Abstract
Breast cancer is a complex and multifactorial disease, and environmental factors have been suggested to increase its risk. However, prior research has largely focused on studying exposures to one factor/contaminant at a time, which does not reflect the real-world environment. Herein, we investigate associations between breast cancer and the Environmental Quality Index (EQI), a comprehensive assessment of five domains of environmental quality (air, water, land, sociodemographic, and built) at the county level. Breast cancer diagnoses for North Carolina women were obtained from the North Carolina Central Cancer Registry (2009-2014) and the county of residence at the time of diagnosis was linked with the EQI. We evaluated the odds of localized, regional, or distant metastatic breast cancer in categories of environmental quality using women with carcinoma in situ as registry-based controls. Overall environmental quality was generally not associated with invasive breast cancer; however, all breast cancer types tended to be inversely associated with land quality, particularly in more rural communities [distant metastatic breast cancer was 5-8% more likely (OR 1.08; 95% CI: 1.02, 1.14, p=0.02) compared to carcinoma in situ]. Cumulatively, our results suggest that some broad measures of environmental quality are associated with invasive breast cancer but that associations vary by environmental domain, cancer stage, subtype, and urbanicity. Our findings suggest that components of land quality (e.g. pesticide applications and animal facilities) warrant additional investigation in relation to invasive breast cancer.
Keywords: North Carolina, County, built, land, environmental exposure, sociodemographic
Introduction
Breast cancer is the most common malignancy among women, and it is estimated that one in eight women will develop invasive breast cancer [1]. Recent studies suggest that the risk of breast cancer include a combination of genetic and environmental factors (reviewed in [2]) with compelling associations for increased risk with exposures to single pesticides, ionizing radiation, solvents and other environmental contaminants [3-14]. However, the specific environmental contributors to disease risk remain poorly characterized and most importantly, there is paucity of studies evaluating the role of everyday environmental exposures which occur simultaneously and as mixtures rather than single agents [15, 16]. Failing to account for cumulative environmental exposure may results in an underestimation of the true impact of the environment on breast cancer risk [17].
Empirically measuring the totality of the environment remains a challenge in epidemiologic research [17] and because demographic characteristics, including race/ethnicity and socioeconomic status [18] are increasingly correlated with higher breast cancer incidence. In particular, African-American and younger women and those residing in rural areas are more likely to be diagnosed with aggressive hormonal subtypes like triple-negative and inflammatory breast cancers [19-27], which are highly invasive and frequently present as late-stage tumors. To address this issue, the concept of environmental quality index (EQI) was developed by the United States Environmental Protection Agency (EPA) [28, 29]. EQI is a publicly available database that combines assessments of environmental factors across the entire United States (2000-2005) into an overall environmental assessment score and scores for five domains of environmental quality (air, water, land, sociodemographic and built environments). These data were used in a recent cancer study across the United States; findings suggest that county-level age-adjusted breast cancer incidence rates are associated with indicators of poor environmental quality, such that areas with worst environmental quality appear to have higher rates of breast cancer [17]. In addition to cancers, the EQI has also been linked to health outcomes such as preterm birth and mortality [30, 31]. Herein, we utilize individual-level data for women diagnosed with breast cancer in North Carolina to build upon the work of Jagai et al. [17] by accounting for confounding individual demographic and lifestyle factors.
Breast cancer is not one disease, but rather distinct subgroups that may be separated by factors such as stage, morphology, histology, gene expression, and hormone receptor status [27, 32-35]. Therefore, to test the hypothesis that the incidence of specific breast cancer stages can vary by demographics (age, race, reproductive age or history, weight, income, location) [27, 36-39] in relation to environmental factors, we utilized EQI datasets to investigate associations between environmental factors and breast cancer (separated as localized, regional or distant metastatic disease) compared to carcinoma in situ.
Materials and Methods
Study population
Breast cancer patient data was obtained from the North Carolina Central Cancer Registry (NC CCR). The NC CCR is a central reporting system for cancer cases that collects all cancer incidence data for the state of North Carolina, in all 100 counties. We selected breast cancer diagnosed between 2009 and 2014 for our analyses (approximately 10 years after the time period that the EQI was constructed to capture). Our analyses included patients diagnosed with localized, regional, or distant metastatic invasive breast cancer as classified in the summary staging defined by the Surveillance, Epidemiology, and End Results (SEER) program 2000 [40]. This staging is based on ICD-10 tumor histology and behavior coding, tumor locality within the breast, and lymph node involvement, including metastatic spread (Supplemental Table 1). Localized, regional, or distant metastatic patients were considered cases in separate datasets. Because only cancer patients are included in the NC CCR, we used carcinoma in situ (includes both ductal and lobular, non-invasive breast cancer) patients as registry-based controls. County at diagnosis, age, and race were available for all breast cancer patients, while BMI and smoking status were also available for many but not all individuals.
Environmental quality index (EQI)
The EQI is available at the county-level and includes a total environmental assessment as well as estimates of environmental quality in five separate domains – air, water, land, sociodemographic, and built. The EQI was developed in four distinct parts, which includes identification of environmental domains, identifying and reviewing sources of data from 2000-2005 for individual factors that would make up each domain, constructing variables based on these data, and reduction of data including compilation into domain-specific and a total EQI score [41]. Factors comprising each of the five domains and utilized in statistical analyses can be found in Supplemental Figure 1 or [41]. Data was finalized in 2014 and is publicly available on the USEPA website at https://edg.epa.gov/EPADataCommons/public/ORD/NFIEERL/EQI/. The EQI is a continuous variable but was categorized into quartiles for these analyses to aid in interpretation.
Statistical Analyses
Generalized estimating equation (GEE) models, which take into account clustering of individuals within counties, were used to examine the relationship between breast cancer and environmental quality. Separate models were constructed comparing localized, regional, or distant metastatic breast cancer to carcinoma in situ patients. Models were constructed for overall and domain-specific EQI. For domain-specific models, the other four domains were included in models as potential confounding variables. Models were also adjusted for individual age, BMI, smoking status, and race. Although age and race data were very complete, BMI and smoking data was missing more frequently. To avoid excluding patients with missing data, an “unknown” category was used for BMI and smoking status. To ensure that the inclusion of this category was not biasing associations, we also conducted complete case analyses (including participants with complete data). Results of these analyses were qualitatively very similar; thus, we primarily present analyses including all patients. All statistical analyses were conducted in SAS (Version 9.4). Although we considered several domains of exposure and outcomes in statistical analyses, we did not perform adjustment for multiple comparisons, as this type of adjustment has not been recommended in the epidemiologic literature [42].
