Abstract
Both antiviral treatment with remdesivir and hemoadsorption using a CytoSorb® adsorption device are applied in the treatment of severe COVID‐19. The CytoSorb® adsorber consists of porous polymer beads that adsorb a broad range of molecules, including cytokines but also several therapeutic drugs. In this study, we evaluated whether remdesivir and its main active metabolite GS‐441524 would be adsorbed by CytoSorb®. Serum containing remdesivir or GS‐441524 was circulated in a custom‐made system containing a CytoSorb® device. Concentrations of remdesivir and GS‐441524 before and after the adsorber were analyzed by liquid chromatography‐tandem mass spectrometry. Measurements of remdesivir in the outgoing tube after the adsorber indicated almost complete removal of remdesivir by the device. In the reservoir, concentration of remdesivir showed an exponential decay and was not longer detectable after 60 mins. GS‐441524 showed a similar exponential decay but, unlike remdesivir, it reached an adsorption–desorption equilibrium at ~48 µg/L. Remdesivir and its main active metabolite GS‐441524 are rapidly eliminated from the perfusate by the CytoSorb® adsorber device in vitro. This should be considered in patients for whom both therapies are indicated, and simultaneous application should be avoided. In general, plasma levels of therapeutic drugs should be closely monitored under concurrent CytoSorb® therapy.
Keywords: COVID‐19, cytokine, hemoadsorption, remdesivir, SARS‐CoV‐2
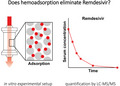
In vitro study evaluating the impact of the CytoSorb(R) adsorber device on Remdesivir and its active metabolite GS‐441524.
Abbreviations
- COVID‐19
coronavirus disease 2019
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
In humans, infection with the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causes coronavirus disease 2019 (COVID‐19), which can lead, in severe cases, to excessive inflammation and ultimately to life‐threatening pneumonia. Antiviral treatment with remdesivir, a nucleoside analogue hampering the viral RNA polymerase, was recommended for hospitalized patients with severe COVID‐19. 1 , 2 Beyond this, extracorporeal cytokine adsorption using a CytoSorb® adsorption device (CytoSorbents Corp., Monmouth Junction, New Jersey, USA) was suggested for mitigation of excessive inflammatory response in COVID‐19. 3 The CytoSorb® adsorber consists of porous polymer beads that adsorb molecules within the 5–55 kDa range, including cytokines, myoglobin, or bilirubin, but also therapeutic drugs. 4 It can be integrated in extracorporeal membrane oxygenation systems or continuous renal replacement therapy circuits and is used for different indications, including intoxications, rhabdomyolysis, liver failure, or septic shock. 4 Recently, the United States Food and Drug Administration has authorized the emergency use of the CytoSorb® adsorber for the treatment of COVID‐19 5 but there are no data available on potential interaction with remdesivir use. Thus, the aim of this study was to investigate whether remdesivir would be adsorbed by CytoSorb®.
2. MATERIAL AND METHODS
Remdesivir is a prodrug that is rapidly metabolized in humans to its main active metabolite GS‐441524. 6 To evaluate the capacity of the CytoSorb® hemoadsorption device to eliminate remdesivir or GS‐441524, we applied a previously published protocol. 7 , 8 A CytoSorb® device was integrated in a custom‐made system using a peristaltic pump (AlphaControl PSP‐V12G, Figure 1) and primed with fetal calf serum (Sigma‐Aldrich, Taufkirchen, Germany). Remdesivir (1200 µg/L) or GS‐441524 (200 µg/L, both Cayman Chemical, Ann Arbor, MI, USA) at concentrations similar to plasma levels observed in humans 6 was dissolved in 2,000 mL serum supplemented with NaF (2.5 g/L) for stabilization. The solutions were circulated from a reservoir at a flow rate of 200 mL/min for 60 mins at 36.5°C in two separate experiments. Samples (1 mL) were taken from the circuit simultaneously before (pre) and after (post) the adsorption device at 0, 5, 10, 15, 30, and 60 mins (Figure 1). The adsorption device was replaced after each experiment. Concentrations of remdesivir (linearity 25–1000 ng/mL, limit of detection 0.54 ng/mL, limit of quantification 1.1 ng/mL) and GS‐441524 (linearity 2.5–100 ng/mL, limit of detection 0.82 ng/mL, limit of quantification 0.84 ng/mL) were analyzed within 4 h by a liquid chromatography‐tandem mass spectrometry method after protein precipitation using D4‐remdesivir as internal standard (modified after 9 ). Half‐lives were calculated by a one‐phase exponential decay model (Y = (Y0‐NS)*exp(−K*X) + NS) using GraphPad Prism 9.0.0.
