Abstract
We realized that tailoring the pore size/geometry and chemistry, by virtue of alkynyl or naphthalene replacing phenyl within a series of isomorphic MOFs, can optimize methane storage working capacities, affording an exceptionally high working capacity of 203 cm3 (STP) cm−3 at 298 K and 5–80 bar.
The exponential increase in global energy usage over the past 50 years has pushed the implementation of a cleaner fuel for replacing conventional petroleum fuels to a level of utmost importance. Natural gas (NG), primarily composed of methane, holds bright promise as a viable alternative fuel due to its abundant reserves, low CO2 emission and high research octane number (RON = 107). However, its widespread use is limited by the relatively low volumetric energy density. Although liquefied NG (LNG) at 113 K and compressed NG (CNG) at 250 bar are already in use, cryogenic and high-pressure storage conditions have severely hampered practical applications. For instance, overhigh pressures required for liquefaction can only be achieved by using complex and expensive multi-stage compressors, leading to some cost, space, and safety issues for use in passenger vehicles. In this regard, adsorbed natural gas (ANG) systems have aroused considerable interest to overcome these issues, which involves filling storage tanks with porous materials to store a high density of methane at modest pressures. To guide the research of the ANG technology, the U.S. Department of Energy (DOE) has set the ambitious volumetric NG storage target of 263 cm3 (STP) cm−3 at room temperature (RT) assuming the adsorbent packing loss is ignored, equivalent to that of CNG at 250 bar and 298 K.1
Extensive research efforts have been devoted to developing new materials with high methane storage capacity in order to meet the challenging storage target. Microporous metal–organic frameworks (MOFs) are known to be very promising for methane storage because of their high surface areas, tunable pores, and versatile chemistry.2–4 A large number of MOFs, such as HKUST-1, UTSA-76, Co(BDP), MOF-5, NU-125, Al-soc-MOF-1, MAF-38, MOF-905, NJU-Bai43, and MFM-115 have been reported as outstanding storage adsorbents, showing some of the highest gravimetric or volumetric CH4 storage capacities.5–11 It is notable that the volumetric working capacity is a key parameter to evaluate the performance of methane storage materials, because it determines the driving range of vehicles powered by natural gas. Since the automobile industry requires an engine inlet pressure of 5 bar, the working capacity (also called deliverable capacity) is defined as the difference of the amount of methane adsorbed between the target storage pressure (generally 35–80 bar) and 5 bar. Prominently, there are two pathways to improve the methane working capacity of a given porous material. On one hand, optimizing pore sizes or functional sites in MOFs can enforce the strength of host–methane and methane–methane interactions (Qst), contributing to high volumetric methane uptake at high pressure.5,6 Nevertheless, overhigh Qst in MOFs leads to a rapid increase in the low-pressure uptake, sometimes delimiting their high working capacity, as exemplified by MOF-74 and PCN-14.6 On the other hand, large-pore MOFs (e.g., MOF-905, Al-soc-MOF-1 and NU-111) commonly show moderate Qst for methane, resulting in a low CH4 adsorption at 5 bar;9,10 however, too weak CH4 affinity delimits the total CH4 uptake at high pressure, thus sacrificing the working capacity in some cases.10 Evidently, it is highly desired to optimize the porous structures with suitable methane binding affinity for balancing the CH4 adsorption amounts between the high and low pressure, thus targeting higher volumetric working capacity.
