced EGFR phosphorylation and regulation of EGFR downstream pathways.58 Several clinical studies have investigated combinations without platinum.59 A combination of weekly paclitaxel and Cx proved feasible and safe as first-line treatment of patients unfit for cisplatin, with an ORR of 54%, and PFS of 4.2 months in a phase II single-arm Spanish study.60 A phase II study called CACTUS is investigating the combination of Cx and nab-paclitaxel in R/M HNSCC. The efficacy of Cx and docetaxel combination was evaluated also in platinum-pretreated patients obtaining in a single arm study a disease control rate (DCR) of 51% and mPFS of 3.1 months, independently from a previous response to platinum.61
Panitumumab
Two clinical studies investigated the use of Panitumumab as a single agent in pretreated HNSCC. An open-label, single-arm, multicenter trial published in 2015 studied panitumumab monotherapy at the dose of 9 mg/kg Q3W (PRISM trial). Only mild activity of panitumumab in this setting was shown with an ORR of 4%, mPFS of 1.4 months.62 Another study investigated the safety and efficacy of a 2-week schedule of panitumumab at 6 mg/kg in the same setting, obtaining similar moderate activity, with an ORR of 6%, a mPFS of 2.6 months, and a mOS of 9.7 months.63 Moreover, the efficacy of Panitumumab in first line R/M HNSCC was assessed by a phase III SPECTRUM trial, evaluating cisplatin and 5-FU with or without the anti-EGFR antibody panitumumab. The benefit seen in EXTREME with anti-EGFR Cx was not reproduced: primary endpoint of improvement in OS was not reached, even if ORR (36% versus 25%, p = 0.0065) and mPFS (5.8 versus 4.6 months, p = 0.0036) were significantly improved with panitumumab. In subgroup analysis, OS was improved only in p16-negative patients (p = 0.0115).56 Different features of panitumumab may be responsible for these differences: lower ADCC-inducing ability, lack of maintenance treatment, and the 3-week schedule. Also, the prevalence of p16-positive tumors in the SPECTRUM trial was higher, maybe due to geographic differences (EXTREME trials enrolled only European patients).
Sym004
Sym004 is a synergistic antibody combination containing two recombinant mAbs, futuximab and modotuximab, which bind to different, non-overlapping epitopes of EGFR, different from the epitopes of Cx and panitumumab. In contrast to single anti-EGFR antibodies, Sym004 induces rapid and highly efficient degradation of EGFR.64 Preclinical studies have shown that the combination of Sym004 and radiation resulted in significant tumor regrowth delay and superior anti-tumor effects compared with treatment with Sym004 or radiation alone in lung and HNC.65 In a proof of concept trial, clinical activity of Sym004 was investigated in 26 patients, including 23 progressing on previous anti EGFR treatment. Even if no objective responses were observed, 50% of patients had stable disease (SD) as best response, with a mPFS and mOS of 82 and 156 days, respectively. This trial revealed modest anti-tumor activity of Sym004 in an extensively pretreated advanced HNSCC population, and, interestingly, paired biopsies showed a significant down-regulation of EGFR in both skin and tumors following exposure to Sym004, supporting the activity of Sym004 in this setting.66
EGFR tyrosine kinase inhibitors
Afatinib
The LUX-Head & Neck 1 trial tested Afatinib in European patients with R/M HNSCC whose disease progressed after first-line platinum regimens versus methotrexate. Afatinib improved the primary endpoint of PFS by 0.9 months (mPFS = 2.6 versus 1.7 months, p = 0.030), with a DCR of 49.1% versus 38.5% of CT, but OS was not significantly different. Prespecified tumor biomarkers analysis identified subgroups of patients achieving increased benefit from target therapy: p16-negative, EGFR-amplified, HER3-low, PTEN-high.67 Similar data were obtained by the more recent LUX-Head & Neck 3 trial, with the same design in Asian population.68 The accrual of trial LUX-Head & Neck 2 trial, comparing Afatinib and placebo after CRT in primary unresected HNSCC patients, was halted due to futility of interim pre-planned analysis.69
Lapatinib
Lapatinib is a reversible dual EGFR and HER2-TKI that has been tested in HNSCC. In a phase II trial on pretreated R/M HNSCC patients, no objective responses were observed with lapatinib, regardless of prior EGFR treatment.70 In L/A unresected HNSCC, Lapatinib combined with cisplatin-based CRT was well tolerated, with increases in ORR at 6 months post-CRT (53% versus 36%), and better PFS benefit in p16-negative disease (>20.4 months versus 10.9),71 but these data were not confirmed by a phase III randomized trial.72
Gefitinib and erlotinib
In refractory metastatic HNC patients, gefitinib and erlotinib showed some benefit in ORR, and mOS ranged from 0% to 15% and 5.9–8.1 months, respectively.73,74 However, gefitinib did not obtain OS benefit versus methotrexate in a phase III trial, also having higher hemorrhagic toxicity events.75 In LA-HNSCC, addition of erlotinib to CRT did not improve PFS and primary endpoint of CR (CR 52 versus 40%, p = 0.08).76 In a first-line setting, adjunction of erlotinib to cisplatin and docetaxel as first-line regiment followed by erlotinib maintenance improved PFS from 4.4 to 6.1 months (p = 0.026), RR (56 versus 44%) and OS from 13.7 to 17.0 months (p = 0.07) in a randomized phase II trial.77 The onset of skin rash was associated with clinical benefit to both agents,78 in the absence of other known molecular predictors of response. A recent meta-analysis provides pooled estimates of the effectiveness and safety of gefitinib-based therapy in patients with advanced HNSCC in comparison with standard regimens. As underlined by the authors, disappointingly, benefits from gefitinib-containing regimens are still negative.79
Vandetanib
Vandetanib is an oral anti-cancer agent that selectively targets vascular endothelial growth factor receptor (VEGFR), EGFR, and rearranged during transfection (RET) tyrosine kinases. Preclinical evidence supports its potential role and clinical activity in HNSCC.80–83 In a phase II randomized trial, R/M HNSCC patients received docetaxel alone or with vandetanib: some trends in clinical benefit were observed, but they did not have enough clinical power to continue accrual.84
Resistance to anti-EGFR in HNSCC
Resistance to targeted therapy can either be primary, meaning that patient do not respond to targeted treatment, or secondary, patients respond to treatment but will eventually develop resistance (Table 2).
