Key Points
Question
What are perceptions of the coronavirus disease 2019 (COVID-19) health risks among ophthalmic patients, and are they associated with patients’ attendance to time-sensitive appointments?
Findings
In this nonvalidated telephone survey of 348 patients at high risk of both reversible and irreversible vision loss from lapses in care, concern regarding COVID-19 exposure during eye clinic visits and diabetic retinopathy diagnosis (vs exudative age-related macular degeneration) were associated with loss to follow-up.
Meaning
This nonvalidated survey suggests that fear of COVID-19 exposure is associated with patients missing ophthalmic care appointments, suggesting the potential importance of clearly conveying infection-control measures.
This survey study uses data from a random sample of patients from 2 tertiary eye care centers to investigate the association of missed ophthalmic appointments with patient fear of contracting the coronavirus disease 2019 virus, SARS-CoV-2.
Abstract
Importance
Patient perceptions regarding the risks of obtaining in-person ophthalmic care during the coronavirus disease 2019 (COVID-19) pandemic may affect adherence to recommended treatment plans and influence visual outcomes. A deeper understanding of patient perspectives will inform strategies to optimize adherence with vision-preserving therapies.
Objective
To evaluate perceptions of COVID-19 exposure risk and their association with appointment attendance among patients at high risk of both reversible and irreversible vision loss from lapses in care.
Design, Setting, and Participants
This survey study included a nonvalidated telephone survey designed in April and May of 2020 and a retrospective medical record review conducted in parallel with survey administration from May 22 to August 18, 2020. Participants were recruited from 2 tertiary eye care centers (Emory Eye Center in Atlanta, Georgia, and W.K. Kellogg Eye Center in Ann Arbor, Michigan). The study included a random sample of patients with diagnoses of exudative age-related macular degeneration (AMD) or diabetic retinopathy (DR) who received an intravitreal injection between January 6 and March 13, 2020, and were scheduled for a second injection between March 13 and May 6, 2020.
Main Outcomes and Measures
Association between perceptions regarding COVID-19 risks and loss to follow-up.
Results
Of 1004 eligible patients, 423 (42%) were successfully contacted, and 348 (82%) agreed to participate (participants’ mean [SD] age, 75 [12] years; 195 women [56%]; 287 White [82%] patients). Respondents had a mean (SD) of 2.7 (1.1) comorbidities associated with severe COVID-19, and 77 (22%) knew someone with COVID-19. Of all respondents, 163 (47%) were very concerned or moderately concerned about vision loss from missed treatments during the pandemic. Although 208 (60%) believed the COVID-19 virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), exposure at the eye clinic was extremely unlikely or unlikely, 49 (14%) believed it was extremely likely or likely. Seventy-eight participants (22%) were lost to follow-up. Concern regarding COVID-19 exposure during clinic visits (odds ratio [OR], 3.9; 95% CI, 1.8-8.4) and diagnosis of DR (vs AMD) (OR, 8.130; 95% CI, 3.367-20.408) were associated with an increase in likelihood of loss to follow-up.
Conclusions and Relevance
Among patients at high risk for vision loss from lapses in care, many expressed concerns regarding the effect of the pandemic on their ability to receive timely care. Survey results suggest that fear of SARS-CoV-2 exposure was associated with a roughly 4-fold increase in the odds of patient loss to follow-up. These results support the potential importance of clearly conveying infection-control measures.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic initially reduced patient volumes by 79% in outpatient eyecare centers across the US, the largest decline of any specialty.1 Volume has since rebounded, but ophthalmologists continued to experience substantial cumulative visit deficits into the summer of 2020.2 In large part, volume was reduced owing to clinicians canceling routine visits in accordance with American Academy of Ophthalmology COVID-19 recommendations.3 There has, however, been an alarming trend of patients not attending retained appointments deemed time-sensitive by their clinician.4 This situation is consistent with larger trends across health care services.5 Anticipating adverse outcomes, public health experts have called for further study into the barriers to health care access during this unprecedented time.6,7,8
Much of retinal care involves administration of in-person, time-sensitive interventions, such as intravitreal injections for exudative age-related macular degeneration (AMD). Emerging data have already demonstrated increased vision-threatening submacular hemorrhage rates in patients with AMD during the pandemic.9 Vision loss from such events not only affects vision-related quality of life but can more broadly affect other health outcomes.10
On the other hand, patients with retinal disease, such as AMD and diabetic retinopathy (DR), are at high risk for severe COVID-19, owing to age and comorbidities. Worldwide, ophthalmologists have thus faced difficult decisions in selecting which patients should visit the clinic for time-sensitive therapy. Over time, clinicians have reached some consensus regarding the importance of continuing retinal care while using best practices to mitigate COVID-19 transmission risk.4 However, there has been limited assessment of patient perspectives. Treatment decisions, which ostensibly intend to provide the best possible patient care, have been made without fully accounting for patient-centered views on the competing risks of vision loss and virus exposure.
