Abstract
Cardiovascular diseases are the most common causes of mortality worldwide. They are frequently the reasons for patient hospitalization, their incapability for work, and disability. These diseases represent a significant socio-economic burden affecting the medical system as well as patients and their families. It has been demonstrated that the etiopathogenesis of cardiovascular diseases is significantly affected by lifestyle, and so modification of the latter is an essential component of both primary and secondary prevention. Cardiac rehabilitation (CR) represents an efficient secondary prevention model that is especially based on the positive effect of regular physical activity. This review presents an overview of basic information on CR with a focus on current trends, such as the issue of the various training modalities, utilization, and barriers to it or the use of telemedicine technologies. Appropriate attention should be devoted to these domains, as CR continues evolving as an effective and readily available intervention in the future.
Keywords: Cardiac rehabilitation, Secondary prevention, Cardiovascular diseases, Utilization, Barriers, Telerehabilitation
Core Tip: Cardiac rehabilitation (CR) is an efficient and cost-effective secondary prevention model. It brings many benefits for patients with cardiovascular diseases but also acts at the medical and social system levels. Despite all of this, CR is highly underutilized across the world. There are problems in inconsistent delivery, deficient reimbursement, and further CR participation barriers in individuals. This article represents a comprehensive overview of the current situation and provides future perspectives, such as use of telerehabilitation.
INTRODUCTION
Cardiovascular diseases (CVDs) cause 31% of all deaths worldwide[1]. This indicator reaches 39%-47% (for females and males, respectively) in Europe[2], and most deaths in the United States are due a CVD[3]. The annual global number of CVD deaths is 17.9 million; these mainly (85%) due to coronary artery disease (CAD) and stroke. Over 75% of mortality occurs in low-middle income countries[1]. It seems that the high mortality in poorer countries is not related to risk factors but might be related to worse access to health care[4]. Although age-standardized death rates show a decreasing trend in most countries, the absolute number of patients increases[3,5].
CVDs constitute a common reason for hospitalization[6] and cause an inability to work[7]. Stroke and CAD are the most frequent causes of disability and are responsible for approximately 20% of age-standardized disability-adjusted life years[8,9]. According to the American Heart Association (AHA), a sum of USD 351 billion (i.e., 14% of total funds spent on health care) was spent on the treatment of CVDs during 2014-2015. This amount is expected to approach USD 1100 billion in 2035[3]. This is a serious issue affecting both the social sphere and the national economy.
There are predispositions and risk factors of CVDs on a behavioral, biological, and social level. The etiology and pathogenesis of these diseases depend appreciably on the style of living. Major risk factors include smoking, lack of physical activity, and unhealthy diets (including excessive alcohol consumption)[1]. Behavioral risk factors can result in hypertension, increased blood sugar/lipid levels, and overweight or obesity[2,3]. Such intermediary factors can be controlled within primary prevention. Determinants also exist at the social, economic, and cultural levels—globalization, urbanization, and population aging[1]. Additional risk factors include male gender, poverty, stress, and genetic predisposition[1]. The occurrence and development of CVDs are also related to psychological disorders such as anxiety, depression, and sleep disorders[10]. A comprehensive approach to cardiac rehabilitation (CR) can contribute favorably to the diagnosis of such conditions and the initiation of treatment[10]. According to the World Health Organization[1,11], up to 80% of premature heart disease, stroke, and diabetes and 75% of recurrent cardiovascular events could be prevented, reinforcing the need for optimized and holistic prevention strategies.
The following text presents a review on CR basics, including its components, prescription, safety, effectiveness, and conventional and alternative training modalities. The next section contains information about CR delivery around the world, its uptake, and potential barriers. Finally, the last part offers strategies to improve CR participation, focusing on telerehabilitation (TR). This work aims to provide a comprehensive overview of contemporary CR and to outline its future perspectives.
CR
Over time, physical activity in reference to heart disease is far from being new. Even in 1772, Heberden observed the beneficial effects in the patient he advised to saw wood for 30 min daily over 6 mo[12,13]. Despite some evidence of these particular physical activity benefits, mobility restriction was imposed on patients with acute cardiac events, often leading to serious deconditioning problems, decline in functional capacity, prolonged hospitalization, and increased mortality[12]. This wrong attitude was furthermore reinforced after the description of myocardial infarction by Herrick in 1912[12,13]. In the 1930s, patients with acute coronary events were still advised to observe 6 wk of bed rest[12]. The “chair” therapy was introduced in the 1940s[14], and a short daily walk of 3-5 mins was allowed 4 wk after the coronary events first in the early 1950s[12]. Concerns about measurable physical invalidism after myocardial infarction were mostly unfounded. Many healed patients had exercise capacity equal to presumably healthy, sedentary middle-aged men[13]. In 1952 the first inpatient exercise training program for patients with CAD was described by Newman et al[15]. Controlled physical activity began during the second week in the hospital and increased until discharge at 6 wk. In the 1960s, progressively earlier mobilization after the acute coronary event was practiced[13]. During the 1970s, the physiological basis of exercise benefits was acknowledged, which led to the development of CR programs[13]. Guidelines for CR were first established by the American College of Sports Medicine[16] and the AHA[17]. Rehabilitation and secondary prevention gained broad support as an essential component of comprehensive care in patients with CAD[13].
CR is defined as a secondary prevention model that reduces mortality and the risk of recurrent events and improves CVD patients' quality of life (QoL)[18-20]. Systematic reviews showed a 20%-25% reduction in all-cause and cardiovascular mortality[21] and a decrease in the risk of recurrent myocardial infarctions by 38%[22]. Shepherd et al[23] pointed to a bilateral relationship between improved QoL and physical activity level. In the setting of CR, hospitalizations for CAD can be reduced by up to 18%[21].
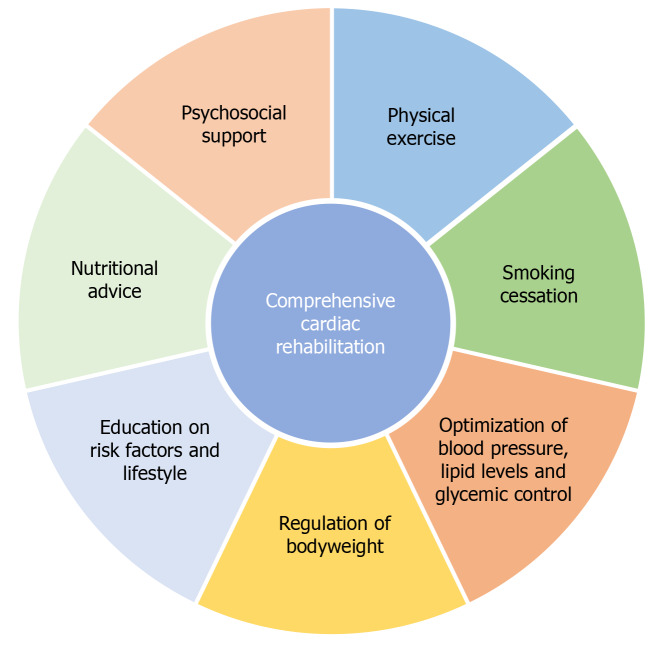
A CR program is primarily based on the favorable effects of physical exercise (exercise-based CR), but comprehensive programs also include educational sessions focusing on risk factors, lifestyle and its modification, nutritional advice, psychological support, and optimized pharmacotherapy (Figure 1). Top-class CR facilities provide this complex area multidisciplinary setting[18,19].
Figure 1.
Comprehensive cardiac rehabilitation and its core components. Programs of cardiac rehabilitation support patients in goals of increasing levels of physical activity, healthy nutrition, optimal adherence to medication, body weight regulation, smoking cessation, and optimal psychosocial well-being, thereby helping them to reduce the risk of recurrent cardiovascular event.
Although representing an important component of follow-up care, CR is provided in < 54% countries worldwide[24,25]. It is particularly low-middle income countries staying behind (about 8% and 28% respectively)[24], despite their broader needs[24,25]. The International Council of Cardiovascular Prevention and Rehabilitation was established in 2011 in response to this situation. This body's primary goal is to join societies (worldwide), support CVDs’ prevention, and promote CR as an essential part of the care[26].
CR consists mainly of the following phases: Hospitalization phase (Phase I); Outpatient phase (Phase II); and Maintenance phase (Phase III/IV)[27-29]. Phase I is initiated early in the hospital (in most cases till 48 h post-operation)[29] and is aimed at patient cardiovascular system adaptation to physical activity and has a crucial role in terms of discharge planning[27,28]. At least as important is the education of patients about risk factors and initiation of their lifestyle changes. A long-term secondary prevention plan is set up during that phase[27,28].
The outpatient CR program that follows (Phase II) lasts typically till week 36 after the CVD event. The main part of the outpatient program is monitored physical exercise (up to 36 training sessions), optimized based on personalized clinical assessment, with input from exercise stress testing (cardiopulmonary exercise testing; e.g., spiroergometry or walk tests)[27,28]. The importance of functional evaluation through exercise testing before starting a training program is strongly emphasized for a physiologically comprehensive exercise intensity prescription[30]. Exercise intensity prescription is a key issue and is directly linked to both the amount of improvement (effectiveness of CR) and the risk of adverse events during exercise (safety)[30]. Phase II is considered crucial concerning the stimulation of favorable lifestyle changes and adherence to secondary prevention principles. This phase is also critical from the participation aspect, which is then mirrored in the maintenance phase[27,28].
The aim of Phase III/IV is to maintain the established lifestyle changes under minimal professional supervision with the target of stabilization of patient's health[27-29].
The main indications of CR (its outpatient phase) include the condition after an acute coronary syndrome, percutaneous coronary intervention, and coronary artery bypass grafting. Chronic coronary syndromes and heart failure (HF) are also indications for CR[27,28,31-34]. Peripheral artery disease of the legs is a less frequent indication[35,36].
The safety of this approach is an essential precondition for a complete CR application. According to the retrospective study by Pavy et al[37], one severe cardiac event was associated with 49565 h of physical exercise and 8484 stress tests. The cardiac arrest rate was calculated to be 1.3 per million hours of training. Low, moderate, or high risk of complications can be estimated based on the patient's overall clinical profile (including a detailed history and physical examination), ejection fraction, functional capacity, the presence of ischemia (electrocardiography ST segment changes; e.g., during exercise stress testing), and any presence of uncontrolled cardiac arrhythmias as well as by other diagnostic tests that could be considered as relevant (on a case-by-case basis by the attending physician)[27,35,38].
The effectiveness of CR has been well described, particularly following coronary revascularization, respectively, in patients with CAD[18,39]. Chronic HF is an increasing health problem. There are two basic types of HF: With reduced ejection fraction (HFrEF, ≤ 40%) and preserved ejection fraction, both resulting in a smaller volume of blood pumped around the body[40]. This diagnosis is associated with a reduced tolerance to exercise, reduced QoL, and increased risk of hospitalization and death[40,41]. In this context, the publications suggest that exercise-based CR is beneficial, but the supporting evidence is less convincing[34,41-43].
According to Long et al[40], exercise-based CR makes little or no difference in all-cause mortality over the short term (but may improve it in the long-term), probably reduces all-cause/HF—specific hospitalization, and may confer a clinically significant improvement in the QoL (mainly in patients with HFrEF). In comparison, Taylor et al[41] show that exercise-based CR did not significantly affect the risk of mortality and hospitalization in patients with HFrEF. The study by Bjarnason-Wehrens et al[42] found similar results but added that programs of CR are likely to improve exercise capacity and QoL in this group of patients. O'Connor et al[43] further came to conflicting results. In the primary analysis, exercise training resulted in nonsignificant reductions in mortality or hospitalization. Nevertheless, after adjusting for highly prognostic predictors, exercise-based CR was associated with modest significant decreases in both all-cause mortality/hospitalization and cardiovascular mortality/HF-specific hospitalization. It seems that CR efficiency in HF also depends on the ejection fraction of the left ventricle[40,41]. In summary, exercise-based CR is an efficient and safe, supported treatment for HF patients[27,42], but further evidence is needed to show better the effects of exercise-based CR, especially among patients with preserved ejection fraction[40].
Comprehensive CR is safe, improves exercise capacity, QoL, and general and mental health in patients implanted with pacemaker or cardioverter-defibrillator[44]. Nevertheless, further extensive studies are needed to evaluate CR's role in this subgroup of patients. CR appears to be also safe in patients following heart transplantation, improves exercise capacity, but has no significant effect on the QoL in the short term[45]. The condition following a valve defect surgery (such as aortic stenosis) is an area where the benefits of CR are examined. CR was found to affect favorably functional capacity and QoL in such patients[46,47]. In addition to the benefits described above, CR also brings about improvements in the symptoms associated with cardiac arrhythmia (atrial fibrillation)[48,49].