Table 2.
Resistance to EGFR inhibition in HNSCC.
| Signalling pathway | Mechanism | Reference |
|---|---|---|
| EGFR family | EGFRvIII | Wheeler et al.85 |
| EGFRK521variant | Braig et al.86 | |
| HER2 activation | Novoplansky et al.87 | |
| Ligand overexpression | Boeckx et al.88 | |
| PIK3CA pathway | PIK3CA mutation | Kyungsuk et al.89 |
| PTEN loss of expression | Da Costa et al.90 | |
| RAS | KRAS mutation | Eze et al.91 |
| HRAS mutation | Puram et al.92 | |
| MET | Expression and activation | Boeckx et al.88 |
EGFR, epidermal growth factor receptor; HNSCC, head and neck cancer squamous cell carcinoma.
Resistance to anti-EGFR targeted therapy may be due to: (1) intrinsic activation of EGFR, (2) activation of an EGFR downstream component, or (3) of another TK receptor such as hepatocyte growth factor (HGF) receptor (MET) (Figure 1).90
(1) Among the first genetic alterations of the EGFR that have been identified, the type-III mutated variant (EGFRvIII), and the EGFRK521 variant correlates with therapeutic resistance to Cx in preclinical models and in clinical trials. Mechanistically, EGFRvIII is characterized by an in-frame deletion from exons 2 through 7 in the extracellular domain, which inhibits EGFR ligands from binding and leads to constitutive activation of its TK domain, while the EGFR K521 variant is characterized by a frequent single nucleotide polymorphism in the extracellular domain that reduces affinity for Cx.85,86 A genetic profiling of HNSCC samples with EGFR activation revealed that EGFR ligands were highly expressed in a Cx resistant subset, suggesting an autocrine sustained EGFR hyperactivity.88
(2) Alternatively, the function of a target gene can be bypassed by activating downstream molecules. Comprehensive genomic analysis of HNSCC revealed frequent alterations in the PI3K pathway, e.g., mutations in PIK3CA, which is the third most commonly mutated gene in HNSCC. Compensatory activation of PI3K downstream pathway is demonstrated by higher PI3K pathway gene expression and efficacy of PI3K inhibitors in Cx-resistant HNC cells.89 In a clinical setting, the addition of PX-866, a PI3K inhibitor, to Cx did not show any significant clinical benefit in R/M HNSCC in a randomized phase II study, but other novel combinations are still under evaluation, such as combinations of Cx with Buparlisib, a pan-PI3K inhibitor [ClinicalTrials.gov identifier: NCT01816984], or with Alpelisib, an α-specific PI3K inhibitor [ClinicalTrials.gov identifier: NCT01602315], and/or with temsirolimus [ClinicalTrials.gov identifier: NCT01256385].
