Key Points
Question
Does irradiation dose escalation to doses known to cure other cancers improve local tumor control and survival for patients with localized pancreatic cancer?
Findings
Ablative radiation therapy after induction chemotherapy in 119 patients with locally advanced unresectable pancreatic cancer was associated with a 2-year local tumor progression rate of only 32.8% and a 2-year overall survival rate of 38% from the time of irradiation.
Meaning
Hypofractionated ablative radiation therapy was associated with durable control of the primary tumor leading to favorable survival outcomes.
This cohort study examines the use of hypofractionated ablative radiation therapy for patients with locally advanced pancreatic cancer treated with a novel radiation planning and delivery technique.
Abstract
Importance
Surgical resection has been considered the only curative option for patients with pancreatic cancer. Nonoperative local treatment options that can provide a similar benefit are needed. Emerging radiation techniques that address organ motion have enabled curative radiation doses to be given in patients with inoperable disease.
Objective
To determine the association of hypofractionated ablative radiation therapy (A-RT) with survival for patients with locally advanced pancreatic cancer (LAPC) treated with a novel radiation planning and delivery technique.
Design, Setting, and Participants
This cohort study included 119 consecutive patients treated with A-RT between June 2016 and February 2019 and enrolled in a prospectively maintained database. Patients were treated with a standardized technique within a large academic cancer center regional network. All patients with localized, unresectable, or medically inoperable pancreatic cancer with tumors of any size and less than 5 cm luminal abutment with the primary tumor were eligible.
Interventions
Ablative RT (98 Gy biologically effective dose) was delivered using standard equipment. Respiratory gating, soft tissue image guidance, and selective adaptive planning were used to address organ motion and limit the dose to surrounding luminal organs.
Main Outcomes and Measures
The primary outcome was overall survival (OS). Secondary outcomes included incidence of local progression and progression-free survival.
Results
Between 2016 and 2019, 119 patients (59 men, median age 67 years) received A-RT, including 99 with T3/T4 and 53 with node-positive disease, with a median carbohydrate antigen 19-9 (CA19-9) level greater than 167 U/mL. Most (116 [97.5%]) received induction chemotherapy for a median of 4 months (0.5-18.4). Median OS from diagnosis and A-RT were 26.8 and 18.4 months, respectively. Respective 12- and 24-month OS from A-RT were 74% (95% CI, 66%-83%) and 38% (95% CI, 27%-52%). Twelve- and 24-month cumulative incidence of locoregional failure were 17.6% (95% CI, 10.4%-24.9%) and 32.8% (95% CI, 21.6%-44.1%), respectively. Postinduction CA19-9 decline was associated with improved locoregional control and survival. Grade 3 upper gastrointestinal bleeding occurred in 10 patients (8%) with no grade 4 to 5 events.
Conclusions and Relevance
This cohort study of patients with inoperable LAPC found that A-RT following multiagent induction therapy for LAPC was associated with durable locoregional tumor control and favorable survival. Prospective randomized trials in patients with LAPC are warranted.
Introduction
Pancreatic cancer is a leading cause of cancer death in the US.1 For patients with localized disease, surgery and systemic therapy provide the best opportunity for durable cancer control. Resection with negative margins is associated with the best outcomes,2 but is often hindered by mesenteric vessel involvement. Long-term survival associated with incompletely resected and unresected tumors has not surpassed a median of 12 months and 2-year rate of 20%. In randomized trials, conventional doses of radiation therapy (RT) as a consolidation strategy after gemcitabine for unresected tumors do not improve patient survival.3,4,5 Multiagent chemotherapy regimens have since been shown to be superior to gemcitabine. However, the importance of local tumor control remains, and is illustrated by the survival limitation in patients with localized tumors who cannot have surgery compared with those who can. Recent technological advancements and innovative solutions for managing internal organ motion have enabled safe RT dose escalation. Early reports of dose escalation to an ablative threshold in small cohorts suggested that controlling the primary tumor can improve survival.6,7 We report mature data on a 119-patient, uniformly treated cohort with regard to ablative RT (A-RT) as well as diagnostic, therapeutic, and supportive care pathways.