This study, performed at 2 geographically distant clinical sites, aimed to improve our understanding of patient attitudes. We surveyed individuals at a high risk of vision loss from missed appointments: those receiving regular intravitreal injections for either exudative AMD or vision-threatening complications of DR. Additionally, we explored whether their attitudes were associated with adherence to recommended treatment regimens. A deeper understanding of patients’ perspectives will inform our strategies to optimize adherence with vision-preserving treatment protocols during the pandemic.
Methods
This survey study included a nonvalidated telephone survey and medical record review conducted at Emory University and the University of Michigan and was approved by each institution’s institutional review board. Patient information was gathered and secured in accordance with the Health Insurance Portability and Accountability Act. All data were deidentified before being shared between institutions. None of these data have been reported previously. Verbal informed consent was obtained from all participants, and the research adhered to the tenets of the Declaration of Helsinki. Patients did not receive compensation or incentives to participate. This study followed the American Association for Public Opinion Research (AAPOR) reporting guideline.
Participant Selection
Local billing databases and electronic health records were queried for patients with exudative AMD (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] H35.32*) or DR (ICD-10 E10.3* or E11.3*) who received an intravitreal injection (Current Procedural Terminology code 67028) at Emory Eye Center or University of Michigan W.K. Kellogg Eye Center between January 6 and March 13, 2020. Health records for 1252 patients were selected at random for review. Of these, 1004 patients (80%) scheduled for a second injection between March 13 and May 6, 2020, were deemed eligible and were called. We intended for a 2:1 patient ratio of AMD to DR. Because this was the first study of this type to our knowledge, there was insufficient background data to perform power calculations. Patients with exudative AMD, being most sensitive to small delays in treatment, were prioritized for inclusion.
Survey Design and Administration
A nonvalidated 13-item telephone survey was developed from April 9 to May 6, 2020, by a team of ophthalmologists across both institutions, including the input of an expert in survey design and administration (P.N.-C.) as well as retina specialists with experience conducting survey research (R.C.R. and N.J.). The survey was piloted on individuals unrelated to the study, face validity was evaluated, and clarifications were made based on feedback.
The survey included questions regarding living setting, degree of concern regarding vision loss due to missed appointments, degree of concern regarding COVID-19 exposure at the eye clinic, employment status, and education level (eFigure in the Supplement). The survey incorporated 2 questions from the National Eye Institute Visual Function Questionnaire 25 regarding patients’ perceptions of their general health and eyesight.11 It also included a time trade-off question for utility assessment to be reported in a subsequent article.
The survey was conducted from May 22 to June 14, 2020, at Emory and from June 30 to August 18, 2020, at Kellogg. Medical students administering the survey were provided a detailed protocol and script including exact phrasing for question and answer options, alternate phrasing for clarification when needed, and how to frame and introduce questions. They practiced and attained proficiency before interviewing participants.
Retrospective Medical Record Review
Demographic information, visual acuity (VA), presence of risk factors for severe COVID-19 infection (older than 65 years, diabetes, respiratory disease, immunosuppression, hypertension, cardiovascular disease, and end-stage kidney disease), clinic location, intravitreal injection regimen, and loss to follow-up (LTFU) status were extracted from the electronic health records from the most recent clinic encounter. This medical record review was performed immediately after survey administration. A patient was defined as LTFU if they canceled or did not attend their appointment and did not have a follow-up visit within 14 days. Clinician-initiated visit cancelations were not classified as LTFU.
Statistical Methods
Descriptive statistics were used to summarize participant characteristics using Microsoft Excel (Microsoft Corp). Stated 95% CIs assumed a normal distribution.
The association of each covariate with LTFU was assessed using multivariate logistic regression analysis using GraphPad Prism, version 8.4 (GraphPad Software). Given the limitations inherent in performing regression analyses with ordinal Likert-item variables, Likert responses were consolidated and binarized for the analysis into prespecified response categories deemed a priori to be clinically meaningful by the investigators (eFigure in the Supplement). The multivariate model was adjusted for age, sex, diagnosis (AMD or DR), institution, and additional covariates associated with either the LTFU outcome a P < .10 in the final model or that accounted for a greater than 20% effect on the parameter estimate for another covariate. Variable selection was performed through an iterative process as previously described.12 A sensitivity analysis separately evaluated survey responses and the LTFU outcome among Black and Latino respondents.
Results
Of 1004 eligible patients, 423 (42%) were successfully contacted and 348 (82%) agreed to participate (mean [SD] age, 75 [12] years; 195 women [56%]; 287 White patients [82%]). A total of 238 participants (68.4%) had AMD and 110 (31.6%) had DR. The AMD:DR patient ratio was 2.16:1 rather than the intended 2:1 owing to variability in who consented to participate. The median interval between each participant’s last visit and their survey date was 30 days (interquartile range [IQR], 12-71 days) (Table 1). A total of 311 respondents (89.4%) lived independently (rather than in assisted living or a nursing home). Seventy-seven (22%) knew someone with COVID-19, with a greater proportion among those with DR (37 of 110 [34%]) than those with AMD (40 of 238 [17%]) (proportion difference [PD], 17%; 95% CI, 7%-27%).
