This decision analytical model uses a hypothetical cohort of men in England to evaluate whether magnetic resonance imaging before biopsy for prostate cancer screening is associated with improvements in benefit-harm and cost-effectiveness profiles compared with biopsy-first screening.
Key Points
Question
Is magnetic resonance imaging (MRI) before biopsy associated with improved benefit-harm and cost-effectiveness profiles compared with biopsy-first screening for prostate cancer using risk-stratified and age-based strategies?
Findings
In this decision analytical model of a hypothetical cohort of 4.48 million men aged 55 to 69 years, MRI-first screening strategies were associated with a more favorable benefit-harm profile and with improved cost-effectiveness compared with biopsy-first strategies. The MRI-first risk-stratified screening strategies were more cost-effective than MRI-first age-based screening and were associated with less overdiagnosis and a comparable number of prevented deaths from prostate cancer.
Meaning
Risk-stratified screening using MRI before biopsy was associated with improvements in the benefit-harm profile and cost-effectiveness compared with biopsy-first screening for prostate cancer, suggesting that this strategy should be evaluated prospectively.
Abstract
Importance
If magnetic resonance imaging (MRI) mitigates overdiagnosis of prostate cancer while improving the detection of clinically significant cases, including MRI in a screening program for prostate cancer could be considered.
Objective
To evaluate the benefit-harm profiles and cost-effectiveness associated with MRI before biopsy compared with biopsy-first screening for prostate cancer using age-based and risk-stratified screening strategies.
Design, Setting, and Participants
This decision analytical model used a life-table approach and was conducted between December 2019 and July 2020. A hypothetical cohort of 4.48 million men in England aged 55 to 69 years were analyzed and followed-up to 90 years of age.
Exposures
No screening, age-based screening, and risk-stratified screening in the hypothetical cohort. Age-based screening consisted of screening every 4 years with prostate-specific antigen between the ages of 55 and 69 years. Risk-stratified screening used age and polygenic risk profiles.
Main Outcomes and Measures
The benefit-harm profile (deaths from prostate cancer, quality-adjusted life-years, overdiagnosis, and biopsies) and cost-effectiveness (net monetary benefit, from a health care system perspective) were analyzed. Both age-based and risk-stratified screening were evaluated using a biopsy-first and an MRI-first diagnostic pathway. Results were derived from probabilistic analyses and were discounted at 3.5% per annum.
Results
The hypothetical cohort included 4.48 million men in England, ranging in age from 55 to 69 years (median, 62 years). Compared with biopsy-first age-based screening, MRI-first age-based screening was associated with 0.9% (1368; 95% uncertainty interval [UI], 1370-1409) fewer deaths from prostate cancer, 14.9% (12 370; 95% UI, 11 100-13 670) fewer overdiagnoses, and 33.8% (650 500; 95% UI, 463 200-907 000) fewer biopsies. At 10-year absolute risk thresholds of 2% and 10%, MRI-first risk-stratified screening was associated with between 10.4% (7335; 95% UI, 6630-8098) and 72.6% (51 250; 95% UI, 46 070-56 890) fewer overdiagnosed cancers, respectively, and between 21.7% fewer MRIs (412 100; 95% UI, 411 400-412 900) and 53.5% fewer biopsies (1 016 000; 95% UI, 1 010 000-1 022 000), respectively, compared with MRI-first age-based screening. The most cost-effective strategies at willingness-to-pay thresholds of £20 000 (US $26 000) and £30 000 (US $39 000) per quality-adjusted life-year gained were MRI-first risk-stratified screening at 10-year absolute risk thresholds of 8.5% and 7.5%, respectively.
Conclusions and Relevance
In this decision analytical model of a hypothetical cohort, an MRI-first diagnostic pathway was associated with an improvement in the benefit-harm profile and cost-effectiveness of screening for prostate cancer compared with biopsy-first screening. These improvements were greater when using risk-stratified screening based on age and polygenic risk profile and may warrant prospective evaluation.
Introduction
The use of multiparametric magnetic resonance imaging (MRI) as a triage test before biopsy in men with a clinical suspicion of prostate cancer has been shown both to be cost-effective1 and to be associated with a number of benefits, including the avoidance of unnecessary biopsies in approximately one-third of men, an improved detection rate of clinically significant cancer, and a reduction in the detection of clinically insignificant cancer.2,3,4,5 Although screening using prostate-specific antigen (PSA) is associated with a 20% reduction in prostate cancer–specific mortality,6 the harms of overdiagnosis and overtreatment are considered to outweigh this benefit in most men.7 As a result, formal, population-based screening is not currently recommended in any jurisdiction. Overdiagnosed cancers are those that in the absence of screening would neither be detected nor impact individuals during their lifetime.8 Offering MRI before biopsy in a population-based screening program for prostate cancer would entail additional cost. However, this cost may be offset by fewer biopsies and a reduction in the number of men diagnosed with prostate cancer, largely by mitigating overdiagnosis.
A previous modeling study9 showed that a risk-stratified screening program based on age and polygenic profile may be more cost-effective and preserve the mortality benefits associated with age-based screening with PSA while reducing the number of cancers overdiagnosed. However, the benefit-harm profile and cost-effectiveness associated with a biopsy-first age-based screening program compared with those associated with biopsy-first risk-stratified screening and whether there are further gains associated with risk-stratified screening coupled with an MRI-first diagnostic pathway are unknown. An assessment of the outcomes associated with MRI using different screening strategies is necessary before designing a prospective evaluation of a prostate cancer screening program. In this decision analytical model, we evaluated MRI as a triage test before biopsy with age-based and polygenic risk–stratified screening strategies and assessed screening strategies associated with the greatest improvements in benefit-harm profiles and cost-effectiveness.