Training modalities
Aerobic endurance exercise is the gold standard of the outpatient training program. While most European and North American guidelines recommend a moderate-high intensity, lower-intensity exercise is preferred in the United Kingdom, Australia, and New Zealand[29]. The latter countries also put less accent on technical exercise stress examination (such as electrocardiography, spiroergometry) while routinely using walk tests (e.g., the 6-min walk test, shuttle walk tests) to assess the functional capacity[29].
Timed walk tests are easy to implement, require fewer resources, do not require expensive equipment, and can be a feasible alternative to evaluate functional capacity[50,51]. In the study by Harris et al[52], results from 6-min walk test highly correlated with peak oxygen uptake (peakVO2) measurement among CR patients. Due to Lelis et al[51], Incremental Shuttle Walk Test (ISWT) can contribute to identifying patients at low risk for a cardiac event during exercise at moderate intensity. Hanson et al[50] provide support for the ISWT as a convenient field test but not as a surrogate to predict symptom-limited exercise test duration for individuals. Recently, however, there has been an increasing emphasis on comprehensive stress assessment prior to and after completing the training program[53] including technical exercise stress testing, especially for high-risk patients[30].
Training sessions should be organized three to five times a week, and each session (main exercise) should last 20-60 min[54]. A training session should include the warm-up, main exercise, and cool-down. Preferred types of exercise include walking, jogging, cycling, or rowing[55]. The optimum exercise intensity lies within the range of 40%-70% of the heart rate (HR) reserve or 50%-80% of the exercise capacity (peak HR, peakVO2) achieved during exercise stress testing[54,55]. The incremental cardiopulmo-nary exercise test allows matching the different physiological responses of different exercise intensity to the individual patient pathophysiological and clinical status, maximizing the benefits from aerobic exercise training in CR[30]. Resistance training is also recommended to supplement CR[56].
Some of the main goals of an optimally set exercise-based CR program are the achievement of improved cardiorespiratory fitness (CRF), exercise tolerance, and QoL[21]. Mounting evidence has firmly established that low levels of CRF are associated with a high risk of CVD and all-cause mortality[57]. Although different CRF parameters decrease with age and are (in terms of absolute values) lower in women, a significant beneficial effect of CR on them has been observed[57-59]. The typically measured parameter is peakVO2 (mL/kg per minute) as an important prognostic indicator. Each metabolic equivalent increased by one unit (1 MET = 3.5 mL/kg per minute) was associated with a total mortality risk reduction by 8%-20%[57,60,61], and CR induced a mean peakVO2 increase by 5.4 mL/kg per minute[62]. Furthermore, the study results by Hartman et al[63] suggest that the changes in oxygen uptake were subject to appreciable heterogeneity, which did not correlate with improvements in other cardiovascular risk factors. In this context, De Schutter et al[64] referred that greater increases of peakVO2 (≥ 2.5 mL/kg per minute) were independently associated with improvements in survival, level of high-density lipoprotein cholesterol, weight loss, and body fat. Efforts are needed to improve CRF in secondary prevention of CVDs.
HIGH-INTENSITY INTERVAL TRAINING
It has been demonstrated that CRF improvement is directly dependent on the intensity of the exercise. A higher intensity induces a higher peakVO2 increase[65]. Such findings constitute the basis for high-intensity interval training (HIIT), in which high-intensity activity intervals alternate with lower-intensity activity (or passive recovery) intervals[66].
Compared to the conventional continuous moderate-intensity training practice, studies have reported on HIIT being more effective in improving CRF while remaining safe[67]. In comparison, Conraads et al[68] observed similar improvements in CRF and peripheral endothelial function following continuous training or HIIT in CAD patients. However, the main limitations reported from the study are the lower mean HR training zone in the HIIT group (88% of peak HR) and the higher mean HR training zone in the moderate-intensity continuous training group (80% of peak HR) than was prescribed. The study by Ellingsen et al[69] reported an average training zone of 90% of peak HR for HIIT, where approximately half of the patients with HFrEF trained below the set target. Both studies pointed to the importance of prescribing and adjusting the HR training zone and exercise intensity according to the patient's subjective perception (e.g., use of The Borg Scale). Ballesta García et al[70] reported a higher efficiency of HIIT in patients with HF compared to patients with CAD and recommended using active recovery intervals. According to Wisløff et al[71], exercise intensity was an essential factor for reversing left ventricular remodeling and improving aerobic capacity, endothelial function, and QoL in patients with post-infarction HF. Apart from the above diagnoses, favorable results were also achieved after heart transplantations, including the potential reduction of anxiety—a frequent health complication in such patients[72,73].
Current HIIT research limitations include that most studies use mostly one training modality, which is not a reflection of the real world where different types of physical activity are common[67]. Besides, future research should also focus on women, as this population is a minority in the studies, and this gender bias represents a limitation in generalizing the results.
RESISTANCE TRAINING
While resistance training was perceived as inappropriate in the past because of fear of provoked complications, this training modality is a recommended component of CR[74]. Current experience shows that a combination of endurance training and resistance training (combined training) is more efficient than either modality on its own[75]. Higher efficiency of combined training was demonstrated on improved CRF and muscle strength. Resistance exercise is an essential supplement for elderly patients in particular, resulting in enhanced self-management and QoL[76,77]. The exercise can be based on using the patient's body weight, elastic bands, free weights, or fitness machines. Intensity should be selected to enable eight to fifteen repetitions, eight to ten exercises in a two to three fashion (30%-60% of one-repetition maximum). The recommended training frequency is two to three times a week[55,56].
ALTERNATIVE TRAINING MODALITIES
Apart from the above-mentioned well-established modalities, research has also focused on other feasible training alternatives. The goal is to set up a selection of options and adapt the CR program to the patient's needs, experience, risk profile, and preferences; the options should be appropriately tailored to the ethnic and cultural area[78].
Some of these alternatives include Tai Chi and yoga. Tai Chi, initially a type of Chinese martial art, includes low-moderate intensity aerobic exercise. Data suggests that this exercise brings about improved CRF, like regular activities of identical intensity. Besides, Tai Chi has favorable psychological impacts, including reduced anxiety and depression[79,80]. However, current Tai Chi experiments were performed predominantly on Chinese people. There are large differences in the environment, figure, and cultural background in different countries, and understanding the Tai-Chi approach may be different. Whether Tai Chi is generally effective on a global scale will need to be further verified in the future[81].
Yoga as a combination of physical and respiratory exercise and meditation can also be a useful CR supplement. Yoga was demonstrated to reduce stress and improve the autonomous nervous system's function, thereby affecting cardiovascular risk factors[82]. An alternative approach through yoga has been shown to improve the subjective assessment of health status, reaching pre-infarct levels[83]. If yoga is found to be effective in CR, it has the potential to transform the care of acute myocardial infarction patients in India and other low-middle income country settings[84].
Dance can also be used to complement CR. Urbano et al[85] reported several favorable effects of social dance or samba lessons—glycemia reduction, lower HR at rest, lower cholesterol, and better relaxation.
García et al[86] compared the effect of conventional and modified tennis training (to control the exercise intensity according to the patient’s functional capacity; e.g., walking slowly, walking fast, jogging, and running) in low-risk patients after acute coronary syndrome. Both groups showed improvements in QoL, lipid profile, and exercise tolerance. The development of modifications to CR programs based on alternative sports activities leads, in particular, to improved exercise adherence[87].
Technological progress also offers the opportunity to make use of virtual reality. New generations of active video games allow the user to interact with the platform through targeted movement exercises. Several significant cardiovascular benefits in this context are demonstrated by the reviews of García-Bravo et al[88] and Ruivo[89]. Besides, playing video games is fun, which improves the motivation to exercise[89]. The use of virtual reality could be considered as a complementary tool of exercise training in CR. However, it is necessary to carry out studies with adequate methodological quality to determine the ideal technological systems, target populations, and clear protocols to study their effects in the short, medium and long-term assessments[90].
The authors agree that further extensive studies about alternative training modalities are needed to support such findings.
RESPIRATORY TRAINING
According to the review by Wang et al[91], inspiratory muscle training and respiratory exercise improved HF patients' cardiovascular function, functional capacity, and QoL. Exercise using the Threshold IMT (inspiratory muscle trainer; Respironics, Murrysville, PA, United States) resulted in a higher strength of the respiratory muscles and reduced the perception of dyspnea and depression in patients with HFrEF[92]. According to Hermes et al[93], a combination of conventional training and inspiratory muscle training was more effective than endurance training and resistance training alone in that it increased the peakVO2 in patients after coronary bypass. On the other hand, this hypothesis has not been proved in HF patients[94]. Adamopoulos et al[95] demonstrated that the addition of inspiratory muscle training to CR resulted in a supplementary improvement in respiratory muscle function, dyspnea, QoL, and inflammatory/cardiac biomarkers but not in cardiopulmonary exercise parameters (e.g., peakVO2, ventilatory threshold, exercise duration) in moderate chronic HF. There is a consensus that the inclusion of respiratory training can support the effect of conventional aerobic exercise and, hence, should become a standard option of CR[94,95].
PATIENTS WITH COMORBIDITIES
CR has been traditionally formed as a single disease service in Europe, North America, and other developed countries. Patients have clearly defined CR indications associated with the primary disease (post-myocardial infarction, coronary revascularization, HF, etc.). However, they usually suffer from comorbidities mostly related to hypertension, atrial fibrillation, and diabetes mellitus (e.g., peripheral vascular disease, chronic kidney disease, and post-stroke condition)[96]. Patients with multimorbidity are at higher risk of disability, loss in health-related QoL, hospitalization, and mortality[96].
To reflect these complex clinical situations and improve acceptance of CR, a model of personalized multimorbidity rehabilitation should be embedded and followed. This model counts with the potential interaction of multiple diseases and their management[96]. For example, it is clinically intuitive to combine chronic obstructive pulmonary disease (COPD) and chronic HF in this model. These diseases have a common condition of dyspnea/disability and co-existing prevalence, so service could be targeted around a common disability rather than the primary organ disease[96,97]. According to Evans et al[97], training program (aerobic exercise and educational lessons) focused on the improvement of exercise performance in COPD and HF patients was effective and feasible. Both groups of patients reached similar results in ISWT and were significantly higher compared to usual care. Smith et al[98] identified the emerging evidence to support policy for the management of patients with multimorbidity and common comorbidities in primary care and community settings.
The challenge for the indication-specific model of CR (and other services; e.g., pulmonary rehabilitation) is to evolve, building on existing successes to address more comprehensively the needs of patients with multimorbidity. Due to a lack of evidence, there is an urgent need for research into the acceptability, efficiency, and cost-effectiveness in these models[96].
UTILIZATION OF CR
Although representing an efficient model of secondary prevention, CR does not find as extensive utilization as would be expected[99]. Only 15%-30% of eligible patients enter the training program[100]. The participation is somewhat better, 40%-50% in high-income countries, where health care is readily available[101,102]. High heterogeneity in the availability and utilization is found in the remaining countries. Generally speaking, CR is inadequately available and not widely used in such countries[103].
Because of various availability and delivery worldwide, it is essential to establish and unify quality indicators at the international level (make a standardization of CR)[104]. The European Preventive Associations has recently defined the minimal and optimal standards, including core components of CR, referral and timing, patient assessment, exercise training parameters, education, long-term strategies, or CR results evaluation[74,105]. Compliance with these standards could improve CR process standardization and, hence, increase the quality of CR[106]. Resources, policy changes, and certification systems are needed to ensure that all programs meet minimum standards for CR delivery, ensuring optimal patient safety and outcomes[107].
Adherence to the rehabilitation program is also an issue. According to the review of Oosenbrug et al[108], patients who enter the program completed a median of 72% of the prescribed training sessions.
Utilization by populations
A lower level of utilization was observed among several subgroups, such as older people[109], patients with comorbidities, unemployed, single, and less-educated individuals[110]. Regarding gender, the level of participation and adherence was significantly lower among women than men[101,108], although their needs are often higher[111]. Shanmugasegaram et al[112] found more severe barriers to CR among rural inhabitants. Among factors that adversely affect utilization are low income and low socioeconomic status[110,112]. This effect was demonstrated not only in individuals but also in the social environment[113]. Edwards et al[114] suggest that a lower level of adherence and completion of the rehabilitation program is associated with depression symptoms. Future research should focus on improving the participation of individuals among these specific subgroups.
BARRIERS TO CR
Factors affecting the entry into CR include, in particular, patient awareness, education of physicians (other healthcare providers), availability, and inclusion of rehabilitation in the comprehensive care scheme, financial support, and timing of referral/ enrolment[87]. As to participation and adherence to the training program, primary barriers include work conflicts, family responsibilities, financial costs, fear of the exercise, lack of motivation, or a long travel distance[102]. Lack of social support and comorbid conditions, such as arthritis, osteoporosis, and urinary incontinence, were also reported in a review focusing on female patients[59].
