Abstract
We report here a scalable, catalytic one-pot approach to enantiopure and unmodified β2-amino acids. A newly developed confined imidodiphosphorimidate (IDPi) catalyzes a broadly applicable reaction of diverse bis-silyl ketene acetals with a silylated aminomethyl ether, followed by hydrolytic workup, to give free β2-amino acids in high yields, purity, and enantioselectivity. Importantly, both aromatic and aliphatic β2-amino acids can be obtained using this method. Mechanistic studies are consistent with the aminomethylation to proceed via silylium-based asymmetric counteranion-directed catalysis (Si-ACDC) and a transition state to explain the enantioselectivity is suggested on the basis of density functional theory calculation.
Among the various classes of amino acids, β2-amino acids hold a particularly prominent place and occur in an increasing number of pharmaceuticals, natural products, and drug candidates.1−11 However, while chemists, in recent years, have delivered several methods toward the asymmetric synthesis of β2-amino acids,12−39 catalytic approaches that directly deliver the free, unmodified amino acid, without requiring separate redox- or protecting group manipulations, to our knowledge, have not yet been developed. Our inspirational blueprint to address this challenge is a hypothetical chiral acid catalyzed direct three-component-Mannich reaction of carboxylic acids with formaldehyde and ammonia (eq 1).
 |
1 |
Unfortunately, except with malonic acid derivatives and nonenantioselectively so,40,41 such a “dream-reaction” has not yet been realized, arguably due to the current inability of chemists to catalytically enolize carboxylic acids.42−44 An attractive, even though less direct alternative would be a Mukaiyama-style reaction of preformed bis-silyl ketene acetals (bis-SKAs) with a formaldehyde imine equivalent. While this transformation has been described in a nonenantioselective fashion,45 asymmetric versions are entirely unknown. Encouraged by our recent studies on silylium-based asymmetric counteranion-directed catalysis (Si-ACDC),46−66 we envisaged to apply this approach to a TMSX*-catalyzed reaction of bis-SKAs with a silylated aminomethyl ether, followed by hydrolytic workup and extraction, which should deliver the free, unmodified β2-amino acids and enable a simple catalyst HX* recovery (eq 2, X*– = enaniopure counteranion). Here we report on the realization of this concept with a general and highly enantioselective imidodiphosphorimidate (IDPi) catalyzed Mukaiyama Mannich-type reaction that delivers free β2-amino acids with either aromatic or aliphatic substituents.
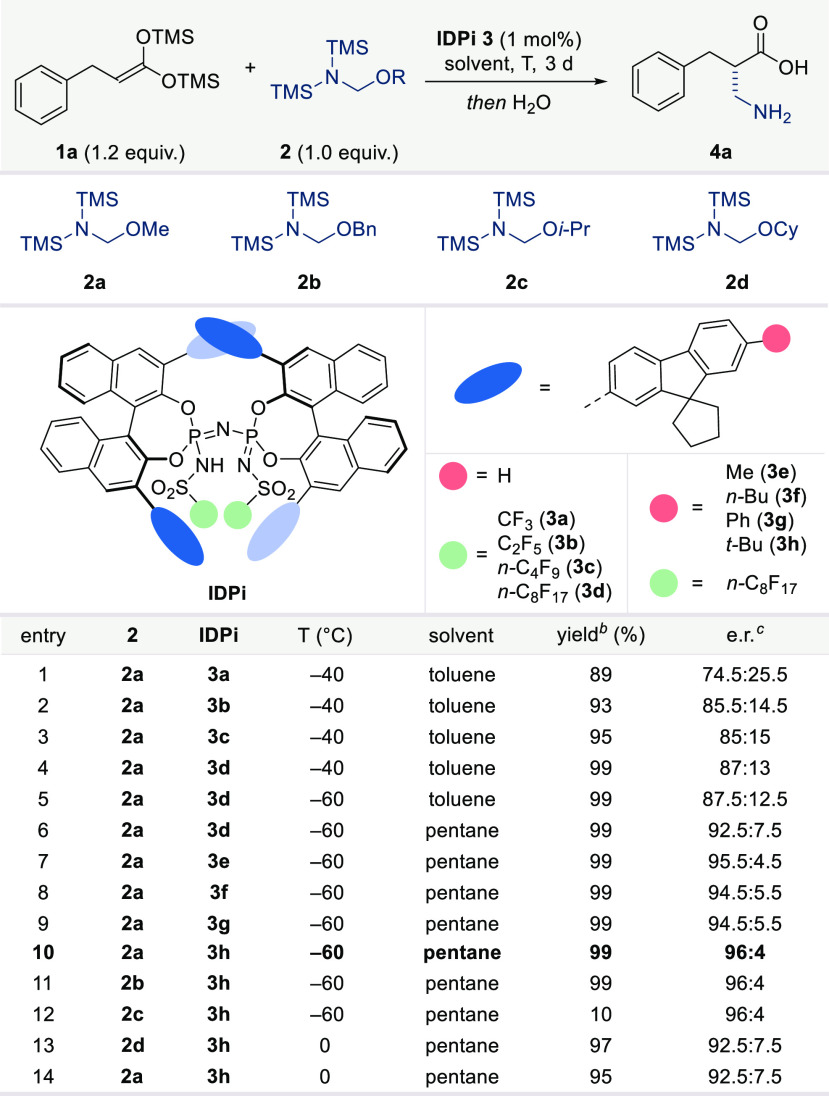
We chose α-benzyl bis-SKA 1a as our model substrate and commercially available α-aminomethyl ether 2a as methylene imine equivalent to initiate our studies (Table 1). An initial catalyst exploration revealed that moderately acidic Brønsted acids, such as chiral phosphoric acids (CPAs),67,68 even upon warming, did not give any of the desired product, while imidodiphosphoric (IDP)69 acids promoted the reaction at 0 °C to give racemic product (see the Supporting Information). In contrast, the much more acidic IDPi catalysts provided both sufficient reactivity and promising enantioselectivity (at −40 °C in toluene). Among