PTEN is a negative regulator of the PI3K pathway, and PTEN loss is detected in about 30% of cases.90 Loss of PTEN expression in tumor samples correlates with longer PFS on Cx therapy, while patients with PTEN high-expressing tumors had improved PFS.91
In colorectal cancer, as an example, KRAS mutations are associated with intrinsic resistance to Cx or panitumumab.11 In HNSCC, the majority of Cx-naïve tumors do not carry RAS mutations, with the exception of 4.3% with HRAS mutations, especially in patients with extensive tobacco exposure,92 while almost half of patients develop acquired RAS mutations as a resistance mechanism to EGFR inhibition.91
(3) MET expression has been associated with resistance to Cx in a preclinical HNSCC model and in a retrospective study.93,94 Novoplansky et al. reported clinical evidence of MET activation by ligand-dependent (mediated by HGF produced by stromal cells) or ligand-independent (MET amplification and activating mutations).87,95 The activation of HER2 signaling has been also associated with Cx resistance, suggesting a potential role for afatinib in this setting.88
Perspectives: role of anti-EGFR drugs in the era of immunotherapy
ICIs in HNSCC
Recently, the introduction of ICIs has changed the standard of care in oncology. ICIs, such as anti-PD-1/PD-L1 (programmed death-ligand 1), are currently approved in various cancer types, including HNSCC.96 In cancers, tumor cells induce immunosuppression through the interaction of PD-L1 expressed on their surface with PD-1 expressed by T-cells, preventing attack from the immune system.97 In particular, last year, two anti-PD-1 antibodies, nivolumab and pembrolizumab, were approved in Europe and Italy for treatment of relapsed metastatic HNSCC; nivolumab is used after progression to platinum CT independently from PD-L1 expression, whereas pembrolizumab is approved in the same setting for PD-L1-positive HNSCC, and also in first line as monotherapy or in combination with platinum-based CT in PD-L1 positive HNSCC. The approval of these drugs has been incredibly fast, based on positive results of three phase III trials – the Checkmate-141 trial for nivolumab, and the Keynote-040 and Keynote-048 trials for pembrolizumab.98 Nivolumab showed improved OS and quality of life in relapsed HNSCC patients, 99,100 compared with standard of care, according to investigators’ choice, especially in the absence of previous exposure to anti-EGFR Cx: nivolumab improved the mOS in patients not pre-treated with Cx by 3.3 months, while the benefit was only 2 months in patients with prior Cx exposure. Similarly, pembrolizumab showed a statistically significant increased OS in recurrent platinum-refractory HNSCC and also in PD-L1 positive HNSCC patients in first-line8: pembrolizumab alone improved OS compared with CT plus Cx (14.9 versus 10.7 months, HR: 0.61, in tumors with PD-L1 expression ⩾20%; 12.3 versus 10.3 months, HR: 0.78, in tumors with PD-L1⩾1%) and was non-inferior in the total population (11.6 versus 10.7, HR: 0.85), while pembrolizumab plus CT was even better in the same settings (OS of 14.7 versus 11 months, HR: 0.6, in tumors with PD-L1⩾20%; 13.6 versus 10.4 months, HR: 0.65, in tumors with PD-L1⩾1%; 13 versus 10.7, HR: 0.77 in the total population).9 Biologically, it is known that combination of immunotherapy with CT is synergistic due to the ability of CT to induce DNA damage and increase innate immunity pathway activation in other cancer types,101–103 and we speculate this may explain the benefit of combination of pembrolizumab with platinum also in this setting. However, we still do not know if the combination of Cx plus immunotherapy plus/minus CT may represent a future promising treatment strategy.
Combination of Cx with ICIs
The biological rational for combining Cx and immunotherapy is still not completely known, but there are multiple hypotheses on the high relevance of the immunologic activity of Cx, based on its peculiar effect of promoting a more permissive anti-tumor immune reaction through NK activation.104 Clinical and preclinical studies have shown that immunoglobulin G1 monoclonal antibodies, such as Cx, have the highest capability for stimulating ADCC as compared with other isotypes. Cx stimulates ADCC by binding its constant region, Fc, with a natural killer (NK) cell receptor, resulting in NK cell activation.104 Active NK cells can carry out their own lytic activity on tumor cells, which causes the release of tumor antigens, resulting in the activation of cytotoxic T cells by the presentation of antigens by macrophages and dendritic cells. In this manner, the crosstalk between immune cells and the continuous release of antigens lead to the activation of both innate and adaptive immune systems. Thus, Cx can potentially augment the activity of PD-1/PD-L1 inhibition by synergistically and fully mobilizing the adaptive and innate immune systems against tumor cells (Table 3). Specifically, combination of Cx plus anti-PD-L1 avelumab is object of study in ongoing prospective trials in various cancer types, like lung cancer,105 CRC106 and HNSCC [ClinicalTrials.gov identifier: NCT03494322] order to evaluate if using two ADCC-inducing mAbs can synergize in terms of beneficial immune effect.107,108 Also the anti-PD-1 pembrolizumab [ClinicalTrials.gov identifier: NCT03082534] in combination with Cx is being evaluated in phase II clinical trials.107
Table 3.
Summary of ongoing clinical trials of combinations including anti-EGFR therapy in HNSCC.
| Combination | Study name | Phase | Treatment arms | Setting | Results |
|---|---|---|---|---|---|
| Cetuximab + Avelumab | REACH | II | Avelumab-cetuximab-radiotherapy versus standard of care (RT plus cetuximab or cisplatin) | LA | Ongoing |
| Cetuximab + RT | PembroRad | II | Pembrolizumab versus Cetuximab combined with RT | LA | Ongoing |
| Cetuximab + Olaparib + RT | [ClinicalTrials.gov identifier: NCT01758731] | I | Cetuximab + olaparib + RT | LA, heavy smokers | Safe profile for MTD of olaparib of 50 mg/die |
| Durvalumab + Cetuximab + RT | DUCRO-HN | I/II | Durvalumab, Cetuximab and RT followed by adjuvant Durvalumab (6 months) | LA | Ongoing |
| Cetuximab + afatinib | [ClinicalTrials.gov identifier: NCT02979977] | II | Cetuximab + afatinib | R/M | Ongoing |
| Monalizumab + cetuximab + anti-PD-L1 | [ClinicalTrials.gov identifier: NCT02643550] | I/II | Monalizumab (anti-NKG2A) + cetuximab + anti-PD-L1 | R/M | Ongoing |
| Avelumab, cetuximab and Palbociclib | [ClinicalTrials.gov identifier: NCT03498378] | I | Avelumab, Cetuximab, and Palbociclib | R/M | Ongoing |
EGFR, epidermal growth factor receptor; HNSCC, head and neck cancer squamous cell carcinoma; LA, locally advanced; MTD, maximum tolerable dose; PD-L1, programmed death-ligand 1; RT, radiotherapy.
