Abstract
Study Objective:
Nonfatal opioid overdose represents an opportunity to engage young adults into medication for opioid use disorder (MOUD). We seek to: (1) describe characteristics of young adults who experience nonfatal overdose and (2) estimate rates of and time to MOUD for young adults relative to 26–45 year olds (yo).
Methods
We conducted a cohort study using retrospective administrative data of 15,281 individuals ages 18–45 who survived an opioid-related overdose in Massachusetts between 2012–2014 using de-identified, individual-level, linked datasets from Massachusetts government agencies. We described patient characteristics stratified by age (18–21, 22–25, and 26–45) and evaluated multivariable Cox proportional hazards models to compare rates of MOUD receipt controlling for age, gender, history of mental health disorders, and addiction treatment.
Results:
Among 4268 young adults in the year following with nonfatal overdose, 28% (n=336/1209) of 18–21 yo received any MOUD, 36% (n=1097/3059) of 22–25 yo received MOUD, and 36% (n=3916/11013) of 26–45 yo received MOUD. For 18–21 yo and 22–25 yo, median time to buprenorphine treatment was 4 months (IQR 1,7; 1,8), 4 months (IQR: 2,8; 2,9) to methadone, and 1 month (IQR:1,1) to naltrexone. Eighteen-twenty one year olds were less likely (AHR 0.60 [95% CI: 0.45, 0.70]) to receive methadone than 22–25 and 26–45 yo. Both 18–21 yo and 22–25 yo were more likely to receive naltrexone (AHR 1.65 [95% CI:1.36, 2.00] and 1.41 [95% CI:1.23, 1.61]) than 26–45 yo.
Conclusions
One in three young adults received MOUD in the 12 months after surviving an overdose. Type of MOUD received appeared to be age-associated. Future research should focus on how medication choice is made and how to optimize the emergency department for MOUD initiation after nonfatal overdose.
Keywords: young adults, nonfatal opioid overdose, medication for opioid use disorder
Introduction
Background
In the US, the age-adjusted opioid-related mortality rate tripled from 1999 to 20161. In Massachusetts, an alarming increase in opioid-related deaths occurred, from 379 in 2000 to an estimated 2149 in 2016, which disproportionately occurred among individuals under 25 years old2. Young adults (18–25 year olds) have been particularly affected by the opioid epidemic3–5. In the U.S. between 2002 and 2013, young adults had a greater increase in prevalence of past-year heroin use disorder (108%) compared to other age groups6,7. Drug overdose deaths nearly quadrupled in the 15–24 year-old age group from 1999 to 20161.
Young adults have distinct developmental differences that predispose them to substance use disorders. During this development period, the reward system and resulting positive reinforcement are relatively more advanced than inhibitory systems, leading to increased vulnerability to risky substance use and addiction8. Clinically, young adults respond to interventions differently than older adults9,10, emphasizing the need to better design appropriate interventions to engage and retain them in treatment. As deaths continue to increase among this age group, opportunities to identify them and engage them are important to recognize.
One such opportunity is presentation to the emergency department for nonfatal opioid overdose. Visits to emergency departments for suspected opioid overdoses increased 30% from July 2016 to September 201711,12. Nonfatal opioid overdose is a significant predictor for recurrent nonfatal opioid overdose and for fatal overdose. Medications for opioid use disorder (MOUD) have been shown to not only improve abstinence and retention in care but also have a positive mortality benefit. Provision of MOUD in the period after an overdose may therefore be a critical strategy to address overdose deaths.
Importance
Given the increasing rate of opioid overdose deaths, the opportunity that surviving an opioid overdose provides, and the challenges of engaging young adults in care, it is important to characterize nonfatal opioid overdose incidence and subsequent treatment engagement, or lack thereof, in this age group. These data can provide a baseline to compare the effectiveness of efforts to improve MOUD initiation in the emergency department. Policymakers can begin to formulate interventions to respond to nonfatal overdose as a sentinel event in a high-risk, hard to engage population that could benefit from targeted prevention and treatment. Given the recent data showing success of initiating MOUD in the emergency department13, a better understanding of treatment patterns after a nonfatal overdose could be an important way to tailor such interventions.
Goals of This Investigation
The aims of this study are to: (1) describe characteristics of young adults (18–25 year olds) who experience nonfatal overdose and (2) estimate the time to MOUD treatment and rates of MOUD treatment to 26–45 year olds in the 12 months following nonfatal overdose.
Materials and Methods
Study Design
We conducted a retrospective cohort study of individuals in Massachusetts, age 18 to 45 years, who had a nonfatal overdose between January 1, 2012 and December 31, 2014.
Data Source
Chapter 55 of the Acts of 2015 (“Chapter 55”) mandated that the Massachusetts Department of Public Health (MDPH) analyze data from several Massachusetts government agencies and allowed for the linkage of these datasets to identify and report on trends among persons who suffered fatal and non-fatal opioid overdose14. The Chapter 55 database includes Massachusetts residents who are 11 years and older and have public or private insurance.
Data from disparate agencies were linked through a ten-level match protocol and subsequently de-identified at MDPH, allowing for this study to examine the full course of patients during the study period from 2011–2015. The ten levels of matches were tested between the datasets in Chapter 55 datasets and identifiers in the All Payers Claim Database (APCD). Data linkage was conducted by the Center for Health Information and Analysis in consultation with MDPH. All matches were deterministic. In order to improve accuracy, no close matches were used. The matching procedure produced matching from 71% to 100%. In order to obtain access to the data, our team submitted a proposal to MDPH for approval. All analyses occurred onsite at MDPH.
To construct the set of variables needed for this study, we used data from the APCD, Massachusetts Department of Public Health Bureau of Substance Addiction Services (BSAS), the Massachusetts Prescription Monitoring Program (PMP), Massachusetts Ambulance Trip Record Information System (MATRIS), and Massachusetts Acute Hospital Case Mix15.
Study Cohort
Individuals entered the cohort when they experienced a nonfatal overdose between January 1, 2012 and December 31, 2014 in Massachusetts providing a full 12 months of observation prior to and after the nonfatal overdose. Each individual contributed only their first non-fatal overdose event in the dataset window. Recurrent non-fatal overdose events were excluded. Nonfatal overdose was identified in two ways. First, any individual who had an ambulance encounter related to opioid overdose was included. The algorithm used to identify opioid-related overdoses in the EMS data resulted from a collaboration between MDPH and the Centers for Disease Control and Prevention (CDC)16. The second was an emergency department, observation, or hospital encounter with an ICD 9 containing a diagnosis code for opioid poisoning (965.00–965.02, 965.09, E85.00-E85.02)17. Visits to Veteran’s Administration hospitals were not included. There were 558 events that were removed from the analysis because death occurred within 30 days of the overdose.