our IDPi libraries, spirocyclopentyl-3-fluorenyl substituted catalysts 3 turned out to be particularly promising in terms of reactivity and enantioselectivity. Extending the perfluoroalkyl sulfonyl chains in the inner core further increased the enantioselectivity (entries 1–4). With catalyst 3d, temperature and solvent were further optimized. Lowering the temperature to −60 °C led to a slight increase in enantioselectivity (entry 5). Importantly, with pentane as the solvent instead of toluene, the enantiomeric ratio significantly increased (entry 6). Furthermore, we tested IDPi catalysts 3e–g, possessing an additional substituent at the fluorenyl group (entries 7–10). Ultimately, we identified the tert-butyl substituted IDPi catalyst 3h as the optimal one, giving an e.r. of 96:4 in almost quantitative yield (entry 10).
Table 1. Reaction Developmenta.
Reactions were conducted on a 0.02 mmol scale: 1a:2 = 1.2:1.
Yields were determined by 1H NMR using mesitylene as internal standard.
After simplified workup, enantiomeric ratios (e.r.) were measured by HPLC. See the Supporting Information for further information.
We also studied the effect of the aminomethyl source on the conversion and stereochemical outcome (entries 11–14). Different ethers 2 with varying leaving groups were examined. Interestingly, while the alkoxy group had only an insignificant effect on the enantiocontrol, isopropyl ether 2c gave only poor conversion at −60 °C (entries 10–12). These results are consistent with the absence of the leaving group of ether 2 in the enantiodetermining step and point toward an efficient association of the bis(silyl)iminium ion with the IDPi anion. This hypothesis could indeed be validated with a remarkably broad scope of both aromatic and aliphatic bis-SKAs (Table 2). Various free β2-amino acids with electronically and sterically diverse substituents were obtained in excellent yields and enantioselectivities. For example, bis-SKAs 1a–c with different methylene tether lengths between a phenyl group and carboxylic acid functionality afforded the desired products in similar excellent yields and enantioselectivities. Similarly, either electron-neutral or electron-donating groups at the β-phenyl ring of the bis-SKA gave the corresponding free β2-amino acids in >90% yields with around 95:5 e.r. (4d–e). Notably, β2-amino acids with electron-withdrawing groups (F, CF3, Cl), either at the ortho-, meta-, or para-position of the β-phenyl ring were generated in >90% yield with higher enantioselectivities (>97:3 e.r.) (4f–j). Other substrates with aromatic and heteroaromatic groups, such as 1k with naphthyl and 1l bearing a thiophenyl substituent, were well tolerated, affording the aminomethylation products 4k and 4l in excellent yield and e.r..
Table 2. Substrate Scopea.

Reactions were conducted on a 0.2 mmol scale: 1:2a = 1.2:1. Isolated yields with e.r. measured by HPLC. For derivatization, see Supporting Information.
e.r. measured by HPLC after derivatization.
3 mol % 3h. BzCl, benzoyl chloride; DCM, dichloromethane; TEA, triethylamine.
Directly aryl-substituted bis-SKAs 1m–q were also examined and proved to be slightly less reactive, requiring 3 mol % of catalyst 3h to furnish the corresponding products in moderate to good yields and excellent enantioselectivities.