Methods
Model Structure
This decision analytical model, conducted between December 2019 and July 2020, used a life-table approach adapted from a model of polygenic risk–stratified screening for prostate cancer.9 This Markov model simulated a hypothetical cohort of men in no screening, age-based screening, and polygenic risk–stratified screening scenarios. The hypothetical cohort consisted of 4.48 million men aged 55 to 69 years, the mean population of men between these ages in England from 2013 to 2016, followed up to 90 years of age.9,10 The University College London Research Ethics Committee would deem this study exempt from ethical review and informed patient consent because it used only openly available data sources. The study followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline.
In the hypothetical age-based screening cohorts, men received PSA testing every 4 years between the ages of 55 and 69 years in accordance with the European Randomized Study of Screening for Prostate Cancer (ERSPC).11 We used age and polygenic profiles to estimate the 10-year absolute risk of developing prostate cancer in the risk-stratified screening cohort. We varied the 10-year absolute risk thresholds at which individuals were eligible for screening from 2% to 10%. The age-specific proportion of men eligible for screening varies by risk; as an example, at a 2% absolute risk threshold, 49% of men would start screening at 55 years of age, with gradually more men being screened by 69 years of age. Only men above the 10-year absolute risk threshold started quadrennial screening with PSA at the age that they reached this threshold, with all screening ending at 69 years of age.
Modeling MRI
In the screened cohorts, men were suspected to have prostate cancer if they had a PSA level ≥3 ng/mL, as per the core analyses of the ERSPC.11 To assess the consequences of MRI for screening, we modeled 2 diagnostic pathways: biopsy first and MRI first. In the modeled biopsy-first screening pathway, men with suspected prostate cancer (PSA level ≥3 ng/mL) next received a diagnostic biopsy. In the modeled MRI-first pathway, all men with a PSA level ≥3 ng/mL were offered an MRI. Those with abnormal MRI findings, defined as a Prostate Imaging–Reporting and Data System12 score ≥3, were subsequently offered a biopsy.
In the MRI-first cohorts, we adjusted incidence, mortality, and the cancer stage at diagnosis to reflect the detectability of clinically significant and insignificant cancers by MRI before biopsy (eAppendix in the Supplement). In the risk-stratified screening cohorts, we multiplied incidence and mortality by the age-specific relative risk of developing cancer in the higher and lower risk groups for each absolute risk threshold. We calculated overdiagnosed cancers by multiplying incident screening-detected cancers by the age-specific proportion estimated to be overdiagnosed,13 adjusting in the MRI-first cohorts for the reduction in clinically insignificant cancers detected by MRI.
Polygenic Risk
The risk of receiving a diagnosis of prostate cancer varies among men. When combined, the 175 susceptibility loci for prostate cancer that have been identified in genome-wide association studies14 define a log-normal relative risk distribution with a variance of 0.68 (further details are given in the eAppendix in the Supplement).9 We used this distribution to evaluate the age-specific proportion of men eligible for screening by risk threshold as well as the proportion of total cancers expected to occur in these men. From this, we derived the age-specific relative risk of developing cancer among those above and below the threshold.
Model Parameters and Outputs
Model parameters are shown in Table 1.2,8,11,15,16,17,18,19,20,21,22,23,24,25,26 Their underlying assumptions have been described previously9 and are available in the eAppendix in the Supplement. We derived estimates of the detectability of MRI for clinically significant and insignificant cancers from the systematic review and meta-analysis of Drost and colleagues2 and of misclassification using data from the Trio study.16 In this context, misclassification occurs when a cancer is reported as clinically insignificant on MRI rather than clinically significant. We generated a life table of prostate cancer incidence and mortality as well as mortality from other causes based on mean data from 2013 to 2016 from the Office for National Statistics.10,27,28
Table 1. Parameters of the Decision Analytical Model.
| Parameter | Central estimate (95% CI) | Parameterization in probabilistic analysesa | Source |
|---|---|---|---|
| Life table | |||
| Relative reduction in prostate cancer–specific mortality with screening | 0.80 (0.72-0.89) | SE = 0.06 | 11 |
| Relative incidence of prostate cancer with screening | 1.23 (1.03-1.48) | SE = 0.18 | 15 |
| Proportion of overdiagnosed cancers | −0.62 + (Age ×0.014) | SE = 0.001 | 8 |
| Relative reduction in advanced cancer at diagnosis if screened | 0.85 (0.72-0.99) | SE = 0.07 | 15 |
| Diagnostic pathway | |||
| Detected using MRI before biopsy | |||
| Reduction in clinically insignificant cancers | 0.92 (0.90-0.94) | SE = 0.01 | 2 |
| Increase in clinically significant cancers | 1.02 (1.01-1.05) | SE = 0.01 | 2 |
| Cancers misclassified by MRI as insignificant, % | 2.76 (2.06-3.46) | SE = 0.004 | 16 |
| Utility values | |||
| General population utility | 0.86 (0.85-0.88) | 0.83 + [Gamma (4, 0.06) × 0.167] | 17 |
| Relative reduction in utility for those with prostate cancer | 0.93 (0.88-1.00)b | 0.88 + [Gamma (5, 0.05) × 0.20] | 18 |
| Costs, GBP in 2020 pricesc | |||
| Prostate-specific antigen testing | 13 (9-18) | α = 33.9; β = 0.4 | 19,20 |
| Polygenic risk stratification | 25 (17-33) | α = 33.9; β = 0.7 | d |
| Multiparametric MRI | 379 (254-504) | α = 33.9; β = 11.2 | 21 |
| Biopsy | 581 (389-772) | α = 33.9; β = 17.1 | 19,22,23 |
| Staging of diagnosed cancer | 545 (365-725) | α = 33.9; β = 16.1 | 19,22,23 |
| Active surveillance | 5052 (3385-6719)e | α = 33.9; β = 149.1 | 19,23,24 |
| Radical prostatectomy | 9808 (6571-13 044) | α = 33.9; β = 289.5 | 19,22,23 |
| Radical radiotherapy | 6462 (4330-8594) | α = 33.9; β = 190.7 | 19,22,23 |
| Brachytherapy | 1832 (1228-2437) | α = 33.9; β = 54.1 | 19,22,23 |
| Chemotherapy | 8911 (5971-11 852) | α = 33.9; β = 263.0 | 19,22,25 |
| Androgen-deprivation therapy | 671 (449-892) | α = 33.9; β = 19.8 | 9,22 |
| Palliation and death from prostate cancer | 8204 (642-24 308)f | α = 1.8; β = 4625.9 | 26 |
Abbreviations: GBP, British pounds; MRI, magnetic resonance imaging.