Independent Variable
The primary independent variable was age group, categorized as 18–21 years, 22–25 years and 26–45 years. Young adulthood is a transitional period where changes in brain function, social capital, and individual responsibility are greater than other periods. Therefore, we subcategorized the age group in order to understand whether the characteristics and medication experience were consistent through the period. This has been shown in prior work that has shown differences between 18–21 yo and 22–25 yo. We chose to use the age group of 26–45 as the comparison group because age 26 is age when brain myelination of the frontal lobe has matured. This is also the age when young adults are no longer eligible to be on their parents’ health insurance. We capped the age group at 45, so that are observations were not affected by the increasing onset of the chronic illnesses of aging, like chronic obstructive pulmonary disease, chronic liver disease from hepatitis C infection and alcohol, and cardiovascular disease.
Covariates
We included in the multivariable models the following covariates: gender (from the APCD); anxiety; and depression. Anxiety and depression were identified through International Classification of Diseases, Ninth and Tenth Revisions (ICD 9, 10) diagnosis codes (anxiety: 300.X, F41.X; depression: 296.2X, 296.3X, 296.99, 300.4, 311, 625.4, F32.X, F33.X, F34.1, F34.8X) and defined as having a claim for these conditions any time between 2011 and 2015. Homelessness was identified using ICD 9 diagnosis code V60.0 or ICD 10 diagnosis code Z590 in the APCD. Receipt of opioid prescriptions in the past 12 months was obtained from the PMP. We included involuntary commitment to substance use treatment through a special statute specific to Massachusetts because of risk to self or others in the prior 12 months before the nonfatal overdose from BSAS. The other covariates were BSAS funded inpatient medical detoxification and residential substance use treatment (defined as any treatment beyond medical detoxification) in the 12 months before nonfatal overdose
Outcomes
The primary outcome was receipt of medication for opioid use disorder (MOUD) defined as follows: buprenorphine obtained from the PMP; oral or injectable naltrexone, obtained from APCD; or methadone treatment as identified in BSAS or APCD data (identified via Healthcare Common Procedure Coding System code H0020). Receipt of MOUD was identified in each month starting with the month of the nonfatal overdose through twelve months afterward.
Statistical Analysis
We used summary statistics to describe characteristics of the cohort. We examined time to receipt of MOUD after nonfatal overdose by estimating Kaplan-Meier survival curves stratified by age groups (i.e., 18–21, 22–25, and 26–45). Individuals were censored at 12 months or at death. We chose 12 months so we would have the same amount of follow up time for all individuals in the cohort. We calculated median time to treatment in months and median duration of medication in months. We developed multivariable Cox proportional hazards models to compare rates of treatment receipt after nonfatal overdose adjusting for sex, anxiety or depression diagnosis, homelessness, past year benzodiazepine prescription, past year opioid use disorder medication treatment, past year detoxification admission, past year residential treatment, and past year involuntary commitment. We used SAS Studio version 3.5 (SAS Institute, Cary, NC).
We received a Not Human Subjects Research determination from the Boston University Medical Campus Institutional Review Committee.
Results
Cohort Characteristics
Of 15,281 individuals between the ages of 18–45 with a non-fatal overdose who encountered medical care, 4268 (28%) were young adults (i.e., age 18–25 years). Greater proportions of young adults were female and had been involuntarily committed in the year prior to the nonfatal overdose. Among 18–21 yo, 10% received buprenorphine (n=118/1209), 7% received naltrexone (n=87/1209), and 4% received methadone (44/1209) in the year preceding nonfatal overdose. Among 22–25 yo, 13% received buprenorphine (410/3059), 8% received naltrexone (232/3059 respectively), and 9% received methadone (n=260/3059) in the year preceding nonfatal overdose. In the year following the nonfatal overdose we observed the following mortality: 3% (n=31) of 18–21 yo, 2% (n=64) of 22–25 yo, 4% (n=398) of 26–45 yo. Other characteristics are shown in Table 1.
Table 1:
Characteristics of individuals ages 18–45 years who survived a nonfatal opioid-related overdose in Massachusetts between 2012–2014 stratified by age group (N=15,281)
| Variables | 18–21 yo N = 1209 | 22–25 yo N = 3059 | 26–45 yo N = 11,013 | |||
|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |
| Female | 43.8% | [41 – 46.6] | 38% | [36.68 – 40.12] | 33.5% | [32.62 – 34.38] |
| Homeless history | 10.7% | [8.96 – 12.44] | 13.7% | [12.48 – 14.92] | 18.3% | [17.58 – 19.02] |
| Incarceration history | 4.0% | [2.9 – 5.1] | 6.7% | [5.81 – 7.59] | 6.3% | [5.85 – 6.75] |
| Involuntary commitment * | 7.5% | [6.02 – 9.98] | 8.2% | [7.23 – 9.17] | 4.2% | [3.83 – 4.57] |
| Anxiety diagnosis, ever | 15.9% | [13.84 – 17.96] | 15.6% | [14.31 – 16.89] | 20.3% | [19.55 – 21.05] |
| Depression diagnosis, ever | 17.7% | [15.55 – 19.85] | 17.5% | [16.15 – 18.85] | 23.3% | [22.51 – 24.09] |
| Past year opioid prescription ** | 31.2% | [28.59 – 33.81] | 40.1% | [38.36 – 41.84] | 39.6% | [38.69 – 40.51] |
| Past year benzodiazepine prescription | 11.2% | [9.42 – 12.98] | 17.4% | [16.06 – 18.74] | 27.5% | [26.67 – 28.33] |
| Past year buprenorphine | 9.8% | [8.12 – 11.48] | 13.4% | [12.19 – 14.61] | 14.3% | [13.65 – 14.95] |
| Past year naltrexone | 7.2% | [5.74 – 8.66] | 7.6% | [6.66 – 8.54] | 4.9% | [4.5 – 5.3] |
| Past year methadone | 3.6% | [2.55 – 4.65] | 8.5% | [7.51 – 9.49] | 13.0% | [12.37 – 13.63] |
| State funded Detoxification program prior to nonfatal overdose in past year | 21.9% | [19.57 – 24.23] | 30.9% | [29.26 – 32.54] | 28.8% | [27.95 – 29.65] |
| State funded Residential Program prior to nonfatal overdose in past year | 9% | [7.39 – 10.61] | 11.4% | [10.27 – 12.53] | 11.3% | [10.71 – 11.89] |
Massachusetts allows for involuntary commitment through the court system to mandate treatment for individuals whose alcohol or substance use presents an acute risk to their health
This does not include buprenorphine
Medication Treatment After Nonfatal Overdose
In the 12 months following a nonfatal overdose, 35% of individuals aged 18–45 received any medication treatment. Of 18–21 yo who had a nonfatal overdose, 28% received any medication treatment (7% methadone, 16% buprenorphine, 10% naltrexone). Of 22–25-year-olds, 36% received any medication treatment (12% methadone, 20% buprenorphine and 10% naltrexone). (Figure 1). The median time to treatment is reported in Table 2. The median time in months treated with buprenorphine was 2 (Interquartile range (IQR) 1,6), 2 (IQR: 1,6), and 3 (IQR: 1,7) months for 18–21 yo, 22–25 yo, and 26–45 yo respectively. The median time in months treated with methadone was 4 (IQR: 2,8) , 4 (IQR: 2,9), and 5 (IQR: 2,9) months for 18–21 yo, 22–25 yo, and 26–45 yo respectively. The median time in months treated with naltrexone was 1 months (IQR: 1,1) for all age groups.