The scope of this transformation also includes simple, aliphatic β2-amino acids. For example, bis-SKAs 1r–u, which were generated from propionic acid, butyric acid, valeric acid, and hexanoic acid, respectively, reacted smoothly, where the enantioselectivities increased with longer alkyl chains. Branched and cyclic alkyl groups (4v–x) and a methoxy- (4y) and an olefin-substituted alkyl chain (4z) were all tolerated and provided the desired products in good to excellent yields and enantioselectivity. Interestingly, the enantiopure bis-SKA 1A and its enantiomer ent-1A reacted to products 4A and 4B in good yields and, in both cases, featuring excellent and catalyst-controlled diastereoselectivity. Limitations of our method include the use of bis-silyl ketene acetals derived from α,α-disubstituted carboxylic acids and of C-substituted imine sources, which display reduced reactivity and lead to lower diastereoselectivity and enantioselectivity (see the Supporting Information).
The absolute configuration of our obtained β2-amino acids was determined from X-ray crystallographic analysis of products 4h, 4i, and 4j. Furthermore, bromoalkyl substituted bis-SKA 1C gave γ-aminobutyric acid uptake inhibitor (S)-(+)-nipecotic acid705 in a one-pot operation in 84% yield and 97:3 e.r. when treating the initial reaction product with triethylamine. The absolute configuration of amino acid 5 was determined by converting it to the corresponding benzamide 6, crystals of which were subjected to an X-ray crystallographic analysis. 1H NMR investigation of the crude reaction mixture revealed the existence of silylated product 4C, confirming that cyclization occurs only upon base treatment. In fact, oligomers were detected with concomitant formation of a small amount of compound 5 if the reaction mixture was treated with only water. Instead, treatment with benzoyl chloride and aqueous potassium carbonate enabled the access to the corresponding α-amidomethylated δ-valerolactone 7.
The practicality of our method was illustrated with two scale-up experiments, involving an extremely concise product purification and catalyst recovery. Using 1 mol % of catalyst 3h, 12 mmol of bis-SKA 1a and 10 mmol of imine precursor 2a gave 1.77 g of the free β2-amino acid 4a in 99% isolated yield with an e.r. of 95.5:4.5. The workup of the reaction mixture included a simple extraction with water and washing with dichloromethane without further purification. Gratifyingly, catalyst 3h could be easily recovered in 96% yield from the organic phase via flash chromatography and acidification. Similarly, 2.84 g of the aliphatic free β2-amino acid 4u was obtained in 98% isolated yield with an e.r. of 95:5 from 20 mmol of reagent 2a using only 0.5 mol % of catalyst 3h, which was recovered in 95% yield from the organic phase after flash chromatography and acidification.
Optionally, the crude products can be readily derivatized in situ into a variety of synthetically useful building blocks such as the corresponding N-Boc- or N-Fmoc-protected β2-amino acids 8 and 9 by treating the reaction mixture with an appropriate derivatization reagent.
On the basis of the observation that the alkyl group of ethers 2 had an insignificant effect on the enantioselectivity (Table 1, entries 11–14), coupled with literature results,45,58−66 we envision a catalytic cycle as shown in Figure 1a. Accordingly, the reaction commences with the in situ silylation of the IDPi catalyst 3 by bis-SKA 1 to furnish the N-silylated catalyst I and/or its diastereomeric O–Si-silatropomers.58−66 α-Aminomethyl ether 2 then reacts with catalyst I, generating the methylene iminium ion-IDPi anion pair II, simultaneously liberating TMSOMe.45 Subsequently, bis-SKA 1 reacts with the cationic methylene iminium ion in the anionic catalyst pocket to give ion pair III. Intra-ion-pair silyl transfer from the cationic product back onto its counteranion then furnishes the silylated product IV and re-establishes the silylated catalyst I. Finally, hydrolytic workup and extraction of the reaction mixture delivers the free β2-amino acid 4. On the basis of a detailed conformational search and subsequent Density Functional Theory (DFT) optimization of ion pair II, we tentatively propose a sterical hindrance-based selectivity model (Figure 1b), where re-facial addition of bis-SKA 1 to methylene iminium-IDPi anion pair II leads to the observed enantiomer (see the Supporting Information).
Figure 1.
(a) Proposed catalytic cycle. (b) Suggested re-facial approach of the SKA onto the DFT optimized iminium-IDPi ion pair II.
We have developed a traceless and scalable approach to enantiopure free β2-amino acids via catalytic asymmetric aminomethylation of bis-silyl ketene acetals. A variety of aromatic and aliphatic bis-SKAs from carboxylic acids with diverse electronics and sterics were tolerated in this transformation and provided the corresponding amino acids in excellent yields and enantioselectivities. The purification process is extremely simple and concise and enables catalyst recovery. We conducted control experiments that are consistent with a mechanism that proceeds via Si-ACDC, while preliminary computational studies suggest steric effects to cause the observed enantioselectivity. As IDPi catalysts are currently being commercialized, the methodology reported here may facilitate the synthesis of pharmaceuticals, natural products, and peptidic foldamers.