Rural-urban sensitivity analysis
Prior research demonstrates that relationships between the EQI and cancer incidence may differ based on urbanicity. To evaluate the possibility of differential impacts in urban and rural datasets, we conducted a series of sensitivity analyses stratifying by urbanity using the rural-urban continuum code RUCCs. RUCC was originally developed as a nine-item categorization code of proximity to/influence of major metropolitan areas in the Surveillance, Epidemiology, and End Results (SEER) program [43], and was restructured into four codes for the EQI [41]. Due to small samples sizes in some categories, codes were condensed into three categories for our analyses as follows: metropolitan urbanized = codes 1 + 2 + 3; non-metro urbanized = 4 + 5; less populated = 6 + 7 + 8 + 9 (Supplemental Table 1). In these analyses, the total EQI and domain-specific EQI were stratified by RUCC categories rather than using the EQI & domains created specifically for each RUCC category in the original EQI composition, in order to combine the more rural categories with limited sample size.
Results
Patients Exhibit Rural-Urban Divide
Among North Carolina women diagnosed with breast cancer between 2009 and 2014, 7975 were diagnosed with carcinoma in situ, 25827 with localized breast cancer, 12371 with regional breast cancer, and 2073 with distant metastatic breast cancer (defined in Supplemental Table 1). A large portion of these patients with breast cancer inhabited metropolitan urbanized areas (75% carcinoma in situ, 74% localized, 72% regional, and 71% distant metastatic cases), with only 7-9% of patients residing in less populated rural areas (Supplemental Table 2). Additionally, there were ‘age at diagnosis’ differences across rural-urban stratifications. Mean age at diagnosis were significantly higher in less populated areas for invasive breast cancers versus carcinomas in situ (e.g., 61.4 years carcinoma in situ, 62.8 years distant metastatic, p=0.03); however, there was no differences in age at diagnosis identified in this dataset related to those residing in metropolitan areas (60.0 years carcinoma in situ, 60.1 years distant metastatic, p=0.40).
Environmental quality varies by rural-urban context
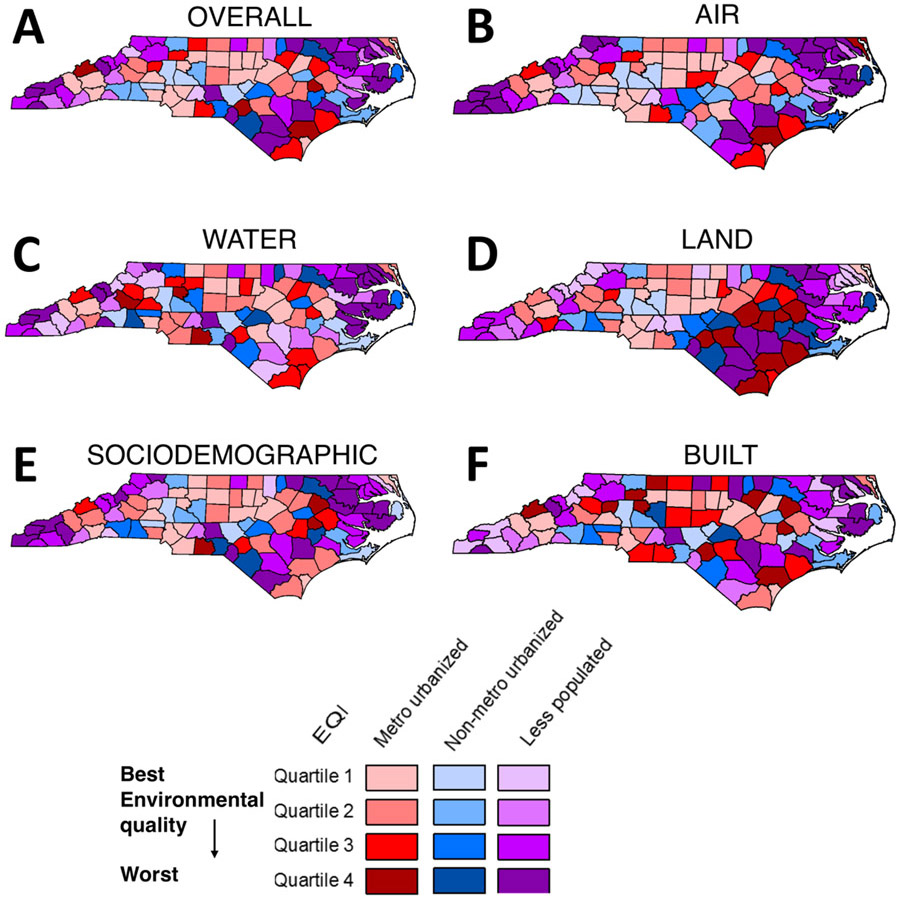
We generated a map of EQI quartiles in ArcGIS, similar to what was presented in the original EQI report [41]. Less populated counties generally had overall worse environmental quality, including when separated by domains (Fig. 1). For example, worst land environmental quality was highly concentrated in the eastern region of the state, which is also a less populated region (Fig. 1). As a trend supporting this geographical observation, breast cancer cases in counties with the first EQI quartile (best environmental quality) for total EQI and all EQI domains were underrepresented in the less populated rural-urban category (Supplemental Table 2). For example, only 7-9% of breast cancer cases regardless of stage were in less populated counties with high overall environmental quality. Conversely, 33-35% were in less populated counties with the worst overall environmental quality.
Figure 1. Environmental Quality Index Stratified by Rural-Urban Categories by County.
Quartiled Environmental Quality Index data by county and by rural-urban category for (A) total EQI and (B) air, (C) water, (D) land, (E) sociodemographic, and (F) built EQI domains. Maps recreated based on the EQI overview report [41].