Table 1. Participant Demographic and Clinical Characteristics.
| Variable | No. (%)a | ||||
|---|---|---|---|---|---|
| All (N = 348) | Site | Condition | |||
| Emory (n = 226) | Kellogg (n = 122) | AMD (n = 238) | DR (n = 110) | ||
| Age, y | |||||
| Mean (SD) | 75 (12) | 74 (13) | 75 (12) | 80 (8) | 62 (11) |
| Median | 77 | 78 | 76 | 81 | 62 |
| Women | 195 (56) | 127 (56) | 68 (56) | 143 (60) | 52 (47) |
| Race/ethnicity | |||||
| White | 287 (82) | 173 (77) | 114 (94) | 223 (94) | 64 (58) |
| Black | 40 (12) | 38 (17) | 2 (2) | 6 (3) | 34 (31) |
| Latino | 7 (2) | 6 (3) | 1 (1) | 2 (1) | 5 (5) |
| Asian | 5 (1) | 2 (1) | 3 (2) | 5 (2) | 0 |
| Currently employed | 60 (17) | 45 (20) | 15 (12) | 20 (8) | 40 (36) |
| Education level | |||||
| Did not finish high school | 17 (5) | 13 (6) | 4 (3) | 14 (6) | 3 (3) |
| Finished high school or some college | 146 (42) | 101 (45) | 45 (37) | 94 (39) | 52 (47) |
| Finished college | 95 (27) | 52 (23) | 43 (35) | 65 (27) | 30 (27) |
| Advanced degree | 90 (26) | 60 (27) | 30 (25) | 65 (27) | 25 (23) |
| Living setting | |||||
| Independently | 311 (89) | 200 (88) | 111 (91) | 211 (89) | 100 (91) |
| Assisted living | 19 (6) | 15 (7) | 4 (3) | 18 (8) | 1 (1) |
| Nursing home | 0 | 0 | 0 | 0 | 0 |
| Living situation | |||||
| Alone | 97 (28) | 63 (28) | 34 (28) | 76 (32) | 21 (19) |
| With partner | 193 (55) | 119 (53) | 74 (60) | 132 (55) | 61 (55) |
| With children but not partner | 34 (10) | 29 (13) | 5 (4) | 20 (8) | 14 (13) |
| Other | 24 (7) | 15 (7) | 9 (7) | 10 (4) | 14 (13) |
| Subjective overall health | |||||
| Excellent or very good | 128 (37) | 90 (40) | 38 (31) | 108 (45) | 20 (18) |
| Good | 128 (37) | 78 (35) | 50 (41) | 80 (34) | 48 (44) |
| Fair or poor | 91 (26) | 58 (25) | 34 (28) | 50 (21) | 42 (38) |
| Personally know someone infected with COVID-19 | 77 (22) | 48 (21) | 29 (24) | 40 (17) | 37 (34) |
| No. of comorbidities | |||||
| Mean (SD) | 2.7 (1.1) | 2.7 (1.1) | 2.7 (1.1) | 2.6 (1.1) | 2.9 (1.2) |
| Median | 3 | 3 | 3 | 3 | 3 |
| Subjective corrected eyesight | |||||
| Excellent or good | 138 (40) | 82 (36) | 56 (46) | 96 (40) | 42 (38) |
| Fair | 139 (40) | 94 (42) | 45 (37) | 89 (37) | 50 (45) |
| Poor, very poor, or blind | 71 (20) | 50 (22) | 21 (17) | 53 (22) | 18 (16) |
| logMAR VA | |||||
| Mean (SD), (Snellen) | 0.55 (0.61), (20/71) | 0.62 (0.66), (20/83) | 0.44 (0.50), (20/55) | 0.60 (0.63), (20/80) | 0.45 (0.55), (20/56) |
| Median, (Snellen) | 0.3, (20/40) | 0.4, (20/50) | 0.3, (20/40) | 0.4, (20/50) | 0.3, (20/40) |
| Injection interval | |||||
| Mean (SD) | 6.8 (2.6) | 6.3 (2.3) | 7.6 (2.9) | 7.1 (2.6) | 6.1 (2.3) |
| Median | 6 | 6 | 7 | 7 | 6 |
| Lost to follow-up | 78 (22) | 56 (25) | 22 (18) | 46 (19) | 32 (29) |
Abbreviations: AMD, age-related macular degeneration; COVID-19, coronavirus disease 2019; DR, diabetic retinopathy; VA, visual acuity.
Values written as No. (%) unless otherwise specified. Counts do not all equal 348 (100%) owing to missing responses in some categories.
Demographic characteristics were similar between institutions except for proportionally more White participants in the Kellogg cohort (114 of 122 [94%]) than the Emory cohort (173 of 226 [77%]) (PD, 17%; 95% CI, 10%-24%). Compared with those with DR, respondents with AMD were more likely to be White (94% vs 58%; PD, 36%; 95% CI, 26%-45%), women (60% vs 47%; PD, 13%; 95% CI, 2%-24%), have fewer COVID-19 risk factor comorbidities (2.6 vs 2.9; mean absolute difference, 0.3; 95% CI, 0.04-0.56), and have worse mean VA (logMAR [Snellen] 0.60 [20/80] vs 0.45 [20/56]; mean absolute difference, 0.15; 95% CI, 0.03-0.29).