Acknowledgments
We thank the generous support from the Max Planck Society, the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, Leibniz Award to B.L.) and under Germany’s Excellence Strategy (EXC 2033-390677874-RESOLV), the European Research Council (ERC, European Union’s Horizon 2020 research and innovation program “C–H Acids for Organic Synthesis, CHAOS” Advanced Grant Agreement No. 694228), and the Horizon 2020 Marie Sklodowska-Curie Postdoctoral Fellowship (to R.M., Grant agreement No. 897130). The authors thank Benjamin Mitschke for his help during the preparation of this manuscript and several members of the group for crowd reviewing. We also thank the technicians of our group and the members of our NMR, MS, and chromatography groups for their excellent service.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c00249.
Experimental details and analytical data for all new compounds, crystallographic data for compounds 4h, 4i, and 4j, HPLC traces, NMR spectra, computational studies, optimized structures, and Cartesian coordinates (PDF)
Accession Codes
CCDC 2056835–2056839 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare the following competing financial interest(s): We have a patent on the catalyst class.
Supplementary Material
References
- Cheng R. P.; Gellman S. H.; DeGrado W. F. β-Peptides: From structure to function. Chem. Rev. 2001, 101, 3219–3232. 10.1021/cr000045i. [DOI] [PubMed] [Google Scholar]
- Lelais G.; Seebach D. β2-Amino acids—Syntheses, occurrence in natural products, and components of β-peptides. Biopolymers 2004, 76, 206–243. 10.1002/bip.20088. [DOI] [PubMed] [Google Scholar]
- Aguilar M. I.; Purcell A. W.; Devi R.; Lew R.; Rossjohn J.; Smitha A. I.; Perlmutter P. β-Amino acid-containing hybrid peptides—New opportunities in peptidomimetics. Org. Biomol. Chem. 2007, 5, 2884–2890. 10.1039/b708507a. [DOI] [PubMed] [Google Scholar]
- Seebach D.; Gardiner J. β-Peptidic peptidomimetics. Acc. Chem. Res. 2008, 41, 1366–1375. 10.1021/ar700263g. [DOI] [PubMed] [Google Scholar]
- Kudo F.; Miyanaga A.; Eguchi T. Biosynthesis of natural products containing β-amino acids. Nat. Prod. Rep. 2014, 31, 1056–1073. 10.1039/C4NP00007B. [DOI] [PubMed] [Google Scholar]
- Adkins J. C.; Noble S. Tiagabine—A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the management of epilepsy. Drugs 1998, 55, 437–460. 10.2165/00003495-199855030-00013. [DOI] [PubMed] [Google Scholar]
- Appella D. H.; Christianson L. A.; Karle I. L.; Powell D. R.; Gellman S. H. β-Peptide foldamers: Robust helix formation in a new family of β-amino acid oligomers. J. Am. Chem. Soc. 1996, 118, 13071–13072. 10.1021/ja963290l. [DOI] [Google Scholar]
- Eddinger G. A.; Gellman S. H. Differential effects of β3- versus β2-amino acid residues on the helicity and recognition properties of bim BH3-derived α/β-peptides. Angew. Chem., Int. Ed. 2018, 57, 13829–13832. 10.1002/anie.201806909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganty S.; Golakoti T.; Heltzel C.; Moore R. E.; Yoshida W. Y. Isolation and structure determination of cryptophycins 38, 326, and 327 from the terrestrial cyanobacterium Nostoc sp GSV 224. J. Nat. Prod. 2004, 67, 1403–1406. 10.1021/np0499665. [DOI] [PubMed] [Google Scholar]
- Neary P.; Delaney C. P. Alvimopan. Expert Opin. Invest. Drugs 2005, 14, 479–488. 10.1517/13543784.14.4.479. [DOI] [PubMed] [Google Scholar]
- Hoy S. M. Netarsudil ophthalmic solution 0.02%: First global approval. Drugs 2018, 78, 389–396. 10.1007/s40265-018-0877-7. [DOI] [PubMed] [Google Scholar]