Individual demographics are associated with breast cancer
Age at diagnosis was significantly associated with all stages of breast cancer, with an increase of 1-3% in invasive breast cancer odds for every 10-year age increment in localized and distant metastatic breast cancers (Table 1, p<0.001). A higher percentage of later-stage breast cancer cases (24% regional, 29% distant metastatic) versus carcinoma in situ patients (20%) were black (Supplemental Table 2), a pattern consistent across rural-urban categories. Black patients had particularly higher odds of regional and distant metastatic breast cancer (Table 1). The highest increase was for distant metastatic breast cancer, where black patients had a 9% increase in odds of having distant metastatic breast cancer versus carcinoma in situ, regardless of rural-urban area (Non-stratified OR 1.09; 95% CI: 1.07, 1.12, p<0.0001). Those who were current smokers or were a former smoker as opposed to having never smoked had significantly increased odds of breast cancer regardless of stage or rural-urban area. This was most apparent for distant metastatic cases, where current smokers as compared to those who had never smoked had 15% increased odds of presenting with distant metastatic breast cancer versus carcinoma in situ (non-stratified OR 1.15; 95% CI: 1.10, 1.21, p<0.0001). Finally, there was no association found between breast cancer versus carcinoma and BMI category, although those with an unknown BMI did have significantly different odds compared to normal weight persons, which differed by breast cancer stage.
Table 1.
Odds ratios (95% CI) for having localized, regional, or distant metastatic breast cancer versus carcinoma in situ based on total environmental quality index and individual covariates age at diagnosis, BMI, smoking status, and race using quartile 1 (worst environmental domain quality) as reference.
| Localized Breast Cancer |
Regional Breast Cancer |
Distant Metastatic Breast Cancer | |
|---|---|---|---|
| EQI | |||
| First Quartile (Best) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Second Quartile | 1.00 (0.97-1.02) | 1.01 (0.98-1.06) | 1.01 (0.98-1.05) |
| Third Quartile | 1.02 (0.99-1.04) | 1.05 (1.01-1.09) | 1.05 (1.01-1.09) |
| Fourth Quartile (Worst) | 0.99 (0.97-1.02) | 1.02 (0.97-1.06) | 1.00 (0.97-1.03) |
| p-trend | 0.488 | 0.042 | 0.054 |
| Age at Diagnosis | |||
| Age (10yr increments) | 1.03 (1.02-1.03) | 0.99 (0.98-0.99) | 1.01 (1.00-1.02) |
| BMI | |||
| Normal weight | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Underweight | 0.99 (0.94-1.04) | 1.01 (0.95-1.07) | 1.13 (1.02-1.26) |
| Overweight | 1.00 (0.98-1.02) | 1.00 (0.97-1.04) | 1.01 (0.97-1.04) |
| Obese | 1.01 (0.99-1.02) | 1.02 (0.99-1.05) | 0.99 (0.96-1.02) |
| Unknown | 0.98 (0.96-1.00) | 0.98 (0.95-1.01) | 1.03 (0.98-1.07) |
| Smoker | |||
| Current smoker | 1.05 (1.03-1.07) | 1.10 (1.07-1.14) | 1.15 (1.10-1.21) |
| Former smoker | 1.02 (1.00-1.03) | 1.04 (1.02-1.06) | 1.00 (0.97-1.03) |
| Never smoked | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Unknown | 1.00 (0.98-1.02) | 0.99 (0.97-1.02) | 0.94 (0.91-0.98) |
| Race | |||
| White | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Black | 0.97 (0.96-0.99) | 1.04 (1.03-1.06) | 1.09 (1.07-1.12) |
| Other | 1.01 (0.98-1.03) | 1.01 (0.96-1.06) | 1.06 (1.00-1.12) |
Bolded values are p<0.05
Environmental quality and breast cancer: Differences by stage, domain, and urbanicity
Models were constructed to estimate odds of having localized, regional, or distant metastatic invasive breast cancer (compared to non-invasive carcinoma in situ) as a function of the environmental quality index (EQI). Odds ratios greater than one thus represent increased odds of having localized, regional, or distant metastatic breast cancer, whereas odds ratios less than one represent greater odds of having carcinoma in situ versus the comparison breast cancer stage. Our results show that environmental quality is associated with breast cancer differentially by breast cancer stage, environmental domain, and rural-urban area.
Taken together, we observed little evidence of association between invasive breast cancer and total environmental quality as measured by the EQI. However, poor total environmental quality was associated with a 5% increase in the odds of having both regional and distant metastatic breast cancer versus carcinoma in situ (OR 1.05; 95% CI: 1.01, 1.09, p=0.003) but in most cases, this association was seen in the third quartile (poor, but not the worst environmental quality) (Table 1, Fig. 2, Fig. 3, and Fig. 4).
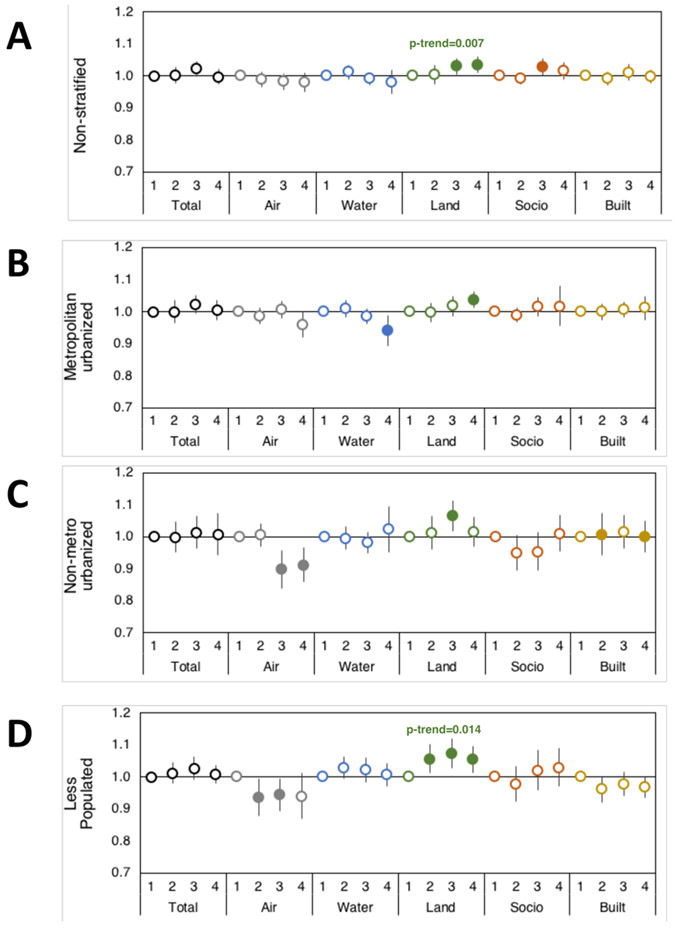
Figure 2. Environmental domain quality and rural-urban effects on odds of localized breast cancer.