Among all participants, 91 (26%) reported fair or poor overall health, and medical record review revealed a mean (SD) of 2.7 (1.1) COVID-19 risk factor comorbidities. Mean (SD) VA was 0.55 (0.61) logMAR (Snellen equivalent, 20/71), and 210 (60%) respondents reported fair, poor, or very poor eyesight.
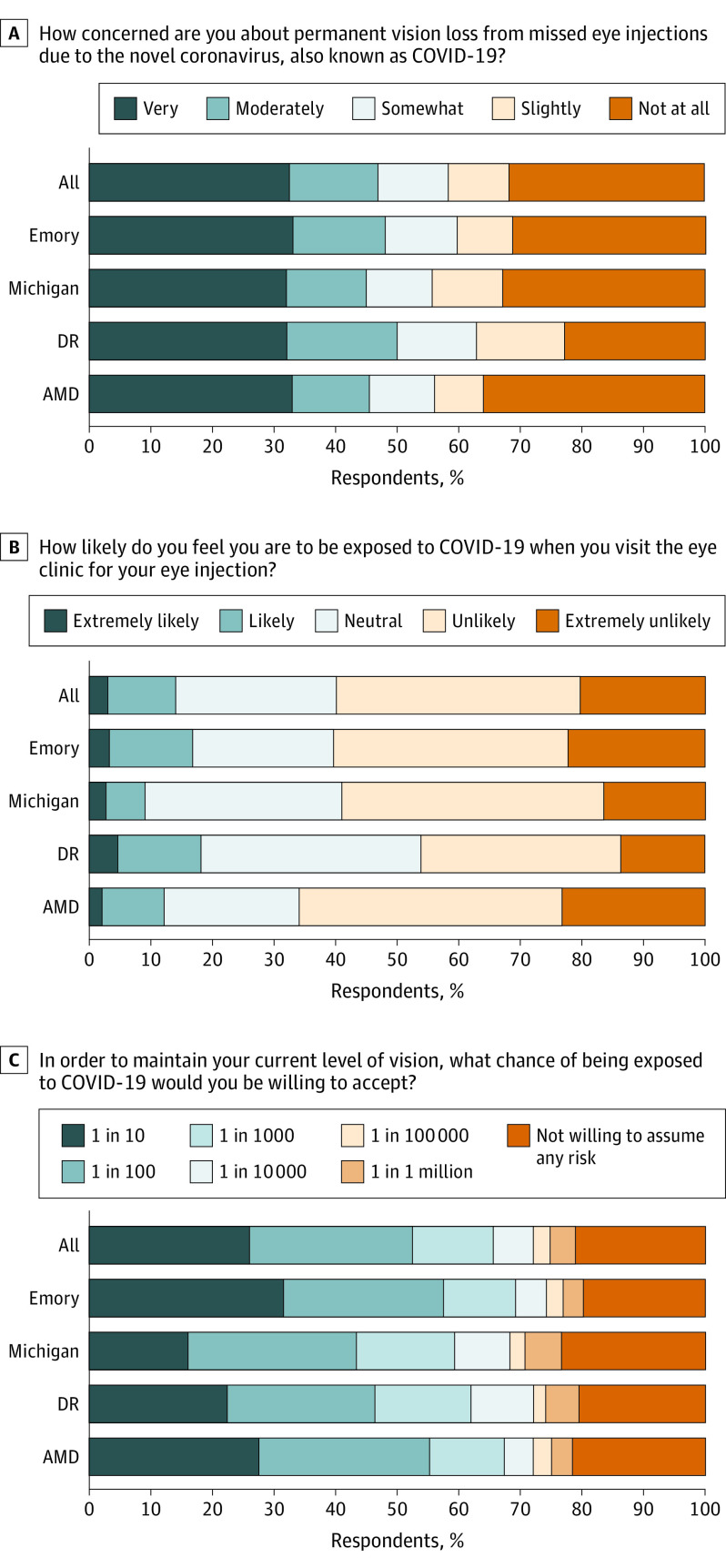
One hundred sixty-three respondents (47%) were very or moderately concerned about vision loss from missed injections during the pandemic (Figure), with similar rates among all subgroups (Table 2). Although 208 patients (60%) believed they were unlikely or extremely unlikely to be exposed to COVID-19 at an eye clinic visit, 49 (14%) believed it was extremely likely or likely. One hundred seventy-nine (51%) patients across both sites would undertake a 1:10 or 1:100 risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure to maintain their current vision, although 95 patients (27%) would only assume a 1:100 000 or lower maximal risk (Table 2).
Figure. Patient Perspectives Regarding the Effect of the Coronavirus Disease 2019 (COVID-19) Pandemic on Their Ophthalmic Care.