The following distributions were used: log-normal for relative reductions and increases, β for proportions, and gamma for utilities and cost. All α and β refer to shape and scale, respectively.
Range.
To convert GBP to US dollars, multiply by 1.36.
Cost of polygenic risk stratification was empirical from personal communication of tariffs applied in the English National Health Service.
The eAppendix in the Supplement and Callender et al9 give further details.
95% credible interval.
Outputs were the number of prostate cancers, deaths from prostate cancer, overdiagnosed cancers, biopsies, MRIs, life-years, quality-adjusted life-years (QALYs), and costs. We modeled costs from the perspective of the National Health Service in 2020 prices and derived the cost of polygenic screening from an empirical estimate. We included the following cost components: screening tests, diagnosis and assessment, treatment, and end-of-life care. We used literature-based estimates of treatment to calculate prostate cancer utilities. We applied a discount of 3.5% to all future costs and benefits, reflecting National Institute for Health and Care Excellence guidance.29
Cost-effectiveness
We used net monetary benefit (NMB) to compare the cost-effectiveness of different screening interventions, calculated by subtracting costs accrued from the QALYs generated by an intervention multiplied by the willingness-to-pay threshold. The willingness-to-pay threshold reflects the value that a health system deems appropriate to pay for 1 year at full health; we used willingness-to-pay thresholds of £20 000 (US $26 000) and £30 000 (US $39 000), the range considered by the National Institute for Health and Care Excellence.29 The screening strategy with the highest NMB for a given willingness to pay was considered the most cost-effective.
Statistical Analysis
We accounted for parameter uncertainty by running each scenario 10 000 times, on each occasion drawing parameter estimates for all variables simultaneously from an underlying distribution (Table 1). We present the mean of these probabilistic analyses throughout unless otherwise stated. We generated 95% uncertainty intervals (UIs) by using the values at the 2.5th and 97.5th centiles of the sorted probabilistic results. To reflect parameter uncertainty in the presentation of the results in the text, we rounded values to 4 significant digits.
We conducted scenario analyses to evaluate the consequences of different assumptions concerning the associations between changes in clinically insignificant and significant cancers detected by MRI, the costs of polygenic testing and MRI, varying overdiagnosis by polygenic risk, and uptake of PSA and risk-stratified screening. We performed all statistical analyses using Python, version 3.7 (Python Software Foundation).
Results
The decision analytical model included a hypothetical cohort of 4.48 million men in England, ranging in age from 55 to 69 years (median, 62 years). The age distribution of the cohort is shown in eFigure 1 in the Supplement.
Comparison of MRI-First Age-based and Risk-stratified Screening Strategies With No Screening
Compared with no screening, MRI-first age-based screening was associated with 36 910 (95% CI, 33 720-40 040) fewer deaths from prostate cancer but 70 640 (95% UI, 63 100-79 070) overdiagnosed cancers, which was 1 in 4 screen-detected cancers (Figure 1). The MRI-first age-based screening strategy was associated with an increase of 994 000 (95% CI, 979 500-1 007 000) MRIs and 667 200 (95% UI, 662 100-669 400) additional biopsies.
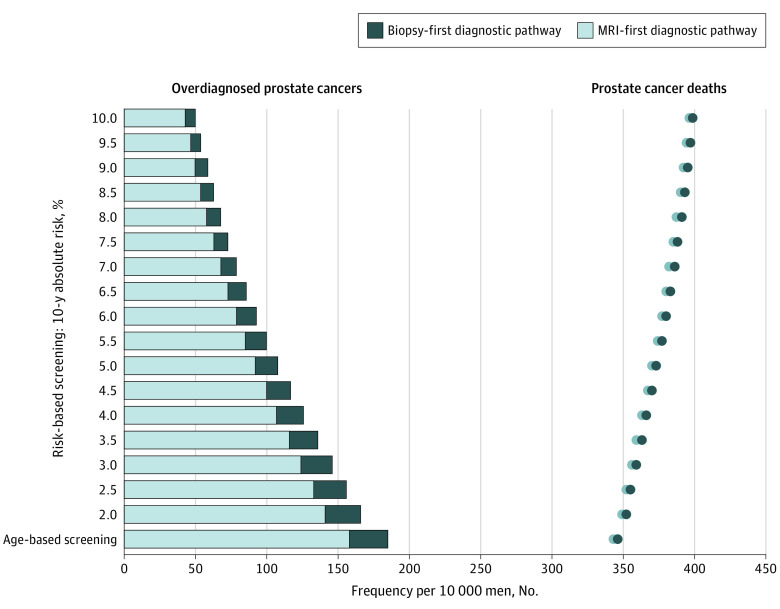
Figure 1. Overdiagnosed Cancers and Deaths From Prostate Cancer by Diagnostic Pathway.