Odds ratios with 95% CI of having localized breast cancer versus carcinoma in situ based on Total and individual environmental quality index domain indices (Air, Water, Land, Sociodemographic (Socio), and Built domains) in (A) non-stratified and urban/rural category strata (B) metropolitan urbanized, (C) non-metro urbanized, and (D) less populated. First quartile EQI (best environmental quality) is reference and models adjusted for individual age, BMI, smoking status, and race. Filled circles represent significant odds ratios at p<0.05, and the p-trend is included where p-trend<0.05.
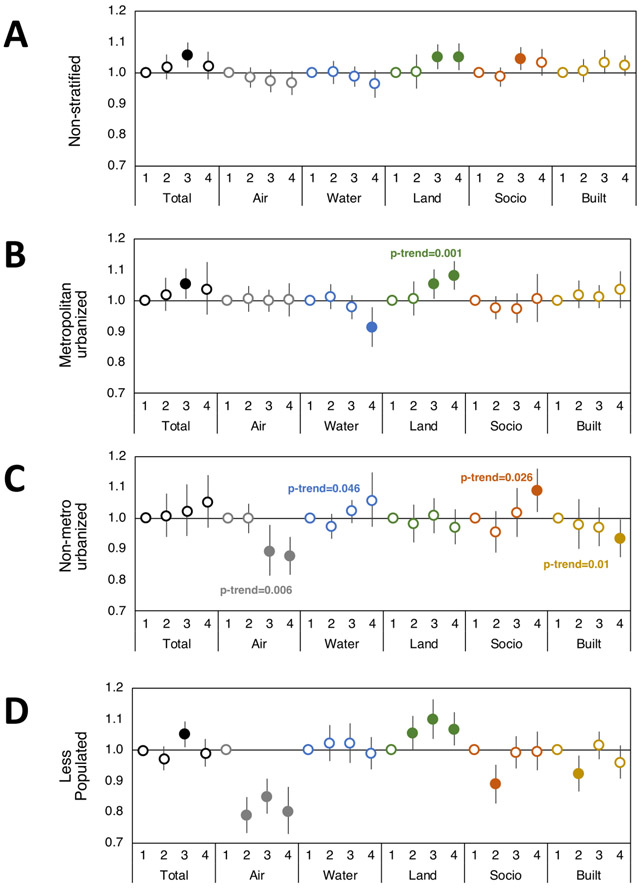
Figure 3. Environmental domain quality and rural-urban effects on odds of regional breast cancer.
Odds ratios with 95% CI of having regional breast cancer versus carcinoma in situ based on Total and individual environmental quality index domain indices (Air, Water, Land, Sociodemographic (Socio), and Built domains) in (A) non-stratified and urban/rural category strata (B) metropolitan urbanized, (C) non-metro urbanized, and (D) less populated. First quartile EQI (best environmental quality) is reference and models adjusted for individual age, BMI, smoking status, and race. Filled circles represent significant odds ratios at p<0.05, and the p-trend is included where p-trend<0.05.
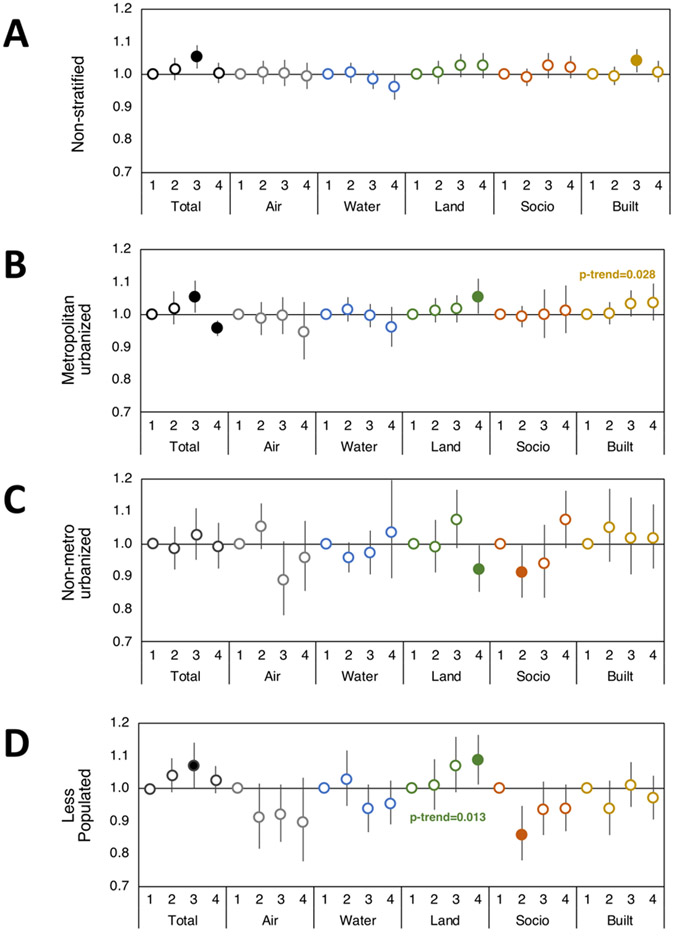
Figure 4. Environmental domain quality and rural-urban effects on odds of distant metastatic breast Cancer.
