Abstract
Proton Magnetic Resonance Spectroscopy (1H-MRS) studies have demonstrated abnormal levels of a variety of neurometabolites in treatment-seeking individuals with moderate-severe Alcohol Use Disorder (AUD) following acute withdrawal. In contrast, few studies have investigated neurochemical changes across early abstinence in less-severe, treatment-naïve AUD. The present study, which represents the primary report of a research grant from ABMRF/The Alcohol Research Fund, measured dorsal anterior cingulate cortex (dACC) GABA, glutamate, and glutamine levels in treatment-naïve AUD (n=23) via three 1H-MRS scans spaced across a planned week of abstinence from alcohol. In addition to AUD participants, twelve light drinkers completed two scans, separated by 48 hours, to ensure that results in AUD were not produced by between-scan differences other than abstinence from alcohol. 1H-MRS spectra were acquired in dACC at each scan using 2D J-resolved Point Resolved Spectroscopy. Linear mixed modeling results demonstrated a significant increase in GABA, but not glutamate or glutamine (ps=0.237–0.626), levels between scans 1 and 2 (+8.88%, p = 0.041), with no difference between scans 2 and 3 (+1.00%, p = 0.836), in AUD but not LD (F=1.24, p=0.290) participants. Exploratory regression analyses tentatively revealed a number of significant prospective associations between changes in glutamine levels and heavy drinking, craving and withdrawal symptoms. Most notably, the present study demonstrated return from abnormally-low to normal GABA levels in treatment-naïve AUD within 3d of their last drink; the pattern of results was consistent with glutamate and glutamine disturbances being exclusive to relatively more-severe AUD.
INTRODUCTION
Preclinical research has long demonstrated that ethanol has acute facilitatory effects on GABAergic synapses (Lovinger, 1997) and acute inhibitory effects on glutamatergic synapses (Lovinger 1989; Lovinger et al., 1990). Compensatory adaptations that develop in response to chronic ethanol administration, and are believed to underlie the behavioral phenomena of ethanol tolerance and withdrawal, include: a) increased presynaptic glutamate release and b) increased postsynaptic function of NR2A and NR2B subunit-containing NMDA receptors, as well as c) increased postsynaptic expression of α4 subunit-containing, yet decreased expression of α1 subunit-containing, GABAA receptors and d) brain-region specific altered presynaptic GABA release and GABAB receptor function (reviewed in Roberto and Varodayan, 2017); investigation of infralimbic mPFC, for example, has revealed decreased presynaptic GABA release following chronic ethanol administration (Pleil et al., 2015). Unlike glutamate and GABA, glutamine, the critical amino acid precursor to both glutamate and GABA (Bak et al., 2006), has rarely been the subject of investigation of preclinical ethanol administration studies. Nevertheless, the available literature suggests increased brain glutamine concentrations in ethanol-fed rats (Lee et al., 2012) and decreased brain glutamine concentrations (Hermann et al., 2012), along with increased packing density of glutamine-synthetase astrocytes (Miguel-Hidalgo, 2006), during acute ethanol withdrawal.
Proton Magnetic Resonance Spectroscopy (1H-MRS) allows for the in vivo measurement of neurometabolite concentrations, including glutamate, using standard MRI hardware, and 1H-MRS studies demonstrating brain glutamate disturbances in individuals with Alcohol Use Disorders (AUD) have been reported for more than a decade now. When 1H-MRS investigations of fronto-cortical glutamate levels in individuals with Alcohol Use Disorder (AUD) are sorted by time since last drink, the literature generally demonstrates that glutamate levels in individuals with AUD are abnormally high during acute withdrawal (Hermann et al., 2012), and abnormally low one-week from last alcohol consumption (Mon et al., 2012, Thoma et al., 2011; c.f., Bauer et al., 2013). Short-term follow-up data from two studies have additionally demonstrated potential normalization of brain glutamate levels approximately 2–5 weeks from last alcohol consumption (Hermann et al., 2012, Mon et al., 2012). Finding a feasible temporal window in which to measure brain glutamate in recently drinking, yet not acutely intoxicated, individuals with moderate-severe AUD has proven extremely challenging because the duration between acute intoxication and acute withdrawal can be brief and variable. One investigation of recently-drinking, heavy drinkers who reported a loss of control over their drinking found that glutamate levels in frontal white matter were significantly reduced relative to both light drinkers and heavy drinkers not reporting loss of control over drinking (Ende et al., 2013).
Advances in MRI technology and 1H-MRS methodology have further enabled accurate, in vivo measurement of GABA and glutamine, concentrations in human brain tissue at commonly available, 3- to 4-Tesla, magnetic field strengths. 1H-MRS studies that have reported brain GABA and glutamine levels have been relatively rare, in part because measurement of these metabolites is more technically challenging relative to glutamate (see Figure 1a). Nonetheless, whereas one early small study supported decreased GABA levels in individuals with AUD relative to controls (Behar et al., 1999), subsequent larger studies have failed to find significant differences in GABA levels between AUD and controls (Mason et al., 2006, Mon et al., 2012, Abe et al., 2013). Though not conducted in individuals with diagnosable AUD, a recent study of “emerging adults” (aged 18–24 years) found significantly decreased ACC GABA levels in binge alcohol drinking vs. light drinking participants (Silveri et al., 2014). Only one published study has reported significant differences in brain glutamine levels between AUD and controls, suggesting significantly higher glutamine levels in AUD participants (Thoma et al., 2011). Although this finding is potentially consistent with findings from the preclinical literature, it is unlikely that the method of 1H-MRS acquisition used in that study (i.e., Point Resolved Spectroscopy; PRESS) is capable of validly quantifying glutamine levels at physiological concentrations and commonly available (e.g., 3- to 4-Tesla) magnetic field strengths (Henry et al., 2011). Fortunately, more advanced 1H-MRS acquisition methods (e.g., 2D J-resolved PRESS), capable of reliably and validly separating glutamate, glutamine, and GABA signals, are now available (Prescot and Renshaw, 2013; see Figure 1b).
Figure 1.