Methods
Consecutive patients with localized unresectable and medically inoperable pancreatic ductal adenocarcinoma (PDAC) who received definitive A-RT at Memorial Sloan Kettering Cancer Center regional network June 2016 to February 2019 were included. Tumors of any size and less than 5 cm luminal abutment were eligible for A-RT. A database of baseline clinical and treatment characteristics, outcomes, and adverse events (AEs) was prospectively maintained at 3- and 6-month intervals. The Memorial Sloan Kettering institutional review board approved the study, and all participants provided written informed consent.
Ablative RT simulation, planning, and delivery were carried out according to the standardized protocol as previously described8 (Supplement). Briefly, fractionation schemes included 75 Gy in 25 fractions (biologically effective dose [BED] = 97.5 Gy) for tumors less than 1 cm from stomach or intestines, and 67.5 Gy in 15 fractions (BED = 97.88 Gy) for tumors of 1 cm or further. Treatments were delivered on Varian linear accelerators with RPM system for respiratory motion management and daily cone-beam computed tomography (CBCT) for image guidance.
Locoregional and distant progression were based on RECIST 1.1 criteria. Adverse events were scored by Common Terminology Criteria for Adverse Events (version 4.0). Statistical methods are detailed in eMethods the Supplement.
Results
One hundred and nineteen consecutive nonmetastatic patients with PDAC were treated with definitive intent June 2016 to February 2019 (eTable 1 in the Supplement). Overall, 99 (83%) had radiographic T3/T4 disease, and 51 (49%) were node-positive. One hundred and sixteen (97.5%) received induction chemotherapy, consisting of mFOLFIRINOX (fluorouracil, oxaliplatin, irinotecan, leucovorin) (n = 66), gemcitabine/nab-paclitaxel (n = 37), or other (n = 13) for a median of 4 months (0.5 months-13 months). Ablative RT consisted of 75 Gy in 25 fractions (n = 96) or 67.5 Gy in 15 fractions (n = 23) with concurrent fluoropyrimidine (n = 107) or other (n = 4) chemotherapy.
At a median follow-up of 18.4 months from A-RT and 24.5 months from diagnosis, 55 patients had died. Median progression-free survival (PFS) and overall survival (OS) from the time of A-RT were 6.3 months (95% CI, 5.26-8.78) and 18.2 months (95% CI, 16.3-26.8), respectively. Median PFS and OS from diagnosis were 13.2 months (95% CI, 11.4-15.7) and 26.8 months (95% CI 21.7-35.7), respectively. The 12- and 24-month OS from the time of A-RT were 74% (95% CI, 66%-83%) and 38% (95% CI, 27%-52%), respectively (Figure 1). The 12- and 24-month cumulative incidence of locoregional progression (LRP) were 17.6% (95% CI, 10.4%-24.9%) and 32.8% (95% CI, 21.6%-44.1%), respectively. Twenty-five patients were alive without radiographic evidence of progression at the time of manuscript preparation. Of 103 patients with data on subsequent systemic chemotherapy, 19 started maintenance therapy and 53 received salvage chemotherapy during the follow-up period.
Figure 1. Overall Survival and Cumulative Incidence of Locoregional Progression.