Odds ratios with 95% CI of having distant metastatic breast cancer versus carcinoma in situ based on Total and individual environmental quality index domain indices (Air, Water, Land, Sociodemographic (Socio), and Built domains) in (A) non-stratified and urban/rural category strata (B) metropolitan urbanized, (C) non-metro urbanized, and (D) less populated. First quartile EQI (best environmental quality) is reference and models adjusted for individual age, BMI, smoking status, and race. Filled circles represent significant odds ratios at p<0.05, and the p-trend is included where p-trend<0.05.
The air environmental quality domain is comprised of criteria and hazardous air pollutants such as particulate matter, ozone, carbon monoxide, and numerous volatile compounds, among others. Air quality tended to be inversely associated with localized and regional breast cancer, with a suggestion of stronger effects in non-metro urbanized and less populated areas. For example, the worst air quality was associated with a 20% decreased odds of regional breast cancer versus carcinoma in situ in less populated regions (OR 0.80; 95% CI: 0.72, 0.88, p<0.0001), and similar associations were seen for localized breast cancer (Supplemental Table 3, Fig. 2, Fig. 3, and Fig. 4).
The water environmental quality domain is comprised of estimates of domestic, agricultural, and industrial water use, drought status, and water quality and contaminant levels in natural sources, precipitation monitors, and public water supplies. In general, water quality associations were null; however, we did see an association in metropolitan urbanized counties for localized and regional breast cancer (Supplemental Table 3, Fig. 2, Fig. 3, and Fig. 4). We caution against over-interpretation of these results since they suggest opposite effects of water environmental quality.
The land environmental quality domain is comprised of agricultural information including crops, livestock, and pesticides used, toxic release and cleanup sites on the National Priority List (NPL), geochemical data (such as arsenic and lead, among others), and areas with potentially elevated indoor radon levels (Supplemental Figure 1). Land environmental quality was consistently associated with increased odds of localized, regional, and distant metastatic breast cancers compared to carcinoma in situ. This association was most consistent in metropolitan urbanized and less populated areas. Overall, patients residing in metropolitan urbanized counties with the worst land environmental quality had 3-5% increased odds of having breast cancer versus carcinoma in situ (localized OR 1.03; 95% CI: 1.01, 1.06, p=0.003; regional OR 1.08; 95% CI: 1.03, 1.12, p<0.001; distant metastatic OR 1.05; 95% CI: 1.00, 1.10, p=0.04), while the risk increased to 5-8% in less populated counties (localized OR 1.05; 95% CI: 1.01, 1.09, p=0.01; regional OR 1.06; 95% CI: 1.01, 1.12, p=0.012; distant metastatic OR 1.08; 95% CI: 1.01, 1.16, p=0.02) (Supplemental Table 3).
The sociodemographic environmental quality domain is comprised of U.S. Census population, housing, and economic data, as well as community and crime data. In the current analysis of NC counties, we observed associations between sociodemographic environmental quality and invasive breast cancer in unadjusted models. However, it should be noted that these associations, after accounting for individual demographic characteristics became statistically insignificant but remained comparable to unadjusted models (Supplemental Table 4). For example, the worst sociodemographic environmental quality increased odds of distant metastatic breast cancer by 10% in non-metro urbanized counties (OR 1.10; 95% CI: 1.00, 1.20, p=0.035), an association which was not significant in adjusted models (adjusted OR 1.07; 95% CI: 0.98, 1.16, p=0.18), suggesting that this environmental domain may be at least partially confounded by individual factors like age, race, BMI, and smoking status that are used to adjust models.
Finally, we assessed the built environmental quality domain, which is comprised of commercial business information, roads, motor vehicle crash fatalities, low-rent and section-eight housing, and public transportation use. Our analysis suggests an inverse association with built environmental quality for regional breast cancer in non-metro urbanized counties (OR 0.93; 95% CI: 0.87, 0.99, p=0.04) and an increased odds of distant metastatic breast cancer for patients residing in counties with the worst built environmental quality, particularly in metropolitan urban areas (p-trend=0.03).
Counties across North Carolina had a wide range of EQI values, representing anywhere from 27-54% the range of EQI values across all counties in the United States, depending on the EQI domain (Supplemental Table 5).
Discussion
Investigating incidence of breast cancers of all types in total has the potential to mask differences in the impact of environmental factors on the development of different subsets of breast cancer, particularly those influencing development of later stages or more aggressive subsets. Hormone receptor subtype information was available for only 68% of patients within the dataset, so summary staging as “localized”, regional”, and “distant” was chosen to differentiate patients as it allows for assessment of increasing invasiveness and severity. Patients with distant metastatic breast cancer have poor prognosis despite aggressive, multidisciplinary treatment regimens compared to carcinoma in situ or early-stage breast cancer [44]. This reinforces the unmet need to identify risk factors associated with advanced breast cancers in order to reduce incidence and improve overall breast cancer survival.
The present study demonstrates the strongest positive association for poor land environmental quality and distant metastatic breast cancer. In particular, patients residing in less populated counties with the worst land environmental quality were 8% more likely to have distant metastatic breast cancer than carcinoma in situ, an association which was greater in rural areas but also persisted in metropolitan areas. This association was also present for localized and regional breast cancer, with 5-6% increased odds in worst land environmental quality rural areas. While 8% is a small increase, a large proportion of the population lives in these communities and at the population level, the impact of an 8% impact is quite substantial. Most importantly, while our data evaluated EQI and breast cancer in the state of North Carolina, greater variation in land quality is present in other areas of the United States (Supplemental Table 5), thereby highlighting the broader applicability of this work. Moreover, our data indicate that the association between distant metastatic breast cancer and broad land environmental quality is dependent on rural-urban context, with the major effects occurring in less populated areas, which suggests the need to critically evaluate specific rural vs urban environmental factors. For example, hog farms and resultant toxic waste lagoons, which frequently are in rural areas associated with land EQI domains, have recently been studied in eastern NC and found to have significant potential human health effects [45]. This underscores the importance of our sensitivity analysis stratifying by rural-urban context and should be used in future studies considering associations between disease and environmental exposures.