Figure 1: Receipt of medication treatment in 12 months following a nonfatal overdose stratified by age groups. Error bars represent 95% CI*.
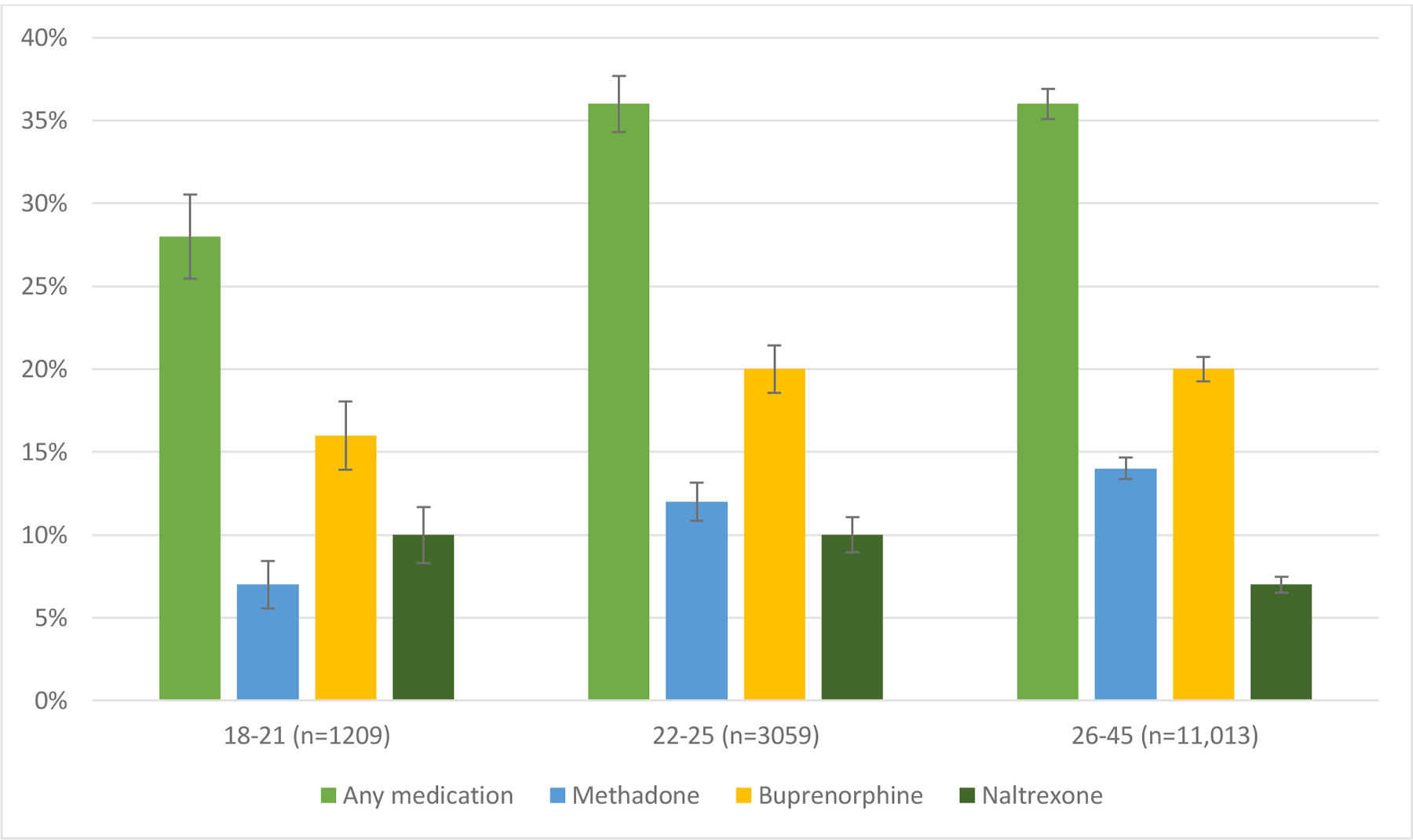
*Individuals could have received more than one kind of medication type
Table 2:
Median time to* medication treatment (tx) in months after nonfatal overdose by age groups (Interquartile Range)
| Age Group | Buprenorphine Time to Tx | Methadone Time to Tx | Naltrexone Time to Tx |
|---|---|---|---|
| 18–21 years | 4 (1,8) | 5 (1,8) | 4 (2,8) |
| 22–25 years | 4 (1,7) | 3 (1,8) | 4 (1,8) |
| 26–45 years | 3 (1,7) | 3 (1,6) | 4 (2,8) |
Median time in months to receipt of medication treatment
The unadjusted survival analysis shows a smaller proportion 18–21 yo received methadone, buprenorphine, or any MOUD overall. (Figures 2a, b, d). A higher proportion received naltrexone. (Figure 2c). However, in the multivariable adjusted Cox regression model, no differences in receipt of any MOUD were detected by age group [Adjusted hazard ratios (AHR) 0.91 (95% Confidence Interval (CI): 0.81, 1.02) and 1.06 (95% CI: 0.99, 1.13) for 18–21 yo and 22–25 yo respectively compared with 26–45 yo]. However, 18–21 yo were less likely (AHR 0.60 [95% CI: 0.45, 0.70]) to receive methadone than 22–25 and 26–45 yo. Both 18–21 