Abstract
Objective
Re-positivity of SARS-CoV-2 in discharged COVID-19 patients have been reported; however, early risk factors for SARS-CoV-2 re-positivity evaluation are limited.
Methods
This is a prospective study, a total of 145 COVID-19 patients were treated and all discharged according to the guideline criteria by Mar 11th 2020. After discharge, clinical visits and viral RT-PCR tests by the second and fourth week follow-up were carried-out. Patient demographic and clinical characteristics and laboratory data on admission and discharge were retrieved, and predictive factors for SARS-CoV-2 re-positivity were analyzed.
Results
13 out of 145 (9.0%) COVID-19 patients were confirmed re-positivity of SARS-CoV-2 by RT-PCR test. The median interval between disease onset to recurrence was 38 days. SARS-CoV-2 re-positive cases were of significantly longer virus shedding duration, notably higher body temperature, heart rate and lower TNF-α and IgG levels on admission. Covariate logistic regression analysis revealed virus shedding duration and IgG levels are independent risk factors for SARS-CoV-2 return positive after discharge.
Conclusion
Longer viral shedding duration and lower IgG levels are risk factors for re-positivity of SARS-CoV-2 for discharged COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, Re-positivity, Risk factor
1. Introduction
The Coronavirus disease 2019 (COVID-19) pandemic is still threatening since December 2019. Great efforts such as quarantine policies and medical cares have been taken to contain the pandemic spreading [1]. A certain proportion of re-positivity of SARS-CoV-2 in COVID-19 patients after discharged during follow-up among different populations have been reported and raised public concerns, though clinical relevance of the re-positivity of SARS-CoV-2 remains unclear [2], [3].
Since Lan et al. [4] firstly reported the repositivity of SARS-CoV-2 among four COVID-19 cases during their convalescent stage at early February 2020, more and more studies focused on the repositivity of SARS-CoV-2 have been carried out [5]. Among different cohorts studied in previous studies, the proportion of patients with SARS-CoV-2 re-positivity after discharge varies from 2.4% to 69.2% [3]. Du et al. [6] reported that 3 out of 126 (2.4%) COVID-19 patients were tested re-positivity of SARS-CoV-2 but asymptomatic after their discharge. In a rather limited size, only 13 cases of Iranian COVID-19 patients, Habibzadeh et al. [7] found 9 (69.2%) of them were SARS-CoV-2 re-positive. In South Korea, with a larger cohort of 8922 COVID-19 patients, 2923 (3.3%) were confirmed to be re-positivity of SARS-CoV-2 [8].
Though most of the recurrence of SARS-CoV-2 among COVID-19 cases after discharge are asymptomatic, public concerns on risk of infectious potential from these recovered COVID-19 patients with SARS-CoV-2 re-positivity is reasonable, as human-to-human transmission of SARS-CoV-2 from asymptomatic patients was emphasized in previous studies [9], [10]. However, early predictive risk factors for re-positivity of SARS-CoV-2 after discharge are unavailable yet.
In this prospective study, among 145 discharged COVID-19 patients, SARS-CoV-2 RT-PCR tests by the second and fourth week during follow-up were carried-out, 13 of them were confirmed to be SARS-CoV-2 re-positive. Patient demographic and clinical characteristics and laboratory data on admission were retrieved, and risk factors for SARS-CoV-2 re-positivity were evaluated.
2. Materials and methods
2.1. Patients and data collection
From 19th January 2020, 145 consecutive laboratory confirmed COVID-19 cases were hospitalized and treated at our officially designated medical center for COVID-19, Taizhou EnZe Medical Group (Center), Zhejiang, China. All of them were discharged by Mar 11th 2020 and no death case was occurred. Nasopharyngeal swab specimens for SARS-CoV-2 detection were tested by the real-time reverse transcriptase-polymerase chain reaction (RT-PCR) kit (Wuhan Easydiagnosis Biomedicine Co. Ltd). Patients with cycle threshold (Ct) values ≤40 were considered SARS-CoV-2 positive. COVID-19 patient diagnosis, treatment and discharge criteria were abided by the Diagnosis and Treatment Plan of Corona Virus Diseases 2019 (Tentative Seventh Edition), National Health Commission (NHC) of the People’s Republic of China [11].