Psychometrically validated scales assessing the utilization have been created, such as Beliefs About Cardiac Rehabilitation[115], Cardiac Rehabilitation Preference Form[116], and Cardiac Rehabilitation Enrolment Obstacles[117]. Their area of use, however, is somewhat limited—Beliefs About Cardiac Rehabilitation is only applicable to enrolment (not participation), Cardiac Rehabilitation Preference Form assesses only patient's perception of the CR program features importance, and Cardiac Rehabilitation Enrolment Obstacles was only validated in a sample of percutaneous coronary intervention patients. The Cardiac Rehabilitation Barriers Scale, serving to identify the barriers to the enrolment/participation at the medical system, health professional, and patient levels is an exception in this respect. The English version includes 21 items (barriers) divided into four subscales: Healthcare system factors; logistical barriers; work/time conflicts; and comorbidities/functional status. The items are assessed on a 5-point Likert scale (1—strongly disagree, 5—strongly agree). A higher score indicates a more substantial barrier to CR[118]. The Cardiac Rehabilitation Barriers Scale has now been translated into 15 languages[119], and new ones are validated on an ongoing basis[120]. According to Ali et al[121], higher adherence/ completion, improved functional status, and reduced depression symptoms correlate with higher patient satisfaction within CR. The Patient Assessment of Chronic Illness Care scale can be used to assess the degree of satisfaction.
The causes of low utilization are very diverse among the different countries[122,123]. While the barriers to CR have been well described in developed countries (North America and Western Europe)[87,100,102], the knowledge is minimal for the rest of the world[123]. Insight into the barriers can help develop strategies aimed at improving the utilization of CR.
STRATEGIES TO IMPROVE UTILIZATION OF CR
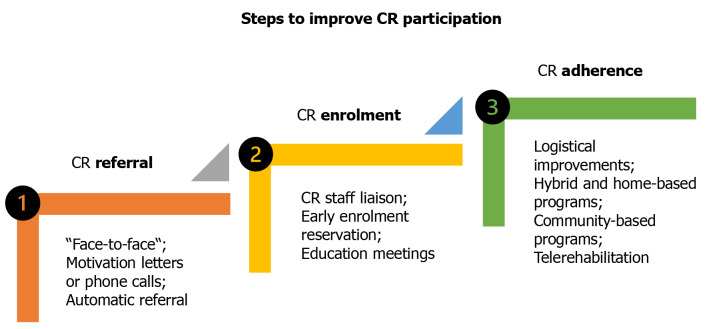
Deficient use of CR is a global problem. So, strategies to improve this, including improvements in participation, are tested particularly in developed countries[124] (Figure 2). However, the countries differ in care availability, training programs, funding patterns, and patient recruitment methods[25,107,125]. For instance, according to the European Cardiac Rehabilitation Inventory Survey[126], only 61% of European countries had national CR associations, and 57% had professional guidelines. As such, both differences and needs should be analyzed.
Figure 2.
Strategies to improve participation in cardiac rehabilitation. The scheme shows efficient strategies for better participation in cardiac rehabilitation. There are three steps for implementation of these—referral, enrolment, and adherence. CR: Cardiac rehabilitation.
CR delivery
A vast majority of the programs are funded by governments and other public sources, private resources (namely by the patient), or health insurance companies. Although financially efficient and even cost-saving[127,128], CR is inconsistently and inadequately reimbursed[127]. Higher funding of services implies a better availability of CR. While the funds often come from national health services in Europe and Central Asia, private systems can play a more critical role in the rest of the world[125].
According to a review by Pesah et al[125], the number of participants per program lies within the range from 129 to 639 annually (median 202, more in Europe than in North America). A larger volume of a program is probably irrelevant against the quality of care provided[129]. Nevertheless, it better covers the capacity needs of CR because it is possible to increase the number of patients per program (not only the number of programs). In this context, Pack et al[130] pointed to the fact that the programs in the United States can cover 58% of eligible patients as a maximum (actual utilization about 34%). So the capacity of CR itself, which is insufficient in several countries, is the next issue for future policy discussion[25].
CR was shown to be available in 54% of countries (median four programs/ country)[25]. Program density concerning the population number can serve as a parameter of CR availability. The estimates are from 0.1 to 6.4 million population per program in the United States and Paraguay. Only one program in the country was identified in Bangladesh, Kenya, and Afghanistan[131]. Individual CR delivery models are being created for low-income countries[132].
In most countries, CR is provided by a multidisciplinary team[125]. According to Supervia et al[107], most programs are led by physicians, and the most common healthcare providers are nurses, nutritional specialists, and physiotherapists. A multidisciplinary team should include the program director and coordinator (cardiologist), nurses, exercise experts (physiotherapists and exercise physiologists), contributing professionals (nutritional specialists, psychologists, pharmacists, and social services experts), and consultant professionals (internists, diabetologists, etc.)[133]. Supervision of exercise training is performed mainly by physiotherapists and nurses[107]. While physiotherapists are a main part of the team in certain regions (e.g., Australia, England, Denmark, Italy, Portugal, Spain, Mexico, Egypt, Qatar, United Arab Emirates, and Canada), exercise physiologists and kinesiologists are more common in some other regions (United States, China, and the Middle East)[125].
On average, 24 training sessions are prescribed per program, though high international and national variability was found[134]. The frequency ranges between one to three sessions a week (average of two sessions a week, 9 wk in total)[125]. A minimum required dose of physical activity must be set to maintain adequate CR effective-ness[135].
CR includes all the major components in most countries, particularly in the high-income ones. Exercise-based programs are most frequently provided. Patient education is frequent in North America and the Middle East, while nutritional advice is provided in East Asia and the Pacific area[125]. Alternative models to center-based programs like home-based or community-based programs are offered infrequently (12% and 10%, respectively)[25]. Mainly in residential areas, is the CR Phase II provided by Austria, Belarus, Croatia, Czech Republic, Finland, France, Germany, Hungary, Iceland, Italy, Lithuania, the Netherlands, Romania, Russia, Serbia, and Spain[125].
The most frequently reported barrier to CR delivery worldwide is a lack of resources[125]. It is unacceptable that Class I/Level A recommendations[28,136] are inadequately funded compared to other similarly graded recommendations for the same indications[125]. Cardiology societies, foundations, and governments should make efforts to achieve better CR reimbursement[127]. The International Council of Cardiovascular Prevention and Rehabilitation recently developed and collected resources for attaining this goal[137].
Globally, the delivery has been described in fewer than one-half of countries providing CR. The least amount of information comes from regions highly burdened by CVDs: East Asia, the Pacific area, Middle East, North Africa, and South Asia. Generally, the data provided are inconsistent, and additional research is needed[25,107,125].
Referral strategies
Santiago de Araújo Pio et al[124] suggest that a referral is more convincing if provided by a health specialist (e.g., nurse or physiotherapist); a physician may play an especially significant role[138]. Information of patients and health professionals (particularly general practitioners and cardiologists) is essential for increasing a level of physical activity within CR. Face-to-face contact is also important[124,138].
Efficient strategies include personal meetings and telephone calls[139], shortening of the time to CR appointment (≤ 10 d)[140], patient navigation/education (e.g., on the bed)[141], text messages[142], and motivation letters based on the Theory of Planned Behaviour[143]. CR enrolment is 27% increased by introducing interventions of that type[99,124].
The importance of initiating appropriate prevention early before hospital discharge cannot be overemphasized, as prevention treatment tends to decrease post-hospitalization[28]. It is necessary to emphasize the preventive measures (e.g., education, referral to CR) directly to the patient, even during the first days after admission/cardiac event, because failure to do so may suggest that these measures are valueless[28,29].
Grace et al[144] determined the attainable limit for enrolment at 70%. Subsequently, Ades et al[145] estimated by calculation that participation improvement from 20% to 70% would save 25000 Lives annually and prevent 180000 hospitalizations every year in the United States.
A system called "automatic referral", based on a patient's electronic record or normal discharge, is also a systematic CR call[146]. Grace et al[147] reported higher effectiveness of the combination of automatic/personal referral (enrollment about 73%) than of each of the strategies (60% and 50% respectively), compared to the standard recommendation (29%). Options also include an early reservation of entry during hospitalization or an educational meeting shortly after discharge from the hospital[148]. An increase in the supply of CR requires decentralization of the exercise training offer. Digital devices and the expansion of community centers can also encourage the practice of physical activity[138].
STRATEGIES FOR IMPROVING ADHERENCE AND COMPLETION
Successful interventions to improve adherence included, among other things, a gender-tailored CR program, because for some patients, public or mixed-gender exercise may represent a serious barrier. In this program, women completed 90% of the prescribed training sessions (77% in the standard program)[149].
Due to review by Room et al[150], focused on behavioural strategies for adherence promotion in older patients, the feedback and monitoring (e.g., individualized graphic feedback on exercise goals and problem-solving support) showed positive outcomes, although there is a lack of evidence to recommend their use currently. According to Lynggaard et al[151], individual patient education based on the Learning and Coping Strategies improved adherence compared to the standard program. This type of intervention appeared to be efficient, particularly in HF patients and low-income and low-educated patients[151]. It is suggested that patients may need ongoing attention and guidance during the outpatient phase of CR but also in the long-term maintenance of their lifestyle changes[150,152].
As it appears, strategies based on unsupervised delivery are most efficient for improving CR adherence. Due to Santiago de Araújo Pio et al[124], such options result in completion improvement by 13%, although utilization measurement in different settings may be difficult to compare and unsupervised delivery is not optimal for all CVD patients (e.g., high-risk patients, especially from the beginning of Phase II)[124].
Home-based program
Among frequent causes of low utilization of center-based programs are logistic barriers, such as a significant travel distance, traffic problems, and time/work conflicts[102]. The home-based program is a feasible solution; it is a CR alternative in a home setting, including exercise, monitoring, control sessions, letters, and telephone calls. Walking is a frequently recommended activity[153,154]. This program's advantages include the possibility of better adaptation to the patient's needs and relatively less time spent because of the patient need and not travel to the rehabilitation site. More detailed benefits and risks of a home-based CR program are listed in Table 1.
Table 1.
Benefits and risks of home-based cardiac rehabilitation program
|
Advantages
|
Disadvantages
|
| Low costs | Lack of "face to face" contact |
| Individual time planning | Deficient supervision and communication |
| More privacy | Absence of social interaction |
| Greater independence and flexibility | Worry about the safety of high-risk patients |
| Minimum time/travel barriers | Lack of published guidelines |
| Integration into daily activities | Lack of legal clarity and accountability |
Home-based program effectiveness has been demonstrated in patients after myocardial infarction and revascularization[21,153], and in HF patients[154]. Like the center-based program, this option has a favorable effect on the risk factors, QoL, and risks of death or a cardiac event[21,153]. Compared to the center-based program, the home-based program was associated with a higher adherence and completion rate[153]. A home-based program may be a reasonable option for selected clinically stable low-moderate risk patients who are eligible for CR but cannot attend a conventional center-based CR program[155].
A hybrid model combining elements (and the assets) of the center-based and home-based program is the next efficient option[156]. According to Imran and colleagues[157,158], hybrid, home-based, and center-based CR induced similar functional capacity improvements, but only home-based programs improved QoL over usual care in HF patients.
TR (remotely monitored training)
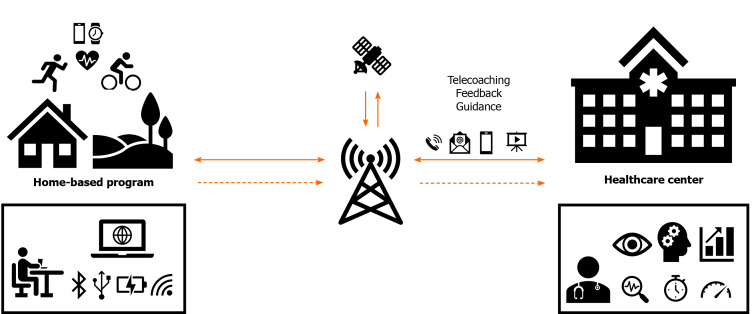
TR is defined as using information and telecommunication technologies to provide health service (rehabilitation) at a large distance. TR includes several approaches, such as remote monitoring, e-learning, and telecoaching[159] (Figure 3). They are approaches that may be used with advantage in home-based or community-based CR programs. Among the most frequently used technologies are smartphones, computers, wearable sensors (for monitoring exercise parameters; e.g., HR and duration of physical activity), and the internet[160,161].
Figure 3.