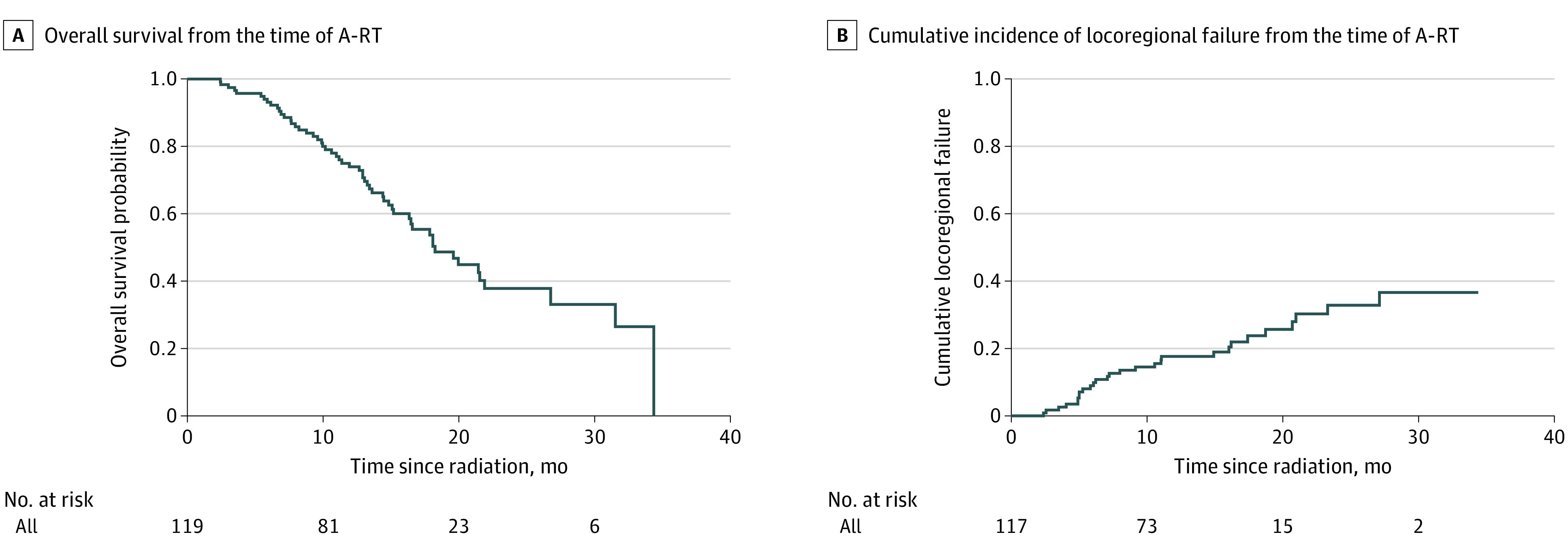
A, Kaplan-Meier estimate of overall survival rates. B, Cumulative incidence of locoregional progression rates. A-RT indicates ablative radiation therapy.
The association of baseline characteristics with OS, PFS, and LRP are shown in eTable 2 in the Supplement. Notably, postinduction reduction in carbohydrate antigen 19-9 (CA19-9) compared with baseline was associated with improved PFS (HR, 1.36; 95% CI, 1.08-1.71; P = .009) and LRP (HR, 1.86; 95% CI, 1.55-2.25; P < .001) on univariate analysis. Using the most optimal cut point for CA19-9 by the maximal χ2 method, 1-year LRP rates were 16.5% vs 44.5% for patients achieving 80th percentile reduction in their CA19-9 vs others (P = .007), with a trend for improved OS of 73% vs 66% (P = .09). On multivariable analysis, only postinduction CA19-9 reduction (HR, 1.33; 95% CI, 1.03-1.70; P = .03) and presence of central tumor high dose (HR, 2.20; 95% CI, 1.12-4.30; P = .02) correlated with improved PFS (Figure 2).
Figure 2. Overall Survival and Cumulative Incidence of Locoregional Progression by CA19-9 Percent Change.