FIGURE 1.
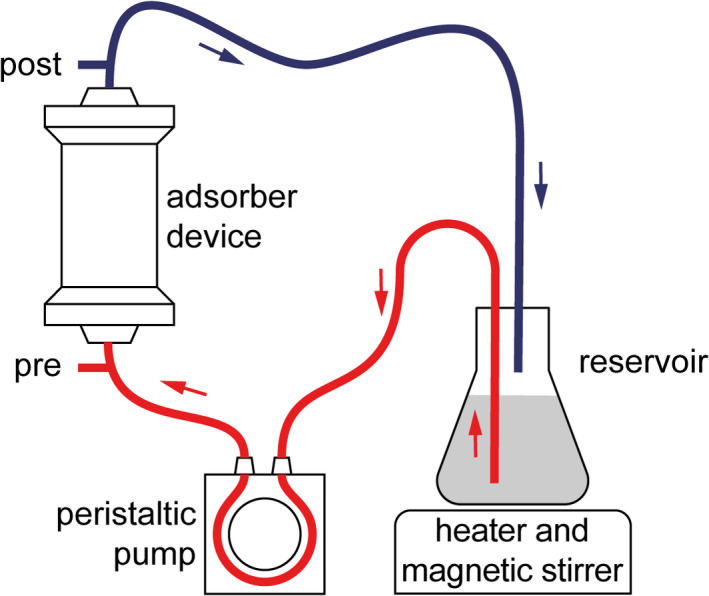
Experimental setup. A CytoSorb® device was integrated in a custom‐made system, and serum containing remdesivir or GS‐441524, respectively, was circulated from a reservoir at a flow rate of 200 mL/min. Samples were taken from the circuit before (pre) and after (post) the adsorber device at 0, 5, 10, 15, 30, and 60 minutes for quantification of remdesivir or GS‐441524
3. RESULTS
At 5, 10, 15, and 30 mins, concentrations of remdesivir in the outgoing tube after the adsorber were below 1% of the pre‐adsorber concentration, indicating almost complete removal of remdesivir by the device. In line with that, serial measurements revealed a one‐phase exponential decay of remdesivir in the reservoir with a calculated half‐life of 7.48 minutes (R2 = .997). After 60 mins, remdesivir was not longer detectable (Figure 2). GS‐441524 showed a similar exponential decay with a calculated half‐life of 8.22 mins (R2 = .972) but, unlike remdesivir, it was not completely removed. We observed an increase in the post‐adsorber concentration of GS‐441524, starting at 5 mins and reaching adsorption–desorption equilibrium at 60 mins (before 49.8 µg/L and after 46.6 µg/L; Figure 2).
FIGURE 2.