Scheme of telerehabilitation (remotely monitored training). The diagram describes remotely monitored exercise training and its potential use within the cardiac rehabilitation home-based program framework.
Reviews comparing the overall efficiency of telemedicine interventions and the conventional outpatient program found similar favorable outcomes, including improved functional capacity and improved QoL. TR is a suitable alternative, especially for patients for whom the center-based program is less available[159,162], and it may increase the capacity of the CR[163].
Physical activity in CR is often assessed using questionnaires or accelerometers[164]. The development of technologies opens new monitoring options. For example, Kraal et al[165] measured HR by using a watch and a chest strap. Song et al[166] combined a chest strap with a smartphone, while Batalik et al[167] used an optical sensor on the wrist. Beatty et al[168] tested the feasibility of using a mobile app, which included setting physical activity targets, monitoring protocols including medical metrics (e.g., body weight, blood pressure, and mental condition), education, reminding, and feedback. Besides, lifestyle-focused text messages have led to improved clinical outcomes in patients with CAD[169]. However, this new alternative's promising results will require more detailed research, including data on the association between improvements in surrogates from technological devices and cardiovascular outcomes. This is an unanswered part of CR with the potential to support the use of remotely monitored training.
TR studies have been performed in almost all cardiac patients (including low- to high-risk patients). A comparable completion of TR interventions with center-based programs supports the assumption for a sufficient alternative method[155]. TR can affect barriers and is particularly suitable for those who face specific barriers and can not participate in center-based programs.
The assessment of the safety aspect of TR is based on the experience of center-based rehabilitation. Adverse events are rare in center-based programs[37]. A review of TR interventions reported no cardiovascular complication or death associated with physical training, mostly in studies that evaluated patients with low-moderate risk of cardiovascular complications[161]. Although patients at higher risk (e.g., HF patients) are already better represented in remote-controlled studies to assess lifestyle changes and psychological interventions, this high-risk population is under-represented in TR exercise intervention studies[170]. For these reasons, it follows that remotely monitored exercise training as part of TR is considered a safe alternative to outpatient CR only for CAD patients with a low-moderate risk of complications[74,161,170].
The discussion on this topic is essential because of the current global situation with the coronavirus disease 2019 (COVID-19) pandemic, where levels of physical activity, social isolation, and a closed CR center are limited[171]. This unprecedented situation does not allow many eligible patients to optimize secondary prevention and practice of physical exercise. This is an even more significant challenge to alternatives, such as TR, to provide the core components of CR for these patients[172]. The call is supported by the European Association of Preventive Cardiology, which considers TR relevant to all CVD patients who can not visit CR centers regularly, also after the end of the COVID-19 pandemic[173].
Community-based program
Optimum treatment of a CVD requires a healthy lifestyle sustained in the long run, including regularly practiced physical activity. Such habits are accepted and strengthened during Phase II of CR[27-29]. However, most outpatient programs are too short (4-12 wk) to fix them in the long-term. Exercise adherence eventually decreases, which may have a negative effect on cardiovascular risk factors[174]. The CR community-based program is an efficient option to bridge the period between Phase II (adoption of the new behavioral patterns) and Phase III/IV (long-term mainten-ance)[174,175].
Community-based programs provide similar services as hospital-based programs, including physical assessment and prescription, exercise, education, nutritional advice, and other comprehensive CR components[174,176]. Sports centers frequently organize such programs under the supervision of physiotherapists or nurses. Their advantages included better availability for patients who do not need to travel long distances to the hospital and potentially higher utilization[174,176]. At the same time, an irreplaceable role in terms of patient supervision, regular clinical assessment, and overall follow-up by a physician is needed[27,28].
According to Mosleh et al[176], community-based programs provide health benefits similar to center-based programs. Mandic et al[174] pointed to a higher attractiveness of community-based programs for older patients, married patients, and patients with musculoskeletal problems who live near community centers. CR programs should also support exercise beyond the regular training sessions to achieve the recommended physical activity level. The assessment of this activity is subject to additional studies. For instance, Alharbi et al[177] validated modern accelerometers to measure the number of steps walked and the moderate-high intensity physical activity duration.
COST-EFFECTIVENESS
The costs associated with the treatment of CVDs are high worldwide and are growing every year[178]. For this reason, the cost of treatment, which allows for the efficient use of resources, is very crucial. Healthcare systems that are limited by budget require information on how best to use resources to increase patients' benefits[178].
Hinde et al[179] examined a CR cost-effectiveness model using the current Cochrane review in relation to socioeconomic status and increased CR utilization. The results confirm the evidence that low-cost CR, which reduces the risk of reinfarction and further hospitalization, is a highly cost-effective intervention. Besides, the research noted low CR costs in a group of patients with low socioeconomic status, which increased the potential health benefits. Using England as a model, authors also estimate the expenditure that could be justified while maintaining CR's cost-effectiveness at £68.4 million per year. There is an apparent reason to support interventions that improve CR utilization. In this context, models of the home-based program have good potential. A study by Taylor et al[180] aimed to assess the long-term cost-effectiveness of adding home-based CR to usual care compared to regular care alone in patients with HFrEF. The intervention analysis was associated with a gain of 0.23 quality-adjusted life year points and increased mean cost of £400 compared to usual care, resulting in a cost per quality-adjusted life year gained of £1720. This cost comparison confirms that home-based CR is a cost-effective treatment option for the patient's whole life.
The above findings should support healthcare providers in funding home-based programs to improve availability and increase the CR's utilization. In summary, as currently delivered, CR is cost-effective in all groups of CVDs due to its low cost and high effectiveness in improving cardiovascular outcomes[179,180].
CARDIO-ONCOLOGY
In recent years, it is possible to observe rapid growth in the field of cardio-oncology. Cancer and CVDs are the leading causes of mortality in developed countries[1,181]. Cancer patients often suffer from cardiovascular complications of treatment and increased cardiovascular risk. The most common factors are reduced CRF, muscle atrophy, hypertension, and smoking[182,183]. Furthermore, the adverse effects of treatment are associated with cardiac dysfunction due to cardiovascular toxicity[183]. Prevention and mitigation of these factors are essential, and a comprehensive CR model can offer such an approach[182].
Physical activity is a significant component in cardiovascular prevention and clinical manifestations of cardiotoxicity[182,183]. According to the AHA[183], an individual, tailor-made exercise program is needed for oncology patients. Physical activity should be adjusted and dosed by the patient's characteristics, medication administered, anamnesis, and exercise response. Moreover, it is necessary to consider the individual training modalities, which can also be prescribed under a patient's preference[183]. In order to make the correct exercise prescription, a cardiological evaluation, including a stress test, is required for these patients[183,184].
Recent studies showed reduced development of cancer progression as well as a reduction in cardiovascular risk following the introduction of a physical exercise[182,183]. Evidence suggested that cancer patients participating in an oncology rehabilitation program had experience improving psychological and physiological parameters[185]. Exercise effectively improved CRF, QoL, and mental well-being and reduced fatigue, anxiety, or depression[184].
The CR model's use provides a very suitable approach to bring rehabilitation to a larger oncology population of survivors[182,185]. However, ensuring routine cardio-oncological rehabilitation is likely to require modifications to current models[184].
CONCLUSION
CVDs pose a severe health problem worldwide and are expected to grow in importance. The secondary prevention CR model is a useful option to reduce mortality and disability. CR brings about many benefits for the individual, including sustained self-sufficiency and improved QoL and acts at the system level, for example, by reducing hospitalizations and cutting overall healthcare costs. Despite all of its benefits, the degree of CR utilization is inadequately low. By analyzing each country's situation, barriers to CR in different population subgroups could be identified, allowing for a tailored utilization of new options emerging from current progress in both science and technology. The findings so identified could thus be used to develop even more efficient CR strategies and potential personalized alternatives.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Czech Society of Cardiology, No. 3673.
Peer-review started: November 1, 2020
First decision: December 30, 2020
Article in press: February 1, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: Czech Republic
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Osailan A S-Editor: Gao CC L-Editor: A P-Editor: Li JH
Contributor Information
Petr Winnige, Department of Public Health, Faculty of Medicine, Masaryk University, Czech Republic, Brno 62500, Jihomoravsky, Czech Republic; Department of Rehabilitation, University Hospital Brno, Brno 62500, Czech Republic.
Robert Vysoky, Department of Public Health, Faculty of Medicine, Masaryk University, Czech Republic, Brno 62500, Jihomoravsky, Czech Republic; Department of Health Promotion, Faculty of Sports Studies, Masaryk University, Brno 62500, Jihomoravsky, Czech Republic.
Filip Dosbaba, Department of Rehabilitation, University Hospital Brno, Brno 62500, Czech Republic.
Ladislav Batalik, Department of Rehabilitation, University Hospital Brno, Brno 62500, Czech Republic. batalik.ladislav@fnbrno.cz.
References
- 1.World Health Organization. Cardiovascular diseases (CVDs). 17 May 2017. [cited October 22, 2020]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 2.Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P European Society of Cardiology. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J. 2020;41:12–85. doi: 10.1093/eurheartj/ehz859. [DOI] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 4.Dagenais GR, Leong DP, Rangarajan S, Lanas F, Lopez-Jaramillo P, Gupta R, Diaz R, Avezum A, Oliveira GBF, Wielgosz A, Parambath SR, Mony P, Alhabib KF, Temizhan A, Ismail N, Chifamba J, Yeates K, Khatib R, Rahman O, Zatonska K, Kazmi K, Wei L, Zhu J, Rosengren A, Vijayakumar K, Kaur M, Mohan V, Yusufali A, Kelishadi R, Teo KK, Joseph P, Yusuf S. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2020;395:785–794. doi: 10.1016/S0140-6736(19)32007-0. [DOI] [PubMed] [Google Scholar]
- 5.European Hearth Network. European cardiovascular disease statistics 2017. [cited October 22, 2020]. Available from: http://www.ehnheart.org/cvd-statistics.html .
- 6.Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999-2011. Circulation. 2014;130:966–975. doi: 10.1161/CIRCULATIONAHA.113.007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Català Tella N, Serna Arnaiz C, Real Gatius J, Yuguero Torres O, Galván Santiago L. Assessment of the length of sick leave in patients with ischemic heart disease. BMC Cardiovasc Disord. 2017;17:32. doi: 10.1186/s12872-016-0460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas H, Diamond J, Vieco A, Chaudhuri S, Shinnar E, Cromer S, Perel P, Mensah GA, Narula J, Johnson CO, Roth GA, Moran AE. Global Atlas of Cardiovascular Disease 2000-2016: The Path to Prevention and Control. Glob Heart. 2018;13:143–163. doi: 10.1016/j.gheart.2018.09.511. [DOI] [PubMed] [Google Scholar]
- 10.Chauvet-Gelinier JC, Bonin B. Stress, anxiety and depression in heart disease patients: A major challenge for cardiac rehabilitation. Ann Phys Rehabil Med. 2017;60:6–12. doi: 10.1016/j.rehab.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Chronic diseases and health promotion. [cited October 22, 2020]. Available from: https://www.who.int/chp/chronic_disease_report/part1/en/index11.html .