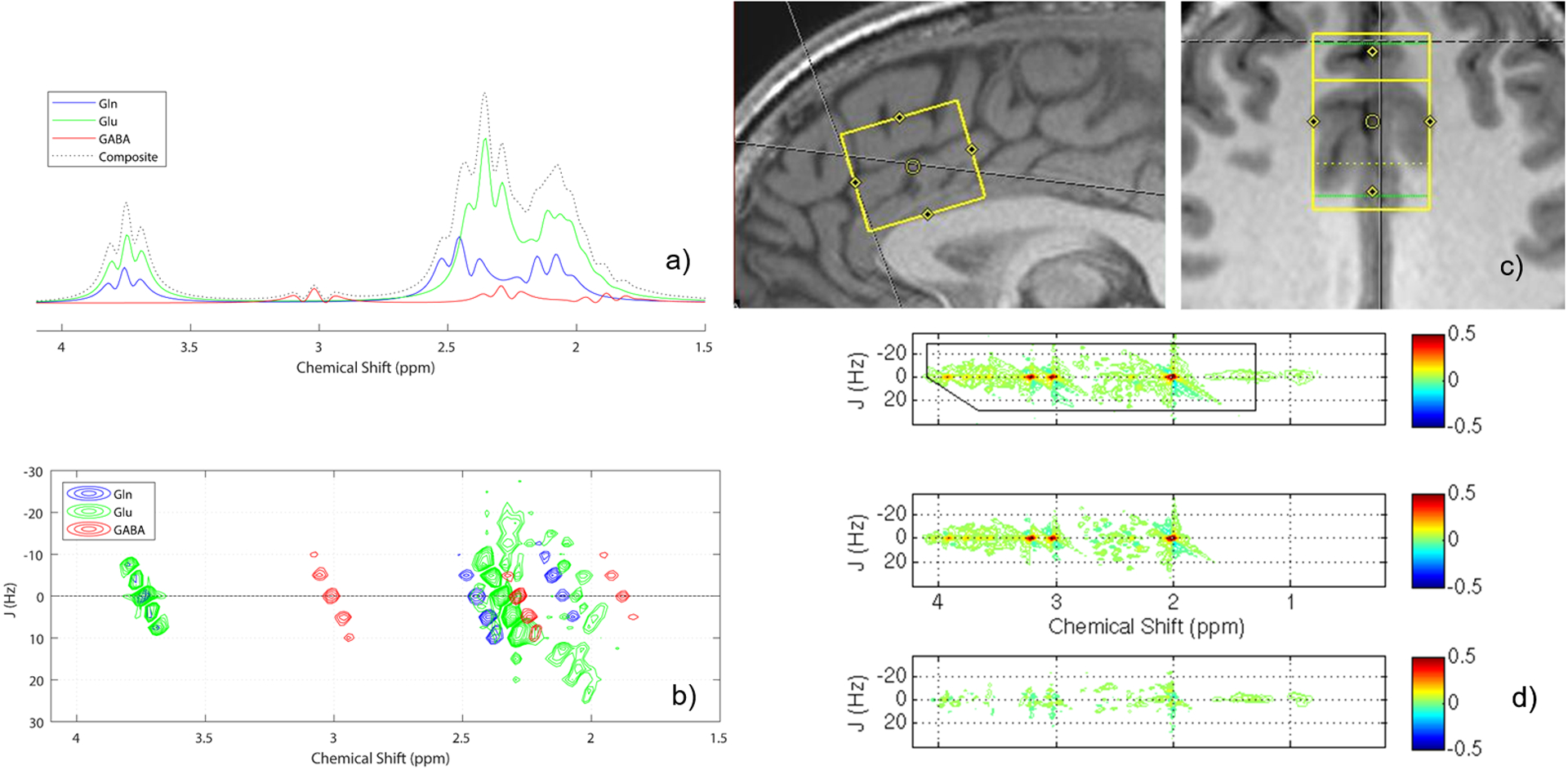
(a) 1D PRESS 1H-MRS spectra (TE = 31 ms) simulated for Gln, Glu, and GABA (B0 = 2.89 T, exponential line broadening = 7 Hz). The data were generated assuming a physiological realistic in vivo metabolite Gln:Glu:GABA ratio of 4:8:1, and the summed composite spectrum also is displayed. (b) 2D J-resolved 1H-MRS spectral fits for Gln, Glu, and GABA, resulting from ProFit analysis of the in vivo dataset presented in (c). Due to unfavorable chemical shift dispersion at 3.0 Tesla, the 1D 1H-MRS spectra show significant peak overlap between Gln and Glu resonances at all chemical shifts (2.0 – 2.5 ppm and 3.75 ppm). For 2D J-resolved 1H-MRS, the encoding of a second frequency dimension acts to separate all J-coupled metabolite resonances over a 2D surface and results in an improved peak separation for Gln and Glu resonances, especially at their 2.0 – 2.5 ppm chemical shift positions. Note that 2D J-resolved 1H-MRS also improves the discrimination of GABA resonances from Glu at ~1.89 ppm when compared to the 1D 1H-MRS situation. (c) Sagittal and axial views of a sample dACC voxel acquisition, and (d) corresponding raw (top), fitted (middle), and residual (bottom) 2D J-resolved 1H-MRS spectra analyzed using Prior Knowledge Fitting (ProFit).
While the 1H-MRS studies reviewed above have been invaluable to our understanding of AUD in humans, the vast majority of them have been cross-sectional (notable exceptions: Hermann et al., 2012, Mon et al., 2012, Mason et al., 2006) and have focused on individuals with severe AUD in treatment. The present study represents an extension of Prisciandaro and colleagues (2016, 2019) reports, which demonstrated a significant inverse correlation between glutamate and recent heavy drinking in treatment-naïve individuals with AUD as well as significantly lower levels of GABA and glutamine in this population relative to light drinkers. Unlike most existing 1H-MRS studies of AUD, the present study focused on non-severe, non-treatment-seeking individuals with AUD, allowing us to determine whether the disturbances in neurometabolite concentrations reported in individuals being treated for severe AUD generalize to this less severe, untreated population. Most importantly, the present study investigated longitudinal changes in glutamate, GABA, and glutamine levels using 2D J-resolved PRESS methods across multiple scans in both AUD and light drinkers, with the timing of AUD scans strategically placed (<1 day, 3 days, and 7 days post-final drink) relative to a monitored episode of abstinence from alcohol.
Given the dearth of 1H-MRS studies that have been conducted in relatively low severity, treatment-naïve AUD, our predictions were necessarily tentative. For example, although research on severe treatment-seeking individuals with AUD would have predicted elevated glutamate levels (corresponding with observable alcohol withdrawal symptoms) at <1d and/or 3d post final drink, followed by decreased glutamate levels at 7d post final drink, the population at hand was unlikely to report significant alcohol withdrawal symptoms (Prisciandaro et al., 2016). In contrast, given that the two available 1H-MRS studies of relatively low severity, treatment-naïve AUD individuals have both found them to have significantly lower levels of prefrontal GABA relative to light drinkers (Prisciandaro et al., 2019, Silveri et al., 2014), we predicted that initial GABA levels would be significantly lower in AUD versus LD and that there would be significant change in GABA levels across scans in AUD but not LD. Prospective associations between changes in GABA, glutamate, and glutamine levels and changes in alcohol consumption, craving, and withdrawal across scan visits were also explored.