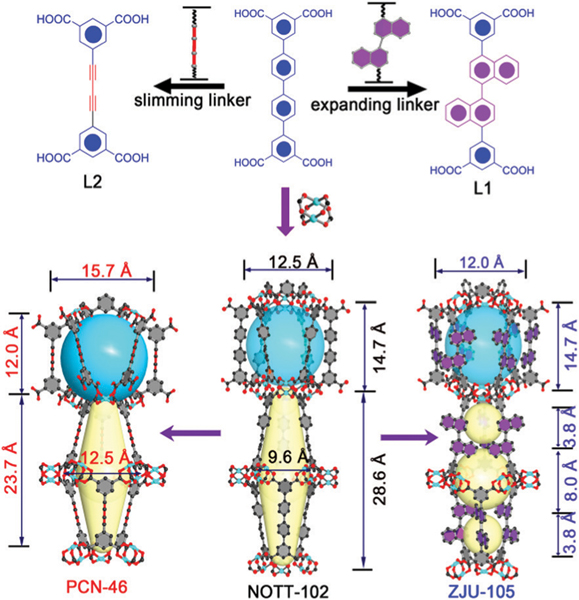
With this in mind, we herein used a crystal-engineering strategy to finely modulate the pore size and chemistry in a family of isomorphic MOFs for methane storage. We chose NOTT-102 as the fundamental framework backbone because of its high methane storage capacity and easily adjustable functionality on the center phenyl rings.14 The use of alkynyl or naphthalene groups to replace the phenyl rings in NOTT-102 afforded two porous materials (namely PCN-46 and ZJU-105), in which the pore size and pore-chemistry can be well modulated (Fig. 1). It was found that the expanded pore size and reduced aromatic CH4-affinity sites in PCN-46a can cause a slight decrease in the low-pressure CH4 uptake while having a negligible effect on the adsorption at 65/80 bar, leading to an exceptionally high volumetric working capacity of 203 cm3 (STP) cm−3 between 5 and 80 bar at 298 K. This value is comparable to the best-performing MOFs reported, such as MFM-115a (208 cm3 (STP) cm−3)12 and MOF-905 (203 cm3 (STP) cm−3).13
Fig. 1.
Comparison of the organic linkers and crystal structures of ZJU-105, NOTT-102, and PCN-46.
The linkers of H4L1 and H4L2 were elaborately designed by using two naphthalene or alkynyl groups replacing the phenyl rings on the linker of NOTT-102 (Fig. 1). Both of the organic linkers were synthesized through a multistep reaction procedure (Schemes S1 and S2, ESI†). A solvothermal reaction of H4L1 with Cu(NO3)2·2.5H2O in a mixture of DMF/H2O (in the presence of HCl) at 80 °C for 3 days afforded blue block crystals of a novel MOF (ZJU-105) with a framework formula of [Cu2(L1)(H2O)2]n. High-quality crystals of PCN-46, [Cu2(L2)(H2O)2]n, were synthesized by a solvothermal reaction of H4L2 with Cu(NO3)2·2.5H2O in a mixture of DMF/EtOH/H2O (in the presence of HNO3) at 85 °C for 1 day. It is worth noting that the sample of PCN-46 in the literature was directly synthesized by using a precursor compound (5-ethynylisophthalic acid) of H4L2 to react with copper salts, which cannot guarantee the high quality of the bulk samples.15 In this work, the phase purity of bulk materials for ZJU-105 and PCN-46 was confirmed by powder X-ray diffraction (PXRD) and the following surface area/pore volume measured (Fig. S10–S15, ESI†).
The crystal structures of the three MOFs investigated are schematically shown in Fig. 1. Structural analysis reveals that both of the ZJU-105 and PCN-46 frameworks are isoreticular to NOTT-102, which consist of paddle-wheel dinuclear Cu2(COO)4 units linked by the carboxylates of L4− linkers to form a 3D NbO-type network.14d As expected, the use of alkynyl or naphthalene groups to replace the phenyl rings on NOTT-102 can finely modulate the pore size/geometry and chemistry. As shown in Fig. 1, the framework of NOTT-102 has two types of cages: one cuboctahedral cage of about 12.5 × 14.7 Å2 and another large irregular elongated cage of ≈9.6 × 28.6 Å2. When the bulky naphthalene rings were incorporated, the pores of ZJU-105 were significantly decreased, wherein the large irregular cage was divided into three small cages with the diameters of 3.8 and 8.0 Å. In contrast, we slim down the organic ligands in PCN-46 by virtue of triple-bond spacers replacing the phenyls in NOTT-102. Relative to NOTT-102, PCN-46 exhibits a similar pore geometry with slightly expanded pore spaces. In addition, the incorporation of alkynyl or naphthalene groups in PCN-46 and ZJU-105 can reduce or increase the aromatic moieties that provide π···CH4 interactions.16 Therefore, the fine-tuned pore size/geometry and aromatic moieties in this series of MOFs can systematically affect the strength of CH4 binding affinities, thus optimizing the target working capacities.