- 12.Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther. 2012;2:38–49. doi: 10.3978/j.issn.2223-3652.2012.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perk J, Mathes P, Gohlke H, Monpere C, Hellemans I, McGee H, Sellier P, Saner H. Cardiovascular Prevention and Rehabilitation. UK: Springer, 2007. [Google Scholar]
- 14.LEVINE SA, LOWN B. The "chair" treatment of acute thrombosis. Trans Assoc Am Physicians. 1951;64:316–327. [PubMed] [Google Scholar]
- 15.Newman LB, Andrews MF, Koblish MO, Baker LA. Physical medicine and rehabilitation in acute myocardial infarction. AMA Arch Intern Med. 1952;89:552–561. doi: 10.1001/archinte.1952.00240040031004. [DOI] [PubMed] [Google Scholar]
- 16.American College of Sports Medicine. Guidelines for Graded Exercise Testing and Exercise Prescription. Philadelphia: Lea and Febiger, 1975. [Google Scholar]
- 17.American Heart Association. The Exercise Standards Book. Dallas: American Heart Association, 1979. [Google Scholar]
- 18.Salzwedel A, Jensen K, Rauch B, Doherty P, Metzendorf MI, Hackbusch M, Völler H, Schmid JP, Davos CH. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: Update of the Cardiac Rehabilitation Outcome Study (CROS-II) Eur J Prev Cardiol. 2020;27:1756–1774. doi: 10.1177/2047487320905719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji H, Fang L, Yuan L, Zhang Q. Effects of Exercise-Based Cardiac Rehabilitation in Patients with Acute Coronary Syndrome: A Meta-Analysis. Med Sci Monit. 2019;25:5015–5027. doi: 10.12659/MSM.917362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabboul NN, Tomlinson G, Francis TA, Grace SL, Chaves G, Rac V, Daou-Kabboul T, Bielecki JM, Alter DA, Krahn M. Comparative Effectiveness of the Core Components of Cardiac Rehabilitation on Mortality and Morbidity: A Systematic Review and Network Meta-Analysis. J Clin Med. 2018;7 doi: 10.3390/jcm7120514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta-analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med. 2005;143:659–672. doi: 10.7326/0003-4819-143-9-200511010-00010. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd CW, While AE. Cardiac rehabilitation and quality of life: a systematic review. Int J Nurs Stud. 2012;49:755–771. doi: 10.1016/j.ijnurstu.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Grace SL, Turk-Adawi KI, Contractor A, Atrey A, Campbell N, Derman W, Melo Ghisi GL, Oldridge N, Sarkar BK, Yeo TJ, Lopez-Jimenez F, Mendis S, Oh P, Hu D, Sarrafzadegan N. Cardiac rehabilitation delivery model for low-resource settings. Heart. 2016;102:1449–1455. doi: 10.1136/heartjnl-2015-309209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turk-Adawi K, Supervia M, Lopez-Jimenez F, Pesah E, Ding R, Britto RR, Bjarnason-Wehrens B, Derman W, Abreu A, Babu AS, Santos CA, Jong SK, Cuenza L, Yeo TJ, Scantlebury D, Andersen K, Gonzalez G, Giga V, Vulic D, Vataman E, Cliff J, Kouidi E, Yagci I, Kim C, Benaim B, Estany ER, Fernandez R, Radi B, Gaita D, Simon A, Chen SY, Roxburgh B, Martin JC, Maskhulia L, Burdiat G, Salmon R, Lomelí H, Sadeghi M, Sovova E, Hautala A, Tamuleviciute-Prasciene E, Ambrosetti M, Neubeck L, Asher E, Kemps H, Eysymontt Z, Farsky S, Hayward J, Prescott E, Dawkes S, Santibanez C, Zeballos C, Pavy B, Kiessling A, Sarrafzadegan N, Baer C, Thomas R, Hu D, Grace SL. Cardiac Rehabilitation Availability and Density around the Globe. EClinicalMedicine. 2019;13:31–45. doi: 10.1016/j.eclinm.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Council of Cardiovascular Prevention and Rehabilitation (ICCPR) Primary goals. 2011 [cited October 26, 2020]. Available from: http://globalcardiacrehab.com/about-us/primary-goals/
- 27.American Association for Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs. 5th ed. US: Human Kinetics, 2013. [Google Scholar]
- 28.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price KJ, Gordon BA, Bird SR, Benson AC. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus? Eur J Prev Cardiol. 2016;23:1715–1733. doi: 10.1177/2047487316657669. [DOI] [PubMed] [Google Scholar]
- 30.Mezzani A, Hamm LF, Jones AM, McBride PE, Moholdt T, Stone JA, Urhausen A, Williams MA European Association for Cardiovascular Prevention and Rehabilitation; American Association of Cardiovascular and Pulmonary Rehabilitation; Canadian Association of Cardiac Rehabilitation. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur J Prev Cardiol. 2013;20:442–467. doi: 10.1177/2047487312460484. [DOI] [PubMed] [Google Scholar]
- 31.Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 32.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 33.Eijsvogels TMH, Maessen MFH, Bakker EA, Meindersma EP, van Gorp N, Pijnenburg N, Thompson PD, Hopman MTE. Association of Cardiac Rehabilitation With All-Cause Mortality Among Patients With Cardiovascular Disease in the Netherlands. JAMA Netw Open. 2020;3:e2011686. doi: 10.1001/jamanetworkopen.2020.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 35.Ambrosetti M, Temporelli PL, Faggiano P, Febo O, Diaco T, Favretto G, Calisi P, Gabriele M, Greco C, Tavazzi L THINKPAD investigators. Lower extremities peripheral arterial disease among patients admitted to cardiac rehabilitation: the THINKPAD registry. Int J Cardiol. 2014;171:192–198. doi: 10.1016/j.ijcard.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 37.Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med. 2006;166:2329–2334. doi: 10.1001/archinte.166.21.2329. [DOI] [PubMed] [Google Scholar]
- 38.Silva AK, Barbosa MP, Bernardo AF, Vanderlei FM, Pacagnelli FL, Vanderlei LC. Cardiac risk stratification in cardiac rehabilitation programs: a review of protocols. Rev Bras Cir Cardiovasc. 2014;29:255–265. doi: 10.5935/1678-9741.20140067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prabhu NV, Maiya AG, Prabhu NS. Impact of Cardiac Rehabilitation on Functional Capacity and Physical Activity after Coronary Revascularization: A Scientific Review. Cardiol Res Pract. 2020;2020:1236968. doi: 10.1155/2020/1236968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, Rees K, Singh SJ, Taylor RS. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019;1:CD003331. doi: 10.1002/14651858.CD003331.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, O'Connor C, Whellan D, Keteyian SJ, Coats A, Davos CH, Dalal HM, Dracup K, Evangelista L, Jolly K, Myers J, McKelvie RS, Nilsson BB, Passino C, Witham MD, Yeh GY, Zwisler AO ExTraMATCH II Collaboration. Impact of exercise-based cardiac rehabilitation in patients with heart failure (ExTraMATCH II) on mortality and hospitalisation: an individual patient data meta-analysis of randomised trials. Eur J Heart Fail. 2018;20:1735–1743. doi: 10.1002/ejhf.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjarnason-Wehrens B, Nebel R, Jensen K, Hackbusch M, Grilli M, Gielen S, Schwaab B, Rauch B German Society of Cardiovascular Prevention and Rehabilitation (DGPR) Exercise-based cardiac rehabilitation in patients with reduced left ventricular ejection fraction: The Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): A systematic review and meta-analysis. Eur J Prev Cardiol. 2020;27:929–952. doi: 10.1177/2047487319854140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iliou MC, Blanchard JC, Lamar-Tanguy A, Cristofini P, Ledru F. Cardiac rehabilitation in patients with pacemakers and implantable cardioverter defibrillators. Monaldi Arch Chest Dis. 2016;86:756. doi: 10.4081/monaldi.2016.756. [DOI] [PubMed] [Google Scholar]
- 45.Anderson L, Nguyen TT, Dall CH, Burgess L, Bridges C, Taylor RS. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst Rev. 2017;4:CD012264. doi: 10.1002/14651858.CD012264.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sibilitz KL, Berg SK, Tang LH, Risom SS, Gluud C, Lindschou J, Kober L, Hassager C, Taylor RS, Zwisler AD. Exercise-based cardiac rehabilitation for adults after heart valve surgery. Cochrane Database Syst Rev. 2016;3:CD010876. doi: 10.1002/14651858.CD010876.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro GS, Melo RD, Deresz LF, Dal Lago P, Pontes MR, Karsten M. Cardiac rehabilitation programme after transcatheter aortic valve implantation vs surgical aortic valve replacement: Systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24:688–697. doi: 10.1177/2047487316686442. [DOI] [PubMed] [Google Scholar]
- 48.Robaye B, Lakiss N, Dumont F, Laruelle C. Atrial fibrillation and cardiac rehabilitation: an overview. Acta Cardiol. 2020;75:116–120. doi: 10.1080/00015385.2019.1565663. [DOI] [PubMed] [Google Scholar]
- 49.Reed JL, Terada T, Chirico D, Prince SA, Pipe AL. The Effects of Cardiac Rehabilitation in Patients With Atrial Fibrillation: A Systematic Review. Can J Cardiol. 2018;34:S284–S295. doi: 10.1016/j.cjca.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 50.Hanson LC, McBurney H, Taylor NF. Is the 10 m incremental shuttle walk test a useful test of exercise capacity for patients referred to cardiac rehabilitation? Eur J Cardiovasc Nurs. 2018;17:159–169. doi: 10.1177/1474515117721129. [DOI] [PubMed] [Google Scholar]
- 51.Lelis JD, Chaves G, Ghisi GLM, Grace SL, Britto RR. Validity of the Incremental Shuttle Walk Test to Assess Exercise Safety When Initiating Cardiac Rehabilitation in Low-Resource Settings. J Cardiopulm Rehabil Prev. 2019;39:E1–E7. doi: 10.1097/HCR.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 52.Harris KM, Anderson DR, Landers JD, Emery CF. Utility of Walk Tests in Evaluating Functional Status Among Participants in an Outpatient Cardiac Rehabilitation Program. J Cardiopulm Rehabil Prev. 2017;37:329–333. doi: 10.1097/HCR.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 53.Zecchin R, Candelaria D, Ferry C, Ladak LA, McIvor D, Wilcox K, Bennett A, Bowen S, Carr B, Randall S, Gallagher R. Development of Quality Indicators for Cardiac Rehabilitation in Australia: A Modified Delphi Method and Pilot Test. Heart Lung Circ. 2019;28:1622–1630. doi: 10.1016/j.hlc.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard D; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115:2675–2682. doi: 10.1161/CIRCULATIONAHA.106.180945. [DOI] [PubMed] [Google Scholar]
- 55.Ancira P, Higgins P. Optimal dose and modality of exercise in patients with coronary artery disease: A review. J Aerobics Fitness. 2016;1:1–5. [Google Scholar]
- 56.Wise FM, Patrick JM. Resistance exercise in cardiac rehabilitation. Clin Rehabil. 2011;25:1059–1065. doi: 10.1177/0269215511423408. [DOI] [PubMed] [Google Scholar]
- 57.Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 58.Jackson AS, Sui X, Hébert JR, Church TS, Blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169:1781–1787. doi: 10.1001/archinternmed.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Witvrouwen I, Van Craenenbroeck EM, Abreu A, Moholdt T, Kränkel N. Exercise training in women with cardiovascular disease: Differential response and barriers - review and perspective. Eur J Prev Cardiol. :2019: 2047487319838221. doi: 10.1177/2047487319838221. [DOI] [PubMed] [Google Scholar]
- 60.Keteyian SJ, Brawner CA, Savage PD, Ehrman JK, Schairer J, Divine G, Aldred H, Ophaug K, Ades PA. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J. 2008;156:292–300. doi: 10.1016/j.ahj.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 61.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous vs moderate intensity aerobic exercise. Am J Cardiol. 2006;97:141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 62.Sandercock G, Hurtado V, Cardoso F. Changes in cardiorespiratory fitness in cardiac rehabilitation patients: a meta-analysis. Int J Cardiol. 2013;167:894–902. doi: 10.1016/j.ijcard.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 63.Hartman YAW, Hopman MTE, Schreuder TH, Verheggen RJHM, Scholten RR, Oudegeest-Sander MH, Poelkens F, Maiorana AJ, Naylor LH, Willems PH, Tack CJ, Thijssen DHJ, Green DJ. Improvements in fitness are not obligatory for exercise training-induced improvements in CV risk factors. Physiol Rep. 2018;6 doi: 10.14814/phy2.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Schutter A, Kachur S, Lavie CJ, Menezes A, Shum KK, Bangalore S, Arena R, Milani RV. Cardiac rehabilitation fitness changes and subsequent survival. Eur Heart J Qual Care Clin Outcomes. 2018;4:173–179. doi: 10.1093/ehjqcco/qcy018. [DOI] [PubMed] [Google Scholar]
- 65.Mitchell BL, Lock MJ, Davison K, Parfitt G, Buckley JP, Eston RG. What is the effect of aerobic exercise intensity on cardiorespiratory fitness in those undergoing cardiac rehabilitation? Br J Sports Med. 2019;53:1341–1351. doi: 10.1136/bjsports-2018-099153. [DOI] [PubMed] [Google Scholar]