A strength of our analysis lies in using North Carolina as a study area, as it has a range of population densities, diverse demographics, and environmental conditions. In maps merging both urbanicity and environmental quality, less populated and/or more rural areas had consistently worse environmental quality across all environmental domains. This is of great interest as recent reports increasingly suggest the role of socioeconomic and environmental as breast cancer risk factors [3-14, 18, 46]. However, association between rural-urban location and breast cancer stage are controversial and less certain [19], and the association between breast cancer stages, urbanicity, and environmental factors has not been studied simultaneously. Our maps showing different patterns in urbanicity and environmental quality reinforces the idea that environmental risk factors may impact breast cancer incidence differentially in urban and rural environments. Additionally, North Carolina has previously been used as a study area to spatially associate urbanicity with receipt of radiation therapy in Medicare receiving breast cancer patients [20] and to study incidence of higher stage basal-like breast cancer risk in premenopausal African American patients [21].
In our unadjusted statistical models, patients residing in a county with the worst sociodemographic environment were 4% more likely to have regional or distant metastatic breast cancer than carcinoma in situ, which increased to 9-10% in non-metro urbanized areas. It has been noted previously that socioeconomic status has significant associations with breast cancer incidence, in both location- and stage-specific models [18, 25, 26]. However, individual factors age, BMI, smoking status, and race accounted for this significance in adjusted models for distant metastatic breast cancer. Individual race as a covariate in adjusted models also had a significant association with distant metastatic breast cancer. Those self-identifying as Black had a 9% greater odds of distant metastatic breast cancer versus carcinoma in situ regardless of rural-urban area. This has been seen in prior studies investigating race/ethnicity associations with spatial incidence and mortality of breast cancer, where non-Whites and more specifically non-Hispanic Blacks are consistently at higher risk for total as well as advanced and late-stage breast cancer subtypes at both state and national levels of analysis [22-24, 27, 47, 48]. Additionally, epidemiological studies report that racial and ethnic minorities, as well as those living in poverty, are exposed to higher levels of various environmental pollutants compared to other populations [49, 50]. This suggests that, after accounting for individual race and other factors, sociodemographic characteristics of a county are less important in distant metastatic breast cancer and strengthens previous studies reporting both individual- and county-based heterogeneity in breast cancer incidence and outcomes [19, 25].
It is also important to note that our study identifies inverse effects for some environmental domains. This requires careful interpretation since the comparison is not between breast cancer and no breast cancer, but rather between invasive breast cancer and carcinoma in situ, i.e. pre-invasive breast cancer. In particular, poor air quality was inversely associated with localized and regional breast cancer in more rural areas, also signifying that it was associated with increased carcinoma in situ versus breast cancer. As a trend, this suggests that contaminants within the air domain that constitute air quality are associated with non-invasive breast cancer rather than any single stage of invasive breast cancer. More work is needed to further elucidate these associations, however, in a recent study, air contamination particulates PM2.5 and NO2 were found to be associated with breast cancer overall and with ductal carcinoma in situ but not invasive breast cancer [51]. Interestingly, associations were seen with invasive breast cancer in certain geographical regions, again indicating that rural-urban sensitivity analysis is paramount to these types of studies of breast cancer and environment associations, including investigations between different breast cancer stages.
Our results should be interpreted in the context of several important limitations. First, not all breast cancer cases may be reported to the NC CCR; however, all health care providers are required by law to report cases to the CCR, so this is not expected to skew results. Additionally, exposure at diagnosis may not be the most etiologically relevant time point. To address this issue, we obtained breast cancer data approximately 10 years after the time period that the EQI is intended to assess. Nonetheless, women in our study population could have moved in the years preceding their diagnosis. While this could result in exposure misclassification, it is likely non-differential with respect to cases status and would likely bias associations toward the null. Another limitation lies in use of carcinoma in situ as controls; ideally, we would have had non-cancer cases for controls if these data were available. If environmental quality was related to both carcinoma in situ and distant metastatic breast cancer, using our method of control selection could mask important trends, as may be the case for our null results. However, it should be noted that this would likely obscure trends, not create them. We further did not perform adjustment for multiple comparisons for statistical significance, but instead chose to focus on comparing patterns and precision of estimates in order to better analyze trends and not over-interpret the significance of results. An important feature of the EQI is that it is available for the entire U.S.; thus, it is feasible to extend our analysis in other geographic areas with cancer registry data and further test the robustness of our findings. Finally, our analysis assigned environmental quality at the county level, which may hide smaller scale trends. Ongoing work is focusing on the acquisition of both patient and environmental data with more granular geographic information, to fully understand the influence of geographically distributed environmental factors on the incidence rates of late-stage invasive breast cancer.
This project provides insight into the association between environmental quality and different stages of invasive breast cancer versus non-invasive carcinoma in situ. We report significant positive association between all stages of breast cancer, particularly distant metastatic breast cancer, and poor land environmental quality, highlighting the need for additional research. Additionally, our work has implications for future epidemiological studies investigating the influence of the environment on disease; our findings suggest that the EQI is a highly relevant measure for controlling for diverse environmental exposures in these studies.
Supplementary Material
Acknowledgements
We thank Dr. Kristen Rappazzo at Environment Protection Agency (EPA, NC) for providing expertise in the Environmental Quality Index and for her insightful review of our manuscript; Brittany Mills for assistance during the Duke University Summer Research Opportunity Program (SROP). In addition, we thank Dr. Soundarya Radhakrishnan, Sohrab Ali, and Dr. Gary Leung at the North Carolina Central Cancer Registry (NCCCR) for providing the data used in our analyses.
Financial Support: This work was supported in part by developmental research funds to G.R.D. and K.H. from the Duke Cancer Institute (Cancer and Environment Program), Duke Environmental Health Scholars Award (predoctoral) to L.M.G, National Institutes of Health under grant P20 CA202925-01A1, the Department of Surgery Bolognesi award to G.R.D.
Footnotes
Conflict of Interest Disclosure: All authors have no relevant disclosures.