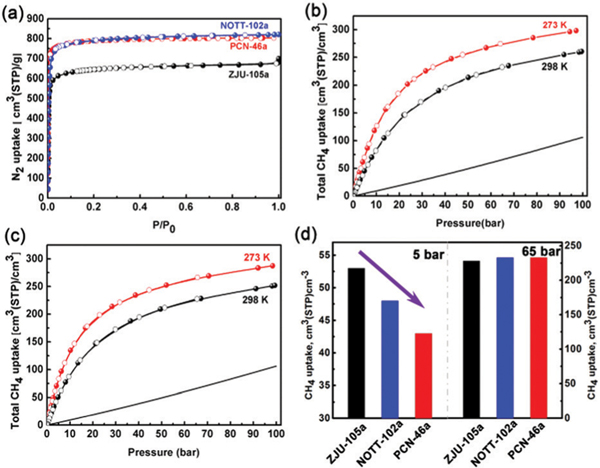
Before methane adsorption measurements, we first measured the N2 adsorption isotherms at 77 K of the activated ZJU-105a and PCN-46a. As shown in Fig. 2a, the N2 isotherms of ZJU-105a and PCN-46a exhibit a reversible type-I character with saturated uptake of 670 and 803 cm3 g−1, respectively. The Brunauer–Emmett–Teller (BET) surface area and pore volume of ZJU-105a were determined to be 2608 m2 g−1 and 1.037 cm3 g−1, respectively, notably lower than those of NOTT-102a (3342 m2 g−1 and 1.268 cm3 g−1) due to the incorporated bulky naphthalene groups. In contrast, PCN-46a with the slimmed alkynyl groups shows a comparable BET surface area and pore volume (3224 m2 g−1 and 1.243 cm3 g−1). We note that these values of PCN-46a are significantly higher than the reported values (2500 m2 g−1 and 1.012 cm3 g−1) in the previous literature,15 and agree well with the theoretical ones (3634 m2 g−1 and 1.266 cm3 g−1) calculated from the crystal structure. This further confirms the high quality of the bulk samples that we synthesized, encouraging us to retest their methane storage capacities.
Fig. 2.
(a) Nitrogen isotherms of ZJU-105a, NOTT-102a and PCN-46a at 77 K. (b) Total volumetric CH4 adsorption isotherms of PCN-46a and (c) ZJU-105a. (d) Comparison of the CH4 adsorption amount of the indicated MOFs at 5 and 65 bar.
Next, we collected the high-pressure methane adsorption isotherms for ZJU-105a and PCN-46a at 273 and 298 K over the pressure range of 0–100 bar. As presented in Fig. 2b and c, ZJU-105a and PCN-46a exhibit high total volumetric methane uptake values of 228 and 233 cm3 (STP) cm−3 at 65 bar and 298 K, respectively. These values are comparable to that of NOTT-102a (233 cm3 (STP) cm−3), indicating that tuning the pore size/geometry in these isomorphic MOFs shows a negligible effect on the total volumetric CH4 uptake at high pressure. This might be attributed to the mutual compensation between the porosity, crystal density and CH4 binding strength. With the pressure increasing to 80 bar, the volumetric uptake of ZJU-105a and PCN-46a can be further increased to 243 and 246 cm3 (STP) cm−3, respectively, achieving 93% of the new DOE’s volumetric target assuming the packing density loss is ignored. These values are higher than those of most of the promising MOFs, such as MOF-905 (228 cm3 (STP) cm−3)13 and Al-soc-MOF-1 (221 cm3 (STP) cm−3),9a and approach those of the best-performing MFM-115 (256 cm3 (STP) cm−3)12 and HKUST-1 (272 cm3 (STP) cm−3).4b
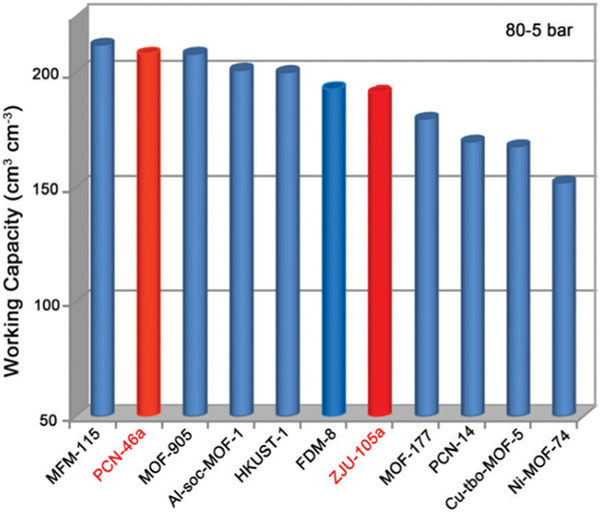