MRI indicates magnetic resonance imaging.
As the risk threshold increased from 2% to 10%, MRI-first risk-stratified screening was associated with a decrease in the ratio of overdiagnosed cancers to prevented deaths from cancer from 1.8 to 1.5; minimizing this ratio improved the benefit-to-harm profile of screening. Compared with no screening at risk thresholds of 2% and 10%, MRI-first risk-stratified screening was associated with between 13 370 (95% CI, 12 640-14 070) and 34 450 (95% CI, 31 590-37 250) fewer deaths from prostate cancer and between 19 390 (95% CI, 17 030-22 180) and 63 300 (95% CI, 56 470-70 970) overdiagnoses. The MRI-first risk-stratified screening programs were associated with needing fewer additional resources because the proportion of men eligible for screening decreased as the risk threshold increased (Table 2). The relative risk of developing cancer compared with the mean risk among those eligible for screening increased as the risk threshold increased. As a result, compared with no screening, MRI-first risk-stratified screening was associated with a greater yield of cancers per MRI and biopsy and, consequently, with fewer scans and biopsies needed.
Table 2. Outcomes Associated With MRI-First Age-based Screening, MRI-First Risk-stratified Screening, and No Screeninga.
| Screening strategy | No. | Costs, millions of GBPd | Cumulative eligible for screening, %e | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer cases | Overdiagnosed cases | Deaths from prostate cancer | MRIs | Biopsies | Life-years | Quality-adjusted life-years | ||||
| Prostateb | Screening detectedc | |||||||||
| No screening | 527 685 | NA | NA | 190 748 | 906 551 | 608 602 | 60 333 037 | 46 694 958 | 3471 | 0 |
| Screening | ||||||||||
| Age based | 625 151 | 285 062 | 70 636 | 153 834 | 1 900 566 | 1 275 766 | 60 458 871 | 46 713 875 | 4382 | 100 |
| Risk stratified, 10-y absolute risk, % | ||||||||||
| 2.0 | 605 904 | 254 286 | 63 302 | 156 299 | 1 488 417 | 999 127 | 60 449 805 | 46 716 499 | 4148 | 73 |
| 2.5 | 598 103 | 238 784 | 59 570 | 157 809 | 1 373 255 | 921 831 | 60 444 508 | 46 716 954 | 4046 | 64 |
| 3.0 | 590 515 | 222 804 | 55 704 | 159 449 | 1 277 309 | 857 432 | 60 438 787 | 46 717 174 | 3958 | 56 |
| 3.5 | 583 369 | 207 038 | 51 871 | 161 134 | 1 198 704 | 804 674 | 60 432 923 | 46 717 192 | 3884 | 49 |
| 4.0 | 576 768 | 191 885 | 48 172 | 162 809 | 1 134 780 | 761 769 | 60 427 103 | 46 717 046 | 3822 | 43 |
| 4.5 | 570 747 | 177 560 | 44 663 | 164 439 | 1 082 950 | 726 981 | 60 421 443 | 46 716 772 | 3770 | 38 |
| 5.0 | 565 301 | 164 160 | 41 368 | 166 004 | 1 040 961 | 698 799 | 60 416 013 | 46 716 399 | 3726 | 33 |
| 5.5 | 560 403 | 151 713 | 38 298 | 167 493 | 1 006 943 | 675 967 | 60 410 854 | 46 715 952 | 3690 | 29 |
| 6.0 | 556 017 | 140 204 | 35 452 | 168 899 | 979 374 | 657 464 | 60 405 983 | 46 715 449 | 3660 | 26 |
| 6.5 | 552 101 | 129 595 | 32 821 | 170 222 | 957 029 | 642 467 | 60 401 405 | 46 714 907 | 3635 | 23 |
| 7.0 | 548 612 | 119 834 | 30 394 | 171 463 | 938 923 | 630 316 | 60 397 116 | 46 714 339 | 3613 | 20 |
| 7.5 | 545 510 | 110 864 | 28 159 | 172 625 | 924 265 | 620 478 | 60 393 107 | 46 713 754 | 3596 | 18 |
| 8.0 | 542 754 | 102 625 | 26 102 | 173 710 | 912 419 | 612 527 | 60 389 365 | 46 713 160 | 3581 | 16 |
| 8.5 | 540 311 | 95 059 | 24 208 | 174 724 | 902 871 | 606 120 | 60 385 875 | 46 712 564 | 3569 | 15 |
| 9.0 | 538 146 | 88 111 | 22 467 | 175 670 | 895 208 | 600 977 | 60 382 622 | 46 711 970 | 3559 | 13 |
| 9.5 | 536 230 | 81 728 | 20 863 | 176 553 | 889 093 | 596 873 | 60 379 591 | 46 711 382 | 3551 | 12 |
| 10.0 | 534 536 | 75 862 | 19 388 | 177 378 | 884 250 | 593 624 | 60 376 768 | 46 710 804 | 3544 | 11 |
Abbreviations: GBP, British pounds; MRI, magnetic resonance imaging; NA, not applicable.
Outcomes among 4.48 million men in hypothetical cohorts followed up to age 90 years.
Prostate cancer cases in the risk-stratified program encompass those detected by screening in high-risk groups between ages 55 and 69 years, those clinically detected in high-risk groups after 69 years of age when screening stopped, and those clinically detected in low-risk groups.