Stacked bar charts show the frequency distribution of responses to 3 Likert-style survey questions in panels A, B, and C, assessing patient perspectives on the effect of the COVID-19 pandemic on their ophthalmic care. Responses to each question are also displayed by clinical site (Emory Eye Center, W.K. Kellogg Eye Center) and disease subgroup. Missing data were excluded from response frequency calculations for this figure. AMD indicates age-related macular degeneration; DR, diabetic retinopathy.
Table 2. Participant Perceptions Regarding Health Risks During the COVID-19 Pandemic.
| Variable | All (n = 348) | Site | P value for Emory vs Kellogg | Condition | P value for AMD vs DR | ||
|---|---|---|---|---|---|---|---|
| Emory (n = 226) | Kellogg (n = 122) | AMD (n = 238) | DR (n = 110) | ||||
| Concern of permanent vision loss due to missed appointments from pandemic, No. (%) | |||||||
| Very or moderately concerned | 163 (47) | 108 (48) | 55 (45) | .62a | 108 (45) | 55 (50) | .27a |
| Somewhat concerned | 39 (11) | 26 (12) | 13 (11) | 25 (11) | 14 (13) | ||
| Slightly or not at all concerned | 145 (42) | 91 (40) | 54 (44) | 104 (44) | 41 (37) | ||
| Perceived likelihood of being exposed to COVID-19 at eye clinic, No. (%) | NA | NA | NA | NA | NA | NA | .001a |
| Extremely likely or likely | 49 (14) | 38 (17) | 11 (9) | .85a | 29 (12) | 20 (18) | .13b |
| Neutral | 91 (26) | 52 (23) | 39 (32) | 52 (22) | 39 (35) | .007b | |
| Unlikely or extremely unlikely | 208 (60) | 136 (60) | 72 (59) | 157 (66) | 51 (46) | .001b | |
| Risk of exposure would be willing to accept to maintain vision, No. (%) | NA | NA | NA | .006a | NA | NA | NA |
| 1 in 10 or 1 in 100 | 179 (51) | 127 (57) | 52 (43) | .016b | 129 (54) | 50 (45) | .27a |
| 1 in 1000 or 1 in 10 000 | 67 (19) | 37 (17) | 30 (25) | .06b | 39 (16) | 28 (25) | |
| 1 in 100 000 or 1 in 1 000 000 | 23 (7) | 13 (6) | 10 (8) | .38b | 15 (6) | 8 (7) | |
| Not willing to assume any risk | 72 (21) | 44 (20) | 28 (23) | .44b | 50 (21) | 22 (20) | |
Abbreviations: AMD, age-related macular degeneration; COVID-19, coronavirus disease 2019; DR, diabetic retinopathy; NA, not applicable.
Mann-Whitney U test used.
χ2 Test for independence used to compare individual responses.
In total, 56 (25%) and 22 (18%) patients from Emory and Kellogg, respectively, were LTFU (PD, 8%; 95% CI, –2% to 16%). Concern regarding COVID-19 exposure had an association with LTFU (odds ratio [OR], 3.9; 95% CI, 1.8-8.4) (Table 3). Diagnosis of DR rather than AMD (OR, 8.1; 95% CI, 3.4-20.4), White race/ethnicity (OR, 4.0; 95% CI, 1.6-11.1), older age (OR, 1.1 for each year of age; 95% CI, 1.03-1.1), and fair or poor self-reported overall health (OR, 2.4; 95% CI, 1.3-4.5) were associated with increased odds of LTFU, whereas living independently (OR, 0.3; 95% CI, 0.1-0.7) was associated with decreased odds of LTFU.
Table 3. Univariate and Multivariate Results Assessing Correlation With Loss to Follow-up.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.021 (0.999-1.044) | .07 | 1.065 (1.029-1.105) | <.001 |
| White race/ethnicity | 1.391 (0.708-2.950) | .36 | 4.006 (1.587-11.055) | .005 |
| Women | 0.828 (0.499-1.375) | .46 | 0.841 (0.465-1.524) | .57 |
| Live independently (vs assisted living) | 0.288 (0.142-0.588) | <.001 | 0.296 (0.129-0.678) | <.001 |
| Live alone | 1.749 (1.018-2.977) | .04 | 1.831 (0.974-3.429) | .06 |
| Finished college or completed advanced degree | 0.613 (0.367-1.017) | .06 | NA | NA |
| Personally know someone infected with COVID-19 | 0.971 (0.516-1.756) | .92 | NA | NA |
| Location (Emory) | 1.506 (0.878-2.657) | .15 | 1.678 (0.899-3.220) | .11 |
| Received treatment at satellite clinic | 1.160 (0.671-1.974) | .59 | NA | NA |
| Self-reported fair or poor health | 2.249 (1.310-4.055) | .003 | 2.417 (1.293-4.512) | .006 |
| No. of comorbidities | 1.438 (1.144-1.821) | .002 | NA | NA |
| Treatment indication of DR (vs AMD) | 1.704 (1.006-2.865) | .046 | 8.130 (3.367-20.408) | <.001 |
| Treated eye ≥3 lines better vision than fellow eye, or treated in both eyes | 0.773 (0.464-1.280) | .32 | NA | NA |
| Self-reported fair, poor, or very poor vision | 2.266 (1.310-4.055) | .004 | NA | NA |
| Very or moderately concerned for permanent vision loss due to missed appointments from pandemic | 1.607 (0.969-2.684) | .07 | 1.755 (0.969-3.219) | .07 |
| Perceived extremely likely or likely to be exposed to COVID-19 at eye clinic | 4.338 (2.300-8.200) | <.001 | 3.900 (1.831-8.403) | <.001 |
| Willing to accept a maximum of 1:100 000 or less risk of exposure to maintain vision | 1.220 (0.700-2.089) | .47 | NA | NA |
Abbreviations: AMD, age-related macular degeneration; COVID-19, coronavirus disease 2019; DR, diabetic retinopathy; NA, not applicable; OR, odds ratio.