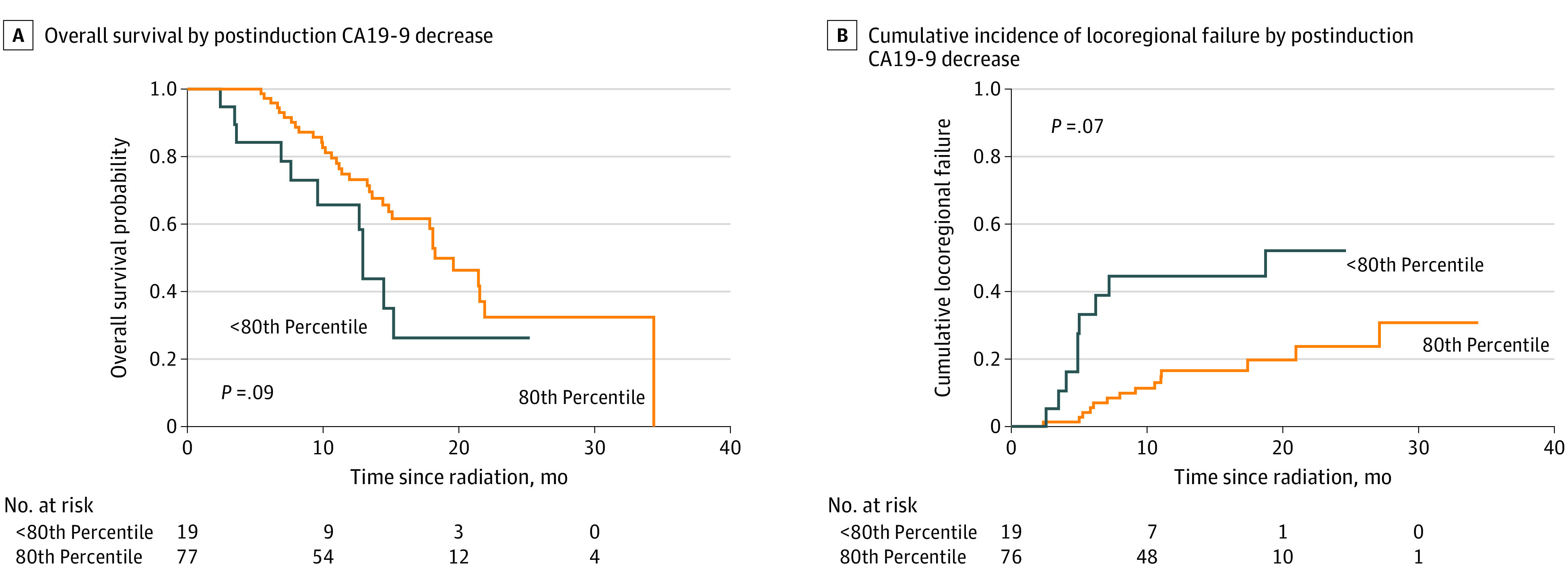
CA19-9 indicates carbohydrate antigen 19-9.
Grade 3 and 4 or higher A-RT-attributable AEs occurred in 16 and 0 patients, respectively (eTable 3 in the Supplement). Ten patients had upper gastrointestinal bleeding, related to anticoagulation in 8 and requiring endoscopic interventions in 2.
Discussion
These data demonstrate that A-RT in anatomically suitable patients with LAPC was associated with durable locoregional tumor control, which may have contributed to a favorable 2-year survival of 38% from the time of A-RT. In comparison, conventional RT has failed to improve OS compared with chemotherapy alone in randomized clinical trials.3,4,5 Median OS from diagnosis has ranged from 8.6 to 15.2 months in patients receiving conventional RT after induction gemcitabine.3,4,5 Importantly, less than 20% of patients receiving conventional RT survived 2 or more years, resulting in lack of LC benchmarks beyond 1 year.
Undoubtedly advances in systemic treatment and patient selection have contributed to the favorable outcomes in this study. FOLFIRINOX and gemcitabine/nab-paclitaxel have been important advances that have improved outcomes in the metastatic and adjuvant settings. Meta-analysis of nonrandomized prospective trials and retrospective studies have also reported encouraging results in patients with localized disease treated with initial FOLFIRINOX. The effect of FOLFIRINOX on mOS (range, 10.0-32.7 months) is confounded by resection rates up to 43% in these reports.9 Surgical resection has until now been the only means of achieving long-term survival.2,10 However, more complex surgical resections have higher perioperative morbidity and mortality rates and inferior long-term survival.11,12,13 Moreover, 2-year local recurrence rates after surgery exceed 30%.14 Ablative RT may offer an alternative in patients who are at higher surgical risk.