In practical applications, the driving range of an ANG vehicle is determined primarily by the working capacity of the adsorbent. We first evaluated the volumetric working capacities of ZJU-105a and PCN-46a to compare with NOTT-102a and some other indicated MOFs (Table 1). As shown in Fig. 2d, due to the smaller pore spaces and more aromatic moieties, ZJU-105a takes up more CH4 (53 cm3 (STP) cm−3) at 5 bar than NOTT-102a (48 cm3 (STP) cm−3). In contrast, PCN-46a shows a lower adsorption at 5 bar (43 cm3 (STP) cm−3) than NOTT-102a. Therefore, PCN-46a exhibits a higher volumetric working capacity of 190 cm3 (STP) cm−3 than NOTT-102a (185 cm3 (STP) cm−3) and ZJU-105a (175 cm3 (STP) cm−3) between 65 and 5 bar at 298 K. It is worth noting that this working capacity of PCN-46a outperforms that of most of the promising MOFs (e.g., NU-125, NU-111, UTSA-20, Al-soc-MOF-1, and MOF-5, Table 1), and is comparable to that of the benchmark HKUST-1 (190 cm3 (STP) cm−3),4a MFM-115 (191 cm3 (STP) cm−3),12 and UTSA-76a (197 cm3 (STP) cm−3).11 When the storage pressure is increased to 80 bar, PCN-46a exhibits an exceptionally high CH4 working capacity of 203 cm3 (STP) cm−3 between 80 and 5 bar at RT (Fig. 3), much higher than that of ZJU-105a (190 cm3 (STP) cm−3) and rivaling the state-of-the-art materials reported, such as MFM-115a (208 cm3 (STP) cm−3)12 and MOF-905 (203 cm3 (STP) cm−3).13
Table 1.
Summary of some promising MOFs for high-pressure CH4 storage
| MOFs | SBETa (m2 g−1) | Total uptakeb cm3/cm3 | Working capacityc cm3/cm3 | Qst (kJ mol−1) |
|---|---|---|---|---|
| PCN-46a | 3224 | 246 (233) | 203 (190) | 14.2 |
| ZJU-105a | 2608 | 243 (228) | 190 (175) | 16.58 |
| MFM-115a12 | 3394 | 256 (238) | 208 (191) | 16.3 |
| MOF-90513 | 3490 | 228 (206) | 203 (181) | 11.7 |
| NOTT-102a14a | 3342 | −(233) | −(185) | 14.90 |
| Al-soc-MOF-19a | 5585 | 221 (197) | 201 (176) | 11.0 |
| HKUST-14a,b | 1850 | 272 (267) | 200 (190) | 17.0 |
| UTSA-7611 | 2820 | −(257) | −(197) | 15.5 |
| MAF-3816a | 2022 | 273 (263) | 197 (187) | 21.6 |
| FDM-89b | 3643 | 215 (193) | 193 (171) | 10.4 |
| MOF-17710a | 4500 | 205 (187) | 185 (167) | 9.9 |
| NU-1254a | 3120 | −(232) | −(183) | 15.1 |
| PCN-144a,b | 2000 | 250 (230) | 178 (157) | 18.7 |
| Cu-tbo-MOF-516b | 3971 | 216 (199) | 175 (158) | 20.4 |
| Ni-MOF-744a,b | 1350 | 267 (251) | 152 (129) | 21.4 |
Calculated from N2 isotherms at 77 K.
At 80(65) bar and 298 K.
The working capacity is defined as the difference in the total uptake values between 80(65) and 5 bar.
Fig. 3.
Comparison of the volumetric CH4 working capacities of PCN-46a and ZJU-105a with some best-performing MOFs reported at 298 K.