and 22–25 yo were more likely to receive naltrexone (AHR 1.65 [95% CI:1.36, 2.00] and 1.41 [95% CI:1.23, 1.61]) than 26–45 yo. There was no difference among receipt of buprenorphine. There was a higher probability of naltrexone receipt in those with past year involuntary commitment, past year detoxification, and past year residential treatment. (Table 3)
Figure 2a:
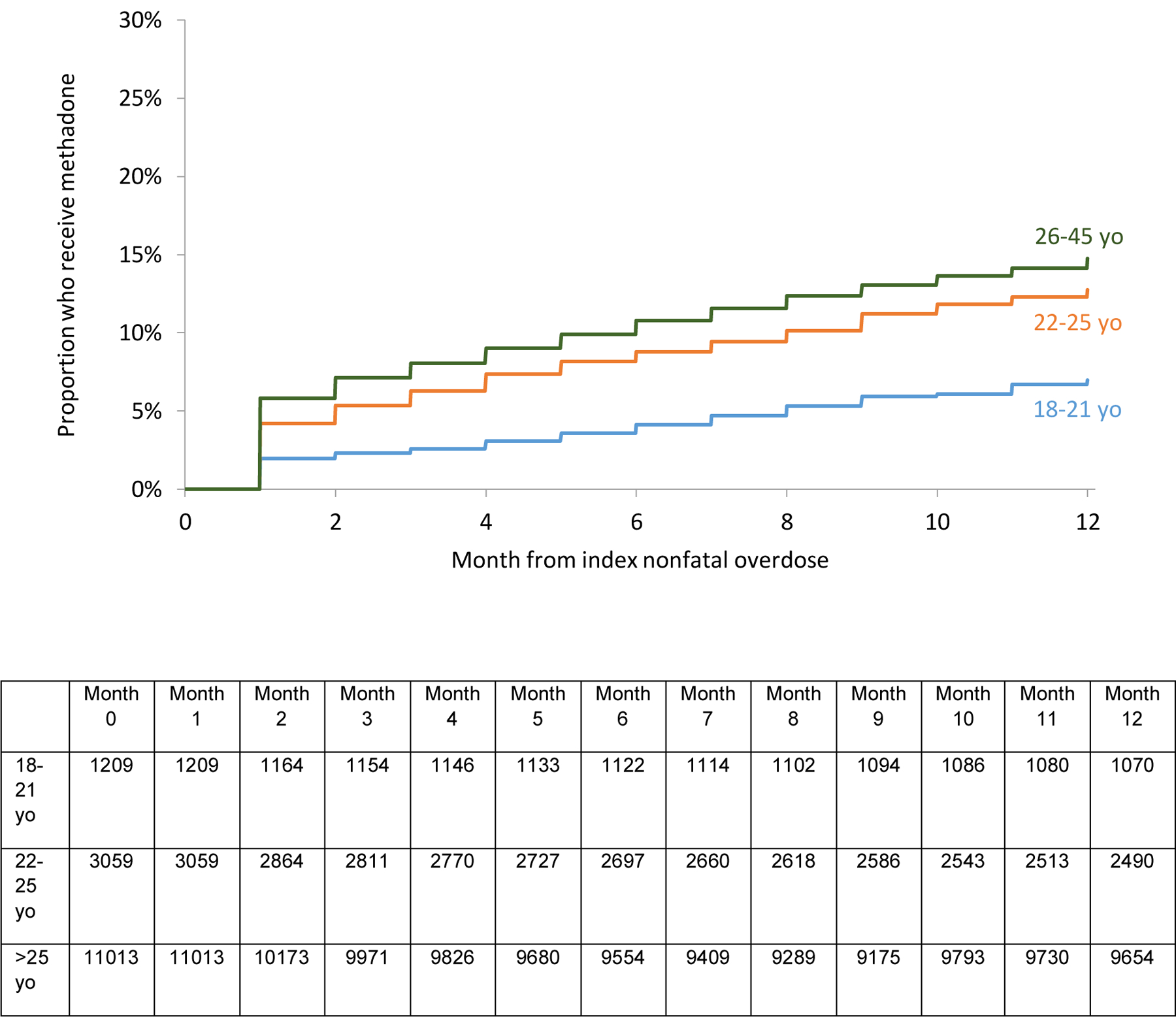
Proportion of 18–45 yo who receive methadone in the 12 months following nonfatal overdose by age groups
Figure 2b:
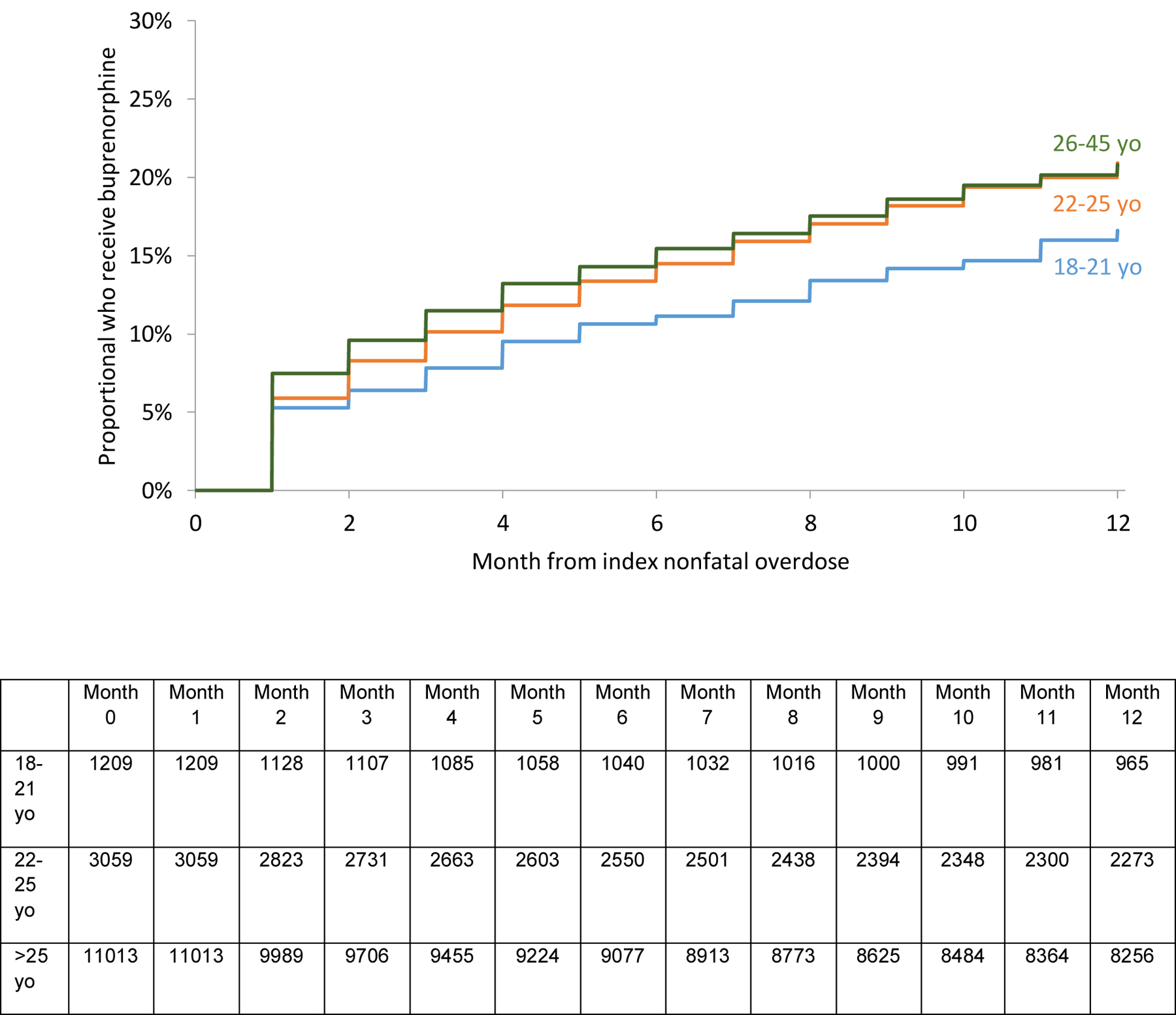
Proportion of 18–45 yo who receive buprenorphine in the 12 months following nonfatal overdose by age groups
Figure 2d:
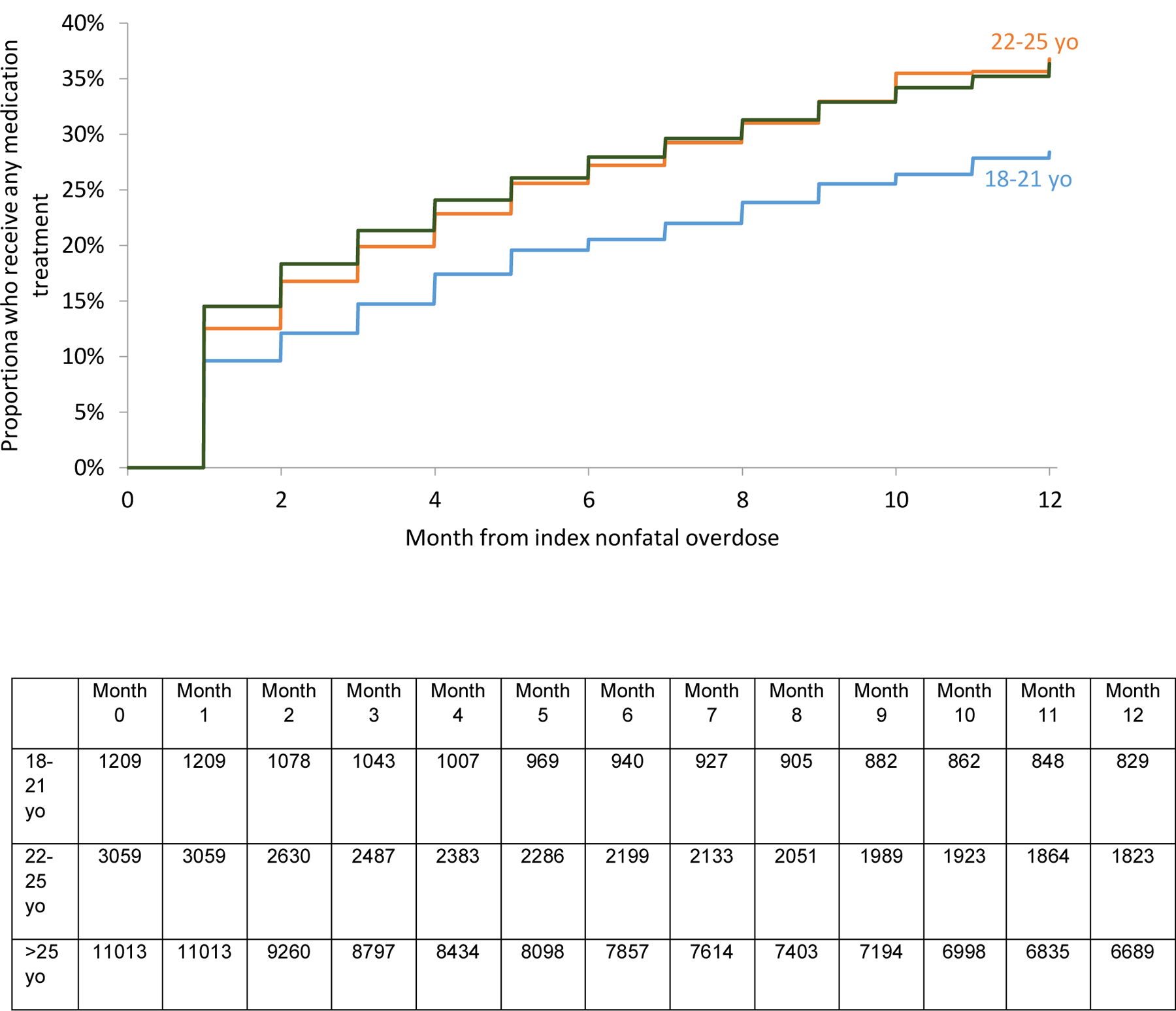
Proportion of 18–45 yo who receive any medication treatment in the 12 months following nonfatal overdose by age groups
Figure 2c:
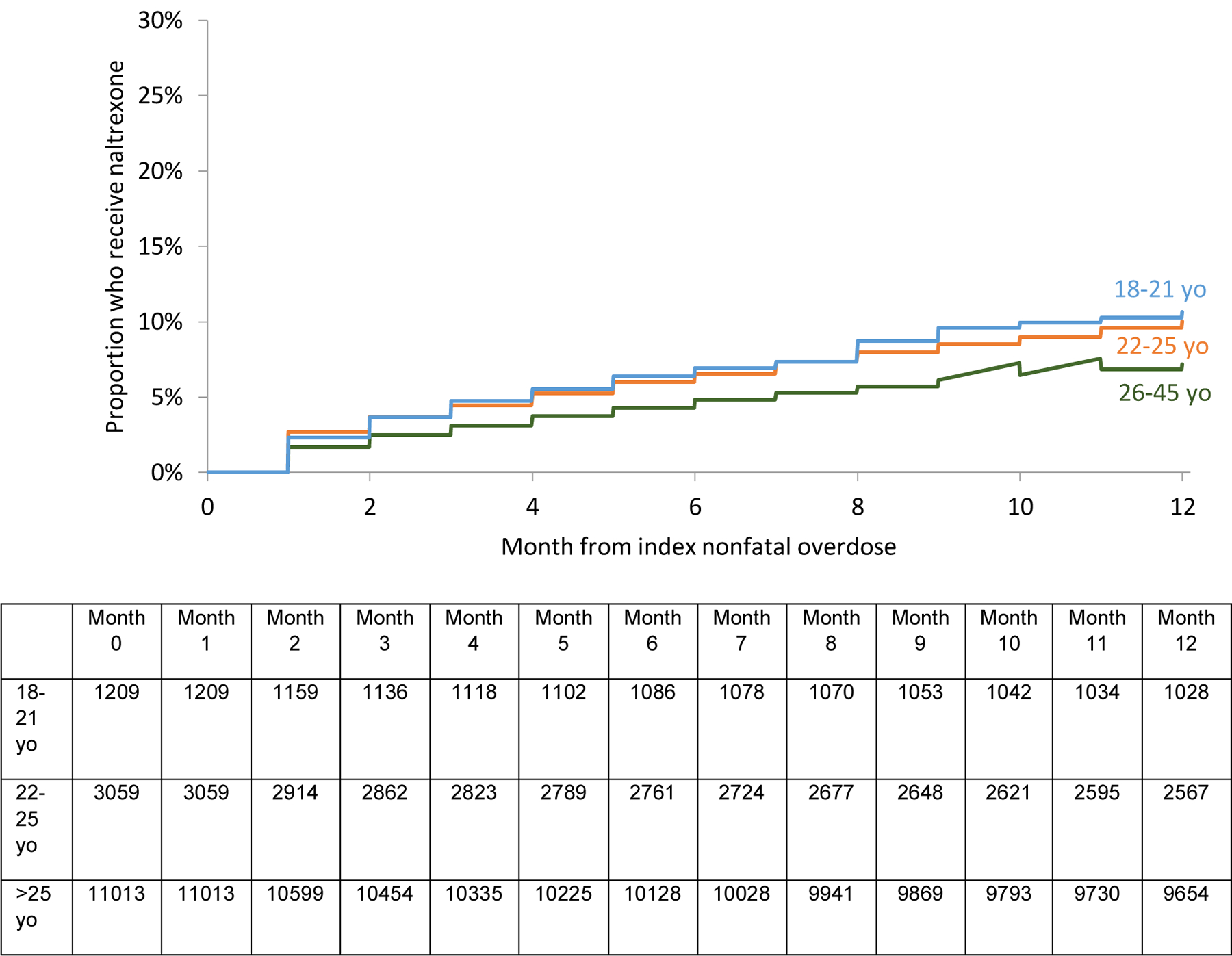
Proportion of 18–45 yo who receive naltrexone in the 12 months following nonfatal overdose by age groups
Table 3:
Adjusted hazard ratio and 95% confidence interval of multivariable Cox proportional hazards models for time to treatment after nonfatal overdose (significant results in bold)
| Characteristic | Buprenorphine | Methadone | Naltrexone | Any Medication Treatment |
|---|---|---|---|---|