METHOD
Participants
Twenty-three individuals with AUD and 12 demographically-matched LD were recruited from community advertisements; an additional 8 LD participants (i.e., females with relatively fewer years of education) were selected from Prisciandaro and colleagues’ LD sample (Prisciandaro et al., 2018) to demographically-match LD and AUD participants for baseline group comparisons. All participants were required to be between the ages of 21 and 40. AUD individuals were also required to meet criteria for DSM-IV diagnostic criteria for alcohol dependence (including the “loss of control over drinking” and/or “inability to cut-down or stop drinking” criteria), to report consuming at least 20 total drinks, with at least one heavy drinking day (i.e., ≥ 5/4 drinks in a day for men/women), in each of the two weeks preceding the study, and to not be actively seeking AUD treatment. Light-drinking individuals were required to not meet DSM-IV diagnostic criteria for alcohol dependence, to report drinking fewer than 14 drinks (7 for women) in each of the two weeks preceding the study, and to have a negative result (i.e., ≤ 1.6 IU) for disialo carbohydrate-deficient transferrin (%dCDT), a biomarker for recent heavy drinking (Helander et al., 2016). LD participants who reported clinically significant alcohol-related problems, or seeking treatment for alcohol abuse, at any time were excluded. For both groups, exclusion criteria included current DSM-IV Axis I disorder other than alcohol dependence or nicotine dependence, positive urine drug or alcohol breath screens on the day of the scan, and/or history of severe alcohol withdrawal (seizure, delirium tremens, need for inpatient or outpatient detoxification), or current alcohol withdrawal symptoms (i.e., Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar; (Sullivan et al., 1989)] > 3). Additional MRI-related exclusion criteria included presence of non-MRI safe objects in the body, claustrophobia, history of traumatic brain injury, or pregnancy.
Procedure
Demographic and diagnostic (via the Structured Clinical Interview for DSM-IV; (First, 1998)) information, along with the Alcohol Dependence Scale (Skinner and Horn, 1984) and the Obsessive Compulsive Drinking Scale (OCDS; Anton et al., 1996), was collected during baseline assessment. Following this assessment, AUD participants were invited to return for 3 MRI scans following (i.e., the next day [approximately 12 hours following], 3 days following, and 7 days following) an anticipated upcoming drinking day. The spacing of appointments was chosen to both roughly match the spacing of rodent scans in Hermann et al., 2012 and to ensure that at least the first 2 scans were squarely within the alcohol consumption detection window of Ethyl glucuronide (EtG, see below; Lowe et al., 2015). Following their initial MRI scan, AUD participants were asked to refrain from drinking until the end of the study. Drinking status was verified via TLFB and urine EtG, with threshold set at 200 ng/ml in order to provide reasonably balanced sensitivity and specificity (Lowe et al., 2015), values at each MRI visit. Specifically, AUD participants were required to have positive EtG and TLFB (past day) measures at scan 1, but negative EtG and TLFB measures at scans 2 and 3. In addition to these assessments, participants were administered a pregnancy test (females), the Alcohol Urge Questionnaire (AUQ; Bohn et al., 1995), and the Alcohol Withdrawal Symptoms Checklist (AWSC; Pittman et al., 2007) at every visit.
LD participants completed the same assessments as AUD participants. Unlike AUD participants, however, LD participants were not restricted in terms of time since last drink preceding their first scan. LD participants recruited for the present study (n=12) additionally completed a second scan, scheduled to mirror the approximately 48-hour interscan interval maintained by AUD participants between scans 1 and 2. Like AUD participants, these LD participants were required to remain abstinent from alcohol between scans 1 and 2. The purpose of this second LD scan was to ensure that results in AUD were not produced by between scan differences other than abstinence from alcohol (e.g., scanner drift, time of day).
All participants completed MRI scans on a 3T Siemens TIM TRIO, including a 2D J-resolved PRESS acquisition (TR/TE=2000/31–229ms; ΔTE=2ms; 4 signal averages per TE step with online averaging; 2D spectral width=2000×500 Hz; 2D matrix size=2048×100; total acquisition time=13:28 min (Prescot and Renshaw, 2013); water unsuppressed 2D 1H-MRS data were also acquired from the ACC voxel with 2 signal averages recorded for each TE step (total acquisition time=3:28 min)) in a 25×25×30mm3 voxel in the dorsal anterior cingulate cortex (dACC; as per Hermann et al., 2012)); see Figure 1c for a sample voxel placement. Prior to MRS acquisition, structural scans were taken for 1H-MRS voxel placement and tissue segmentation (TR/TE=1900/2.26ms; FOV=256mm2; flip angle=9°; spatial resolution=1.0 × 1.0 × 1.0 mm).
1H-MRS Post-processing
Skull stripping and whole brain tissue-type segmentation were performed on MP-RAGE images using the FSL BET and FAST tools (Smith et al., 2004). In-house MATLAB functions were used to extract the 3D volume corresponding to the positioned MRS voxel to obtain within-voxel gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) tissue content for each subject. Eddy currents and residual water were removed using in-house MATLAB functions. Subsequently, the ProFit algorithm was applied using software-supplied 2D basis sets (Schulte and Boesiger, 2006). Prior to Fourier transformation, the raw 2D matrix was zero-filled. Cramer-Rao Lower Bound (CRLB) values, which reflect the uncertainty of estimated model parameters, were provided by the ProFit software. Estimated metabolite peak areas were normalized to the unsuppressed water signal. Finally, metabolite/water ratios were corrected for within-voxel CSF fraction (Prescot and Renshaw, 2013); see Figure 1d for a sample fitted 2D spectrum corresponding to the acquisition depicted in Figure 1c.
Analytic Plan
First, analyses were conducted using AUD (n=23) and LD (n=20) baseline and scan 1 data to evaluate the replicability of results reported in Prisciandaro and colleagues (2016, 2019). Specifically, following characterization of demographic and clinical variables between groups, general linear models (GLM) were evaluated, one per estimated metabolite (glutamate, glutamine, GABA), with group (AUD vs. LD) included as a between-subjects factor. Within-voxel gray matter tissue fraction, expressed as the ratio of gray matter to brain matter (i.e., GM/[GM + WM] = GMBM), was entered as a covariate in each model to adjust coefficients for individual differences in within-voxel tissue composition. Finally, demographic variables that differed between AUD and LD participants at baseline (p < 0.10) were also considered as covariates. Following these analyses, within-group correlations were calculated between glutamate levels and number of heavy drinking days, with heavy drinking days defined as ≥ 4 drinks/day for women and ≥ 5 drinks/day for men (NIAAA, 2006), over the preceding 14 days.
Second, within-subject, between-scan changes in dACC GABA, glutamate, and glutamine concentrations were examined with scan/visit number included as a within-subject factor using linear mixed modeling. This analysis was conducted first for AUD participants, followed by LD participants recruited for the present study (n=12), because we lacked sufficient statistical power to formally evaluate potential between-group differences in within-subject metabolite change (i.e., a group by scan/visit interaction). Within-subject F-statistics with corresponding p < 0.10 values for a given metabolite were followed by pairwise comparisons (evaluated at p < 0.05). Bayes factors were calculated for non-significant findings to aid in interpretation. With the null test value set to “0,” convincing evidence of lack of association/difference was reflected by Bayes factors (i.e., K) > 3.16 (i.e., > 101/2; Rouder et al., 2009). An alpha level of p < 0.05 was adopted across models.