To gain better insight into the origin of different volumetric low-pressure uptake values and working capacities of ZJU-105a and PCN-46a, we first calculated their isosteric heats of adsorption (Qst) from the temperature-dependent isotherms using the virial method (Fig. S22–S24, ESI†). The initial Qst of ZJU-105a was calculated to be 16.58 kJ mol−1, much higher than those of NOTT-102a (14.90 kJ mol−1) and PCN-46a (14.2 kJ mol−1). Due to the same framework backbone and topology, these MOFs have a similar density of open metal sites. The incorporation of naphthalene groups in ZJU-105a can not only notably reduce the pore sizes but also offer more aromatic moieties to interact with CH4 molecules via π···CH4 interactions,16 contributing to the enhanced CH4 affinity and thus a higher CH4 adsorption at low pressure of 5 bar than that of NOTT-102a. In contrast, the use of alkynyl groups to replace the benzene rings in PCN-46a can slightly expand the pore sizes with no aromatic moieties, resulting in lower CH4 affinity and uptake at 5 bar. Therefore, PCN-46a shows the lowest low-pressure CH4 uptake among these three MOFs, thus affording the highest volumetric working capacity.
We have also studied the CO2 and H2 uptake properties of ZJU-105a and PCN-46a over wide temperature and pressure ranges (Fig. S18–S21, ESI†). ZJU-105a and PCN-46a exhibit high total volumetric H2 storage capacities of 48 g L−1 (6.78 wt%) and 50 g L−1 (7.47 wt%) at 65 bar and 77 K, meeting the DOE’s targets of 40 g L−1 (5.5 wt%), albeit at 77 K rather than at ambient temperature. These volumetric values are comparable to those of some of the benchmark MOFs (Table S4, ESI†). The volumetric and gravimetric working capacities of PCN-46a, calculated from (Tmin = 77 K, Pmax = 65 bar) to (Tmax = 160 K, Pmin = 5 bar), are 47.4 g L−1 and 7.1 wt%, rivaling the best-performing material IRMOF-20 (47 g L−1 and 8.4 wt%).17 In addition, ZJU-105a and PCN-46a also exhibit high volumetric CO2 storage capacities of 328 and 309 cm3 (STP) cm−3 (Table S5, ESI†). Finally, we calculated the Qst values of H2 and CO2 for both MOFs. As shown in Fig. S25–S30 (ESI†), the Qst values of ZJU-105a for both H2 and CO2 are much higher than those of PCN-46a, consistent with those for CH4, further confirming that PCN-46a has a weaker interaction with various gas molecules.
In summary, we herein realized that the systematical tuning of the pore size and chemistry in a series of isomorphic MOFs based on NOTT-102 can effectively regulate the methane storage and working capacities. The use of alkynyl and naphthalene replacing phenyl in the NOTT-102 backbone has enabled a gradually decreased low-pressure CH4 uptake from ZJU-105a to PCN-46a, combined with a comparable total storage capacity at high pressure. PCN-46a thus exhibits the highest working capacity of 203 cm3 (STP) cm−3 (at 5–80 bar and 298 K) among the isomorphic MOFs, making it among the best performing porous materials for CH4 storage. This work may provide some guidance for the development of new MOFs with common alkynyl connectivity instead of phenyl to reduce low-pressure CH4 absorption and thus to improve the volumetric working capacity.
Supplementary Material
Acknowledgments
This research was supported by the “National 1000 Young Talent Program”, the “Zhejiang University 100 Talent Program”, and the National Natural Science Foundation of China (51803179).