| 26–45 years (ref) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) |
| 18–21 years | 0.99 (0.85,1.14) | 0.60 (0.45,0.70) | 1.65 (1.36, 2.00) | 0.91 (0.81, 1.02) |
| 22–25 years | 1.10 (0.99,1.19) | 0.91 (0.81,1.02) | 1.41 (1.23, 1.61) | 1.06 (0.99, 1.13) |
| Female | 0.86 (0.80,0.93) | 1.45 (1.32,1.58) | 0.94 (0.83, 1.06) | 1.01 (0.95, 1.07) |
| Homeless history | 1.08 (0.98,1.18) | 1.36 (1.23,1.51) | 1.06 (0.92, 1.23) | 1.14 (1.07, 1.26) |
| Involuntary commitment | 1.08 (0.94,1.25) | 0.89 (0.75,1.07) | 1.48 (1.22, 1.80) | 1.02 (0.92, 1.13) |
| Past year anxiety | 1.08 (0.98,1.20) | 0.95 (0.84,1.08) | 1.24 (1.05,1.46) | 1.06 (0.98, 1.15) |
| Past year depression | 0.99 (0.90, 1.10) | 0.96 (0.85,1.08) | 1.23 (1.05,1.44) | 1.07 (0.99, 1.15) |
| Past year prescription for benzodiazepines | 1.28 (1.18,1.40) | 0.99 (0.89,1.10) | 0.90 (0.78,1.04) | 1.10 (1.03, 1.17) |
| Past year medication treatment for opioid use disorder | 3.04 (2.79, 3.32) | 3.71 (3.36,4.10) | 1.09 (0.92, 1.27) | 4.16 (3.89, 4.45) |
| Past year state funded admission for detoxification | 1.08 (0.99,1.17) | 1.50 (1.36,1.65) | 1.62 (1.43,1.85) | 1.30 (1.26, 1.38) |
| Past year state funded residential treatment | 1.18 (1.06,1.32) | 1.06 (0.93,1.21) | 1.40 (1.19,1.64) | 1.13 (1.04, 1.23) |
Limitations
This study used data from Chapter 55 of the Acts of 2015 of individuals who experienced a nonfatal opioid-related overdose. This dataset could not identify individuals who survived an overdose but did not have an ambulance or hospital encounter. But we were able to include all overdose-related acute hospital discharges and ambulance encounters across all providers in Massachusetts. It is possible that not every individual had a known diagnosis of OUD prior to the non-fatal overdose. However, opioid overdose is almost always a qualifying criterion for opioid use disorder and thus, receipt of MOUD. It is not possible to confirm adherence completely to medication based on administrative data, but high concordance between self-report, electronic pharmacy records, and medication lids has been demonstrated in other studies18. Also, data are not clustered by hospital center or provider. It is likely that there are some locations across the state that provide coordination of care and linkage of treatment than others. As noted in the methods, we excluded individuals who had a death within 30 days of the overdose. We have included a table in the appendix with age and other demographic data on these individuals. It’s important to note that exclusion of them introduces survivor bias.