Third, exploratory prospective associations between GABA, glutamate, and glutamine levels and self-reported alcohol drinking, craving, and withdrawal symptoms were estimated using linear regression methods: 1) Historical measures of drinking (TLFB) and alcohol craving (OCDS, total score, minus alcohol consumption items 7 and 8) collected at baseline were examined as predictors of change in GABA, glutamate, and glutamine levels between scans 1 and 2 (i.e., scan 2 level minus scan 1 level), controlling for scan 1 metabolite levels. 2) Change in GABA, glutamate, and glutamine levels between scans 1 and 2 were, in turn, explored as predictors of change in self-reported craving (AUQ) and withdrawal symptoms (AWSC) between scans 2 and 3 (i.e., scan 3 AUQ/AWSC minus scan 2 AUQ/AWSC), controlling for scan 2 AUQ/AWSC scores.
RESULTS
Baseline Comparison of AUD vs. LD
See Table 1 for demographic and alcohol use characteristics of the sample. AUD (n=23) and LD (n=20) participants did not significantly differ on any demographic variable (notwithstanding trend-level [0.05 > p < 0.10] differences on age and education), but differed significantly on alcohol use characteristics, as would be expected. CRLBs for metabolite concentration estimates were < 20% and did not significantly differ between AUD (GABA M[SD]% = 7.6[3.9], glutamate M[SD]% = 1.5[0.3], glutamine M[SD]% = 6.2[2.9]) and LD (GABA M[SD]% = 5.9[1.8], glutamate M[SD]% = 1.6[0.8], glutamine M[SD]% = 5.5[2.5]) groups (GABA p = 0.168, glutamate p = 0.547, glutamine p = 0.520). There was a significant difference in average within-voxel gray matter to brain matter tissue fraction (i.e., gray matter / [gray matter / white matter]) between AUD (62.41%) and LD (64.70%; t [41] = 2.66, p=0.011).
Table 1.
Demographic and alcohol use characteristics of the sample
| Variable | Alcohol Use Disorder (n = 23) No. (%) of participants / Mean (SD) |
Light Drinker (n = 20) No. (%) of participants / Mean (SD) |
p |
|---|---|---|---|
| Gender (male) | 18 (78.3%) | 12 (60.0%) | 0.193 |
| Smoking Status (non-smoker) | 22 (95.7%) | 20 (100.0%) | 0.345 |
| Race (Caucasian) | 20 (87.0%) | 15 (75.0%) | 0.469 |
| Age | 27.00 (5.98) | 24.30 (3.16) | 0.065 |
| Education (no. of years) | 14.30 (2.12) | 15.30 (1.26) | 0.068 |
| Alcohol Dependence Scale | 11.95 (5.13) | 2.80 (2.88) | <0.001 |
| # Heavy drinking days (past 14) | 7.81 (3.20) | 0.47 (0.77) | <0.001 |
| Drinks per drinking day | 7.23 (3.37) | 2.24 (1.17) | <0.001 |
| Days since last drink | 1.00 (0.00) | 5.10 (7.64) | 0.027 |
| OCDS (total) | 4.09 (3.13) | 0.50 (1.00) | <0.001 |
| Alcohol Urge Questionnaire | 18.35 (9.11) | 10.30 (2.43) | <0.001 |
| Alcohol Withdrawal Symptoms Checklist | 3.39 (4.69) | 0.00 (0.00)a | <0.001 |
Note:
The Alcohol Withdrawal Symptoms Checklist was only administered to the subsample of n=12 Light Drinker participants who were invited to complete a second scan. “Smoker” was defined as an individual who reports smoking ≥ 10 cigarettes/day. OCDS scores were calculated following removal of alcohol consumption items (i.e., items 7 and 8). Significant p values (i.e., p < 0.05) have been bolded.
Age was not significantly associated with metabolite concentrations (p > 0.10) in any of the GLM models and was therefore removed from all final models. In contrast, education was significantly associated with GABA (F=4.97, p=0.031), but not glutamate or glutamine (p > 0.1), concentrations and was therefore retained in the final GABA model. Also, GMBM was significantly associated with glutamate (F=6.86, p=0.012), but not GABA or glutamine (p > 0.1), concentrations and was therefore retained in the final glutamate model. As predicted, dACC GABA levels were significantly different between AUD (M=0.55, SD=0.13) and LD (M=0.61, SD=0.10; Cohen’s D=0.52, F=4.81, p=0.034) participants. However, AUD and LD participants did not differ in terms of their dACC glutamate (AUD: M=4.29, SD=0.65; LD: M=4.19, SD=0.68; Cohen’s D=0.15, F=2.18, p=0.148) or glutamine (AUD: M=1.00, SD=0.21; LD: M=1.01, SD=0.19; Cohen’s D=0.05, F=0.08, p=0.781) levels. Association between number of heavy drinking days in the preceding 14 days was significantly inversely associated with glutamate concentrations in AUD (r = −0.55, p = 0.012; Figure 2), but not LD (r = −0.14, p = 0.561), participants. In sum, AUD participants demonstrated significantly lower levels of GABA, but not glutamate nor glutamine, relative to LD participants.
Figure 2.