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic supplementary information (ESI) available: Synthesis, PXRD, sorption data, NMR, optical images and structural data for PCN-46 and ZJU-105. CCDC 1943325. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc06239d
Notes and references
- 1.He Y, Zhou W, Qian G and Chen B, Chem. Soc. Rev, 2014, 43, 5657. [DOI] [PubMed] [Google Scholar]
- 2.(a) Furukawa H, Cordova KE, O’Keeffe M and Yaghi OM, Science, 2013, 341, 1230444; [DOI] [PubMed] [Google Scholar]; (b) Li B, Wen H-M, Cui Y, Zhou W, Qian G and Chen B, Adv. Mater, 2016, 28, 8819. [DOI] [PubMed] [Google Scholar]
- 3.(a) Wang H and Li J, Acc. Chem. Res, 2019, 52, 1968; [DOI] [PubMed] [Google Scholar]; (b) Nugent P, Belmabkhout Y, Burd SD, Cairns AJ, Luebke R, Forrest K, Pham T, Ma S, Space B, Wojtas L, Eddaoudi M and Zaworotko M, Nature, 2013, 495, 80; [DOI] [PubMed] [Google Scholar]; (c) Wang H, Zhu Q-L and Xu Q, Chem, 2017, 2, 52; [Google Scholar]; (d) Li B, Wen H-M, Yu Y, Cui Y, Zhou W, Chen B and Qian G, Mater. Today Nano, 2018, 2, 21; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wen H-M, Liao C, Li L, Wu H, Zhou W, Hu J and Chen B, J. Mater. Chem. A, 2019, 7, 3128. [Google Scholar]
- 4.(a) Peng Y, Krungleviciute V, Eryazici I, Hupp JT, Farha OK and Yildirim T, J. Am. Chem. Soc, 2013, 135, 11887; [DOI] [PubMed] [Google Scholar]; (b) Mason JA, Veenstra M and Long JR, Chem. Sci, 2014, 5, 32; [Google Scholar]; (c) Makal TA, Li J-R, Lu W and Zhou H-C, Chem. Soc. Rev, 2012, 41, 7761; [DOI] [PubMed] [Google Scholar]; (d) Li B, Wen H-M, Zhou W, Xu JQ and Chen B, Chem, 2016, 1, 557. [Google Scholar]
- 5.(a) Hulvey Z, Vlaisavljevich B, Mason JA, Tsivion E, Dougherty TP, Bloch ED, Head-Gordon M, Smit B, Long JR and Brown CM, J. Am. Chem. Soc, 2015, 137, 10816; [DOI] [PubMed] [Google Scholar]; (b) Chen C-X, Wei Z-W, Jiang J-J, Zheng S-P, Wang H-P, Qiu Q-F, Cao C-C, Fenske D and Su C-Y, J. Am. Chem. Soc, 2017, 139, 6034; [DOI] [PubMed] [Google Scholar]; (c) Zhang M, Zhou W, Pham T, Forrest KA, Liu W, He Y, Wu H, Yildirim T, Chen B, Space B, Pan Y, Zaworotko MJ and Bai J, Angew. Chem., Int. Ed, 2017, 56, 11426. [DOI] [PubMed] [Google Scholar]
- 6.(a) Wu H, Zhou W and Yildirim T, J. Am. Chem. Soc, 2009, 131, 4995; [DOI] [PubMed] [Google Scholar]; (b) Ma S, Sun D, Simmons JM, Collier CD, Yuan D and Zhou H-C, J. Am. Chem. Soc, 2008, 130, 1012. [DOI] [PubMed] [Google Scholar]
- 7.(a) Liang C-C, Shi Z-L, He C-T, Tan J, Zhou H-D, Zhou H-L, Lee Y and Zhang Y-B, J. Am. Chem. Soc, 2017, 139, 13300; [DOI] [PubMed] [Google Scholar]; (b) Tran LD, Feldblyum JI, Wong-Foy AG and Matzger AJ, Langmuir, 2015, 31, 2211; [DOI] [PubMed] [Google Scholar]; (c) Li L, Bell JG, Tang S, Lv X, Wang C, Xing Y, Zhao X and Thomas KM, Chem. Mater, 2014, 26, 4679; [Google Scholar]; (d) Tian T, Zeng Z, Vulpe D, Casco ME, Divitini G, Midgley PA, Silvestre-Albero J, Tan JC, Moghadam PZ and Fairen-Jimenez D, Nat. Mater, 2018, 17, 174. [DOI] [PubMed] [Google Scholar]