The database did not include good indicators for race, human immunodeficiency virus (HIV), and hepatitis C virus (HCV), and socioeconomic status. These factors have been previously associated with opioid-related treatment and overdose and should be better characterized in future studies. Of note, we did not include insurance status because insurance coverage was between 96–97% during the study period19. Our data and our analyses are limited to the years 2011–2015, which included the time period in Massachusetts when fentanyl emerged as a major driver of overdose deaths. Massachusetts was one of the first states affected by fentanyl, and thus, the 2011–2015 timeframe reflects what has happened nationally more recently20. Furthermore, Massachusetts has been an early adopter of near universal healthcare coverage, increased access to medication treatment, and naloxone for overdose prevention, which means that the care environment in Massachusetts represents what other states have been evolving to.2,21,22. An additional limitation is that we only calculated the time in treatment within the 12 month window of the study. Although the median times were all less than 12 months, it would be interesting to look in future studies beyond 12 months of treatment to identify potential differences by age. Finally, as the data are from Massachusetts residents, the results may not be fully reflective of other populations.
Discussion
In this study of individuals ages 18–45 who survived opioid overdose in Massachusetts between 2012–2014, approximately one in three young adults received evidence-based, recommended medication treatment with buprenorphine, naltrexone, or methadone in the subsequent 12 months. The median time to all types of medication treatment was between three to five months for all age groups with time in treatment highest for those receiving methadone and buprenorphine. Young adults were more likely to receive naltrexone than older adults and younger young adults (age 18–21) were less likely to receive methadone.
These data highlight a missed opportunity to engage all adults, including young adults in treatment after nonfatal overdose. The median time to treatment found in this study was at least four months which underscores substantial room for improvement in the timing required to engage them in care. For young adults, providing timely treatment after a near fatal event offers a chance for earlier intervention and prevention of the long-term physical and social consequences of ongoing substance use. The stakes are high, because the mortality is high – 2% or more of individuals in each age group who survive an opioid overdose die within 12 months23. Despite the increased efforts to initiate buprenorphine in the ED since the D’Onofrio study was published, there is no evidence or clinical guidance for administering buprenorphine in the midst of naloxone-precipitated withdrawal24. In D’Onofrio’s study, only 8% of the participants had presented with an overdose. More work is needed to demonstrate the feasibility and safety on MOUD immediately after an overdose.
In addition, we found variation in type of medication received by age group and the consequences of that variation may have important implications. Young adults in the 18–21 yo group were less likely than the older young adults (i.e., 22 to 25 yo) to receive methadone following a nonfatal overdose, even though the evidence for methadone treatment is the best established among all three FDA approved medications. Methadone has been shown to improve retention, decrease risk for HIV, and most importantly to decrease risk for death25,26. There are potential barriers to treating young adults with methadone; it is both associated with significant stigma and also federal rules that severely limit access to methadone to individuals <18 years may make methadone a less recognized option for young adults. However, in this study, similar to other studies, methadone had the best retention in treatment for all age groups27. There was no significant difference in receipt of buprenorphine among young adults. Nonetheless, the overall proportion of people receiving buprenorphine was still less than 20%. Expansion of MOUD is a critical component of federal and state responses to the rising opioid-related overdose rate in the United States and this study demonstrates that for all age groups, there continues to be a wide treatment gap that must be bridged.
We also found that young adults had a higher probability of receiving naltrexone in the 12 months following nonfatal overdose than older adults. However, the median time receiving it was only one month. The effectiveness of a medication is limited to the time people take it, therefore improving medication retention is a crucial challenge for individuals prescribed naltrexone. Naltrexone is the least studied of the three medications indicated for OUD. Further studies should examine how young adult patients and their providers make decisions regarding which MOUD to use including what structural factors (e.g., state regulations or insurance coverage) contribute to MOUD selection. These findings further underscore the need for a more nuanced understanding of how medication choices are being made by patients and providers.
In summary, this study documents low proportions of young adults who receive MOUD after a nonfatal overdose and further advances the evidence base of the types of, time to and duration of medication treatment received by young adults as compared to older adult groups. Knowing that young adults respond to interventions and treatment differently than older adults9 is an important step in improving care for this population. The differences in rate of treatment receipt, types of medication treatment and duration of medication treatment between 18- to 21-year-olds and 22- to 25-year-olds suggests that even within the young adult population, tailored interventions for each age group may be required to best engage them.
Future studies should seek to understand how young adults and providers choose MOUD and demonstrate the safety and feasibility of MOUD initiation post-overdose in the emergency department. As the U.S. continues to experience increasing opioid-related deaths, strategies to ensure that all medications are available to all people, regardless of age are needed and the emergency department can be a critical link in identification and engagement for this highest-risk population.