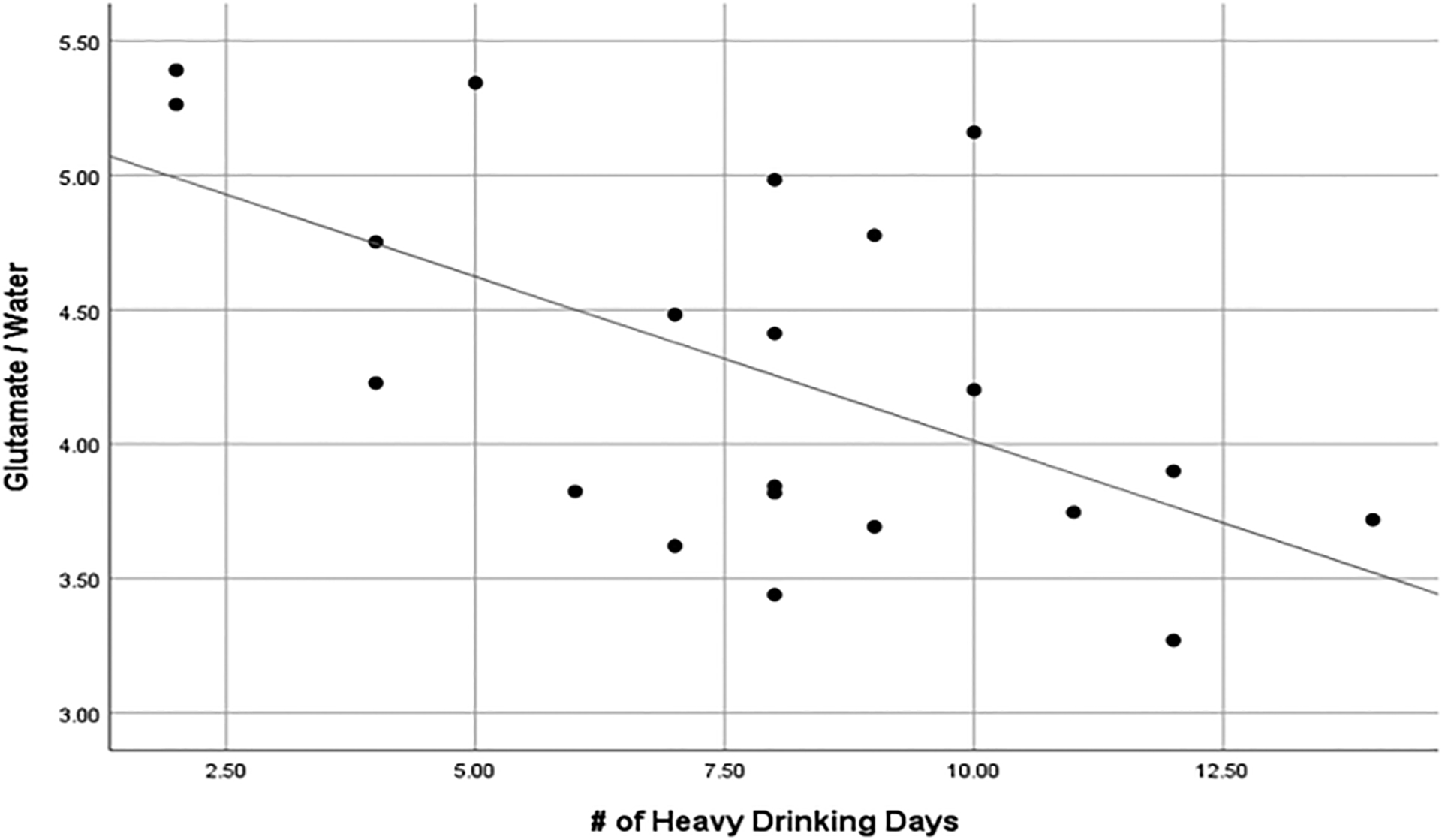
Scatterplot with linear fit line of the significant inverse association between number of heavy drinking days (in the 14 days preceding scan 1) and dACC glutamate/water levels in AUD participants (n=23) <1d following their last drink.
Between-scan Change in GABA, Glutamate, and Glutamine Levels
All AUD (n=23) and LD (n=12) participants completed both scans 1 and 2. However, 2 AUD participants were lost to follow-up between scans 2 and 3. All available data were utilized in estimated linear mixed models. Only GABA passed the necessary threshold required to evaluate pairwise (between-scan) comparisons in AUD participants (F=2.89, p=0.077). These comparisons revealed a significant difference in GABA levels between scan 1 and scan 2 (mean Δ = +0.05 [+8.88%], p = 0.041), no difference between scan 2 and 3 (mean Δ < +0.01 [+1.00%], p = 0.836), and a trend-level difference between scan 1 and scan 3 (mean Δ = +0.06 [+10.00%], p = 0.087). Concerning this latter finding, although the between-subject variability of GABA levels at scan 3 was not larger than the variability at scans 1 or 2, the between-subject variability of changes in GABA levels between scans 1 and 3 (SDΔ = 0.14) was larger than the variability of changes in GABA levels between scans 1 and 2 (SDΔ = 0.11); the largest contributor to this phenomenon was most likely variability in changes (both increases and decreases) in GABA levels between scans 2 and 3 (SDΔ = 0.14).
There was no evidence for significant between-scan change in LD participants’ GABA levels (F=1.24, p=0.290). As anticipated, days since last drink was not significantly associated with GABA levels in LD participants (p = 0.14). Additionally, the Bayes factor representing the difference in GABA levels between AUD and LD participants at scan 2 (i.e., K = 3.66) further supported the interpretation that AUD participants’ GABA levels normalized by 3 days following their last drink. Although, as discussed earlier, the degree of between-scan change in GABA levels experienced by AUD versus LD participants was not formally tested, it is important to note that mean change in GABA levels from scan 1 to scan 2 differed in direction as well as degree between AUD (i.e., significant increase) and LD (i.e., non-significant decrease) participants. A plot of GABA means (by participant group and scan number) with standard error bars is provided in Figure 3, and a plot of glutamate and glutamine means with standard error bars is provided in the accompanying Online Supplement.
Figure 3.
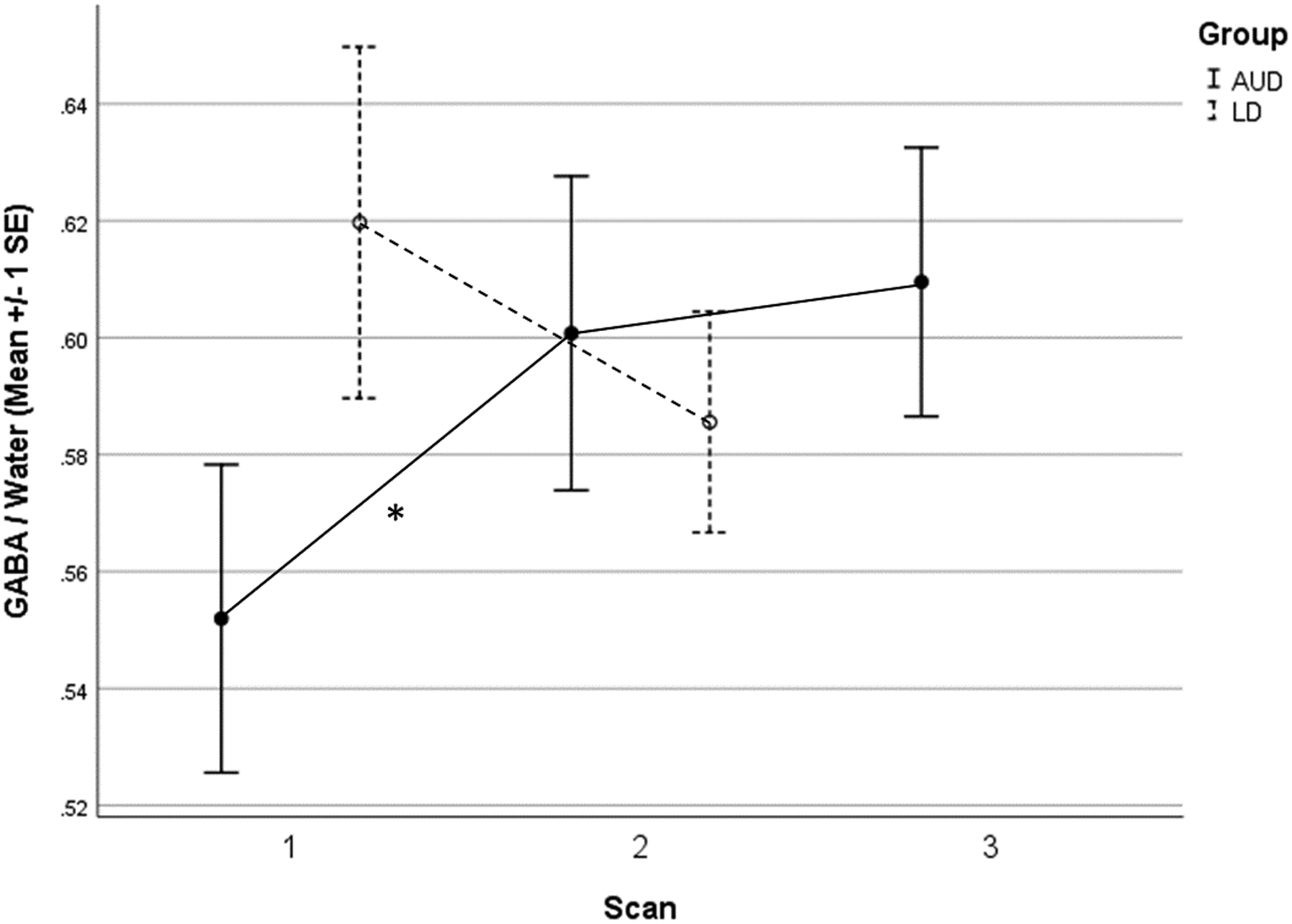
Average (+/− 1 SE) dACC GABA/water levels in AUD (n=23) and LD (n=12) participants across three 1H-MRS scans: <1d, 3d, and 7d following final alcohol consumption in AUD participants, with equivalent inter-scan interval between scans 1 and 2 in LD participants.