Other combination strategies under clinical investigations in HNSCC includes combination of Cx with DNA damaging agents, like RT-Cx [ClinicalTrials.gov identifier: NCT02999087, NCT02707588] and PARP inhibitors,109,110 palbociclib (Table 3).
Conclusion
Squamous cell carcinoma represents the main histologic type of HNSCC with alcohol, tobacco, and HPV as well defined risk factors. Nowadays, the prognosis is still poor, especially in the R/M disease, with a mOS of 1 year. In this scenario, the role of the EGFR pathway in HNSCC has been established as the most feasible and effective molecular signal to target, with Cx playing a remarkable role. In this review, we have analyzed the current role of anti-EGFR drugs in the treatment of HNSCC, discussing available data on efficacy and safety results from clinical trials, and on novel prognostic and predictive factors and mechanisms of resistance. We strongly believe that more attention is needed on the mechanisms of intrinsic and acquired resistance, regarding the role of the “beyond-Cx” EGFR inhibition and the “beyond-EGFR targeting” effects of Cx. Clinical trials evaluating the combination of Cx with immunotherapy or other targeted agents are ongoing in order to identify potential novel therapeutic strategies. In particular, given the ascending role of ICIs in the clinical scenario of HNSCC and the biological rational for combining Cx and immunotherapy, we foresee that these combinations may be of great interest. Moreover, despite the relative low incidence of HNSCC compared with other malignancies, the majority of HNC patients’ tumor lesions are easily accessible and tissue sample affordable, thus making HNSCC the perfect candidate for translational biomarker research. Hopefully, these studies will help in building a biomarker-driven approach for novel combinations, including Cx, in the real-world scenario of HNSCC patients.
Footnotes
Conflict of interest statement: FC: Advisory Boards: Roche, Amgen, Merck, Pfizer, Sanofi, Bayer, Servier, BMS, Cellgene, Lilly; Institutional Research Grants: Bayer, Roche, Merck, Amgen, AstraZeneca, Ipsen. FM: Advisory Boards: MSD, Lilly; Institutional Research Grants: AstraZeneca.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Morena Fasano  https://orcid.org/0000-0003-0782-8778
https://orcid.org/0000-0003-0782-8778
Giuseppe Viscardi  https://orcid.org/0000-0003-4473-9387
https://orcid.org/0000-0003-4473-9387
Domenico Testa  https://orcid.org/0000-0001-7176-3652
https://orcid.org/0000-0001-7176-3652
Contributor Information
Morena Fasano, Department of Precision Medicine, Medical Oncology, University of Campania Luigi Vanvitelli. Via Sergio Pansini 5, Naples, 80131, Italy.
Carminia Maria Della Corte, Department of Precision Medicine, Medical Oncology, University of Campania Luigi Vanvitelli, Naples, Italy.
Giuseppe Viscardi, Department of Precision Medicine, Medical Oncology, University of Campania Luigi Vanvitelli, Naples, Italy.
Raimondo Di Liello, Department of Precision Medicine, Medical Oncology, University of Campania Luigi Vanvitelli, Naples, Italy.
Fernando Paragliola, Department of Precision Medicine, Medical Oncology, University of Campania Luigi Vanvitelli, Naples, Italy.
Francesca Sparano, Department of Precision Medicine, Medical Oncology, University of Campania Luigi Vanvitelli, Naples, Italy.
Maria Lucia Iacovino, Department of Precision Medicine, Medical Oncology, University of Campania Luigi Vanvitelli, Naples, Italy.
Anna Castrichino, Centro polidiagnostico Gammacord-Sanniotac, Benevento, Italy.
Francesca Doria, Centro radiologico Vega, Centro radiologico fisica e terapia fisica Morrone, Caserta, Italy.
Antonello Sica, Department of Precision Medicine, Medical Oncology, University of Campania Luigi Vanvitelli, Naples, Italy.
Floriana Morgillo, Department of Precision Medicine, Medical Oncology, University of Campania Luigi Vanvitelli, Naples, Italy.
Giuseppe Colella, Maxillo-Facial Surgery Department, University of Campania Luigi Vanvitelli, Naples, Italy.
Giampaolo Tartaro, Maxillo-Facial Surgery Department, University of Campania Luigi Vanvitelli, Naples, Italy.
Salvatore Cappabianca, Department of Precision Medicine, Radiology Unit, University of Campania Luigi Vanvitelli, Naples, Italy.
Domenico Testa, Department of Anesthesiology, Surgical and Emergency Science, Clinic of Otorhinolaryngology, Head and Neck Surgery Unit, University of Campania Luigi Vanvitelli, Naples, Italy.
Gaetano Motta, Department of Anesthesiology, Surgical and Emergency Science, Clinic of Otorhinolaryngology, Head and Neck Surgery Unit, University of Campania Luigi Vanvitelli, Naples, Italy.