- Seebach D.; Beck A. K.; Capone S.; Deniau G.; Groselj U.; Zass E. Enantioselective preparation of β2-amino acid derivatives for β-peptide synthesis. Synthesis 2009, 2009, 1–32. 10.1055/s-0028-1087490. [DOI] [Google Scholar]
- Noda H.; Shibasaki M. Recent advances in the catalytic asymmetric synthesis of β2- and β2,2-amino acids. Eur. J. Org. Chem. 2020, 2020, 2350–2361. 10.1002/ejoc.201901596. [DOI] [Google Scholar]
- Bower J. F.; Williams J. M. J. Palladium catalysed asymmetric allylic substitution. Routes to β-amino acids. Synlett 1996, 1996, 685–686. 10.1055/s-1996-5560. [DOI] [Google Scholar]
- Schleich S.; Helmchen G. Pd-catalyzed asymmetric allylic alkylation of 3-acetoxy-N-(tert-butyloxycarbonyl)-1,2,3,6-tetrahydropyridine—Preparation of key intermediates for natural product synthesis. Eur. J. Org. Chem. 1999, 1999, 2515–2521. . [DOI] [Google Scholar]
- Davies H. M. L.; Venkataramani C. Catalytic enantioselective synthesis of β2-amino acids. Angew. Chem., Int. Ed. 2002, 41, 2197–2199. . [DOI] [PubMed] [Google Scholar]
- Rimkus A.; Sewald N. First synthesis of β2-homoamino acid by enantioselective catalysis. Org. Lett. 2003, 5, 79–80. 10.1021/ol027252k. [DOI] [PubMed] [Google Scholar]
- Eilitz U.; Lessmann F.; Seidelmann O.; Wendisch V. Stereoselective synthesis of β2-amino acids by Michael addition of diorgano zinc reagents to nitro acrylates. Tetrahedron: Asymmetry 2003, 14, 189–191. 10.1016/S0957-4166(02)00788-7. [DOI] [Google Scholar]
- Duursma A.; Minnaard A. J.; Feringa B. L. Highly enantioselective conjugate addition of dialkylzinc reagents to acyclic nitroalkenes: a catalytic route to β2-amino acids, aldehydes, and alcohols. J. Am. Chem. Soc. 2003, 125, 3700–3701. 10.1021/ja029817d. [DOI] [PubMed] [Google Scholar]
- Sammis G. M.; Jacobsen E. N. Highly enantioselective, catalytic conjugate addition of cyanide to α,β-unsaturated Imides. J. Am. Chem. Soc. 2003, 125, 4442–4443. 10.1021/ja034635k. [DOI] [PubMed] [Google Scholar]
- Sibi M. P.; Tatamidani H.; Patil K. Enantioselective rhodium enolate protonations. A new methodology for the synthesis of β2-amino acids. Org. Lett. 2005, 7, 2571–2573. 10.1021/ol050630b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. M. L.; Ni A. W. Enantioselective synthesis of β-amino esters and its application to the synthesis of the enantiomers of the antidepressant Venlafaxine. Chem. Commun. 2006, 3110–3112. 10.1039/B605047F. [DOI] [PubMed] [Google Scholar]
- Huang H. M.; Liu X. C.; Deng J.; Qiu M.; Zheng Z. Rhodium-catalyzed enantioselective hydrogenation of β-phthalimide acrylates to synthesis of β2-amino acids. Org. Lett. 2006, 8, 3359–3362. 10.1021/ol0612399. [DOI] [PubMed] [Google Scholar]
- Qiu L. Q.; Prashad M.; Hu B.; Prasad K.; Repic O.; Blacklock T. J.; Kwong F. Y.; Kok S. H. L.; Lee H. W.; Chan A. S. C. Enantioselective hydrogenation of α-aminomethylacrylates containing a free N–H group for the synthesis of β-amino acid derivatives. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 16787–16792. 10.1073/pnas.0704461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J.; Hu X. P.; Huang J. D.; Yu S. B.; Wang D. Y.; Duan Z. C.; Zheng Z. Enantioselective synthesis of β2-amino acids via rh-catalyzed asymmetric hydrogenation with BoPhoz-type ligands: important influence of an N–H proton in the ligand on the enantioselectivity. J. Org. Chem. 2008, 73, 2015–2017. 10.1021/jo702510m. [DOI] [PubMed] [Google Scholar]
- Guo Y. J.; Shao G. A.; Li L. N.; Wu W. H.; Li R. H.; Li J. J.; Song J. A.; Qiu L. Q.; Prashad M.; Kwong F. Y. A general approach to the synthesis of β2-amino acid derivatives via highly efficient catalytic asymmetric hydrogenation of α-aminomethylacrylates. Adv. Synth. Catal. 2010, 352, 1539–1553. 10.1002/adsc.201000122. [DOI] [Google Scholar]