Unlike for GABA, there was no evidence for significant between-scan change in glutamate or glutamine levels in either AUD (glutamate: F=0.65, p=0.532; glutamine: F=0.48, p=0.626) or LD (glutamate: F=0.58, p=0.461; glutamine: F=1.57, p=0.237) participants. Furthermore, Bayes factors for differences in AUD participants’ glutamate (i.e., K1:2 = 6.05, K2:3 = 5.09) and glutamine (i.e., K1:2 = 5.34, K2:3 = 5.16) levels between each pair of scans uniformly supported the interpretation that AUD participants’ glutamate and glutamine levels did not change between scans. A plot of glutamate and glutamine means (by participant group and scan number) with standard error bars is provided in the accompanying Online Supplement.
Exploratory Prospective Associations between GABA, Glutamate, and Glutamine and Alcohol Drinking, Craving, and Withdrawal Symptoms
Baseline levels of heavy drinking (TLFB; β= −0.43, t=2.86, p=0.010) and alcohol craving (OCDS; β= −0.43, t=2.51, p=0.021) significantly predicted change in glutamine levels between scan 1 and scan 2, controlling for scan 1 glutamine levels. In contrast, baseline levels of alcohol craving failed to predict change in GABA (β= 0.35, t=1.86, p=0.078) or glutamate (β= 0.11, t=0.67, p=0.511) levels, and baseline levels of heavy drinking similarly failed to predict change in GABA (β= −0.24, t=1.12, p=0.279) or glutamate (β= −0.09, t=0.43, p=0.670) levels, between scan 1 and scan 2, controlling for baseline levels.
Averaging across AUD participants, neither alcohol craving (AUQ: F=0.61, p=0.551) nor withdrawal symptoms (AWSC: F=0.27, p=0.768) significantly changed across scans. Nonetheless, change in glutamine levels between scan 1 and scan 2 significantly predicted change in self-reported craving (AUQ; β= −0.43, t=2.18, p=0.043) and alcohol withdrawal symptom (AWSC; β= −0.46, t=2.23, p=0.038) scores between scan 2 and scan 3. In contrast, change in GABA levels between scan 1 and scan 2 failed to predict change in self-reported craving (β= −0.24, t=1.12, p=0.276) or alcohol withdrawal symptoms (β= −0.08, t=0.34, p=0.740), and change in glutamate levels between scan 1 and scan 2 similarly failed to predict change in self-reported craving (β= −0.23, t=1.07, p=0.297) or alcohol withdrawal symptoms (β= 0.25, t=1.12, p=0.276), between scan 2 and scan 3. In sum, glutamine, but not GABA nor glutamate, was prospectively associated with alcohol drinking, craving, and withdrawal symptoms in these exploratory analyses.
DISCUSSION
The primary finding from the present study was that dACC GABA levels in relatively low-severity, treatment-naïve AUD individuals a) were abnormally low <1d post final alcohol consumption (i.e., at ‘scan 1’, consistent with Silveri et al., 2014, Prisciandaro et al., 2019, and Pleil et al., 2015), b) significantly increased, approximately 10% on average, between <1d and 3d (i.e., at ‘scan 2’) post final drink to light drinker levels, then c) remained essentially unchanged between days 3 and 7 (i.e., at ‘scan 3’). This finding suggests a relatively early normalization of disrupted prefrontal GABA levels following termination of drinking in relatively low-severity AUD. Consistent with this finding, Gomez and colleagues (2012) reported significant (M = 13%) transient decreases in occipital GABA levels with intravenous ethanol administration in light drinkers. As noted by Gomez and colleagues (2012), alcohol-induced suppression of brain GABA levels may be mediated by the suppressing effects of alcohol on glutamic acid decarboxylase activity (the primary GABA synthesizing enzyme, Seilicovich et al., 1985). It is unclear how this phenomenon may translate to individuals with severe AUD, as we are not aware of any 1H-MRS studies that have reported brain GABA levels within the first week or two of abstinence in that population.
In contrast to GABA, mean-level changes in dACC glutamate levels across scans were not detected in either AUD or light drinkers. As noted earlier, in both preclinical and clinical studies, the most dramatic disturbances in glutamate levels have been found in states of acute alcohol withdrawal (Hermann et al., 2012), and alcohol withdrawal symptoms themselves have been proposed to primarily be the direct result of a hyper-glutamatergic state caused by acute abstinence in individuals with moderate-severe AUD (Roberto and Varodayan, 2017). In the present study, self-reported alcohol withdrawal symptoms were very minimal and did not change over the course of the week of monitored abstinence. As such, results from the present study may suggest that a substantial hyper-glutamatergic state does not occur with abstinence in relatively low-severity AUD. Although this may explain why elevated glutamate levels were not found in the present study, several studies have additionally found moderate-severe AUD individuals not experiencing acute withdrawal symptoms (e.g., 1-week post abstinence) to have significantly lower levels of prefrontal glutamate relative to controls (Mon et al., 2012, Thoma et al., 2011, Ende et al., 2013). It is possible that such glutamate deficiencies are only found in heavier-drinking AUD individuals than were included in the present study. In support of this explanation, we replicated our previous finding of a significant, inverse association between recent heavy drinking and dACC glutamate levels (Prisciandaro et al., 2016) in the present sample. In other words, AUD individuals who reported relatively more heavy drinking days in the 2-weeks preceding their first scan had significantly lower levels of dACC glutamate. Other potential explanations for our failure to find significant changes in dACC glutamate during early abstinence, that are commonly found in studies of treatment-seeking AUD individuals, may be found in the characteristically older age, which confers both increased years of alcohol abuse and an increased number of lifetime withdrawal episodes, and increased rate of cigarette smoking found in treatment-seeking AUD individuals (Meyerhoff et al., 2013).