Ablative RT combines stereotactic technology for precise RT delivery with innovative solutions for internal organ motion and radiation science-informed fractionation to achieve an ablative dose to the target while sparing the adjacent luminal gastrointestinal tract. Incremental dose escalation using this approach in 47 patients with LAPC resulted in improved OS compared with conventional RT (36% vs 19%) at 2 years.7 A multi-institutional study of similarly ablative dosing in 24 patients delivered with magnetic resonance image-guidance, an emerging technology with a distinct solution for organ motion management, resulted in a 2-year OS of 49%, further reaffirming the benefit of dose escalation.6 In our study, 119 patients were selected after initial chemotherapy based on anatomical suitability and treated uniformly with the same ablative technique. Treatment was otherwise according to the same institutional paradigm throughout the study period, with outcomes and AEs longitudinally entered into a prospectively maintained database. As such, this is the largest and most homogeneous cohort, providing a unique opportunity to evaluate the efficacy of A-RT.
Limitations
The limitations of this study include its retrospective, nonrandomized, single institution design, possible selection bias, heterogeneity of induction chemotherapy regimen and duration, lack of a direct comparison RT group, and the possible confounding effect of salvage chemotherapy on survival.
Ablative RT (98 Gy BED) following induction systemic therapy for LAPC was safe and associated with durable local tumor control, which could have contributed to a longer survival duration.
eMethods
eTable 1. Demographic, Clinical, and Treatment Characteristics of Patients
eTable 2. Univariate and multivariate analyses for Overall Survival, Progression-free Survival, and Locoregional Progression
eTable 3. Toxicity
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. doi: 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16(1):11-26. doi: 10.1038/s41571-018-0112-1 [DOI] [PubMed] [Google Scholar]
- 3.Hammel P, Huguet F, van Laethem JL, et al. ; LAP07 Trial Group . Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315(17):1844-1853. doi: 10.1001/jama.2016.4324 [DOI] [PubMed] [Google Scholar]
- 4.Loehrer PJ Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29(31):4105-4112. doi: 10.1200/JCO.2011.34.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19(9):1592-1599. doi: 10.1093/annonc/mdn281 [DOI] [PubMed] [Google Scholar]
- 6.Rudra S, Jiang N, Rosenberg SA, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8(5):2123-2132. doi: 10.1002/cam4.2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan S, Chadha AS, Suh Y, et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94(4):755-765. doi: 10.1016/j.ijrobp.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reyngold M, Parikh P, Crane CH. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol. 2019;14(1):95. doi: 10.1186/s13014-019-1309-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17(6):801-810. doi: 10.1016/S1470-2045(16)00172-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neoptolemos JP, Palmer DH, Ghaneh P, et al. ; European Study Group for Pancreatic Cancer . Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011-1024. doi: 10.1016/S0140-6736(16)32409-6 [DOI] [PubMed] [Google Scholar]
- 11.Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg. 2011;254(6):882-893. doi: 10.1097/SLA.0b013e31823ac299 [DOI] [PubMed] [Google Scholar]
- 12.Bhayani NH, Enomoto LM, James BC, et al. Multivisceral and extended resections during pancreatoduodenectomy increase morbidity and mortality. Surgery. 2014;155(3):567-574. doi: 10.1016/j.surg.2013.12.020 [DOI] [PubMed] [Google Scholar]
- 13.Kulemann B, Hoeppner J, Wittel U, et al. Perioperative and long-term outcome after standard pancreaticoduodenectomy, additional portal vein and multivisceral resection for pancreatic head cancer. J Gastrointest Surg. 2015;19(3):438-444. doi: 10.1007/s11605-014-2725-8 [DOI] [PubMed] [Google Scholar]
- 14.Cloyd JM, Crane CH, Koay EJ, et al. Impact of hypofractionated and standard fractionated chemoradiation before pancreatoduodenectomy for pancreatic ductal adenocarcinoma. Cancer. 2016;122(17):2671-2679. doi: 10.1002/cncr.30117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Demographic, Clinical, and Treatment Characteristics of Patients
eTable 2. Univariate and multivariate analyses for Overall Survival, Progression-free Survival, and Locoregional Progression
eTable 3. Toxicity