Fortunato Ciardiello, Department of Precision Medicine, Medical Oncology, University of Campania Luigi Vanvitelli, Naples, Italy.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systemic review. Cancer Epidemiol Biomarkers Prev 2005; 14: 467–475. [DOI] [PubMed] [Google Scholar]
- 3. Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol 2006; 24: 2606–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grégoire V, Lefebvre JL, Licitra L, et al. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21: 184–186. [DOI] [PubMed] [Google Scholar]
- 5. Argiris A, Harrington KJ, Tahara M, et al. Evidence-based treatment options in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Front Oncol 2017; 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vermorken JB, Mesia R, Rivera F, et al. Platinum-based CT plus Cx in head and neck cancer. N Engl J Med 2008; 359: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 7. Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016; 375: 1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab vs methotrexate, docetaxel, or Cx for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019; 393: 156–167. [DOI] [PubMed] [Google Scholar]
- 9. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with CTvsCx with CT for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019; 394: 1915–1928. [DOI] [PubMed] [Google Scholar]
- 10. Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 1995; 19: 183–232. [DOI] [PubMed] [Google Scholar]
- 11. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008; 358: 1160–1174. [DOI] [PubMed] [Google Scholar]
- 12. Zimmermann M, Zouhair A, Azria D, et al. The epidermal growth factor receptor (EGFR) in head and neck cancer: its role and treatment implications. Radiat Oncol 2006; 1: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tweardy DJ. Elevated levels of transforming growth factor a and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res 1993; 53: 3579–3584. [PubMed] [Google Scholar]
- 14. Grandis JR, Melhem MF, Barnes EL, et al. Quantitative immunohistochemical analysis of transforming growth factor- α and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer 1996; 78: 1284–1292. [DOI] [PubMed] [Google Scholar]
- 15. Temam S, Kawaguchi H, El-Naggar AK, et al. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J Clin Oncol 2007; 25: 2164–2170. [DOI] [PubMed] [Google Scholar]
- 16. Chung CH, Ely K, McGavran L, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol 2006; 24: 4170–4176. [DOI] [PubMed] [Google Scholar]
- 17. Bossi P, Resteghini C, Paielli N, et al. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget 2016; 7: 74362–74379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hama T, Yuza Y, Saito Y, et al. Prognostic significance of epidermal growth factor receptor phosphorylation and mutation in head and neck squamous cell carcinoma. Oncologist 2009; 14: 900–908. [DOI] [PubMed] [Google Scholar]
- 19. Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res 2002; 62: 7350–7356. [PubMed] [Google Scholar]
- 20. Pectasides E, Rampias T, Kountourakis P, et al. Comparative prognostic value of epidermal growth factor quantitative protein expression compared with FISH for head and neck squamous cell carcinoma. Clin Cancer Res 2011; 17: 2947–2954. [DOI] [PubMed] [Google Scholar]
- 21. Mirghani H, Amen F, Moreau F, et al. Oropharyngeal cancers: relationship between epidermal growth factor receptor alterations and human papillomavirus status. Eur J Cancer 2014; 50: 1100–1111. [DOI] [PubMed] [Google Scholar]
- 22. Zaravinos A. An updated overview of HPV-associated head and neck carcinomas. Oncotarget 2014; 5: 3956–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenthal DI, Harari PM, Giralt J, et al. Association of human papillomavirus and p16 status with outcomes in the imcl-9815 phase III registration trial for patients with locoregionally advanced oropharyngeal squamous cell carcinoma of the head and neck treated with RT with or without C. J Clin Oncol 2016; 34: 1300–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kogashiwa Y, Inoue H, Kuba K, et al. Prognostic role of epiregulin/amphiregulin expression in R/M head and neck cancer treated with Cx. Head Neck 2018; 40: 2424–2431. [DOI] [PubMed] [Google Scholar]
- 25. Klinghammer K, Knödler M, Schmittel A, et al. Association of epidermal growth factor receptor polymorphism, skin toxicity, and outcome in patients with squamous cell carcinoma of the head and neck receiving Cx-docetaxel treatment. Clin Cancer Res 2010; 16: 304–310. [DOI] [PubMed] [Google Scholar]
- 26. Uozumi S, Enokida T, Suzuki S, et al. Predictive value of Cx-induced skin toxicity in recurrent or metastatic squamous cell carcinoma of the head and neck. Front Oncol 2018; 8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Troiani T, Napolitano S, Della Corte CM, et al. Therapeutic value of EGFR inhibition in CRC and NSCLC: 15 years of clinical evidence. ESMO Open 2016; 1: e000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fasano M, Corte CM, Della, Califano R, et al. Type III or allosteric kinase inhibitors for the treatment of non-small cell lung cancer. Expert Opin Investig Drugs 2014; 23: 809–821. [DOI] [PubMed] [Google Scholar]
- 29. Milenic DE, Wong KJ, Baidoo KE, et al. Cx: preclinical evaluation of a monoclonal antibody targeting EGFR for radioimmunodiagnostic and radioimmunotherapeutic applications. Cancer Biother Radiopharm 2008; 23: 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldstein NI, Prewett M, Zuklys K, et al. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res 1995; 1: 1311–1318. [PubMed] [Google Scholar]