Finally, mean-level changes in glutamine levels across scans were not detected, However, exploratory regression analyses revealed a number of interesting prospective associations observed between changes in glutamine levels between scans and self-reported alcohol consumption, craving, and withdrawal symptoms. Specifically, both recent heavy drinking and alcohol craving at baseline significantly predicted changes in dACC glutamine levels between scans 1 and 2, such that AUD individuals with relatively lower levels of heavy drinking/craving at baseline were more likely to experience relatively larger increases in glutamine levels (bringing them in range with light drinkers). Conversely, changes in glutamine levels between scans 1 and 2 significantly predicted changes in self-reported alcohol craving and withdrawal symptoms between scans 2 and 3, such that individuals with relatively larger increases in glutamine levels between scans 1 and 2 were more likely to experience relatively larger decreases in craving and withdrawal symptoms between scans 2 and 3. Though intriguing, given the exploratory nature of the analyses that revealed these associations, they should be considered tentative until replicated.
Unlike Prisciandaro and colleagues (2019), no differences were found in glutamine levels between AUD and light drinkers at the initial scan. Although we can only speculate as to why this finding did not replicate in the present sample, we would concentrate initial speculation on the primary difference between the two studies: number of days since last drink at baseline. Specifically, whereas the present study scanned all AUD participants within 24 hours of their last drink, Prisciandaro and colleagues (2019) scanned AUD participants within an average (SD) of 2.45 (1.23) days since their last drink. Further studies would be needed to determine whether dACC glutamine levels exhibit dynamics during early abstinence that eluded the experimental design of the present study.
Although the present study benefited from a number of strengths (e.g., 3 scans/AUD participant, biochemical verification of abstinence, valid measurement of brain glutamine levels), results should nonetheless be interpreted in light of several limitations. First, although consistent with similar 1H-MRS studies of AUD, the present study included relatively small samples, particularly concerning LD participants. Second, although days since last drink was not significantly associated with GABA levels in LD participants, comparison between AUD and LD participants would have been cleaner if both groups had been matched on number of days since last drink at the initial scan. Third, although the 2D J-resolved PRESS 1H-MRS sequence used in the present study allowed for simultaneous acquisition of GABA, glutamate, and glutamine data, existing analytic methods available for the sequence did not allow for separation of co-edited macromolecules from the observed GABA signal. It is important to note that the most common implementation of the MEGA-PRESS sequence similarly does not allow for separation of macromolecules from the GABA signal. Also, it is unlikely that this limitation substantially impacted our findings, as most analyses were within-subjects, with each AUD participant acting as his/her own control.
These limitations notwithstanding, the present study significantly extends the literature by demonstrating roughly 10% average increases in dACC GABA levels over the course of 7d of abstinence from alcohol in relatively low-severity, treatment-naïve individuals with AUD. Results from the present study may also suggest that prefrontal glutamine levels may serve as important predictors of changes in alcohol craving and withdrawal symptoms across early abstinence from alcohol, and that significantly abnormal glutamate and glutamine levels typically linked to alcohol withdrawal symptoms may only be found in individuals with relatively more-severe AUD. Further research is needed to examine the reproducibility of these findings.
Supplementary Material
Figure 4.
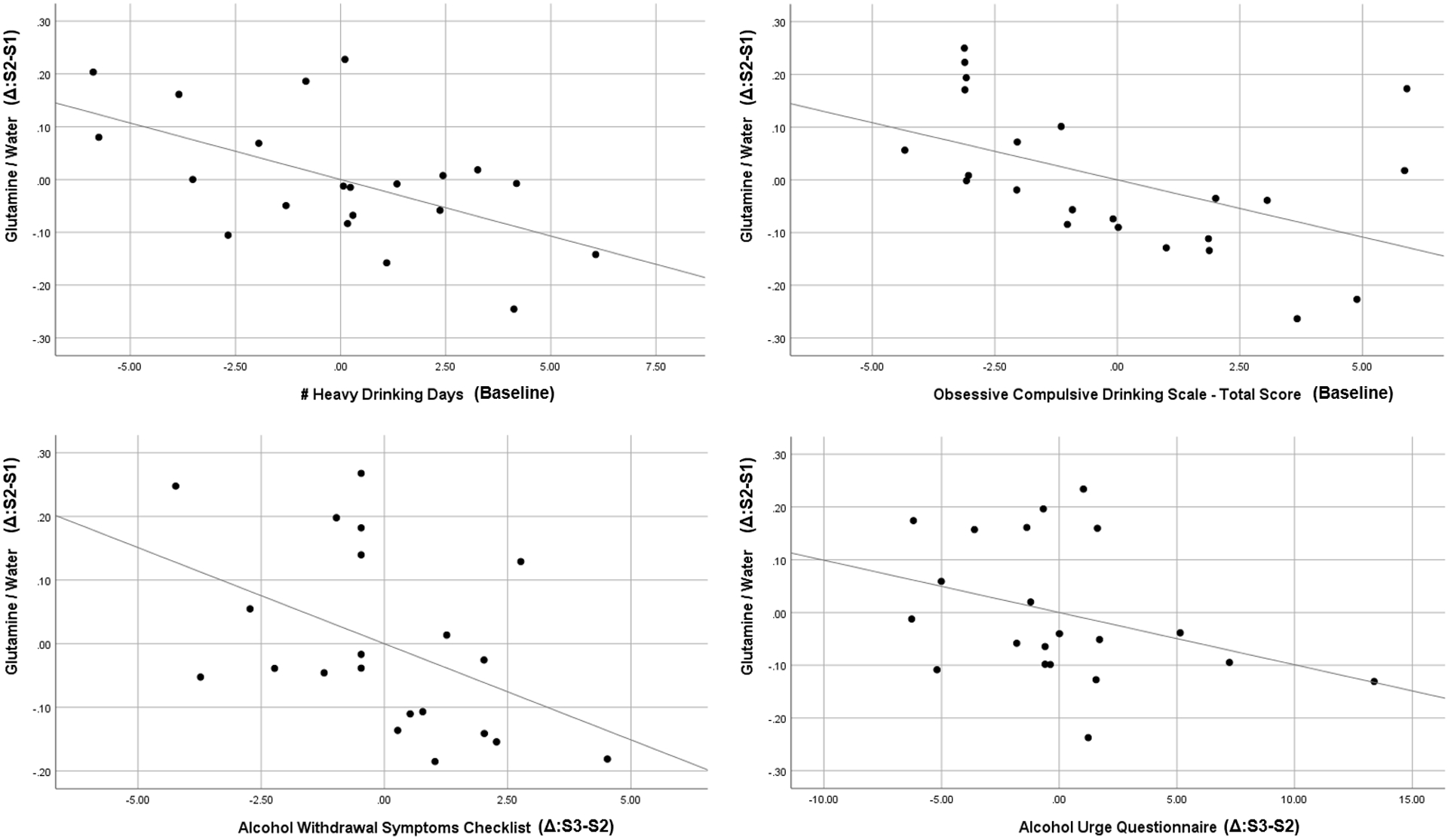
Partial regression plots (i.e., controlling for temporally-preceding levels/scores) for associations between changes in glutamine/water levels between scans 1 and 2 (y-axis, all panels) and a) number of heavy drinking days preceding baseline (top left panel), b) Obsessive Compulsive Drinking Scale total scores at baseline (top right panel), c) changes in Alcohol Withdrawal Symptoms Checklist scores between scans 2 and 3 (bottom left panel), and d) changes in Alcohol Urge Questionnaire scores between scans 2 and 3 (bottom right panel).