- Li L. N.; Chen B.; Ke Y. Y.; Li Q.; Zhuang Y.; Duan K.; Huang Y. C.; Pang J. Y.; Qiu L. Q. Highly efficient synthesis of heterocyclic and alicyclic β2-amino acid derivatives by catalytic asymmetric hydrogenation. Chem. - Asian J. 2013, 8, 2167–2174. 10.1002/asia.201300339. [DOI] [PubMed] [Google Scholar]
- Remarchuk T.; Babu S.; Stults J.; Zanotti-Gerosa A.; Roseblade S.; Yang S. H.; Huang P.; Sha C. B.; Wang Y. C. An efficient catalytic asymmetric synthesis of a β2-amino acid on multikilogram scale. Org. Process Res. Dev. 2014, 18, 135–141. 10.1021/op4002966. [DOI] [Google Scholar]
- Li S. K.; Xiao T. F.; Li D. D.; Zhang X. M. First iridium-catalyzed highly enantioselective hydrogenation of β-nitroacrylates. Org. Lett. 2015, 17, 3782–3785. 10.1021/acs.orglett.5b01758. [DOI] [PubMed] [Google Scholar]
- Jian J. H.; Hsu C. L.; Syu J. F.; Kuo T. S.; Tsai M. K.; Wu P. Y.; Wu H. L. Access to β2-amino acids via enantioselective 1,4-arylation of β-nitroacrylates catalyzed by chiral rhodium catalysts. J. Org. Chem. 2018, 83, 12184–12191. 10.1021/acs.joc.8b00586. [DOI] [PubMed] [Google Scholar]
- Kang Z. H.; Wang Y. H.; Zhang D.; Wu R. B.; Xu X. F.; Hu W. H. Asymmetric counter-anion-directed aminomethylation: synthesis of chiral β-amino acids via trapping of an enol intermediate. J. Am. Chem. Soc. 2019, 141, 1473–1478. 10.1021/jacs.8b12832. [DOI] [PubMed] [Google Scholar]
- Lin W. L.; Zhang K. F.; Baudoin O. Regiodivergent enantioselective C–H functionalization of Boc-1,3-oxazinanes for the synthesis of β2- and β3-amino acids. Nat. Catal. 2019, 2, 882–888. 10.1038/s41929-019-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y.; Gellman S. H. Enantioselective organocatalytic aminomethylation of aldehydes: a role for ionic interactions and efficient access to β2-amino acids. J. Am. Chem. Soc. 2006, 128, 6804–6805. 10.1021/ja061731n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiderska M. A.; Stewart J. D. Asymmetric bioreductions of β-nitro acrylates as a route to chiral β2-amino acids. Org. Lett. 2006, 8, 6131–6133. 10.1021/ol062612f. [DOI] [PubMed] [Google Scholar]
- Martin N. J. A.; Cheng X.; List B. Organocatalytic asymmetric transferhydrogenation of β-nitroacrylates: accessing β2-amino acids. J. Am. Chem. Soc. 2008, 130, 13862–13863. 10.1021/ja8069852. [DOI] [PubMed] [Google Scholar]
- Bernal P.; Fernandez R.; Lassaletta J. M. Organocatalytic Asymmetric Cyanosilylation of Nitroalkenes. Chem. - Eur. J. 2010, 16, 7714–7718. 10.1002/chem.201001107. [DOI] [PubMed] [Google Scholar]
- Tite T.; Sabbah M.; Levacher V.; Briere J. F. Organocatalysed decarboxylative protonation process from Meldrum’s acid: enantioselective synthesis of isoxazolidinones. Chem. Commun. 2013, 49, 11569–11571. 10.1039/c3cc47695b. [DOI] [PubMed] [Google Scholar]
- Xu J. F.; Chen X. K.; Wang M.; Zheng P. C.; Song B. A.; Chi Y. R. Aminomethylation of enals through carbene and acid cooperative catalysis: concise access to β2-amino acids. Angew. Chem., Int. Ed. 2015, 54, 5161–5165. 10.1002/anie.201412132. [DOI] [PubMed] [Google Scholar]
- Wang K.; Yu J.; Shao Y.; Tang S.; Sun J. Forming all-carbon quaternary stereocenters by organocatalytic aminomethylation: concise access to β2,2-amino acids. Angew. Chem., Int. Ed. 2020, 59, 23516–23520. 10.1002/anie.202009892. [DOI] [PubMed] [Google Scholar]
- Mannich C.; Ganz E. β-Aminodicarboxylic acids and aminopolycarboxylic acids. Ber. Dtsch. Chem. Ges. B 1922, 55B, 3486–3504. 10.1002/cber.19220551017. [DOI] [Google Scholar]