Among 47 respondents self-identifying as Black or Latino, 9 (19%) were LTFU as compared with 67 of 287 (23%) White respondents (PD, −4%; 95% CI, −16% to 8%). Fifteen Black or Latino respondents (32%), compared with 58 White respondents (20%), knew someone with COVID-19 (PD, 12%; 95% CI, –2% to 26%). Twenty-three Black respondents (49%), compared with 134 White respondents (47%), were very or moderately concerned about vision loss from missed injections during the pandemic (PD, 2%; 95% CI, −13% to 18%), and 15 (32%) compared with 31 White respondents (11%) felt they were extremely likely or likely to be exposed to COVID-19 during eye clinic visits (PD, 21%; 95% CI, 7%-35%). In this subgroup, the adjusted OR for the association of perceived high exposure risk on the LTFU outcome was 2.0 (95% CI, 0.33-12.25).
Discussion
Since the onset of the COVID-19 pandemic, ophthalmologists have grappled with whether and how to continue administering time-sensitive, vision-preserving care in the face of potentially lethal SARS-CoV-2 transmission events. This study adds the patient perspective to the discourse. We found that a large proportion of patients were concerned the pandemic would affect their retinal care, and most were willing to assume some risk of COVID-19 exposure in order to maintain continuity of care. However, a subset of patients felt they were likely or extremely likely to be exposed to SARS-CoV-2 during clinic visits for intravitreal injections, and fear of exposure was associated with markedly increased odds of LTFU. These findings have important public health implications and highlight potential avenues to address in order to avoid suboptimal visual outcomes.
Nearly half (163 of 348 [47%]) of respondents were very or moderately concerned they would incur permanent vision loss from missed injections during the pandemic (Figure). At the same time, 49 respondents (14%) perceived that routine eye clinic visits were risky, believing they were likely or extremely likely to be exposed to COVID-19 during them. This proportion was markedly higher (32%) among respondents identifying as Black or Latino. Weighing these competing risks, over half of respondents (179 of 348 [51%]) were willing to assume odds of 1:100 or even higher of SARS-CoV-2 exposure during clinic visits to maintain their current vision.
Seventy-eight participants (22%) were LTFU. Of all covariates assessed, diagnosis of DR (vs AMD), White race/ethnicity, and patient perception of high exposure risk during clinic visits were associated with the greatest increase in odds of LTFU. Of these covariates, only patient exposure risk perception represents a potentially modifiable factor. The strong association between exposure risk concern and LTFU has important implications for how we communicate our risk mitigation strategies.
Older age and subjectively worse health status were also associated with increased LTFU. Interestingly, objective health status assessment by number of high-risk comorbidities for COVID-19 had no such association. Additionally, although 77 respondents (22%) personally knew someone affected by COVID-19, this was not associated with LTFU.
Responses were similar between Emory and Kellogg, despite many differences between the sites. These clinics are not only geographically distant, but also had differing experiences with the pandemic. The Detroit, Michigan, metropolitan region, near Kellogg, experienced an initial surge that overwhelmed health systems. Michigan residents were under stay-at-home orders over twice as long as Georgia residents, and there were higher rates of COVID-19 prevalence in Michigan than Georgia during survey administration (828 per 100 000 during July-August in Michigan; 450 per 100 000 during May-June in Georgia).13,14,15
One year into the COVID-19 pandemic, we have a better understanding regarding transmission dynamics. It is unlikely we can assure zero risk of COVID-19 exposure in health care facilities, and we cannot control potential exposures during patient transportation to a facility. However, emerging data suggest that with appropriate precautions, risk of transmission within health care facilities is low.16 In 1 study, there were no documented transmission events among 142 patients and 11 staff members exposed to a symptomatic COVID-19–positive retina specialist in an outpatient clinic.17 These findings mirror those from a widely publicized study of 2 symptomatic COVID-19–positive hair stylists, in which none of their 139 clients developed COVID-19 in the setting of a universal mask policy.18 Additional studies across multiple sites suggest low transmission rates in COVID-19 hospital wards with the use of appropriate safety measures.16
Given these data, we believe it is imperative for health care professionals to adopt and display best practices for infection control. Such practices have been enumerated by bodies such as the Centers for Disease Control and Prevention19 and the American Academy of Ophthalmology20 and include prescreening of patients, clinicians, and staff for symptoms or exposures to COVID-19; optimizing ventilation; universal masking; physical distancing; robust hand and equipment sanitation protocols; and a reduction in clinical volume, patient contacts, and the time patients spend in the clinic.