- 66.Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med. 2012;42:587–605. doi: 10.2165/11631910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 67.Hannan AL, Hing W, Simas V, Climstein M, Coombes JS, Jayasinghe R, Byrnes J, Furness J. High-intensity interval training vs moderate-intensity continuous training within cardiac rehabilitation: a systematic review and meta-analysis. Open Access J Sports Med. 2018;9:1–17. doi: 10.2147/OAJSM.S150596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conraads VM, Pattyn N, De Maeyer C, Beckers PJ, Coeckelberghs E, Cornelissen VA, Denollet J, Frederix G, Goetschalckx K, Hoymans VY, Possemiers N, Schepers D, Shivalkar B, Voigt JU, Van Craenenbroeck EM, Vanhees L. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol. 2015;179:203–210. doi: 10.1016/j.ijcard.2014.10.155. [DOI] [PubMed] [Google Scholar]
- 69.Ellingsen Ø, Halle M, Conraads V, Støylen A, Dalen H, Delagardelle C, Larsen AI, Hole T, Mezzani A, Van Craenenbroeck EM, Videm V, Beckers P, Christle JW, Winzer E, Mangner N, Woitek F, Höllriegel R, Pressler A, Monk-Hansen T, Snoer M, Feiereisen P, Valborgland T, Kjekshus J, Hambrecht R, Gielen S, Karlsen T, Prescott E, Linke A SMARTEX Heart Failure Study (Study of Myocardial Recovery After Exercise Training in Heart Failure) Group. High-Intensity Interval Training in Patients With Heart Failure With Reduced Ejection Fraction. Circulation. 2017;135:839–849. doi: 10.1161/CIRCULATIONAHA.116.022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ballesta García I, Rubio Arias JÁ, Ramos Campo DJ, Martínez González-Moro I, Carrasco Poyatos M. High-intensity Interval Training Dosage for Heart Failure and Coronary Artery Disease Cardiac Rehabilitation. A Systematic Review and Meta-analysis. Rev Esp Cardiol (Engl Ed) 2019;72:233–243. doi: 10.1016/j.rec.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 71.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training vs moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 72.Nytrøen K, Rolid K, Andreassen AK, Yardley M, Gude E, Dahle DO, Bjørkelund E, Relbo Authen A, Grov I, Philip Wigh J, Have Dall C, Gustafsson F, Karason K, Gullestad L. Effect of High-Intensity Interval Training in De Novo Heart Transplant Recipients in Scandinavia. Circulation. 2019;139:2198–2211. doi: 10.1161/CIRCULATIONAHA.118.036747. [DOI] [PubMed] [Google Scholar]
- 73.Yardley M, Gullestad L, Bendz B, Bjørkelund E, Rolid K, Arora S, Nytrøen K. Long-term effects of high-intensity interval training in heart transplant recipients: A 5-year follow-up study of a randomized controlled trial. Clin Transplant. 2017;31 doi: 10.1111/ctr.12868. [DOI] [PubMed] [Google Scholar]
- 74.Ambrosetti M, Abreu A, Corrà U, Davos CH, Hansen D, Frederix I, Iliou MC, Pedretti RF, Schmid JP, Vigorito C, Voller H, Wilhelm M, Piepoli MF, Bjarnason-Wehrens B, Berger T, Cohen-Solal A, Cornelissen V, Dendale P, Doehner W, Gaita D, Gevaert AB, Kemps H, Kraenkel N, Laukkanen J, Mendes M, Niebauer J, Simonenko M, Zwisler AO. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. :2020: 2047487320913379. doi: 10.1177/2047487320913379. [DOI] [PubMed] [Google Scholar]
- 75.Schroeder EC, Franke WD, Sharp RL, Lee DC. Comparative effectiveness of aerobic, resistance, and combined training on cardiovascular disease risk factors: A randomized controlled trial. PLoS One. 2019;14:e0210292. doi: 10.1371/journal.pone.0210292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khadanga S, Savage PD, Ades PA. Resistance Training for Older Adults in Cardiac Rehabilitation. Clin Geriatr Med. 2019;35:459–468. doi: 10.1016/j.cger.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xanthos PD, Gordon BA, Kingsley MI. Implementing resistance training in the rehabilitation of coronary heart disease: A systematic review and meta-analysis. Int J Cardiol. 2017;230:493–508. doi: 10.1016/j.ijcard.2016.12.076. [DOI] [PubMed] [Google Scholar]
- 78.Clark RA, Conway A, Poulsen V, Keech W, Tirimacco R, Tideman P. Alternative models of cardiac rehabilitation: a systematic review. Eur J Prev Cardiol. 2015;22:35–74. doi: 10.1177/2047487313501093. [DOI] [PubMed] [Google Scholar]
- 79.Liu T, Chan AW, Liu YH, Taylor-Piliae RE. Effects of Tai Chi-based cardiac rehabilitation on aerobic endurance, psychosocial well-being, and cardiovascular risk reduction among patients with coronary heart disease: A systematic review and meta-analysis. Eur J Cardiovasc Nurs. 2018;17:368–383. doi: 10.1177/1474515117749592. [DOI] [PubMed] [Google Scholar]
- 80.Yang YL, Wang YH, Wang SR, Shi PS, Wang C. The Effect of Tai Chi on Cardiorespiratory Fitness for Coronary Disease Rehabilitation: A Systematic Review and Meta-Analysis. Front Physiol. 2017;8:1091. doi: 10.3389/fphys.2017.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng D, Wang B, Li Q, Guo Y, Wang L. Research on Function and Mechanism of Tai Chi on Cardiac Rehabilitation. Chin J Integr Med. 2020;26:393–400. doi: 10.1007/s11655-020-3262-9. [DOI] [PubMed] [Google Scholar]
- 82.Guddeti RR, Dang G, Williams MA, Alla VM. Role of Yoga in Cardiac Disease and Rehabilitation. J Cardiopulm Rehabil Prev. 2019;39:146–152. doi: 10.1097/HCR.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 83.Prabhakaran D, Chandrasekaran AM, Singh K, Mohan B, Chattopadhyay K, Chadha DS, Negi PC, Bhat P, Sadananda KS, Ajay VS, Singh K, Praveen PA, Devarajan R, Kondal D, Soni D, Mallinson P, Manchanda SC, Madan K, Hughes AD, Chathurvedi N, Roberts I, Ebrahim S, Reddy KS, Tandon N, Pocock S, Roy A, Kinra S Yoga-CaRe Trial Investigators. Yoga-Based Cardiac Rehabilitation After Acute Myocardial Infarction: A Randomized Trial. J Am Coll Cardiol. 2020;75:1551–1561. doi: 10.1016/j.jacc.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chandrasekaran AM, Kinra S, Ajay VS, Chattopadhyay K, Singh K, Singh K, Praveen PA, Soni D, Devarajan R, Kondal D, Manchanda SC, Hughes AD, Chaturvedi N, Roberts I, Pocock S, Ebrahim S, Reddy KS, Tandon N, Prabhakaran D Yoga-CaRe Trial Team. Effectiveness and cost-effectiveness of a Yoga-based Cardiac Rehabilitation (Yoga-CaRe) program following acute myocardial infarction: Study rationale and design of a multi-center randomized controlled trial. Int J Cardiol. 2019;280:14–18. doi: 10.1016/j.ijcard.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 85.Urbano I, Marques ACS, Milanez M. Dance as a Supplementary Instrument for Cardiac Rehabilitation: An Integrative Literature Review. Int J Art. 2018;7:17–29. [Google Scholar]
- 86.García JP, Giraldo VM, Barrado JJ, Casasola CD. Tennis training sessions as a rehabilitation instrument for patients after acute myocardial infarction. J Sports Sci Med. 2013;12:316–322. [PMC free article] [PubMed] [Google Scholar]
- 87.Clark AM, King-Shier KM, Duncan A, Spaling M, Stone JA, Jaglal S, Angus J. Factors influencing referral to cardiac rehabilitation and secondary prevention programs: a systematic review. Eur J Prev Cardiol. 2013;20:692–700. doi: 10.1177/2047487312447846. [DOI] [PubMed] [Google Scholar]
- 88.García-Bravo S, Cuesta-Gómez A, Campuzano-Ruiz R, López-Navas MJ, Domínguez-Paniagua J, Araújo-Narváez A, Barreñada-Copete E, García-Bravo C, Flórez-García MT, Botas-Rodríguez J, Cano-de-la-Cuerda R. Virtual reality and video games in cardiac rehabilitation programs. A systematic review. Disabil Rehabil. :2019: 1–10. doi: 10.1080/09638288.2019.1631892. [DOI] [PubMed] [Google Scholar]
- 89.Ruivo JA. Exergames and cardiac rehabilitation: a review. J Cardiopulm Rehabil Prev. 2014;34:2–20. doi: 10.1097/HCR.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 90.García-Bravo S, Cano-de-la-Cuerda R, Domínguez-Paniagua J, Campuzano-Ruiz R, Barreñada-Copete E, López-Navas MJ, Araujo-Narváez A, García-Bravo C, Florez-Garcia M, Botas-Rodríguez J, Cuesta-Gómez A. Effects of Virtual Reality on Cardiac Rehabilitation Programs for Ischemic Heart Disease: A Randomized Pilot Clinical Trial. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17228472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang MH, Yeh ML. Respiratory training interventions improve health status of heart failure patients: A systematic review and network meta-analysis of randomized controlled trials. World J Clin Cases. 2019;7:2760–2775. doi: 10.12998/wjcc.v7.i18.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bosnak-Guclu M, Arikan H, Savci S, Inal-Ince D, Tulumen E, Aytemir K, Tokgözoglu L. Effects of inspiratory muscle training in patients with heart failure. Respir Med. 2011;105:1671–1681. doi: 10.1016/j.rmed.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 93.Hermes BM, Cardoso DM, Gomes TJ, Santos TD, Vicente MS, Pereira SN, Barbosa VA, Albuquerque IM. Short-term inspiratory muscle training potentiates the benefits of aerobic and resistance training in patients undergoing CABG in phase II cardiac rehabilitation program. Rev Bras Cir Cardiovasc. 2015;30:474–481. doi: 10.5935/1678-9741.20150043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neto MG, Martinez BP, Conceição CS, Silva PE, Carvalho VO. Combined Exercise and Inspiratory Muscle Training in Patients With Heart Failure: A SYSTEMATIC REVIEW AND META-ANALYSIS. J Cardiopulm Rehabil Prev. 2016;36:395–401. doi: 10.1097/HCR.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 95.Adamopoulos S, Schmid JP, Dendale P, Poerschke D, Hansen D, Dritsas A, Kouloubinis A, Alders T, Gkouziouta A, Reyckers I, Vartela V, Plessas N, Doulaptsis C, Saner H, Laoutaris ID. Combined aerobic/inspiratory muscle training vs. aerobic training in patients with chronic heart failure: The Vent-HeFT trial: a European prospective multicentre randomized trial. Eur J Heart Fail. 2014;16:574–582. doi: 10.1002/ejhf.70. [DOI] [PubMed] [Google Scholar]
- 96.Taylor RS, Singh S. Personalised rehabilitation for cardiac and pulmonary patients with multimorbidity: Time for implementation? Eur J Prev Cardiol. :2020: 2047487320926058. doi: 10.1177/2047487320926058. [DOI] [PubMed] [Google Scholar]
- 97.Evans RA, Singh SJ, Collier R, Loke I, Steiner MC, Morgan MD. Generic, symptom based, exercise rehabilitation; integrating patients with COPD and heart failure. Respir Med. 2010;104:1473–1481. doi: 10.1016/j.rmed.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 98.Smith SM, Wallace E, O'Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2016;3:CD006560. doi: 10.1002/14651858.CD006560.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santiago de Araújo Pio C, Beckie TM, Varnfield M, Sarrafzadegan N, Babu AS, Baidya S, Buckley J, Chen SY, Gagliardi A, Heine M, Khiong JS, Mola A, Radi B, Supervia M, Trani MR, Abreu A, Sawdon JA, Moffatt PD, Grace SL. Promoting patient utilization of outpatient cardiac rehabilitation: A joint International Council and Canadian Association of Cardiovascular Prevention and Rehabilitation position statement. Int J Cardiol. 2020;298:1–7. doi: 10.1016/j.ijcard.2019.06.064. [DOI] [PubMed] [Google Scholar]
- 100.Neubeck L, Freedman SB, Clark AM, Briffa T, Bauman A, Redfern J. Participating in cardiac rehabilitation: a systematic review and meta-synthesis of qualitative data. Eur J Prev Cardiol. 2012;19:494–503. doi: 10.1177/1741826711409326. [DOI] [PubMed] [Google Scholar]
- 101.Samayoa L, Grace SL, Gravely S, Scott LB, Marzolini S, Colella TJ. Sex differences in cardiac rehabilitation enrollment: a meta-analysis. Can J Cardiol. 2014;30:793–800. doi: 10.1016/j.cjca.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 102.Clark AM, King-Shier KM, Thompson DR, Spaling MA, Duncan AS, Stone JA, Jaglal SB, Angus JE. A qualitative systematic review of influences on attendance at cardiac rehabilitation programs after referral. Am Heart J 2012; 164: 835-845. :e2. doi: 10.1016/j.ahj.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 103.Ragupathi L, Stribling J, Yakunina Y, Fuster V, McLaughlin MA, Vedanthan R. Availability, Use, and Barriers to Cardiac Rehabilitation in LMIC. Glob Heart 2017; 12: 323-334. :e10. doi: 10.1016/j.gheart.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 104.Moghei M, Oh P, Chessex C, Grace SL. Cardiac Rehabilitation Quality Improvement: A NARRATIVE REVIEW. J Cardiopulm Rehabil Prev. 2019;39:226–234. doi: 10.1097/HCR.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 105.British Association for Cardiovascular Prevention and Rehabilitation. Cardiovascular Disease Prevention and Rehabilitation 2017. [cited October 28, 2020]. Available from: https://www.bacpr.com/resources/BACPR_Standards_and_Core_Components_2017.pdf .