Appendix 1:
Characteristics of individuals ages 18–45 years with a fatal opioid-related overdose in Massachusetts between 2012–2014 stratified by age group (N=558)
| Variables | 18–21 yo N=20 | 22–25 yo N=61 | 26–45 yo N=477 |
|---|---|---|---|
| % | % | % | |
| Female | 35.0% | 18.0% | 29.4% |
| Homeless history | 0.0% | 3.3% | 3.4% |
| Incarceration history | 5.0% | 8.2% | 7.8% |
| Involuntary commitment * | 10.0% | 8.2% | 3.4% |
| Anxiety diagnosis, ever | 10.0% | 29.5% | 20.3% |
| Depression diagnosis, ever | 15.0% | 32.8% | 24.9% |
| Past year opioid prescription ** | 35.0% | 18.0% | 44.4% |
| Past year benzodiazepine prescription | 15.0% | 16.4% | 35.0% |
| Past year buprenorphine | 15.0% | 9.8% | 16.6% |
| Past year naltrexone | 5.0% | 11.5% | 5.0% |
| Past year methadone | 0.0% | 9.8% | 12.8% |
| State funded Detoxification program prior to nonfatal overdose in past year | 15.0% | 19.7% | 23.3% |
| State funded Residential Program prior to nonfatal overdose in past year | 5.0% | 4.9% | 7.3% |
Massachusetts allows for involuntary commitment through the court system to mandate treatment for individuals whose alcohol or substance use presents an acute risk to their health
This does not include buprenorphine
Footnotes
Declarations of competing interests: none
References
- 1.Hedegaard H Drug Overdose Deaths in the United States, 1999–2017. 2018;(329):8. [PubMed] [Google Scholar]
- 2.Massachusetts Department of Public Health. Data Brief: Opioid-related Overdose Deaths among Massachusetts Residents (2018–11). November 2018. https://archives.lib.state.ma.us/handle/2452/796040. Accessed January 8, 2019. [Google Scholar]
- 3.Martins SS, Sarvet A, Santaella-Tenorio J, Saha T, Grant BF, Hasin DS. Changes in US Lifetime Heroin Use and Heroin Use Disorder: Prevalence From the 2001–2002 to 2012–2013 National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(5):445–455. doi: 10.1001/jamapsychiatry.2017.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC. Today’s Heroin Epidemic. Centers for Disease Control and Prevention. https://www.cdc.gov/vitalsigns/heroin/index.html. Published July 7, 2015. Accessed January 8, 2019. [Google Scholar]
- 5.Jones CM. The paradox of decreasing nonmedical opioid analgesic use and increasing abuse or dependence - An assessment of demographic and substance use trends, United States, 2003–2014. Addict Behav. 2017;65:229–235. doi: 10.1016/j.addbeh.2016.08.027 [DOI] [PubMed] [Google Scholar]
- 6.Jones CM, Logan J, Gladden M, Bohm M. Vital Signs: Demographic and Substance Use Trends Among Heroin Users - United States, 2002–2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719–725. [PMC free article] [PubMed] [Google Scholar]
- 7.Trends in Heroin Use in the United States: 2002 to 2013. https://www.samhsa.gov/data/sites/default/files/report_1943/ShortReport-1943.html. Accessed January 8, 2019. [PubMed]
- 8.Sussman S, Arnett JJ. Emerging Adulthood: Developmental Period Facilitative of the Addictions. Evaluation & the Health Professions. 2014;37(2):147–155. doi: 10.1177/0163278714521812 [DOI] [PubMed] [Google Scholar]
- 9.Satre DD, Mertens J, Areán PA, Weisner C. Contrasting outcomes of older versus middle-aged and younger adult chemical dependency patients in a managed care program. Journal of Studies on Alcohol. 2003;64(4):520–530. [DOI] [PubMed] [Google Scholar]
- 10.McCambridge J, Strang J. The efficacy of single-session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: results from a multi-site cluster randomized trial. Addiction. 2004;99(1):39–52. [DOI] [PubMed] [Google Scholar]
- 11.Vivolo-Kantor AM, Seth P, Gladden RM, et al. Vital Signs: Trends in Emergency Department Visits for Suspected Opioid Overdoses — United States, July 2016–September 2017. MMWR Morbidity and Mortality Weekly Report. 2018;67(9):279–285. doi: 10.15585/mmwr.mm6709e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houry DE, Haegerich TM, Vivolo-Kantor A. Opportunities for Prevention and Intervention of Opioid Overdose in the Emergency Department. Ann Emerg Med. 2018;71(6):688–690. doi: 10.1016/j.annemergmed.2018.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Onofrio G, Chawarski MC, O’Connor PG, et al. Emergency Department-Initiated Buprenorphine for Opioid Dependence with Continuation in Primary Care: Outcomes During and After Intervention. J GEN INTERN MED. 2017;32(6):660–666. doi: 10.1007/s11606-017-3993-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapter 55: AN ACT REQUIRING CERTAIN REPORTS FOR OPIATE OVERDOSES.; 2015. https://malegislature.gov/Laws/SessionLaws/Acts/2015/Chapter55. Accessed April 23, 2019.
- 15.Baker CD, Polito KE, Sudders M, Bharel M. An Assessment of Fatal and Nonfatal Opioid Overdoses in Massachusetts (2011–2015).; 2017. [Google Scholar]
- 16.Green CA, Perrin NA, Janoff SL, Campbell CI, Chilcoat HD, Coplan PM. Assessing the accuracy of opioid overdose and poisoning codes in diagnostic information from electronic health records, claims data, and death records. Pharmacoepidemiol Drug Saf. 2017;26(5):509–517. doi: 10.1002/pds.4157 [DOI] [PubMed] [Google Scholar]
- 17.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345(oct03 3):e5945–e5945. doi: 10.1136/bmj.e5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Adherence: Comparison of Methods to Assess Medication Adherence and Classify Nonadherence. Annals of Pharmacotherapy. 2009;43(3):413–422. doi: 10.1345/aph.1L496 [DOI] [PubMed] [Google Scholar]
- 19.Health Insurance Coverage of the Total Population. The Henry J Kaiser Family Foundation. November 2018. https://www.kff.org/other/state-indicator/total-population/. Accessed March 7, 2019.
- 20.Rudd RA. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morbidity and mortality weekly report. 2016;65. [DOI] [PubMed] [Google Scholar]
- 21.Opioid-Related Overdose Deaths, All Intents, MA Residents - Demographic Data Highlights. Massachusetts Department of Public Health; 2018. https://www.mass.gov/files/documents/2018/08/22/Opioid-related%20Overdose%20Deaths%20Demographics%20-%20August%202018.pdf. [Google Scholar]
- 22.Center for Health Information and Analysis. ACCESS TO SUBSTANCE USE DISORDER TREATMENT IN MASSACHUSETTS. mass.gov. https://www.mass.gov/files/documents/2016/08/nr/csat-access-to-substance-use-disorder-treatment-in-mass.pdf. Published April 2015. Accessed April 23, 2019. [Google Scholar]
- 23.Larochelle MR, Bernson D, Land T, et al. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality: A Cohort Study. Annals of Internal Medicine. 2018;169(3):137. doi: 10.7326/M17-3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636–1644. doi: 10.1001/jama.2015.3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. April 2017:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matson SC, Hobson G, Abdel-Rasoul M, Bonny AE. A Retrospective Study of Retention of Opioid-Dependent Adolescents and Young Adults in an Outpatient Buprenorphine/Naloxone Clinic: Journal of Addiction Medicine 2014;8(3):176–182. doi: 10.1097/ADM.0000000000000035 [DOI] [PubMed] [Google Scholar]
- 27.Olsen Y, Sharfstein JM. Confronting the stigma of opioid use disorder--and its treatment. JAMA. 2014;311(14):1393–1394. doi: 10.1001/jama.2014.2147 [DOI] [PubMed] [Google Scholar]