- Rodionow W. M.; Malewinskaja E. T. On the presentation of aryl-β-amino fatty acids (I announcement). Ber. Dtsch. Chem. Ges. B 1926, 59, 2952–2958. 10.1002/cber.19260591147. [DOI] [Google Scholar]
- Tanaka T.; Yazaki R.; Ohshima T. Chemoselective catalytic α-oxidation of carboxylic acids: Iron/alkali metal cooperative redox active catalysis. J. Am. Chem. Soc. 2020, 142, 4517–4524. 10.1021/jacs.0c00727. [DOI] [PubMed] [Google Scholar]
- Morita Y.; Yamamoto T.; Nagai H.; Shimizu Y.; Kanai M. Chemoselective boron-catalyzed nucleophilic activation of carboxylic acids for Mannich-type reactions. J. Am. Chem. Soc. 2015, 137, 7075–7078. 10.1021/jacs.5b04175. [DOI] [PubMed] [Google Scholar]
- Stivala C. E.; Zakarian A. Highly enantioselective direct alkylation of arylacetic acids with chiral lithium amides as traceless auxiliaries. J. Am. Chem. Soc. 2011, 133, 11936–11936. 10.1021/ja205107x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano K.; Morimoto T.; Sekiya M. Primary aminomethylation at the α-position of carboxylic-acids and esters. Trimethylsilyl triflate-catalyzed reaction of ketene silyl acetals with N,N-bis(trimethylsilyl)methoxymethylamine. Chem. Pharm. Bull. 1985, 33, 2228–2234. 10.1248/cpb.33.2228. [DOI] [Google Scholar]
- Garcia-Garcia P.; Lay F.; Garcia-Garcia P.; Rabalakos C.; List B. A powerful chiral counteranion motif for asymmetric catalysis. Angew. Chem., Int. Ed. 2009, 48, 4363–4366. 10.1002/anie.200901768. [DOI] [PubMed] [Google Scholar]
- Mahlau M.; List B. Asymmetric counteranion-directed catalysis: Concept, definition, and applications. Angew. Chem., Int. Ed. 2013, 52, 518–533. 10.1002/anie.201205343. [DOI] [PubMed] [Google Scholar]
- Gandhi S.; List B. Catalytic asymmetric three-component synthesis of homoallylic amines. Angew. Chem., Int. Ed. 2013, 52, 2573–2576. 10.1002/anie.201209776. [DOI] [PubMed] [Google Scholar]
- Wang Q. G.; Leutzsch M.; van Gemmeren M.; List B. Disulfoninnide-catalyzed asymmetric synthesis of β3-amino esters directly from N-Boc-amino sulfones. J. Am. Chem. Soc. 2013, 135, 15334–15337. 10.1021/ja408747m. [DOI] [PubMed] [Google Scholar]
- Wang Q. G.; van Gemmeren M.; List B. Asymmetric disulfonimide-catalyzed synthesis of δ-amino-β-ketoester derivatives by vinylogous Mukaiyama-Mannich reactions. Angew. Chem., Int. Ed. 2014, 53, 13592–13595. 10.1002/anie.201407532. [DOI] [PubMed] [Google Scholar]
- James T.; van Gemmeren M.; List B. Development and applications of disulfonimides in enantioselective review organocatalysis. Chem. Rev. 2015, 115, 9388–9409. 10.1021/acs.chemrev.5b00128. [DOI] [PubMed] [Google Scholar]
- Tap A.; Blond A.; Wakchaure V. N.; List B. Chiral allenes via alkynylogous Mukaiyama aldol reaction. Angew. Chem., Int. Ed. 2016, 55, 8962–8965. 10.1002/anie.201603649. [DOI] [PubMed] [Google Scholar]
- Zhang Z. P.; Bae H. Y.; Guin J.; Rabalakos C.; van Gemmeren M.; Leutzsch M.; Klussmann M.; List B. Asymmetric counteranion-directed Lewis acid organocatalysis for the scalable cyanosilylation of aldehydes. Nat. Commun. 2016, 7, 12478. 10.1038/ncomms12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzenmeier T.; van Gemmeren M.; Xie Y. W.; Hofler D.; Leutzsch M.; List B. Asymmetric Lewis acid organocatalysis of the Diels-Alder reaction by a silylated C–H acid. Science 2016, 351, 949–952. 10.1126/science.aae0010. [DOI] [PubMed] [Google Scholar]
- Mandrelli F.; Blond A.; James T.; Kim H.; List B. Deracemizing α-branched carboxylic acids by catalytic asymmetric protonation of bis-silyl ketene acetals with water or methanol. Angew. Chem., Int. Ed. 2019, 58, 11479–11482. 10.1002/anie.201905623. [DOI] [PubMed] [Google Scholar]