- 8.(a) Mason JA, Oktawiec J, Taylor MK, Hudson MR, Rodriguez J, Bachman JE, Gonzalez MI, Cervellino A, Guagliardi A, Brown CM, Llewellyn PL, Masciocchi N and Long JR, Nature, 2015, 527, 357; [DOI] [PubMed] [Google Scholar]; (b) Yang Q-Y, Lama P, Sen S, Lusi M, Chen KJ, Gao W-Y, Shivanna M, Pham T, Hosono N, Kusaka S, Perry J. J. t., Ma S, Space B, Barbour LJ, Kitagawa S and Zaworotko MJ, Angew. Chem., Int. Ed, 2018, 57, 5684; [DOI] [PubMed] [Google Scholar]; (c) Kundu T, Shah BB, Bolinois L and Zhao D, Chem. Mater, 2019, 31, 2842. [Google Scholar]
- 9.(a) Alezi D, Belmabkhout Y, Suyetin M, Bhatt PM, Weseliński J, Solovyeva V, Adil K, Spanopoulos I, Trikalitis PN, Emwas A-H and Eddaoudi M, J. Am. Chem. Soc, 2015, 137, 13308; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tu B, Diestel L, Shi Z-L, Bandara WRLN, Chen Y, Lin W, Zhang Y-B, Telfer SG and Li Q, Angew. Chem., Int. Ed, 2019, 131, 5402; [DOI] [PubMed] [Google Scholar]; (c) Wilmer CE, Farha OK, Yildirim T, Eryazici I, Krungleviciute V, Sarjeant AA, Snurr RQ and Hupp JT, Energy Environ. Sci, 2013, 6, 1158. [Google Scholar]
- 10.(a) Gándara F, Furukawa H, Lee S and Yaghi OM, J. Am. Chem. Soc, 2014, 136, 5271; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Peng Y, Srinivas G, Wilmer CE, Eryazici I, Snurr RQ, Hupp JT, Yildirim T and Farha OK, Chem. Commun, 2013, 49, 2992; [DOI] [PubMed] [Google Scholar]; (c) Yuan D, Zhao D, Sun D and Zhou H-C, Angew. Chem., Int. Ed, 2010, 49, 5357. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Wen H-M, Wang H, Wu H, Tyagi M, Yildirim T, Zhou W and Chen B, J. Am. Chem. Soc, 2014, 136, 6207. [DOI] [PubMed] [Google Scholar]
- 12.Yan Y, Kolokolov DI, Silva I. d., Stepanov AG, Blake AJ, Dailly A, Manuel P, Tang CC, Yang S and Schröder M, J. Am. Chem. Soc, 2017, 139, 13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang J, Furukawa H, Zhang Y-B and Yaghi OM, J. Am. Chem. Soc, 2016, 138, 10244. [DOI] [PubMed] [Google Scholar]
- 14.(a) Wen H-M, Li B, Li L, Lin R-B, Zhou W, Qian G and Chen B, Adv. Mater, 2018, 30, 1704792; [DOI] [PubMed] [Google Scholar]; (b) He Y, Zhou W, Yildirim T and Chen B, Energy Environ. Sci, 2013, 6, 2735; [Google Scholar]; (c) Yan Y, Juricek M, Coudert FX, Vermeulen NA, Grunder S, Dailly A, Lewis W, Blake AJ, Stoddart JF and Schröder M, J. Am. Chem. Soc, 2016, 138, 3371; [DOI] [PubMed] [Google Scholar]; (d) Lin X, Telepeni I, Blake AJ, Dailly A, Brown CM, Simmons JM, Zoppi M, Walker GS, Thomas KM, Mays TJ, Hubberstey P, Champness NR and Schröder M, J. Am. Chem. Soc, 2009, 131, 2159. [DOI] [PubMed] [Google Scholar]
- 15.Zhao D, Yuan D, Yakovenko A and Zhou H-C, Chem. Commun, 2010, 46, 4196. [DOI] [PubMed] [Google Scholar]
- 16.(a) Lin J-M, He C-T, Liu Y, Liao P-Q, Zhou D-D, Zhang J-P and Chen X-M, Angew. Chem., Int. Ed, 2016, 55, 4752; [DOI] [PubMed] [Google Scholar]; (b) Spanopoulos I, Tsangarakis C, Klontzas E, Tylianakis E, Froudakis G, Adil K, Belmabkhout Y, Eddaoudi M and Trikalitis PN, J. Am. Chem. Soc, 2016, 138, 1568; [DOI] [PubMed] [Google Scholar]; (c) Wu H, Simmons JM, Liu Y, Brown CM, Wang X-S, Ma S, Peterson VK, Southon PD, Kepert CJ, Zhou H-C, Yildirim T and Zhou W, Chem. – Eur. J, 2010, 16, 5205. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed A, Liu Y, Purewal J, Tran LD, Wong-Foy AG, Veenstra M, Matzger AJ and Siegel DJ, Energy Environ. Sci, 2017, 10, 2459. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.