References
- 1.Siegel RL, Miller KD, & Jemal A , Cancer statistics, 2016. CA: a cancer journal for clinicians, 2016. 66(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Gray JM, Rasanayagam S, Engel C, & Rizzo J, State of the evidence 2017: an update on the connection between breast cancer and the environment. Environmental health perspectives, 2017. 16(1): p. 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pestana D, Teixeira D, Faria A, Domingues V, Monteiro R, & Calhau C, Effects of environmental organochlorine pesticides on human breast cancer: putative involvement on invasive cell ability. Environmental toxicology, 2015. 30(2): p. 168–176. [DOI] [PubMed] [Google Scholar]
- 4.He TT, Zuo AJ, Wang JG, & Zhao P, Organochlorine pesticides accumulation and breast cancer: A hospital-based case–control study. Tumor Biology, 2017. 39(5): p. 1010428317699114. [DOI] [PubMed] [Google Scholar]
- 5.Eldakroory SA, Morsi DE, Abdel-Rahman RH, Roshdy S, Gouida MS, & Khashaba EO, Correlation between toxic organochlorlne pesticides and breast cancer. Human & experimental toxicology, 2017. 36(12): p. 1326–1334. [DOI] [PubMed] [Google Scholar]
- 6.Engel LS, Werder E, Satagopan J, Blair A, Hoppin JA, Koutros S, Lerro CC, Sandler DP, Alavanja MC, & Beane Freeman LE, Insecticide use and breast cancer risk among farmers’ wives in the Agricultural Health Study. Environmental health perspectives, 2017. 125(9): p. 097002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellsworth RE, Kostyniak PJ, Chi LH, Shriver CD, Costantino NS, & Ellsworth DL, Organochlorine pesticide residues in human breast tissue and their relationships with clinical and pathological characteristics of breast cancer. Environmental toxicology, 2018. 33(8): p. 876–884. [DOI] [PubMed] [Google Scholar]
- 8.Clapp RW, Jacobs MM, & Loechler EL, Environmental and occupational causes of cancer: new evidence 2005-2007. Reviews on environmental health, 2008. 23(1): p. 1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, & Rudel RA, Environmental pollutants and breast cancer: epidemiologic studies. Cancer: Interdisciplinary International Journal of the American Cancer Society, 2007. 109: p. 2667–2711. [DOI] [PubMed] [Google Scholar]
- 10.Zheng T, Holford TR, Mayne ST, Tessari J, Owens PH, Zahm SH, Zhang B, Dubrow R, Ward B, Carter D, & Boyle P, Environmental exposure to hexachlorobenzene (HCB) and risk of female breast cancer In Connecticut. Cancer Epidemiology and Prevention Biomarkers, 1999. 8(5): p. 407–411. [PubMed] [Google Scholar]
- 11.Ben-Jonathan N, Endocrine Disrupting Chemicals and Breast Cancer: The Saga of Bisphenol A, in Estrogen Receptor and Breast Cancer. 2019, Humana Press, Cham. p. 343–377. [Google Scholar]
- 12.Parada H Jr, Gammon MD, Ettore HL, Chen J, Calafat AM, Neugut AI, Santella RM, Wolff MS, & Teitelbaum SL, Urinary concentrations of environmental phenols and their associations with breast cancer incidence and mortality following breast cancer. Environment international, 2019. 130: p. 104890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White AJ, O’brien KM, Niehoff NM, Carroll R, & Sandler DP, Metallic air pollutants and breast cancer risk In a nationwide cohort study. Epidemiology, 2019. 30(1): p. 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeves KW, Díaz Santana M, Manson JE, Hankinson SE, Zoeller RT, Bigelow C, Sturgeon SR, Spiegelman D, Tinker L, Luo J, & Chen B, Urinary phthalate blomarker concentrations and postmenopausal breast cancer risk. JNCI: Journal of the National Cancer Institute, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauer SJ, Tarpley M, Shah I, Save AV, Lyerly HK, Patierno SR, Williams KP, & Devi GR, Bisphenol A activates EGFR and ERK promoting proliferation, tumor spheroid formation and resistance to EGFR pathway Inhibition In estrogen receptor-negative Inflammatory breast cancer cells. Carcinogenesis, 2017. 38(3): p. bgx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gearhart-Serna LM, Jayasundara N, Tacam M, Di Giulio R, & Devi GR, Assessing Cancer Risk Associated with Aquatic Polycyclic Aromatic Hydrocarbon Pollution Reveals Dietary Routes of Exposure and Vulnerable Populations. Journal of environmental and public health, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagai JS, Messer LC, Rappazzo KM, Gray CL, Grabich SC, & Lobdell DT, County-level cumulative environmental quality associated with cancer Incidence. Cancer, 2017. 123(15): p. 2901–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry KA, Sherman R, Farber S, Cockburn M, Goldberg DW, & Stroup AM, The joint effects of census tract poverty and geographic access on late-stage breast cancer diagnosis In 10 US States. Health & place, 2013. 21(110–121). [DOI] [PubMed] [Google Scholar]
- 19.Sealy-Jefferson S, Roseland ME, Cote ML, Lehman A, Whitsel EA, Mustafaa FN, Booza J, & Simon MS, Rural–Urban Residence and Stage at Breast Cancer Diagnosis Among Postmenopausal Women: The Women's Health Initiative. Journal of Women's Health, 2019. 28(2): p. 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler SB, Kuo TM, Durham D, Frizzelle B, Reeder-Hayes K, & Meyer AM, Effects of distance to care and rural or urban residence on receipt of radiation therapy among North Carolina Medicare enrollees with breast cancer. North Carolina medical journal, 2014. 75(4): p. 239–246. [DOI] [PubMed] [Google Scholar]
- 21.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, & Deming SL, Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Journal of the American Medical Association 2006. 295(21): p. 2492–2502. [DOI] [PubMed] [Google Scholar]