- Zhang Z. P.; Klussmann M.; List B. Kinetic study of disulfonimide-catalyzed cyanosilylation of aldehydes by using a method of progress rates. Synlett 2020, 31, 1593–1597. 10.1055/s-0040-1707129. [DOI] [Google Scholar]
- Wakchaure V. N.; Obradors C.; List B. Chiral bronsted acids catalyze asymmetric additions to substrates that are already protonated: Highly enantioselective disulfonimide-catalyzed Hantzsch ester reductions of NH-imine hydrochloride salts. Synlett 2020, 31, 1707–1712. 10.1055/s-0040-1706413. [DOI] [Google Scholar]
- Kaib P. S. J.; Schreyer L.; Lee S.; Properzi R.; List B. Extremely active organocatalysts enable a highly enantioselective addition of allyltrimethylsilane to aldehydes. Angew. Chem., Int. Ed. 2016, 55, 13200–13203. 10.1002/anie.201607828. [DOI] [PubMed] [Google Scholar]
- Lee S.; Kaib P. S. J.; List B. Asymmetric catalysis via cyclic, aliphatic oxocarbenium ions. J. Am. Chem. Soc. 2017, 139, 2156–2159. 10.1021/jacs.6b11993. [DOI] [PubMed] [Google Scholar]
- Bae H. Y.; Hofler D.; Kaib P. S. J.; Kasaplar P.; De C. K.; Dohring A.; Lee S.; Kaupmees K.; Leito I.; List B. Approaching sub-ppm-level asymmetric organocatalysis of a highly challenging and scalable carbon-carbon bond forming reaction. Nat. Chem. 2018, 10, 888–894. 10.1038/s41557-018-0065-0. [DOI] [PubMed] [Google Scholar]
- Gatzenmeier T.; Kaib P. S. J.; Lingnau J. B.; Goddard R.; List B. The catalytic asymmetric Mukaiyama-Michael reaction of silyl ketene acetals with α,β-unsaturated methyl esters. Angew. Chem., Int. Ed. 2018, 57, 2464–2468. 10.1002/anie.201712088. [DOI] [PubMed] [Google Scholar]
- Lee S.; Bae H. Y.; List B. Can a ketone be more reactive than an aldehyde? Catalytic asymmetric synthesis of substituted tetrahydrofurans. Angew. Chem., Int. Ed. 2018, 57, 12162–12166. 10.1002/anie.201806312. [DOI] [PubMed] [Google Scholar]
- Gatzenmeier T.; Turberg M.; Yepes D.; Xie Y. W.; Neese F.; Bistoni G.; List B. Scalable and highly diastereo- and enantioselective catalytic Diels-Alder reaction of α,β-unsaturated methyl esters. J. Am. Chem. Soc. 2018, 140, 12671–12676. 10.1021/jacs.8b07092. [DOI] [PubMed] [Google Scholar]
- Schreyer L.; Kaib P. S. J.; Wakchaure V. N.; Obradors C.; Properzi R.; Lee S.; List B. Confined acids catalyze asymmetric single aldolizations of acetaldehyde enolates. Science 2018, 362, 216–219. 10.1126/science.aau0817. [DOI] [PubMed] [Google Scholar]
- Schreyer L.; Properzi R.; List B. IDPi catalysis. Angew. Chem., Int. Ed. 2019, 58, 12761–12777. 10.1002/anie.201900932. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Bae H. Y.; Leutzsch M.; Kennemur J. L.; Becart D.; List B. The silicon-hydrogen exchange reaction: A catalytic sigma-bond metathesis approach to the enantioselective synthesis of enol silanes. J. Am. Chem. Soc. 2020, 142, 13695–13700. 10.1021/jacs.0c06677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T. Stronger bronsted acids. Chem. Rev. 2007, 107, 5744–5758. 10.1021/cr068374j. [DOI] [PubMed] [Google Scholar]
- Terada M. Binaphthol-derived phosphoric acid as a versatile catalyst for enantioselective carbon–carbon bond forming reactions. Chem. Commun. 2008, 4097–4112. 10.1039/b807577h. [DOI] [PubMed] [Google Scholar]
- Čorić I.; List B. Asymmetric spiroacetalization catalysed by confined Bronsted acids. Nature 2012, 483, 315–319. 10.1038/nature10932. [DOI] [PubMed] [Google Scholar]
- Takahashi K.; Miyoshi S.; Kaneko A.; Copenhagen D. R. Actions of nipecotic acid and SKF89976A on GABA transporter in cone-driven horizontal cells dissociated from the catfish retina. Jpn. J. Physiol. 1995, 45, 457–473. 10.2170/jjphysiol.45.457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.