- 31. Wu X, Rubin M, Fan Z, et al. Involvement of p27KIP1 in G1 arrest mediated by an anti-epidermal growth factor receptor monoclonal antibody. Oncogene 1996; 12: 1397–1403. [PubMed] [Google Scholar]
- 32. Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology 2010; 77: 400–410. [DOI] [PubMed] [Google Scholar]
- 33. Markovic A, Chung CH. Current role of EGF receptor monoclonal antibodies and tyrosine kinase inhibitors in the management of head and neck squamous cell carcinoma. Expert Rev Anticancer Ther 2012; 12: 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Cruijsen H, Giaccone G, Hoekman K. Epidermal growth factor receptor and angiogenesis: opportunities for combined anticancer strategies. Int J Cancer 2005; 117: 883–888. [DOI] [PubMed] [Google Scholar]
- 35. Bowles DW, Mcdermott JD, Jimeno A. Novel treatments for head and neck squamous cell carcinoma: preclinical identification and clinical investigation. Futur Oncol 2014; 10: 1065–1080. [DOI] [PubMed] [Google Scholar]
- 36. Overdijk M, Verploegen S, Van den Brakel J, et al. Role of ADCC in the in vivo antitumor effects of zalutumumab, a human anti-EGF receptor antibody. J Clin Oncol 2010; 28: e13102. [Google Scholar]
- 37. Schneider-Merck T, Lammerts van Bueren JJ, Berger S, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol 2010; 184: 512–520. [DOI] [PubMed] [Google Scholar]
- 38. Stamos J, Sliwkowski MX, Eigenbrot C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem 2002; 277: 46265–46272. [DOI] [PubMed] [Google Scholar]
- 39. Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004; 101: 13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cassell A, Grandis JR. Investigational EGFR-targeted therapy in head and neck squamous cell carcinoma. Expert Opin Investig Drugs 2010; 19: 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Myllynen L, Kwiatkowski M, Gleißner L, et al. Quantitative proteomics unveiled: regulation of DNA double strand break repair by EGFR involves PARP1. Radiother Oncol 2015; 116: 423–430. [DOI] [PubMed] [Google Scholar]
- 42. Kriegs M, Kasten-Pisula U, Riepen B, et al. Radiosensitization of HNSCC cells by EGFR inhibition depends on the induction of cell cycle arrests. Oncotarget 2016; 7: 45122–45133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012; 343: 342–350. [DOI] [PubMed] [Google Scholar]
- 44. Macha MA, Rachagani S, Qazi AK, et al. Afatinib radiosensitizes head and neck squamous cell carcinoma cells by targeting cancer stem cells. Oncotarget 2017; 8: 20961–20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rusnak DW, Mullin RJ, Alligood KJ, et al. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res 2001; 61: 7196–7203. [PubMed] [Google Scholar]
- 46. Bonner JA, Harari PM, Giralt J, et al. RT plus Cx for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354: 567–578. [DOI] [PubMed] [Google Scholar]
- 47. Bonner JA, Harari PM, Giralt J, et al. RT plus Cx for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between Cx-induced rash and survival. Lancet Oncol 2010; 11: 21–28. [DOI] [PubMed] [Google Scholar]
- 48. Magrini SM, Buglione M, Corvò R, et al. Cx and RTvs cisplatin and RT for locally advanced head and neck cancer: a randomized phase II trial. J Clin Oncol 2016; 34: 427–435. [DOI] [PubMed] [Google Scholar]
- 49. Petrelli F, Coinu A, Riboldi V, et al. Concomitant platinum-based CT or Cx with RT for locally advanced head and neck cancer: a systematic review and meta-analysis of published studies. Oral Oncol 2014; 50: 1041–1048. [DOI] [PubMed] [Google Scholar]
- 50. Huang J, Zhang J, Shi C, et al. Survival, recurrence and toxicity of HNSCC in comparison of a RT combination with cisplatin vsCx: a meta-analysis. BMC Cancer 2016; 16: 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without Cx for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014; 32: 2940–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gillison ML, Trotti AM, Harris J, et al. RT plus Cx or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 2019; 393: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mehanna H, Robinson M, Hartley A, et al. RT plus cisplatin or Cx in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 2019; 393: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kies MS, Holsinger FC, Lee JJ, et al. Induction CT and Cx for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol 2010; 28: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lefebvre JL, Pointreau Y, Rolland F, et al. Induction CT followed by either chemoRT or bioRT for larynx preservation: the TREMPLIN randomized phase II study. J Clin Oncol 2013; 31: 853–859. [DOI] [PubMed] [Google Scholar]
- 56. Vermorken JB, Stöhlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol 2013; 14: 697–710. [DOI] [PubMed] [Google Scholar]
- 57. Guigay J, Fayette J, Dillies AF, et al. Cx, docetaxel, and cisplatin as first-line treatment in patients with recurrent or metastatic head and neck squamous cell carcinoma: a multicenter, phase II GORTEC study. Ann Oncol 2015; 26: 1941–1947. [DOI] [PubMed] [Google Scholar]
- 58. Hanauske AR, Depenbrock H, Shirvani D, et al. Effects of the microtubule-disturbing agents docetaxel (taxotere®), vinblastine and vincristine on epidermal growth factor-receptor binding of human breast cancer cell lines in vitro. Eur J Cancer 1994; 30: 1688–1694. [DOI] [PubMed] [Google Scholar]
- 59. Navolanic PM, Lee JT, McCubrey JA. Docetaxel cytotoxicity is enhanced by inhibition of the Raf/MEK/ERK signal transduction pathway. Cancer Biol Ther 2003; 2: 677–678. [PubMed] [Google Scholar]