While adopting such practices, it is important for health care professionals to communicate these efforts with patients on local and national levels. The retina community has not always succeeded in delivering public health messages. A prior study demonstrated that 17% of individuals with AMD did not know that potential blindness ensues from their disease, and 83% did not know it is a chronic condition.21 Retina specialists should endeavor to convey the vision-threatening nature of these diagnoses and the need for long-term consistent management with time-sensitive therapies. In the case of COVID-19, the American Academy of Ophthalmology developed a patient-facing online resource, “Eye Health During COVID-19,” providing guidance for ophthalmic patients and specifically addressing the questions of patients with AMD.22 Additional guidelines for other types of medical care have been produced by the Centers for Disease Control and Prevention and specialty associations. Clinics should proactively disseminate this information, because many clinic patients may not routinely access such internet-based resources.
Strengths and Limitations
This study has some particular strengths. Although vaccines and COVID-19 treatments offer hope of returning to normal operations, our findings are likely to remain relevant. There is no guarantee that such innovations will eradicate disease transmission, and some believe SARS-CoV-2 may remain endemic.23 Additionally, globalization trends in recent decades have been accompanied by an increase in the frequency of pandemics.24 We believe that our findings will have relevance for similar events in the future and may also help inform health care providers in other specialties. Additionally, by querying patients with both AMD and DR at 2 separate locations with differing pandemic experiences, our findings have broadly applicable relevance.
This study has some limitations. Our understanding of COVID-19 and its transmission dynamics has grown at a rapid pace, and this study presents patient perspectives at 1 time point. However, the survey was conducted after Georgia and Michigan initiated reopening plans and at least 2 months after the March 11, 2020, World Health Organization recognition of the pandemic, allowing time for patient perspectives to evolve in parallel with increased scientific data on virus transmission and treatment.
The results of this nonvalidated telephone survey administered by medical students calling on behalf of the participant’s eye center may be subject to response, acquiescence, and social desirability biases. Additionally, although medical students were trained for the study, they are not trained interviewers. We endeavored to reduce these biases through the use of neutral language and avoidance of having the treating ophthalmologists administer surveys.
Participant numeracy may limit the inferences that can be drawn from the survey item that queried subjects regarding the odds of virus exposure they were willing to accept to maintain their vision. However, the interviewers were permitted to restate this question in multiple ways when requested. Although the wording of the survey was revised with input from individuals unrelated to the study, its clarity is limited by lack of input from the patient population studied.
When comparing outcomes among clinical sites (Emory vs Kellogg), we note some key differences among clinical characteristics between these cohorts, most prominently race/ethnicity. We have attempted to adjust for confounding by race in the multivariate regression model for the LTFU outcome.
Patients were deemed LTFU if they missed a scheduled appointment by 14 or more days. This was based on our clinical experience and literature suggesting that VA outcomes in exudative AMD may suffer from short lapses in care.25 However, there is no standardized definition of LTFU, and definitions vary widely.25 Future research should consider defining longer durations of time as LTFU. Finally, the decision to pool the ordinal Likert-item variables into prespecified groupings gave us less power to detect differences between groups.
Conclusions
The retina community has developed a consensus that it is appropriate to continue time-sensitive care during the COVID-19 pandemic. This survey study demonstrated that many patients share our concern for loss of vision from undertreatment. However, some patients feared a high risk of COVID-19 exposure during visits to the eye clinic, which has the potential to adversely affect patient adherence and visual outcomes. During uncertain times, we should proactively address unwarranted patient fears. In particular, we should communicate that the very low risk of a COVID-19 transmission event should be balanced with the risk of vision loss from treatment delays in a variety of ophthalmic pathologies. We can thus limit the collateral effect of this pandemic on patients’ ophthalmic health.