Screening-detected cancers were cancers detected with screening between the ages of 55 and 69 years.
To convert GBP to US dollars, multiply by 1.36.
Cumulative proportion of the population above the risk threshold and therefore eligible for screening between the ages of 55 and 69 years (more details are given in eTable 1 in the Supplement). The no-screening scenario assumes that men with clinically suspected cancer will have an MRI before biopsy in accordance with 2019 National Institute for Health and Care Excellence guidelines.29
Comparison of MRI-First and Biopsy-First Age-based Screening
In comparison with a biopsy-first diagnostic approach, MRI-first age-based screening was associated with 0.9% (1368; 95% UI, 1370-1409) fewer deaths from prostate cancer, 14.9% (12 370; 95% UI, 11 100-13 670) fewer overdiagnosed cancers, and 33.8% (650 500; 95% UI, 463 200-907 000) fewer biopsies. This translated into an associated increase of 0.03% (15 840; 95% UI, 11 170-25 850) total QALYs and 0.008% (4600; 95% UI, 4602-4772) total life-years (Figure 1, Table 2, and eTable 2 in the Supplement) and an associated decrease in the ratio of overdiagnosis to prevented deaths from prostate cancer from 2.2 to 1.9. The costs associated with MRI-first compared with biopsy-first age-based screening were lower despite an associated 4.8-fold increase in the number of MRIs (1.5 million; 95% UI, 1.47 million-1.53 million).
Comparison of MRI-First and Biopsy-First Risk-stratified Screening
In comparison with biopsy-first risk-stratified screening, MRI-first risk-stratified screening was associated with fewer deaths from prostate cancer, fewer overdiagnosed cancers, just less than half the number of biopsies, and an increase in QALYs gained at a lower cost (Table 2 and eTable 2 in the Supplement). At a 10-year absolute risk threshold of 2%, MRI-first risk-stratified screening was associated with a 3.9-fold greater number of scans required (1 102 000; 95% UI, 1 074 000-1 136 000) compared with a biopsy-first risk-stratified screening program, decreasing to a 2.6-fold increase (545 900; 95% UI, 503 000-596 300) at a risk threshold of 10%.
Comparison of MRI-First Age-based Screening With MRI-First Risk-stratified Screening
Compared with MRI-first age-based screening, MRI-first risk-stratified screening was associated with fewer harms (overdiagnoses and biopsies) and lower costs but with more deaths from prostate cancer. At 10-year absolute risk thresholds of 2% and 10%, MRI-first risk-stratified screening was associated with between 10.4% (7335; 95% UI, 6630-8098) and 72.6% (51 250; 95% UI, 46 070-56 890) fewer overdiagnosed cancers, respectively, and between 21.7% fewer MRIs (412 100; 95% UI, 411 400-412 900) and 53.5% fewer biopsies (1 016 000; 95% UI, 1 010 000-1 022 000), respectively, compared with MRI-first age-based screening (Table 2). In comparison with MRI-first age-based screening, MRI-first risk-stratified screening was associated with more QALYs at all risk thresholds below 7.5% and with progressively lower costs as the risk threshold increased from 2.0% to 10.0%. However, MRI-first risk-stratified screening was associated with between 1.6% (2465; 95% UI, 2133-2794) and 15.3% (23 540; 95% UI, 21 080-25 970) more deaths from prostate cancer at risk thresholds of 2.0% and 10.0%, respectively.
Cost-effectiveness
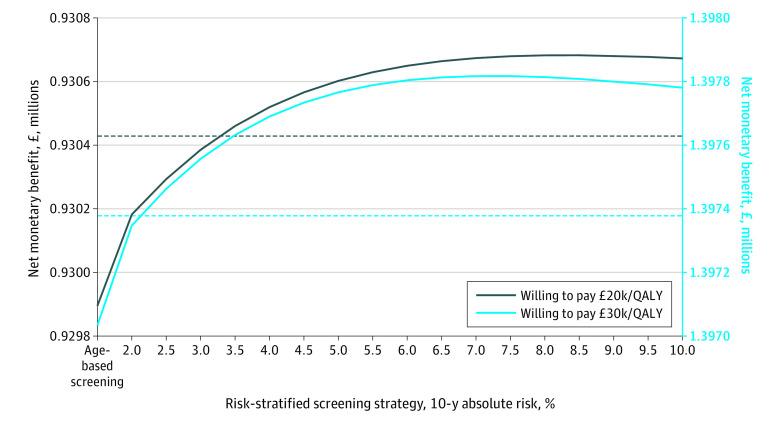
All MRI-first risk-stratified screening scenarios at thresholds of 3.5% or greater were associated with an NMB greater than that of no screening at a willingness-to-pay threshold of £20 000 (US $26 000) (Figure 2; cost-effectiveness acceptability curves are shown in eFigure 2 in the Supplement). MRI-first age-based screening was associated with the lowest NMB and was the least cost-effective MRI-first screening strategy at willingness-to-pay thresholds of both £20 000 (US $26 000) and £30 000 (US $39 000) per QALY gained. The strategies associated with the highest NMB at willingness-to-pay thresholds of £20 000 (US $26 000) and £30 000 (US $39 000) per QALY gained were MRI-first risk-stratified screening at a risk threshold of 8.5% and 7.5%, respectively (the cost-effectiveness acceptability frontier is presented in eFigure 3 in the Supplement, and the NMB of MRI-first and biopsy-first screening scenarios are given in eFigure 4 in the Supplement).