- 60. Hitt R, Irigoyen A, Cortes-Funes H, et al. Phase II study of the combination of Cx and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol 2012; 23: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 61. Knoedler M, Gauler TC, Gruenwald V, et al. Phase II study of Cx in combination with docetaxel in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck after platinum-containing therapy: a multicenter study of the Arbeitsgemeinschaft Internistische Onkologie. Oncol 2013; 84: 284–289. [DOI] [PubMed] [Google Scholar]
- 62. Rischin D, Spigel DR, Adkins D, et al. PRISM: phase 2 trial with panitumumab monotherapy as second-line treatment in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Head and Neck 2014; 36: 1391. [DOI] [PubMed] [Google Scholar]
- 63. Siano M, Molinari F, Martin V, et al. Multicenter phase II study of panitumumab in platinum pretreated, advanced head and neck squamous cell cancer. Oncologist 2017; 22: 782-e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pedersen MW, Jacobsen HJ, Koefoed K, et al. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res 2010; 70: 588–597. [DOI] [PubMed] [Google Scholar]
- 65. Huang S, Peet CR, Saker J, et al. Sym004, a novel anti-EGFR antibody mixture, augments radiation response in human lung and head and neck cancers. Mol Cancer Ther 2013; 12: 2772–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Machiels JP, Specenier P, Krauß J, et al. A proof of concept trial of the anti-EGFR antibody mixture Sym004 in patients with squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 2015; 76: 13–20. [DOI] [PubMed] [Google Scholar]
- 67. Machiels JPH, Haddad RI, Fayette J, et al. Afatinib vs methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol 2015; 16: 583–594. [DOI] [PubMed] [Google Scholar]
- 68. Guo Y, Ahn M-J, Chan A, et al. Afatinib vs methotrexate as second-line treatment in Asian patients with recurrent or metastatic squamous cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 3): an open-label, randomised phase III trial. Ann Oncol 2019; 30: 1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Burtness B, Haddad R, Dinis J, et al. LUX-head and neck 2: randomized, double-blind, placebo-controlled, phase III trial of afatinib as adjuvant therapy after chemoradiation (CRT) in primary unresected, high/intermediate-risk, squamous cell cancer of the head and neck (HNSCC) patients (pts). J Clin Oncol 2017; 35: 6001. [Google Scholar]
- 70. De Souza JA, Davis DW, Zhang Y, et al. A phase II study of lapatinib in R/M squamous cell carcinoma of the head and neck. Clin Cancer Res 2012; 18: 2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Harrington K, Berrier A, Robinson M, et al. Randomised phase II study of oral lapatinib combined with chemoRT in patients with advanced squamous cell carcinoma of the head and neck: rationale for future randomised trials in human papilloma virus-negative disease. Eur J Cancer 2013; 49: 1609–1618. [DOI] [PubMed] [Google Scholar]
- 72. Harrington K, Temam S, Mehanna H, et al. Postoperative adjuvant lapatinib and concurrent chemoRT followed by maintenance lapatinib monotherapy in high-risk patients with resected squamous cell carcinoma of the head and neck: a phase III, randomized, double-blind, placebo-controlled stu. J Clin Oncol 2015; 33: 4202–4209. [DOI] [PubMed] [Google Scholar]
- 73. Wheeler RH, Jones D, Sharma P, et al. Clinical and molecular phase II study of gefitinib in patients (pts) with recurrent squamous cell cancer of the head and neck (H&N Ca). J Clin Oncol 2005; 23: 5531. [Google Scholar]
- 74. Soulieres D, Senzer NN, Vokes EE, et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 2004; 22: 77–85. [DOI] [PubMed] [Google Scholar]
- 75. Stewart JSW, Cohen EEW, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 2009; 27: 1864–1871. [DOI] [PubMed] [Google Scholar]
- 76. Martins RG, Parvathaneni U, Bauman JE, et al. Cisplatin and RT with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: a randomized phase II trial. J Clin Oncol 2013; 31: 1415–1421. [DOI] [PubMed] [Google Scholar]
- 77. William WN, Tsao AS, Feng L, et al. Single arm, phase II study of cisplatin, docetaxel, and erlotinib in patients with recurrent and/or metastatic head and neck squamous cell carcinomas. Oncologist 2018; 23: 526-e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Soulieres D. Identifying predictive and surrogate markers of erlotinib antitumor activity other than rash. Oncology (Williston Park) 2003; 17: 29–33. [PubMed] [Google Scholar]
- 79. Tang X, He J, Li B, et al. Efficacy and safety of gefitinib in patients with advanced head and neck squamous cell carcinoma: a meta-analysis of randomized controlled trials. J Oncol 2019; 2019: 6273438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res 2007; 5: 203–220. [DOI] [PubMed] [Google Scholar]
- 81. Gustafson D, Frederick B, Merz A, et al. Dose scheduling of the dual VEGFR and EGFR tyrosine kinase inhibitor vandetanib (ZD6474, Zactima) in combination with RT in EGFR-positive and EGFR-null human head and neck tumor xenografts. Cancer Chemother Pharmacol 2008; 61: 179–188. [DOI] [PubMed] [Google Scholar]
- 82. Sano D, Matsumoto F, Valdecanas DR, et al. Vandetanib restores head and neck squamous cell carcinoma cells’ sensitivity to cisplatin and radiation in vivo and in vitro. Clin Cancer Res 2011; 17: 1815–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Papadimitrakopoulou VA, Frank SJ, Cohen EW, et al. Phase I study of vandetanib with radiation therapy with or without cisplatin in locally advanced head and neck squamous cell carcinoma. Head Neck 2016; 38: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Limaye S, Riley S, Zhao S, et al. A randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN). Oral Oncol 2013; 49: 835–841. [DOI] [PubMed] [Google Scholar]