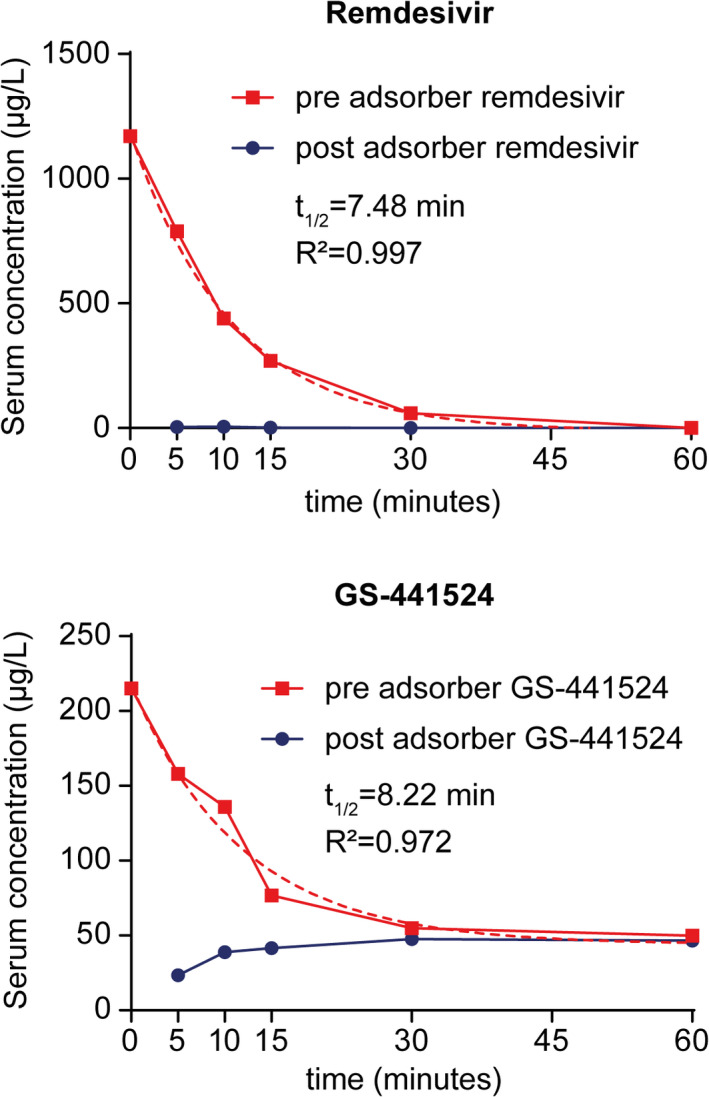
Adsorption of remdesivir or GS‐441524 in vitro. Concentrations of remdesivir or GS‐441524 in samples taken from the circuit before (pre) and after (post) the adsorber device were quantified by liquid chromatography with mass spectrometry detection. Dashed lines indicate calculated half‐life (t1/2)
4. DISCUSSION
Our results show that remdesivir and GS‐441524 are rapidly eliminated from the perfusate by the CytoSorb® adsorption device in an in vitro model. These observations should be considered when treating COVID‐19 patients with cytokine adsorption and remdesivir.
While remdesivir is rapidly metabolized, its main active metabolite GS‐441524 has a long plasma half‐life of ~24.5 h in humans. 6 Though metabolism of remdesivir in vivo is complex, our results suggest that concurrent adsorption therapy with CytoSorb® could significantly limit systemic exposure and thus antiviral effectiveness of GS‐441524. We observed a saturation plateau of GS‐441524 at ~48 ng/ml (~0.16 µM), which might be explained by a lower affinity to the adsorber of GS‐441524 compared to remdesivir. The remaining circulating levels were significantly below the effective antiviral concentration of GS‐441524 against SARS‐CoV‐2 (EC‐50 0.47 ‐ 1.09 µM) that has been determined in vitro. 10
The prognostic value of both, remdesivir and hemoadsorption, in the treatment of COVID‐19 is still under investigation. While a benefit of early use of remdesivir in hospitalized patients with severe symptoms is plausible, the benefit in later disease stages remains questionable at this time. 1 , 2 In contrast, hemoadsorption therapy may be limited to critical cases of COVID‐19 that are associated with a significant increase in cytokine levels. This, however, remains to be confirmed. 3
Possible interactions of different treatment regimen in COVID‐19 demand careful consideration. This is particularly true for antiviral drugs, for which significant drug–drug interactions have been described, 11 but also includes non‐pharmacological therapies. Our data suggest that concurrent use of remdesivir and hemoadsorption should rather be avoided and support the view that sequential use at different stages of COVID‐19 may be preferred. The results presented here complement previous reports describing desired or unwanted adsorption of various molecules by the CytoSorb® device. 7 , 8 , 12 , 13 , 14 , 15 In vitro data are available on a broad range of therapeutic drugs that are commonly used in intensive care medicine (Table 1). Drugs that are adsorbed differ in molecule size, solubility, and protein binding, making it difficult to make predictions about a particular substance. Therefore, the influence of hemoadsorption therapy on therapeutic drugs should be systematically assessed in vivo and closely monitored during clinical application.