Figure 2. Net Monetary Benefit Associated With Age-based, Risk-stratified, and No-Screening Strategies Evaluated With a Magnetic Resonance Imaging–First Diagnostic Pathway.
The grey dashed line represents no screening at a willingness to pay of £20 000 (US $26 000); and the blue dashed line, at £30 000 (US $39 000). To convert British pounds to US dollars, multiply by 1.36. QALY indicates quality-adjusted life-year.
Scenario Analyses
In the Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? (PRECISION) trial,30 MRI before biopsy was associated with a 13% (95% CI, 7%-19%) reduction in clinically insignificant cancers detected and a 12% (95% CI, 4%-20%) increase in clinically significant cancers detected. Using these parameters, MRI-first screening strategies were associated with an improvement in the benefit-harm profile and NMB (eFigure 5 in the Supplement). However, age-based screening was associated with reduced cost-effectiveness compared with risk-stratified screening (eTable 5 and eFigure 6 in the Supplement). The cost-effectiveness of MRI-first screening strategies was insensitive to the cost of an MRI scan (baseline of £380 [US $494] to £100 [US $130]) (eFigure 7 in the Supplement). In contrast, MRI-first risk-stratified screening strategies were sensitive to the cost of risk stratification (varied from £25 [US $33] to £100 [US $130]) (eFigure 8 in the Supplement). A 75% uptake of PSA screening was associated with greater cost-effectiveness; however, MRI-first risk-stratified screening was insensitive to a 75% (baseline 100%) uptake of polygenic risk stratification (eFigure 9 in the Supplement). Overdiagnosis has been shown to vary inversely by polygenic risk8; in this scenario, the ratio of prevented deaths from prostate cancer to overdiagnosed cancers was associated with further improvement in risk-stratified screening (eFigure 10 in the Supplement).
Discussion
This decision analytical model showed that an MRI-first diagnostic pathway was associated with an improved benefit-harm profile for prostate cancer screening compared with a biopsy-first diagnostic pathway. This improvement was associated with a reduction in biopsies, overdiagnoses, and deaths from prostate cancer. Moreover, an MRI-first approach was associated with more QALYs at reduced costs compared with a biopsy-first diagnostic pathway.
In addition, we showed that these benefits were greater when risk-stratified screening was combined with an MRI-first diagnostic pathway. By tailoring screening to men at higher absolute risk of developing prostate cancer, MRI-first risk-stratified screening was associated with preventing a number of deaths from prostate cancer comparable with the number of deaths prevented by MRI-first age-based screening. MRI-first risk-stratified screening was also associated with a 10.4% to 72.6% lower probability of overdiagnosis and 21.7% to 53.5% fewer unnecessary biopsies as well as an improvement in the cost-effectiveness of a screening program (Figure 1, Figure 2, and Table 2). Increasing the risk threshold was associated with a lower ratio of overdiagnosed cancers to deaths from prostate cancer. Eligibility for screening became more strict as the risk threshold increased, such that there were fewer screening-detected and potentially overdiagnosed cancers.
Of all strategies studied, MRI-first risk-stratified screening at a 10-year absolute risk threshold of 3.5% was associated with the greatest number of QALYs gained, beyond which the QALYs gained diminished (Table 2 and eTable 2 in the Supplement). As the risk threshold increased, risk-stratified screening was associated with a greater decrease in costs compared with QALYs generated, such that the NMB associated with MRI-first risk-stratified screening began to plateau at a risk threshold of approximately 7% to 8%. This reflects an association with a lower proportion of men expected to be overdiagnosed and with a decrease in the number of men who would be eligible for screening as the risk threshold increased. The benefits associated with screening (mortality reduction and QALYs gained) decreased as the risk threshold increased because the proportion of men eligible for screening became increasingly smaller (Table 2). As a result, the ideal risk threshold for screening would represent a balance between that which minimizes overdiagnosis and maximizes mortality reduction and QALYs gained at an acceptable cost-effectiveness ratio.31
There are several ways that a risk-stratified screening program might be further tailored. The screening interval could be varied by risk if the sojourn time—the time that a cancer remains in a detectable but preclinical state—differs by risk level. This strategy might reduce the number of interval cancers and improve the mortality reduction of the program. To our knowledge, there currently are no data on how the sojourn time varies with risk level; thus, this should be the subject of future work. Alternative strategies also include varying the starting and stopping ages for screening by risk level. Different prognostic markers, such as the 4-kallikrein score as a triage test before biopsy, also warrant comparative analyses.32
Strengths and Limitations
This study has strengths. To our knowledge, there are no comparable models of prostate cancer incorporating MRI and no trials of MRI-first screening. Using a life-table approach, we based the model on well-validated population data, allowing us to calibrate the model (eFigures 11 and 12 in the Supplement), minimize assumptions, and maximize model clarity while using probabilistic analyses to account for parameter uncertainty. We used meta-analyses as the basis of the inputs when possible, accounted for misclassification of cancers by MRI, and ran sensitivity analyses to explore alternative scenarios. Rather than making assumptions regarding the association between polygenic risk and indolent and nonindolent cancers, we used age-specific probabilities of overdiagnosis for transparency. To reflect a countrywide screening program involving radiological centers with varying degrees of experience with MRI, we used conservative baseline estimates of the detection level of MRI for clinically significant and insignificant cancers. Centers with substantial experience with MRI have shown greater reductions in detection of clinically insignificant cancers and greater increases in detection of clinically significant cancers.5 In addition, we used NMB to facilitate the comparison of multiple alternatives and to avoid assumptions of which pairwise comparisons are most appropriate.33
This study also has limitations. We extrapolated the detection rate of MRI for clinically suspected cancer to a screened hypothetical cohort. Magnetic resonance imaging has been shown to distinguish between clinically significant and insignificant cancers.2,3 However, the proportion of cancers deemed clinically insignificant that will progress to become clinically significant and the implications of an MRI-first diagnostic pathway for long-term prostate cancer outcomes remain unknown. In addition, risk-stratified screening may be associated with greater reductions in overdiagnosis and mortality than was found in our study. In the absence of screening data, we assumed in base-case analyses that overdiagnosis and mortality would not differ from that reported in age-based screening trials. The base-case model may therefore underestimate the reduction in overdiagnosis (sensitivity analyses are provided in eFigure 9 in the Supplement), and the assumption that risk-stratified screening would not be associated with a lower relative risk of death from prostate cancer among those screened may not hold.34
Conclusions
In this decision analytical model of a hypothetical cohort of men, an MRI-first diagnostic pathway was associated with an improved benefit-harm profile and cost-effectiveness of screening for prostate cancer. The improvement associated with an MRI-first pathway was greater with risk-stratified screening based on age and polygenic risk profile. Prospective evaluation of an MRI-first risk-stratified screening program, including implementation research, appears to be needed.