- 22.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, & Nyante S, Epidemiology of basal-like breast cancer. Breast cancer research and treatment, 2008. 109(1): p. 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bambhroliya AB, Burau KD, & Sexton K, Spatial analysis of county-level breast cancer mortality in Texas. Journal of environmental and public health, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mobley L, Kuo TM, Scott L, Rutherford Y, & Bose S , Modeling geospatial patterns of late-stage diagnosis of breast cancer in the US. International journal of environmental research and public health, 2017. 14(5): p. 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster TF, Hoffman K, Weinberg J, Vieira V, & Aschengrau A, Community-and individual-level socioeconomic status and breast cancer risk: multilevel modeling on Cape Cod, Massachusetts. Environmental health perspectives, 2008. 116(8): p. 1125–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott L, Mobley LR, & ll’yasova D , Geospatial Analysis of Inflammatory Breast Cancer and Associated Community Characteristics in the United States. International journal of environmental research and public health, 2017. 14(4): p. 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, & Henley SJ, Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. JNCI: Journal of the National Cancer Institute, 2015. 107(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messer LC, et al. , Construction of an environmental quality index for public health research. Environ Health, 2014. 13(1): p. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messer LC, Jagai JS, Rappazzo KM, & Lobdell DT, Construction of an environmental quality index for public health research. Environmental Health, 2014. 13(1): p. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jian Y, Messer LC, Jagai JS, Rappazzo KM, Gray CL, Grabich SC, & Lobdell DT, Associations between Environmental Quality and Mortality in the Contiguous United States, 2000–2005. Environmental health perspectives, 2016. 125(3): p. 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grabich SC, Rappazzo KM, Gray CL, Jagai JS, Jian Y, Messer LC, & Lobdell DT, Additive Interaction between Heterogeneous Environmental Quality Domains (Air, Water, Land, Sociodemographic, and Built Environment) on Preterm Birth. Frontiers in public health, 2016. 4: p. 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alizart M, Saunus J, Cummings M, & Lakhani SR, Molecular classification of breast carcinoma. Diagnostic Histopathology, 2012. 18(3): p. 97–103. [Google Scholar]
- 33.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, & Gräf S, The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature, 2012. 486(7403): p. 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao Z, Shi A, Lu C, Song T, Zhang Z, & Zhao J, Breast cancer: epidemiology and etiology. Cell biochemistry and biophysics, 2015. 72(2): p. 333–338. [DOI] [PubMed] [Google Scholar]
- 35.Inic Z, Zegarac M, Inic M, Markovic I, Kozomara Z, Djurisic I, Inic I, Pupic G, & Jancic S, Difference between luminal A and luminal B subtypes according to Ki-67, tumor size, and progesterone receptor negativity providing prognostic information. Clinical Medicine Insights: Oncology, 2014. 8: p. CMO-S18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, Hewitt SM, Anderson WF, Szeszenia-Dąbrowska N, Bardin-Mikolajczak A, & Zatonski W, Differences In risk factors for breast cancer molecular subtypes In a population-based study. Cancer Epidemiology and Prevention Biomarkers, 2007. 16(3): p. 439–443. [DOI] [PubMed] [Google Scholar]
- 37.Akinyemiju TF, Pisu M, Waterbor JW, & Altekruse SF, Socioeconomic status and Incidence of breast cancer by hormone receptor subtype. Springerplus, 2015. 4(1): p. 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turkoz FP, Solak M, Petekkaya I, Keskin O, Kertmen N, Sarici F, Arik Z, Babacan T, Ozisik Y, & Altundag K, Association between common risk factors and molecular subtypes In breast cancer patients. The Breast, 2013. 22(3): p. 344–350. [DOI] [PubMed] [Google Scholar]
- 39.Anderson KN, Schwab RB, & Martinez ME, Reproductive risk factors and breast cancer subtypes: A review of the literature. Breast Cancer Res Treat, 2014. 144: p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young JL Jr, Roffers SD, Ries LAG, Fritz AG, & Hurlbut AA (eds), SEER Summary Staging Manual - 2000: Codes and Coding Instructions. 2001, National Cancer Institute: Bethesda, MD. [Google Scholar]
- 41.US Environmental Protection Agency, Environmental Quality Index Overview Report. US EPA Office of Research and Development. EPA/600/R-14/305. 2014. [Google Scholar]
- 42.Rothman KJ, No adjustments are needed for multiple comparisons. Epidemiology, 1990: p. 43–46. [PubMed] [Google Scholar]
- 43.Surveillance, Epidemiology, and End Results (SEER) Program, in Research Data (1975-2016), D. National Cancer Institute, Surveillance Research Program, Editor. released April 2019, based on the November 2018 submission: www.seer.cancer.gov. [Google Scholar]
- 44.Carter CL, Allen C, & Henson DE, Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer, 1989. 63(1): p. 181–187. [DOI] [PubMed] [Google Scholar]
- 45.Kravchenko J, Rhew SH, Akushevich I, Agarwal P, & Lyerly HK, Mortality and Health Outcomes in North Carolina Communities Located in Close Proximity to Hog Concentrated Animal Feeding Operations. North Carolina Medical Journal, 2018. 79(5): p. 278–288. [DOI] [PubMed] [Google Scholar]
- 46.McLafferty S, Rural-Urban Disparities in Breast Cancer: Six Suppositions and Future Directions, in Geospatial Approaches to Energy Balance and Breast Cancer. 2019, Springer, Cham. p. 379–398. [Google Scholar]
- 47.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, & Jemal A, Breast cancer statistics, 2015: Convergence of incidence rates between black and white women: Breast cancer statistics, 2015. CA: a cancer journal for clinicians, 2016. 66(1): p. 31–42. [DOI] [PubMed] [Google Scholar]
- 48.DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, & Jemal A, Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities: Cancer statistics for African Americans, 2016. CA: a cancer journal for clinicians, 2016. 66(4): p. 290–308. [DOI] [PubMed] [Google Scholar]
- 49.Brulle RJ, & Pellow DN, Human health and environmental inequalities. Annu. Rev. Public Health, 2006. 27(103–124). [DOI] [PubMed] [Google Scholar]
- 50.Rauh VA, Landrigan PJ, & Claudio L, Housing and health: Intersection of poverty and environmental exposures. Annals of the New York Academy of Sciences, 2008. 1136(1): p. 276–288. [DOI] [PubMed] [Google Scholar]
- 51.White AJ, Keller JP, Zhao S, Carroll R, Kaufman JD, & Sandler DP, Air Pollution, Clustering of Particulate Matter Components, and Breast Cancer in the Sister Study: A US-Wide Cohort. Environmental health perspectives, 2019. 127(10): p. 107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.