TABLE 1.
Removal of therapeutic drugs by the CytoSorb® adsorption device in vitro
| Compound (PubChem ID) | Compound class | Molecular weight (g/mol) | Plasma protein binding | Water solubility | Adsorption in vitro | Ref. |
|---|---|---|---|---|---|---|
| Gentamicin (3467) | Aminoglycoside antibiotic | 477.6 | Low | Soluble | Poor | [8] |
| Amikacin (37768) | Aminoglycoside antibiotic | 585.6 | Low | Soluble | Poor | [8] |
| Netilmicin (441306) | Aminoglycoside antibiotic | 475.6 | Low | Soluble | Poor | [8] |
| Tobramycin (36294) | Aminoglycoside antibiotic | 467.5 | Low | Soluble | Poor | [8] |
| Phenytoin (1775) | Hydantoin derivate antiepileptic | 252.3 | High | Insoluble | Poor | [8] |
| Phenobarbital (4763) | Barbituric acid derivate | 232.2 | Moderate | Low | Moderate | [8] |
| Vancomycin (14969) | Glycopeptide antibiotic | 1449.2 | Moderate | Soluble | Significant | [8] |
| Teicoplanin (133065662) | Glycopeptide antibiotic | 1879.7 | High | Soluble | Significant | [8] |
| Digoxin (2724385) | Cardiac glycoside | 780.9 | Low | Low | Significant | [8] |
| Carbamazepine (2554) | Dibenzoazepine antiepileptic | 236.3 | High | Low | Significant | [8] |
| Valproic acid (3121) | Propylpentanoic acid derivate antiepileptic | 144.2 | High | Low | Significant | [8] |
| Theophylline (2153) | Xanthine derivative | 180.2 | Moderate | Low | Significant | [8] |
| Tacrolimus (445643) | Macrolide derivate | 804.0 | High | Insoluble | Significant | [8] |
| Everolimus (6442177) | Macrolide derivate | 958.2 | High | Low | Significant | [8] |
| Dabigatran (216210) | Thrombin inhibitor | 471.5 | Low | Low | Significant | [12] |
| Edoxaban (10280735) | Factor Xa inhibitor | 548.1 | Moderate | Low | Significant | [13] |
| Rivaroxaban (9875401) | Factor Xa inhibitor | 435.9 | High | Insoluble | Significant | [14] |
| Ticagrelor (9871419) | Adenosine nucleotide analogue platelet inhibitor | 522.6 | High | Low | Significant | [15] |
| Remdesivir (121304016) | Adenosine nucleotide analogue antiviral | 602.6 | Moderate to high | Insoluble | Significant | Biever et al |
| GS‐441524 (44468216) | Adenosine nucleotide analogue antiviral | 291.3 | Low | Insoluble | Significant | Biever et al |
Chemical properties were derived from PubChem database (https://pubchem.ncbi.nlm.nih.gov).
In summary, remdesivir and its main active metabolite GS‐441524 are rapidly eliminated from the perfusate by the CytoSorb® adsorber device in vitro. This should be considered in COVID‐19 patients for whom both therapies are indicated, simultaneous application should rather be avoided. Plasma levels of therapeutic drugs should be closely monitored under concurrent CytoSorb® therapy.
ACKNOWLEDGEMENTS
None.
CONFLICTS OF INTEREST
Paul Biever and Alexander Supady received speakers' honoraria from CytoSorbents, the manufacturer of the CytoSorb® device. The Department of Cardiology and Angiology I received a research grant from CytoSorbents not related to the present study.