- 106.Abreu A, Frederix I, Dendale P, Janssen A, Doherty P, Piepoli MF, Völler H Secondary Prevention and Rehabilitation Section of EAPC Reviewers: Marco Ambrosetti; Davos CH. Standardization and quality improvement of secondary prevention through cardiovascular rehabilitation programmes in Europe: The avenue towards EAPC accreditation programme: A position statement of the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology (EAPC) Eur J Prev Cardiol. :2020: 2047487320924912. doi: 10.1177/2047487320924912. [DOI] [PubMed] [Google Scholar]
- 107.Supervia M, Turk-Adawi K, Lopez-Jimenez F, Pesah E, Ding R, Britto RR, Bjarnason-Wehrens B, Derman W, Abreu A, Babu AS, Santos CA, Jong SK, Cuenza L, Yeo TJ, Scantlebury D, Andersen K, Gonzalez G, Giga V, Vulic D, Vataman E, Cliff J, Kouidi E, Yagci I, Kim C, Benaim B, Estany ER, Fernandez R, Radi B, Gaita D, Simon A, Chen SY, Roxburgh B, Martin JC, Maskhulia L, Burdiat G, Salmon R, Lomelí H, Sadeghi M, Sovova E, Hautala A, Tamuleviciute-Prasciene E, Ambrosetti M, Neubeck L, Asher E, Kemps H, Eysymontt Z, Farsky S, Hayward J, Prescott E, Dawkes S, Santibanez C, Zeballos C, Pavy B, Kiessling A, Sarrafzadegan N, Baer C, Thomas R, Hu D, Grace SL. Nature of Cardiac Rehabilitation Around the Globe. EClinicalMedicine. 2019;13:46–56. doi: 10.1016/j.eclinm.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oosenbrug E, Marinho RP, Zhang J, Marzolini S, Colella TJ, Pakosh M, Grace SL. Sex Differences in Cardiac Rehabilitation Adherence: A Meta-analysis. Can J Cardiol. 2016;32:1316–1324. doi: 10.1016/j.cjca.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 109.Doll JA, Hellkamp A, Ho PM, Kontos MC, Whooley MA, Peterson ED, Wang TY. Participation in Cardiac Rehabilitation Programs Among Older Patients After Acute Myocardial Infarction. JAMA Intern Med. 2015;175:1700–1702. doi: 10.1001/jamainternmed.2015.3819. [DOI] [PubMed] [Google Scholar]
- 110.Ruano-Ravina A, Pena-Gil C, Abu-Assi E, Raposeiras S, van 't Hof A, Meindersma E, Bossano Prescott EI, González-Juanatey JR. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int J Cardiol. 2016;223:436–443. doi: 10.1016/j.ijcard.2016.08.120. [DOI] [PubMed] [Google Scholar]
- 111.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 112.Shanmugasegaram S, Oh P, Reid RD, McCumber T, Grace SL. Cardiac rehabilitation barriers by rurality and socioeconomic status: a cross-sectional study. Int J Equity Health. 2013;12:72. doi: 10.1186/1475-9276-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bachmann JM, Huang S, Gupta DK, Lipworth L, Mumma MT, Blot WJ, Akwo EA, Kripalani S, Whooley MA, Wang TJ, Freiberg MS. Association of Neighborhood Socioeconomic Context With Participation in Cardiac Rehabilitation. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edwards BL, Sydeman SJ. Depression Is Associated With Reduced Outpatient Cardiac Rehabilitation Completion Rates: A SYSTEMATIC LITERATURE REVIEW AND META-ANALYSIS. J Cardiopulm Rehabil Prev. 2019;39:365–372. doi: 10.1097/HCR.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 115.Cooper AF, Weinman J, Hankins M, Jackson G, Horne R. Assessing patients' beliefs about cardiac rehabilitation as a basis for predicting attendance after acute myocardial infarction. Heart. 2007;93:53–58. doi: 10.1136/hrt.2005.081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fernandez RS, Salamonson Y, Juergens C, Griffiths R, Davidson P. Validation of the revised cardiac rehabilitation preference form in patients with post-percutaneous coronary intervention. J Cardiopulm Rehabil Prev. 2007;27:390–394. doi: 10.1097/01.HCR.0000300267.92516.23. [DOI] [PubMed] [Google Scholar]
- 117.Fernandez RS, Salamonson Y, Juergens C, Griffiths R, Davidson P. Development and preliminary testing of the Cardiac Rehabilitation Enrolment Obstacles (CREO) scale: implications for service development. Eur J Cardiovasc Nurs. 2008;7:96–102. doi: 10.1016/j.ejcnurse.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 118.Shanmugasegaram S, Gagliese L, Oh P, Stewart DE, Brister SJ, Chan V, Grace SL. Psychometric validation of the cardiac rehabilitation barriers scale. Clin Rehabil. 2012;26:152–164. doi: 10.1177/0269215511410579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.York University. CRBS instructions and languages/translations. [cited October 29, 2020]. Available from: https://sgrace.info.yorku.ca/cr-barriers-scale/crbs-instructions-and-languages-translations/
- 120.Winnige P, Batalik L, Filakova K, Hnatiak J, Dosbaba F, Grace SL. Translation and validation of the cardiac rehabilitation barriers scale in the Czech Republic (CRBS-CZE): Protocol to determine the key barriers in East-Central Europe. Medicine (Baltimore) 2020;99:e19546. doi: 10.1097/MD.0000000000019546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ali S, Chessex C, Bassett-Gunter R, Grace SL. Patient satisfaction with cardiac rehabilitation: association with utilization, functional capacity, and heart-health behaviors. Patient Prefer Adherence. 2017;11:821–830. doi: 10.2147/PPA.S120464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Im HW, Baek S, Jee S, Ahn JM, Park MW, Kim WS. Barriers to Outpatient Hospital-Based Cardiac Rehabilitation in Korean Patients With Acute Coronary Syndrome. Ann Rehabil Med. 2018;42:154–165. doi: 10.5535/arm.2018.42.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chai LS, Siop S, Putit Z, Lim L, Gunggu A, Tie SF. Translation, Adaptation, and Validation of the Malay Version of the Cardiac Rehabilitation Barriers Scale. J Nurs Res. 2020;28:e64. doi: 10.1097/jnr.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 124.Santiago de Araújo Pio C, Chaves GS, Davies P, Taylor RS, Grace SL. Interventions to promote patient utilisation of cardiac rehabilitation. Cochrane Database Syst Rev. 2019;2:CD007131. doi: 10.1002/14651858.CD007131.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pesah E, Supervia M, Turk-Adawi K, Grace SL. A Review of Cardiac Rehabilitation Delivery Around the World. Prog Cardiovasc Dis. 2017;60:267–280. doi: 10.1016/j.pcad.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 126.Bjarnason-Wehrens B, McGee H, Zwisler AD, Piepoli MF, Benzer W, Schmid JP, Dendale P, Pogosova NG, Zdrenghea D, Niebauer J, Mendes M Cardiac Rehabilitation Section European Association of Cardiovascular Prevention and Rehabilitation. Cardiac rehabilitation in Europe: results from the European Cardiac Rehabilitation Inventory Survey. Eur J Cardiovasc Prev Rehabil. 2010;17:410–418. doi: 10.1097/HJR.0b013e328334f42d. [DOI] [PubMed] [Google Scholar]
- 127.Babu AS, Lopez-Jimenez F, Thomas RJ, Isaranuwatchai W, Herdy AH, Hoch JS, Grace SL in conjunction with the International Council of Cardiovascular Prevention and Rehabilitation (ICCPR) Advocacy for outpatient cardiac rehabilitation globally. BMC Health Serv Res. 2016;16:471. doi: 10.1186/s12913-016-1658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wong WP, Feng J, Pwee KH, Lim J. A systematic review of economic evaluations of cardiac rehabilitation. BMC Health Serv Res. 2012;12:243. doi: 10.1186/1472-6963-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Doherty P, Harrison AS, Knapton M, Dale V. Observational study of the relationship between volume and outcomes using data from the National Audit of Cardiac Rehabilitation. Open Heart. 2015;2:e000304. doi: 10.1136/openhrt-2015-000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pack QR, Squires RW, Lichtman SW, Lopez-Jimenez F, Rodriguez-Escudero JP, Zysek VN, Thomas RJ. Abstract 180: What is the potential capacity for increasing cardiac rehabilitation utilization in the United States? Circulation: Cardiovascular Quality and Outcomes 2013; 6: A180. [Google Scholar]
- 131.Turk-Adawi K, Sarrafzadegan N, Grace SL. Global availability of cardiac rehabilitation. Nat Rev Cardiol. 2014;11:586–596. doi: 10.1038/nrcardio.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grace SL, Turk-Adawi KI, Contractor A, Atrey A, Campbell NR, Derman W, Ghisi GL, Sarkar BK, Yeo TJ, Lopez-Jimenez F, Buckley J, Hu D, Sarrafzadegan N. Cardiac Rehabilitation Delivery Model for Low-Resource Settings: An International Council of Cardiovascular Prevention and Rehabilitation Consensus Statement. Prog Cardiovasc Dis. 2016;59:303–322. doi: 10.1016/j.pcad.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 133.Piepoli MF, Corrà U, Adamopoulos S, Benzer W, Bjarnason-Wehrens B, Cupples M, Dendale P, Doherty P, Gaita D, Höfer S, McGee H, Mendes M, Niebauer J, Pogosova N, Garcia-Porrero E, Rauch B, Schmid JP, Giannuzzi P. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur J Prev Cardiol. 2014;21:664–681. doi: 10.1177/2047487312449597. [DOI] [PubMed] [Google Scholar]
- 134.Chaves G, Turk-Adawi K, Supervia M, Santiago de Araújo Pio C, Abu-Jeish AH, Mamataz T, Tarima S, Lopez Jimenez F, Grace SL. Cardiac Rehabilitation Dose Around the World: Variation and Correlates. Circ Cardiovasc Qual Outcomes. 2020;13:e005453. doi: 10.1161/CIRCOUTCOMES.119.005453. [DOI] [PubMed] [Google Scholar]
- 135.Santiago de Araújo Pio C, Marzolini S, Pakosh M, Grace SL. Effect of Cardiac Rehabilitation Dose on Mortality and Morbidity: A Systematic Review and Meta-regression Analysis. Mayo Clin Proc. 2017;92:1644–1659. doi: 10.1016/j.mayocp.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 136.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV American College of Cardiology Foundation. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:3097–3137. doi: 10.1161/CIR.0b013e3182776f83. [DOI] [PubMed] [Google Scholar]
- 137.International Council of Cardiovascular Prevention and Rehabilitation. Advocacy. [cited October 29, 2020]. Available from: http://globalcardiacrehab.com/advocacy/
- 138.Zores F, Iliou MC, Gellen B, Kubas S, Berthelot E, Guillo P, Bauer F, Lamblin N, Bosser G, Damy T, Cohen-Solal A, Beauvais F. Physical activity for patients with heart failure: Position paper from the heart failure (GICC) and cardiac rehabilitation (GERS-P) Working Groups of the French Society of Cardiology. Arch Cardiovasc Dis. 2019;112:723–731. doi: 10.1016/j.acvd.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 139.Cossette S, Frasure-Smith N, Dupuis J, Juneau M, Guertin MC. Randomized controlled trial of tailored nursing interventions to improve cardiac rehabilitation enrollment. Nurs Res. 2012;61:111–120. doi: 10.1097/NNR.0b013e318240dc6b. [DOI] [PubMed] [Google Scholar]
- 140.Pack QR, Mansour M, Barboza JS, Hibner BA, Mahan MG, Ehrman JK, Vanzant MA, Schairer JR, Keteyian SJ. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: a randomized, single-blind, controlled trial. Circulation. 2013;127:349–355. doi: 10.1161/CIRCULATIONAHA.112.121996. [DOI] [PubMed] [Google Scholar]
- 141.Scott LB, Gravely S, Sexton TR, Brzostek S, Brown DL. Examining the effect of a patient navigation intervention on outpatient cardiac rehabilitation awareness and enrollment. J Cardiopulm Rehabil Prev. 2013;33:281–291. doi: 10.1097/HCR.0b013e3182972dd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, Smith C, Walters DL. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart. 2014;100:1770–1779. doi: 10.1136/heartjnl-2014-305783. [DOI] [PubMed] [Google Scholar]
- 143.Wyer SJ, Earll L, Joseph S, Harrison J, Giles M, Johnston M. Increasing attendance at a cardiac rehabilitation programme: An intervention study using the Theory of Planned Behaviour. Coronaty Health Care. 2001;5:154–159. [Google Scholar]