Funding Sources:
This research was supported by a grant to Dr. Prisciandaro from ABMRF/The Alcohol Research Fund. Dr. Anton was supported by NIAAA K05 AA017435. The authors have no conflicts of interest to report.
REFERENCES
- Abe C, Mon A, Durazzo TC, Pennington DL, Schmidt TP, Meyerhoff DJ (2013) Polysubstance and alcohol dependence: unique abnormalities of magnetic resonance-derived brain metabolite levels. Drug and alcohol dependence 130:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS (2006) The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. Journal of neurochemistry 98:641–653. [DOI] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P (2013) Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38:1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OA, Shulman GI, Navarro V, Petrakis IL, Charney DS, Krystal JH (1999) Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. The American journal of psychiatry 156:952–954. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism, clinical and experimental research 19:600–606. [DOI] [PubMed] [Google Scholar]
- Ende G, Hermann D, Demirakca T, Hoerst M, Tunc-Skarka N, Weber-Fahr W, Wichert S, Rabinstein J, Frischknecht U, Mann K, Vollstadt-Klein S (2013) Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcoholism, clinical and experimental research 37:1643–1649. [DOI] [PubMed] [Google Scholar]
- First MB (1998) Structured clinical interview for DSM-IV axis I disorders : patient edition (February 1996 final), SCID-I/P. Biometrics Research Dept., New York State Psychiatric Institute: New York, N.Y. [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, Koretski J, Guidone E, Jiang L, Petrakis IL, Pittman B, Krystal JH, Mason GF (2012) Intravenous ethanol infusion decreases human cortical gamma-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biological psychiatry 71:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander A, Wielders J, Anton R, Arndt T, Bianchi V, Deenmamode J, Jeppsson JO, Whitfield JB, Weykamp C, Schellenberg F (2016) Standardisation and use of the alcohol biomarker carbohydrate-deficient transferrin (CDT). Clinica chimica acta; international journal of clinical chemistry 459:19–24. [DOI] [PubMed] [Google Scholar]
- Henry ME, Lauriat TL, Shanahan M, Renshaw PF, Jensen JE (2011) Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: a phantom study at 4 Tesla. Journal of magnetic resonance (San Diego, Calif : 1997) 208:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH (2012) Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biological psychiatry 71:1015–1021. [DOI] [PubMed] [Google Scholar]
- Lee DW, Kim SY, Lee T, Nam YK, Ju A, Woo DC, You SJ, Han JS, Lee SH, Choi CB, Kim SS, Shin HC, Kim HY, Kim DJ, Rhim HS, Choe BY (2012) Ex vivo detection for chronic ethanol consumption-induced neurochemical changes in rats. Brain research 1429:134–144. [DOI] [PubMed] [Google Scholar]
- Lovinger DM (1997) Alcohols and neurotransmitter gated ion channels: past, present and future. Naunyn-Schmiedeberg’s archives of pharmacology 356:267–282. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF (1989) Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science (New York, NY) 243:1721–1724. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF (1990) NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. The Journal of neuroscience : the official journal of the Society for Neuroscience 10:1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe JM, McDonell MG, Leickly E, Angelo FA, Vilardaga R, McPherson S, Srebnik D, Roll J, Ries RK (2015) Determining ethyl glucuronide cutoffs when detecting self-reported alcohol use in addiction treatment patients. Alcoholism, clinical and experimental research 39:905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, Epperson CN, Rothman DL, Krystal JH (2006) Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biological psychiatry 59:85–93. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Durazzo TC, Ende G (2013) Chronic alcohol consumption, abstinence and relapse: brain proton magnetic resonance spectroscopy studies in animals and humans. Current topics in behavioral neurosciences 13:511–540. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ (2006) Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol and alcoholism (Oxford, Oxfordshire) 41:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Meyerhoff DJ (2012) Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug and alcohol dependence 125:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA (2006) Focus on Young Adult Drinking. In: Alcohol Alert. [Google Scholar]
- Pittman B, Gueorguieva R, Krupitsky E, Rudenko AA, Flannery BA, Krystal JH (2007) Multidimensionality of the Alcohol Withdrawal Symptom Checklist: a factor analysis of the Alcohol Withdrawal Symptom Checklist and CIWA-Ar. Alcoholism, clinical and experimental research 31:612–618. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL (2015) Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology 99:735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescot AP, Renshaw PF (2013) Two-dimensional J-resolved proton MR spectroscopy and prior knowledge fitting (ProFit) in the frontal and parietal lobes of healthy volunteers: assessment of metabolite discrimination and general reproducibility. Journal of magnetic resonance imaging : JMRI 37:642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, Anton RF (2016) Associations Between Recent Heavy Drinking and Dorsal Anterior Cingulate N-Acetylaspartate and Glutamate Concentrations in Non-Treatment-Seeking Individuals with Alcohol Dependence. Alcoholism, clinical and experimental research 40:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, Anton RF (2019) Brain Glutamate, GABA, and Glutamine Levels and Associations with Recent Drinking in Treatment-Naive Individuals with Alcohol Use Disorder Versus Light Drinkers. Alcoholism, clinical and experimental research 43:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Varodayan FP (2017) Synaptic targets: Chronic alcohol actions. Neuropharmacology 122:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G (2009) Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review 16:225–237. [DOI] [PubMed] [Google Scholar]
- Schulte RF, Boesiger P (2006) ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR in biomedicine 19:255–263. [DOI] [PubMed] [Google Scholar]
- Seilicovich A, Duvilanski B, Gonzalez NN, Rettori V, Mitridate de Novara A, Maines VM, Fiszer de Plazas S (1985) The effect of acute ethanol administration on GABA receptor binding in cerebellum and hypothalamus. European journal of pharmacology 111:365–368. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Cohen-Gilbert J, Crowley DJ, Rosso IM, Jensen JE, Sneider JT (2014) Altered anterior cingulate neurochemistry in emerging adult binge drinkers with a history of alcohol-induced blackouts. Alcoholism, clinical and experimental research 38:969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Horn JL (1984) In: The Alcohol Dependence Scale (ADS) User’s Guide. Addiction Research Foundation: Toronto, Canada. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 Suppl 1:S208–219. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British journal of addiction 84:1353–1357. [DOI] [PubMed] [Google Scholar]
- Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, Bogenschutz M, Lysne P, Tonigan S, Kalyanam R, Gasparovic C (2011) Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