eAppendix.
eTable 1. Age-specific cumulative proportion eligible for screening by 10-year absolute risk threshold
eTable 2. Outcomes of biopsy-first no screening and biopsy-first screening for prostate cancer
eTable 3. Outcomes of biopsy-first no screening and biopsy-first screening for prostate cancer per 10,000 men
eTable 4. Outcomes of MRI-first no screening and MRI-first screening for prostate cancer per 10,000 men
eFigure 1. Population age distribution
eFigure 2. Cost-effectiveness acceptability curves showing MRI-first screening strategies
eFigure 3. Cost-effectiveness acceptability frontier showing MRI-first screening strategies
eFigure 4. Net monetary benefit of MRI-first and biopsy-first strategies
eTable 5. Outcomes of no screening and MRI-first screening for prostate cancer per 10,000 men under assumptions from the PRECISION trial
eFigure 5. Benefit/harm profile per 10,000 men with MRI-first screening using parameter estimates from the PRECISION trial
eFigure 6. Net monetary benefit per 10,000 men of MRI-first screening and no screening using parameter estimates from the PRECISION trial
eFigure 7. Net monetary benefit per 10,000 men of MRI-first screening and no screening at different costs of MRI
eFigure 8. Net monetary benefit per 10,000 men of MRI-first screening and no screening at different costs of polygenic risk stratification
eFigure 9. Net monetary benefit per 10,000 men of MRI-first screening and no screening at 75% uptake of PSA screening and polygenic risk stratification
eFigure 10. Overdiagnosed cancers and prostate cancer deaths prevented per 10,000 men of MRI-first risk-stratified compared to MRI-first age-based screening when overdiagnosis varies by polygenic risk
eFigure 11. Observed vs predicted incidence rate
eFigure 12. Observed vs predicted mortality rate
eReferences
References
- 1.Faria R, Soares MO, Spackman E, et al. Optimising the diagnosis of prostate cancer in the era of multiparametric magnetic resonance imaging: a cost-effectiveness analysis based on the Prostate MR Imaging Study (PROMIS). Eur Urol. 2018;73(1):23-30. doi: 10.1016/j.eururo.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drost FH, Osses DF, Nieboer D, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev. 2019;4(1):CD012663. doi: 10.1002/14651858.CD012663.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elwenspoek MMC, Sheppard AL, McInnes MDF, et al. Comparison of multiparametric magnetic resonance imaging and targeted biopsy with systematic biopsy alone for the diagnosis of prostate cancer: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198427. doi: 10.1001/jamanetworkopen.2019.8427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767-1777. doi: 10.1056/NEJMoa1801993 [DOI] [PMC free article] [PubMed]
- 5.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. ; PROMIS study group . Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815-822. doi: 10.1016/S0140-6736(16)32401-1 [DOI] [PubMed] [Google Scholar]
- 6.Hugosson J, Roobol MJ, Månsson M, et al. A 16-yr follow-up of the European Randomized Study of Screening for Prostate Cancer. Eur Urol. 2019;76(1):43-51. doi: 10.1016/j.eururo.2019.02.009 [DOI] [PMC free article] [PubMed]
- 7.Grossman DC, Curry SJ, Owens DK, et al. ; US Preventive Services Task Force . Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(18):1901-1913. doi: 10.1001/jama.2018.3710 [DOI] [PubMed] [Google Scholar]
- 8.Pashayan N, Duffy SW, Neal DE, et al. Implications of polygenic risk-stratified screening for prostate cancer on overdiagnosis. Genet Med. 2015;17(10):789-795. doi: 10.1038/gim.2014.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callender T, Emberton M, Morris S, et al. Polygenic risk-tailored screening for prostate cancer: a benefit-harm and cost-effectiveness modelling study. PLoS Med. 2019;16(12):e1002998. doi: 10.1371/journal.pmed.1002998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Office for National Statistics . England: population estimates. Accessed December 2019. https://www.nomisweb.co.uk/query/select/getdatasetbytheme.asp?opt=3&theme=&subgrp=
- 11.Schröder FH, Hugosson J, Roobol MJ, et al. ; ERSPC Investigators . Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384(9959):2027-2035. doi: 10.1016/S0140-6736(14)60525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging–Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69(1):16-40. doi: 10.1016/j.eururo.2015.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pashayan N, Duffy SW, Pharoah P, et al. Mean sojourn time, overdiagnosis, and reduction in advanced stage prostate cancer due to screening with PSA: implications of sojourn time on screening. Br J Cancer. 2009;100(7):1198-1204. doi: 10.1038/sj.bjc.6604973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matejcic M, Saunders EJ, Dadaev T, et al. ; PRACTICAL (Prostate Cancer Association Group to Investigate Cancer-Associated Alterations in the Genome) Consortium . Germline variation at 8q24 and prostate cancer risk in men of European ancestry. Nat Commun. 2018;9(1):4616. doi: 10.1038/s41467-018-06863-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilic D, Djulbegovic M, Jung JH, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362:k3519. doi: 10.1136/bmj.k3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahdoot M, Wilbur AR, Reese SE, et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med. 2020;382(10):917-928. doi: 10.1056/NEJMoa1910038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health. 2011;14(4):539-545. doi: 10.1016/j.jval.2010.10.029 [DOI] [PubMed] [Google Scholar]