AUTHOR CONTRIBUTIONS
Paul Biever, Dawid L. Staudacher, Merja A. Neukamm, Christoph Bode, Alexander Supady, and Achim Lother contributed to conception and design of the study and interpreted data. Michaela J. Sommer, Hannah Triebel, and Achim Lother performed experiments, and acquired and analyzed data. Paul Biever, Dawid L. Staudacher, Alexander Supady, and Achim Lother drafted the manuscript. Michaela J. Sommer, Hannah Triebel, Merja A. Neukamm, and Christoph Bode revised the manuscript. All authors gave final approval of the version to be published.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.
ETHICS STATEMENT
Not applicable.
Alexander Supady and Achim Lother contributed equally.
Funding information
The authors received no specific funding for this work.
REFERENCES
- 1. National Institutes of Health COVID‐19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines. https://files.covid19treatmentguidelines.nih.gov/guidelines/section/section_100.pdf. Accessed January 19, 2021. [PubMed]
- 2. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID‐19. https://www.idsociety.org/globalassets/idsa/practice‐guidelines/covid‐19/treatment/idsa‐covid‐19‐gl‐tx‐and‐mgmt‐v3.6.0.pdf. Accessed January 19, 2021. [DOI] [PMC free article] [PubMed]
- 3. Rieder M, Wengenmayer T, Staudacher D, et al. Cytokine adsorption in patients with severe COVID‐19 pneumonia requiring extracorporeal membrane oxygenation. Crit Care. 2020;24(1):435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poli EC, Rimmele T, Schneider AG. Hemoadsorption with CytoSorb® . Intensive Care Med. 2019;45(2):236‐239. [DOI] [PubMed] [Google Scholar]
- 5. United States Food and Drug Administration: CytoSorb® Emergency Use Authorization for Use in Patients with COVID‐19 Infection. https://www.fda.gov/media/136866/download. Accessed January 19, 2021.
- 6. Humeniuk R, Mathias A, Cao H, et al. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID‐19, in healthy subjects. Clin Transl Sci. 2020;13:896‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lang CN, Sommer MJ, Neukamm MA, et al. Use of the CytoSorb adsorption device in MDMA intoxication: a first‐in‐man application and in vitro study. Intensive Care Med Exp. 2020;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reiter K, Bordoni V, Dall'Olio G, et al. In vitro removal of therapeutic drugs with a novel adsorbent system. Blood Purif. 2002;20:380‐388. [DOI] [PubMed] [Google Scholar]
- 9. Alvarez JC, Moine P, Etting I, Annane D, Larabi IA. Quantification of plasma remdesivir and its metabolite GS‐441524 using liquid chromatography coupled to tandem mass spectrometry. Application to a Covid‐19 treated patient. Clin Chem Lab Med. 2020;58:1461‐1468. [DOI] [PubMed] [Google Scholar]
- 10. Pruijssers AJ, George AS, Schafer A, et al. Remdesivir inhibits SARS‐CoV‐2 in human lung cells and chimeric SARS‐CoV expressing the SARS‐CoV‐2 RNA polymerase in mice. Cell Rep. 2020;32:107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rezaee H, Pourkarim F, Pourtaghi‐Anvarian S, Entezari‐Maleki T, Asvadi‐Kermani T, Nouri‐Vaskeh M. Drug‐drug interactions with candidate medications used for COVID‐19 treatment: an overview. Pharmacology research & perspectives. 2021;9:e00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Angheloiu AA, Angheloiu GO. Removal of dabigatran using sorbent hemadsorption. Int J Cardiol. 2019;293:73‐75. [DOI] [PubMed] [Google Scholar]
- 13. Angheloiu AA, Tan Y, Ruse C, Shaffer SA, Angheloiu GO. In‐Vitro sorbent‐mediated removal of edoxaban from human plasma and albumin solution. Drugs in R&D. 2020;20:217‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koertge A, Wasserkort R, Wild T, Mitzner S. Extracorporeal hemoperfusion as a potential therapeutic option for critical accumulation of rivaroxaban. Blood Purif. 2018;45:126‐128. [DOI] [PubMed] [Google Scholar]
- 15. Angheloiu GO, Gugiu GB, Ruse C, Pandey R, Dasari RR, Whatling C. Ticagrelor removal from human blood. JACC Basic Transl Sci. 2017;2:135‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