- 85. Wheeler SE, Egloff AM, Wang L, et al. Challenges in EGFRvIII detection in head and neck squamous cell carcinoma. PLoS One 2015; 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Braig F, Kriegs M, Voigtlaender M, et al. Cx Resistance in head and neck cancer is mediated by EGFR-K521 polymorphism. Cancer Res 2017; 77: 1188–1199. [DOI] [PubMed] [Google Scholar]
- 87. Novoplansky O, Fury M, Prasad M, et al. MET activation confers resistance to Cx, and prevents HER2 and HER3 upregulation in head and neck cancer. Int J Cancer 2019; 145: 748–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Boeckx C, Baay M, Wouters A, et al. Anti-epidermal growth factor receptor therapy in head and neck squamous cell carcinoma: focus on potentialmolecularmechanisms of drug resistance. Oncologist 2013; 18: 850–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kyungsuk J, Hyunseok K, Ranee M. Targeting phosphoinositide 3-kinase (PI3K) in head and neck squamous cell carcinoma (HNSCC). Cancers Head Neck 2018; 19: 795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. da Costa AABA, Costa FD, Araújo DV, et al. The roles of PTEN, cMET, and p16 in resistance to Cx in head and neck squamous cell carcinoma. Med Oncol 2018; 36: 8. [DOI] [PubMed] [Google Scholar]
- 91. Eze N, Lee JW, Yang DH, et al. PTEN loss is associated with resistance to Cx in patients with head and neck squamous cell carcinoma. Oral Oncol 2019; 91: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Puram S V, Rocco JW. Molecular aspects of head and neck cancer therapy. Hematol Oncol Clin North Am 2015; 29: 971–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hartmann S, Bhola NE, Grandis JR. HGF/Met signaling in head and neck cancer: impact on the tumor microenvironment. Clin Cancer Res 2016; 22: 4005–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Madoz-Gúrpide J, Zazo S, Chamizo C, et al. Activation of MET pathway predicts poor outcome to Cx in patients with recurrent or metastatic head and neck cancer. J Transl Med 2015; 13: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Papaccio F, Della Corte CM, Viscardi G, et al. HGF/MET and the immune system: relevance for cancer immunotherapy. Int J Mol Sci 2018; 19: 3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. National Comprehensive Cancer Network. Head and neck cancer, https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed 5 February 2020).
- 97. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest 2015; 125: 3335–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pai SI, Faivre S, Licitra L, et al. Comparative analysis of the phase III clinical trials of anti-PD1 monotherapy in head and neck squamous cell carcinoma patients (CheckMate 141 and KEYNOTE 040). J Immunother Cancer 2019; 7: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Harrington KJ, Ferris RL, Blumenschein G, Jr, et al. Nivolumab vs standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol 2017; 18: 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ferris RL, Licitra L, Fayette J, et al. Nivolumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: efficacy and safety in checkmate 141 by prior Cx use. Clin Cancer Res 2019; 25: 5221–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Della Corte CM, Sen T, Gay CM, et al. STING pathway expression identifies NSCLC with an immune-responsive phenotype. J Thorac Oncol. Epub ahead of print 15 February 2020. DOI: 10.1016/j.jtho.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sen T, Della Corte CM, Milutinovic S, et al. Combination treatment of the oral CHK1 inhibitor, SRA737, and low-dose gemcitabine enhances the effect of programmed death Ligand 1 blockade by modulating the immune microenvironment in SCLC. J Thorac Oncol 2019; 14: 2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Parkes EE, Walker SM, Taggart LE, et al. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst 2016; 109: djw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sparano F, Barra G, Della Corte CM, et al. Evaluation of antibody-dependent cell cytotoxicity (ADCC) in lung cancer cell lines treated with combined anti-EGFR and anti-PD-L1 therapy. Ann Oncol 2019; 30: v772–v773. [Google Scholar]
- 105. Fasano M, DellaCorte CM, Di Liello R, et al. Induction of natural killer antibody-dependent cell cytotoxicity and of clinical activity of cetuximab plus avelumab in non-small cell lung cancer. ESMO Open (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Martinelli E. Oral presentation “CAVE-Colon”. In: GOIM Conference, XXXIII meeting of Researchers, Bari, February 2020. [Google Scholar]
- 107. Ferris RL, Lenz HJ, Trotta AM, et al. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev 2018; 63: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Forster MD, Sacco JJ, Kong AH, et al. EACH: a randomised phase II study evaluating the safety and anti-tumour activity of the combination of avelumab and Cx relative to avelumab monotherapy in R/M head and neck squamous cell cancer. J Clin Oncol 2019; 37(Suppl. 15): TPS6091. [Google Scholar]
- 109. Nowsheen S, Bonner JA, LoBuglio AF, et al. Cx augments cytotoxicity with poly (ADP-Ribose) polymerase inhibition in head and neck cancer. PLoS One 2011; 6: e24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Karam SD, Reddy K, Blatchford PJ, et al. Final report of a phase I trial of olaparib with Cx and radiation for heavy smoker patients with locally advanced head and neck cancer. Clin Cancer Res 2018; 24: 4949–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]