- 18.Heijnsdijk EA, Wever EM, Auvinen A, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med. 2012;367(7):595-605. doi: 10.1056/NEJMoa1201637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UK Department of Health . NHS reference costs 2015 to 2016. Accessed July 19, 2018. https://www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016
- 20.Curtis L, Burns A. Unit costs of health and social care 2016.Accessed July 19, 2018. https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2016/
- 21.National Institute for Health and Care Excellence . Resource impact report: prostate cancer: diagnosis and management (update) (NG131). May 2019. Accessed December 15, 2019. https://www.nice.org.uk/guidance/ng131/resources/resource-impact-report-pdf-6779259325
- 22.National Collaborating Centre for Cancer . Prostate cancer: diagnosis and treatment: clinical guideline 20. 14. Accessed July 20, 2018. https://www.nice.org.uk/guidance/ng131/evidence/full-guideline-january-2014-6781033549?tab=evidence
- 23.National Institute for Health and Care Excellence . Prostate cancer: NICE pathway. Accessed July 20, 2018. https://pathways.nice.org.uk/pathways/prostate-cancer#path=view%3A/pathways/prostate-cancer/prostate-cancer-overview.xml&content=view-index
- 24.Hamdy FC, Donovan JL, Lane JA, et al. ; ProtecT Study Group . 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424. doi: 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Health and Care Excellence . Docetaxel for the treatment of hormone-refractory metastatic prostate cancer: technology appraisal guidance (TA101). June 28, 2006. Accessed July 30, 2018. https://www.nice.org.uk/guidance/ta101
- 26.Round J, Jones L, Morris S. Estimating the cost of caring for people with cancer at the end of life: a modelling study. Palliat Med. 2015;29(10):899-907. doi: 10.1177/0269216315595203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Office for National Statistics. Cancer registration statistics, England. Accessed June 18, 2018. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancerregistrationstatisticscancerregistrationstatisticsengland
- 28.Office for National Statistics . Mortality statistics. Accessed July 16, 2018. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathregistrationssummarytablesenglandandwalesreferencetables
- 29.National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. National Institute for Health and Care Excellence; 2013. [PubMed] [Google Scholar]
- 30.Kasivisvanathan V, Rannikko AS, Borghi M, et al. ; PRECISION Study Group Collaborators . MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767-1777. doi: 10.1056/NEJMoa1801993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pashayan N, Morris S, Gilbert FJ, Pharoah PDP. Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer: a life-table model. JAMA Oncol. 2018;4(11):1504-1510. doi: 10.1001/jamaoncol.2018.1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darst BF, Chou A, Wan P, et al. The four-kallikrein panel is effective in identifying aggressive prostate cancer in a multiethnic population. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1381-1388. doi: 10.1158/1055-9965.EPI-19-1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. 4th ed. Oxford University Press; 2015.
- 34.Seibert TM, Fan CC, Wang Y, et al. ; PRACTICAL Consortium . Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ. 2018;360:j5757. doi: 10.1136/bmj.j5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix.
eTable 1. Age-specific cumulative proportion eligible for screening by 10-year absolute risk threshold
eTable 2. Outcomes of biopsy-first no screening and biopsy-first screening for prostate cancer
eTable 3. Outcomes of biopsy-first no screening and biopsy-first screening for prostate cancer per 10,000 men
eTable 4. Outcomes of MRI-first no screening and MRI-first screening for prostate cancer per 10,000 men
eFigure 1. Population age distribution
eFigure 2. Cost-effectiveness acceptability curves showing MRI-first screening strategies
eFigure 3. Cost-effectiveness acceptability frontier showing MRI-first screening strategies
eFigure 4. Net monetary benefit of MRI-first and biopsy-first strategies
eTable 5. Outcomes of no screening and MRI-first screening for prostate cancer per 10,000 men under assumptions from the PRECISION trial
eFigure 5. Benefit/harm profile per 10,000 men with MRI-first screening using parameter estimates from the PRECISION trial
eFigure 6. Net monetary benefit per 10,000 men of MRI-first screening and no screening using parameter estimates from the PRECISION trial
eFigure 7. Net monetary benefit per 10,000 men of MRI-first screening and no screening at different costs of MRI
eFigure 8. Net monetary benefit per 10,000 men of MRI-first screening and no screening at different costs of polygenic risk stratification
eFigure 9. Net monetary benefit per 10,000 men of MRI-first screening and no screening at 75% uptake of PSA screening and polygenic risk stratification
eFigure 10. Overdiagnosed cancers and prostate cancer deaths prevented per 10,000 men of MRI-first risk-stratified compared to MRI-first age-based screening when overdiagnosis varies by polygenic risk
eFigure 11. Observed vs predicted incidence rate
eFigure 12. Observed vs predicted mortality rate
eReferences