eFigure. Survey Regarding Time-Sensitive Eye Care During the COVID-19 Pandemic
References
- 1.Mehrotra A, Chernew M, Linetsky D, Hatch H, Cutler D. The impact of the COVID-19 pandemic on outpatient visits: a rebound emerges. Published May 19, 2020. Accessed July 28, 2020. https://www.commonwealthfund.org/publications/2020/apr/impact-covid-19-outpatient-visits
- 2.Mehrotra A, Chernew M, Linetsky D, Hatch H, Cutler D. The impact of the COVID-19 pandemic on outpatient visits: practices are adapting to the new normal. Published June 25, 2020. Accessed July 28, 2020. https://www.commonwealthfund.org/publications/2020/jun/impact-covid-19-pandemic-outpatient-visits-practices-adapting-new-normal
- 3.American Academy of Ophthalmology. Recommendations for urgent and nonurgent patient care. Published March 18, 2020. Accessed July 28, 2020. https://www.aao.org/headline/new-recommendations-urgent-nonurgent-patient-care
- 4.American Society of Retina Specialists . COVID-19 Survey July 14, 2020. Published July 2020. Accessed August 29, 2020. https://www.asrs.org/content/documents/covid19july14survey.pdf
- 5.Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19-related concerns—United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi: 10.15585/mmwr.mm6936a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang T. Plug COVID-19 research gaps in detection, prevention and care. Nature. 2020;583(7816):333. doi: 10.1038/d41586-020-02004-1 [DOI] [PubMed] [Google Scholar]
- 7.Elam AR, Ehrlich JR, Lee P. Insights into eye care practice during COVID-19. JAMA Ophthalmol. 2020;138(9):988-989. doi: 10.1001/jamaophthalmol.2020.3244 [DOI] [PubMed] [Google Scholar]
- 8.Mandavilli A. ‘The biggest monster’ is spreading. and it’s not the coronavirus. Published August 3, 2020. Accessed August 29, 2020. https://www.nytimes.com/2020/08/03/health/coronavirus-tuberculosis-aids-malaria.html
- 9.Romano F, Monteduro D, Airaldi M, et al. Increased number of submacular hemorrhages as a consequence of coronavirus disease 2019 lockdown. Ophthalmol Retina. 2020;4(12):1209-1210. doi: 10.1016/j.oret.2020.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morse AR, Seiple W, Talwar N, Lee PP, Stein JD. Association of vision loss with hospital use and costs among older adults. JAMA Ophthalmol. 2019;137(6):634-640. doi: 10.1001/jamaophthalmol.2019.0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD; National Eye Institute Visual Function Questionnaire Field Test Investigators . Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050-1058. doi: 10.1001/archopht.119.7.1050 [DOI] [PubMed] [Google Scholar]
- 12.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgia Department of Public Health . Georgia Department of Public Health daily status report. Accessed August 29, 2020. https://dph.georgia.gov/covid-19-daily-status-report
- 14.United States Census Bureau . QuickFacts: Georgia; Michigan. Accessed August 29, 2020. https://www.census.gov/quickfacts/fact/table/GA,MI/PST045219
- 15.Michigan.gov. Coronavirus—Michigan data. Updated January 28, 2020. Accessed August 29, 2020. https://www.michigan.gov/coronavirus/0,9753,7-406-98163_98173---,00.html
- 16.Wong SCY, Kwong RT-S, Wu TC, et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020;105(2):119-127. doi: 10.1016/j.jhin.2020.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saban O, Levy J, Chowers I. Risk of SARS-CoV-2 transmission to medical staff and patients from an exposure to a COVID-19-positive ophthalmologist. Graefes Arch Clin Exp Ophthalmol. 2020;258(10):2271-2274. doi: 10.1007/s00417-020-04790-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrix MJ, Walde C, Findley K, Trotman R. Absence of apparent transmission of SARS-CoV-2 from two stylists after exposure at a hair salon with a universal face covering policy—Springfield, Missouri, May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(28):930-932. doi: 10.15585/mmwr.mm6928e2 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention . Information for healthcare professional about coronavirus (COVID-19). Published February 11, 2020. Accessed August 12, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/index.html
- 20.American Academy of Ophthalmology. Important coronavirus updates for ophthalmologists. Published May 11, 2020. Accessed August 12, 2020. https://www.aao.org/headline/alert-important-coronavirus-context
- 21.Müller S, Ehlken C, Bauer-Steinhusen U, et al. Treatment of age-related neovascular macular degeneration: the patient’s perspective. Graefes Arch Clin Exp Ophthalmol. 2017;255(11):2237-2246. doi: 10.1007/s00417-017-3739-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seddon JM, Khurana R. Coronavirus and your macular degeneration care. Published March 27, 2020. Accessed August 12, 2020. https://www.aao.org/eye-health/tips-prevention/coronavirus-macular-degeneration-avastin-injection
- 23.Osterholm M, Moore K, Ostrowsky, J et al. COVID-19 The Center for Infectious Disease Research and Policy Viewpoint Part 1: The Future of the COVID-19 Pandemic: Lessons Learned from Pandemic Influenza. University of Minnesota; 2020. [Google Scholar]
- 24.Smith KF, Goldberg M, Rosenthal S, et al. Global rise in human infectious disease outbreaks. J R Soc Interface. 2014;11(101):20140950. doi: 10.1098/rsif.2014.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramakrishnan MS, Yu Y, VanderBeek BL. Association of visit adherence and visual acuity in patients with neovascular age-related macular degeneration: secondary analysis of the comparison of age-related macular degeneration treatment trial. JAMA Ophthalmol. 2020;138(3):237-242. doi: 10.1001/jamaophthalmol.2019.4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Survey Regarding Time-Sensitive Eye Care During the COVID-19 Pandemic