- 144.Grace SL, Chessex C, Arthur H, Chan S, Cyr C, Dafoe W, Juneau M, Oh P, Suskin N. Systematizing inpatient referral to cardiac rehabilitation 2010: Canadian Association of Cardiac Rehabilitation and Canadian Cardiovascular Society joint position paper endorsed by the Cardiac Care Network of Ontario. Can J Cardiol. 2011;27:192–199. doi: 10.1016/j.cjca.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 145.Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, Shepard DS, Thomas RJ. Increasing Cardiac Rehabilitation Participation From 20% to 70%: A Road Map From the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. 2017;92:234–242. doi: 10.1016/j.mayocp.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grace SL, Evindar A, Kung TN, Scholey PE, Stewart DE. Automatic referral to cardiac rehabilitation. Med Care. 2004;42:661–669. doi: 10.1097/01.mlr.0000129901.05299.aa. [DOI] [PubMed] [Google Scholar]
- 147.Grace SL, Russell KL, Reid RD, Oh P, Anand S, Rush J, Williamson K, Gupta M, Alter DA, Stewart DE Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med. 2011;171:235–241. doi: 10.1001/archinternmed.2010.501. [DOI] [PubMed] [Google Scholar]
- 148.Grace SL, Angevaare KL, Reid RD, Oh P, Anand S, Gupta M, Brister S, Stewart DE CRCARE Investigators. Effectiveness of inpatient and outpatient strategies in increasing referral and utilization of cardiac rehabilitation: a prospective, multi-site study. Implement Sci. 2012;7:120. doi: 10.1186/1748-5908-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Beckie TM, Beckstead JW. Predicting cardiac rehabilitation attendance in a gender-tailored randomized clinical trial. J Cardiopulm Rehabil Prev. 2010;30:147–156. doi: 10.1097/HCR.0b013e3181d0c2ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Room J, Hannink E, Dawes H, Barker K. What interventions are used to improve exercise adherence in older people and what behavioural techniques are they based on? BMJ Open. 2017;7:e019221. doi: 10.1136/bmjopen-2017-019221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lynggaard V, Nielsen CV, Zwisler AD, Taylor RS, May O. The patient education - Learning and Coping Strategies - improves adherence in cardiac rehabilitation (LC-REHAB): A randomised controlled trial. Int J Cardiol. 2017;236:65–70. doi: 10.1016/j.ijcard.2017.02.051. [DOI] [PubMed] [Google Scholar]
- 152.Janssen V, De Gucht V, van Exel H, Maes S. A self-regulation lifestyle program for post-cardiac rehabilitation patients has long-term effects on exercise adherence. J Behav Med. 2014;37:308–321. doi: 10.1007/s10865-012-9489-y. [DOI] [PubMed] [Google Scholar]
- 153.Taylor RS, Dalal H, Jolly K, Zawada A, Dean SG, Cowie A, Norton RJ. Home-based vs centre-based cardiac rehabilitation. Cochrane Database Syst Rev. :2015: CD007130. doi: 10.1002/14651858.CD007130.pub3. [DOI] [PubMed] [Google Scholar]
- 154.Zwisler AD, Norton RJ, Dean SG, Dalal H, Tang LH, Wingham J, Taylor RS. Home-based cardiac rehabilitation for people with heart failure: A systematic review and meta-analysis. Int J Cardiol. 2016;221:963–969. doi: 10.1016/j.ijcard.2016.06.207. [DOI] [PubMed] [Google Scholar]
- 155.Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, Sanderson BK, Whooley MA. Home-Based Cardiac Rehabilitation: A Scientific Statement From the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140:e69–e89. doi: 10.1161/CIR.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 156.Chindhy S, Taub PR, Lavie CJ, Shen J. Current challenges in cardiac rehabilitation: strategies to overcome social factors and attendance barriers. Expert Rev Cardiovasc Ther. 2020;18:777–789. doi: 10.1080/14779072.2020.1816464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Imran HM, Baig M, Erqou S, Taveira TH, Shah NR, Morrison A, Choudhary G, Wu WC. Home-Based Cardiac Rehabilitation Alone and Hybrid With Center-Based Cardiac Rehabilitation in Heart Failure: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2019;8:e012779. doi: 10.1161/JAHA.119.012779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Imran HM, Baig M, Shah NR, Erqou S, Wu WH. Abstract 12595: Hybrid cardiac rehabilitation an alternative to center-based cardiac rehabilitation: A systemic review and meta-analysis. Circulation. 2019;140 doi: 10.1161/JAHA.119.012779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Frederix I, Vanhees L, Dendale P, Goetschalckx K. A review of telerehabilitation for cardiac patients. J Telemed Telecare. 2015;21:45–53. doi: 10.1177/1357633X14562732. [DOI] [PubMed] [Google Scholar]
- 160.Huang K, Liu W, He D, Huang B, Xiao D, Peng Y, He Y, Hu H, Chen M, Huang D. Telehealth interventions vs center-based cardiac rehabilitation of coronary artery disease: A systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22:959–971. doi: 10.1177/2047487314561168. [DOI] [PubMed] [Google Scholar]
- 161.Batalik L, Filakova K, Batalikova K, Dosbaba F. Remotely monitored telerehabilitation for cardiac patients: A review of the current situation. World J Clin Cases. 2020;8:1818–1831. doi: 10.12998/wjcc.v8.i10.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jin K, Khonsari S, Gallagher R, Gallagher P, Clark AM, Freedman B, Briffa T, Bauman A, Redfern J, Neubeck L. Telehealth interventions for the secondary prevention of coronary heart disease: A systematic review and meta-analysis. Eur J Cardiovasc Nurs. 2019;18:260–271. doi: 10.1177/1474515119826510. [DOI] [PubMed] [Google Scholar]
- 163.Chan C, Yamabayashi C, Syed N, Kirkham A, Camp PG. Exercise Telemonitoring and Telerehabilitation Compared with Traditional Cardiac and Pulmonary Rehabilitation: A Systematic Review and Meta-Analysis. Physiother Can. 2016;68:242–251. doi: 10.3138/ptc.2015-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kaminsky LA, Brubaker PH, Guazzi M, Lavie CJ, Montoye AH, Sanderson BK, Savage PD. Assessing Physical Activity as a Core Component in Cardiac Rehabilitation: A POSITION STATEMENT OF THE AMERICAN ASSOCIATION OF CARDIOVASCULAR AND PULMONARY REHABILITATION. J Cardiopulm Rehabil Prev. 2016;36:217–229. doi: 10.1097/HCR.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 165.Kraal JJ, Peek N, Van den Akker-Van Marle ME, Kemps HM. Effects of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: short-term results of the FIT@Home study. Eur J Prev Cardiol. 2014;21:26–31. doi: 10.1177/2047487314552606. [DOI] [PubMed] [Google Scholar]
- 166.Song Y, Ren C, Liu P, Tao L, Zhao W, Gao W. Effect of Smartphone-Based Telemonitored Exercise Rehabilitation among Patients with Coronary Heart Disease. J Cardiovasc Transl Res. 2020;13:659–667. doi: 10.1007/s12265-019-09938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Batalik L, Dosbaba F, Hartman M, Batalikova K, Spinar J. Benefits and effectiveness of using a wrist heart rate monitor as a telerehabilitation device in cardiac patients: A randomized controlled trial. Medicine (Baltimore) 2020;99:e19556. doi: 10.1097/MD.0000000000019556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beatty AL, Magnusson SL, Fortney JC, Sayre GG, Whooley MA. VA FitHeart, a Mobile App for Cardiac Rehabilitation: Usability Study. JMIR Hum Factors. 2018;5:e3. doi: 10.2196/humanfactors.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dorje T, Zhao G, Tso K, Wang J, Chen Y, Tsokey L, Tan B, Scheer A, Jacques A, Li Z, Wang R, Chow CK, Ge J, Maiorana A. Smartphone and social media-based cardiac rehabilitation and secondary prevention in China (SMART-CR/SP): A parallel-group, single-blind, randomised controlled trial. Lancet Digit Health. 2019;1:363–374. doi: 10.1016/S2589-7500(19)30151-7. [DOI] [PubMed] [Google Scholar]
- 170.Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102:1183–1192. doi: 10.1136/heartjnl-2015-308966. [DOI] [PubMed] [Google Scholar]
- 171.Peçanha T, Goessler KF, Roschel H, Gualano B. Social isolation during the COVID-19 pandemic can increase physical inactivity and the global burden of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2020;318:H1441–H1446. doi: 10.1152/ajpheart.00268.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Besnier F, Gayda M, Nigam A, Juneau M, Bherer L. Cardiac Rehabilitation During Quarantine in COVID-19 Pandemic: Challenges for Center-Based Programs. Arch Phys Med Rehabil. 2020;101:1835–1838. doi: 10.1016/j.apmr.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Scherrenberg M, Wilhelm M, Hansen D, Völler H, Cornelissen V, Frederix I, Kemps H, Dendale P. The future is now: a call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. :2020: 2047487320939671. doi: 10.1177/2047487320939671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mandic S, Rolleston A, Hately G, Reading S. Chapter 14 - Community-Based Maintenance Cardiac Rehabilitation. Lifestyle in Heart Health and Disease, 2018: 187-198. [Google Scholar]
- 175.Hansen D, Dendale P, Raskin A, Schoonis A, Berger J, Vlassak I, Meeusen R. Long-term effect of rehabilitation in coronary artery disease patients: randomized clinical trial of the impact of exercise volume. Clin Rehabil. 2010;24:319–327. doi: 10.1177/0269215509353262. [DOI] [PubMed] [Google Scholar]
- 176.Mosleh SM, Bond CM, Lee AJ, Kiger A, Campbell NC. Effects of community based cardiac rehabilitation: Comparison with a hospital-based programme. Eur J Cardiovasc Nurs. 2015;14:108–116. doi: 10.1177/1474515113519362. [DOI] [PubMed] [Google Scholar]
- 177.Alharbi M, Bauman A, Neubeck L, Gallagher R. Validation of Fitbit-Flex as a measure of free-living physical activity in a community-based phase III cardiac rehabilitation population. Eur J Prev Cardiol. 2016;23:1476–1485. doi: 10.1177/2047487316634883. [DOI] [PubMed] [Google Scholar]
- 178.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hinde S, Bojke L, Harrison A, Doherty P. Improving cardiac rehabilitation uptake: Potential health gains by socioeconomic status. Eur J Prev Cardiol. 2019;26:1816–1823. doi: 10.1177/2047487319848533. [DOI] [PubMed] [Google Scholar]
- 180.Taylor RS, Sadler S, Dalal HM, Warren FC, Jolly K, Davis RC, Doherty P, Miles J, Greaves C, Wingham J, Hillsdon M, Abraham C, Frost J, Singh S, Hayward C, Eyre V, Paul K, Lang CC, Smith K. The cost effectiveness of REACH-HF and home-based cardiac rehabilitation compared with the usual medical care for heart failure with reduced ejection fraction: A decision model-based analysis. Eur J Prev Cardiol. 2019;26:1252–1261. doi: 10.1177/2047487319833507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.World Health Organization. Cancer. 12 September 2018. [cited January 5, 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer .
- 182.Venturini E, Iannuzzo G, D'Andrea A, Pacileo M, Tarantini L, Canale ML, Gentile M, Vitale G, Sarullo FM, Vastarella R, Di Lorenzo A, Testa C, Parlato A, Vigorito C, Giallauria F. Oncology and Cardiac Rehabilitation: An Underrated Relationship. J Clin Med. 2020;9 doi: 10.3390/jcm9061810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, La Gerche A, Ligibel JA, Lopez G, Madan K, Oeffinger KC, Salamone J, Scott JM, Squires RW, Thomas RJ, Treat-Jacobson DJ, Wright JS American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Peripheral Vascular Disease. Cardio-Oncology Rehabilitation to Manage Cardiovascular Outcomes in Cancer Patients and Survivors: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e997–e1012. doi: 10.1161/CIR.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.D'Ascenzi F, Anselmi F, Fiorentini C, Mannucci R, Bonifazi M, Mondillo S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur J Prev Cardiol. 2019:2047487319874900. doi: 10.1177/2047487319874900. [DOI] [PubMed] [Google Scholar]
- 185.Dittus KL, Lakoski SG, Savage PD, Kokinda N, Toth M, Stevens D, Woods K, OʼBrien P, Ades PA. Exercise-based oncology rehabilitation: leveraging the cardiac rehabilitation model. J Cardiopulm Rehabil Prev. 2015;35:130–139. doi: 10.1097/HCR.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]