Diagnosis and classification of COVID-19 as following (1) Mild cases who have mild clinical symptoms but no pneumonia manifestation in imaging. (2) Ordinary cases who have symptoms (fever, sore throat, runny nose, respiratory tract symptoms, etc) and pneumonia manifestation in imaging. (3) Severe cases who have any of the following symptoms: respiratory distress (respiratory rate ≥ 30 breaths/minute), and/or SpO2 ≤ 93%, and/or PaO2/FiO2 ≤ 300 mmHg (1 mmHg = 0.133 kPa), and/or pulmonary lesions increased > 50% within 24–48 h in imaging. (4) Critical cases who have any of the following symptoms: respiratory failure need mechanical ventilation, and/or shock, and/or complicated with other organ failure and ICU treatment.
Discharge standards for COVID-19 patients as they meet all of following criteria (1) body temperature return to normal ≥ 3 days; and (2) respiratory symptoms significantly improved; and (3) significant absorption of acute lesion in imaging; and (4) SARS-CoV-2 is negative in both nasopharyngeal and anal swabs for two consecutive times (≥24-hour interval).
Follow-up by the second and fourth week after COVID-19 patient discharge was recommended, which require them to return clinical visit and physical examination and routine laboratory tests will be performed. Basic characteristics of patients and their clinical information were retrieved from electronic medical history record. SARS-CoV-2 re-positivity defined as positive by RT-PCR for discharged COVID-19 patients during follow-up with nasopharyngeal swab samples, and consecutive RT-PCR test at an interval of 24-hour–48-hour are performed during the re-admission.
SARS-CoV-2 viral shedding duration was defined by an interval from the day SARS-CoV-2 confirmed positive to the first day when SARS-CoV-2 returned to negative (at least two consecutive negative RT-PCR results with sampling interval at least 24-hour) during hospitalization [12]. Written informed consent was obtained from all patients or guardian, and the protocol of this study was approved by the Ethics committee of the Taizhou Hospital of Zhejiang Province (#K20200111).
2.2. Statistical methods
Continuous variables were presented as median and range or mean ± standard deviation (SD), and comparison between groups were analyzed with Mann-Whitney U test. Categorical variables were presented as as counts and percentages, and difference between groups were analyzed with χ2 test. Covariate binary logistic regression analysis with forward conditional method (co-variables with p < 0.200 were included) were performed, and receiver operating characteristics (ROC) was analyzed with the SPSS v.13.0 (SPSS, Inc., Chicago, IL, USA). Optimal cut-off value was determined by Youden's index. A p < 0.05 (two-sided) was considered statistically significant.
3. Results
3.1. Patients with SARS-CoV-2 re-positivity after discharge
Of 145 COVID-19 patients (77 male and 68 female; median age: 47 years), there were 105 ordinary cases (grouped as non-severe), 39 severe cases and one critical case (grouped as severe). Antiviral treatment were prescribed to all patients and traditional Chinese medicine treatments were prescribed to 135 of these patients, and glucocorticoids treatments were prescribed to 47 patients. All of them were discharged by Mar 11th 2020.
By May 26th, 13 out of these 145 patients (9.0%; 7 male and 6 female; median age: 54 years) were re-admitted to our hospital due to re-positivity of SARS-CoV-2 during follow-up viral test, but all of them without COVID-19 symptoms. Of them, 12 patients whose SARS-CoV-2 returned to be positive by the 1st follow-up and one patient by the 2nd follow-up viral test after discharge. Among 13 COVID-19 patients with SARS-CoV-2 re-positivity, the median interval from symptom onset to SARS-CoV-2 re-positivity is 38 days (range: 26 days to 65 days). The detail clinical features on admission of these COVID-19 patients with SARS-CoV-2 negative and re-positivity after discharge were shown in Table 1 .
Table 1.
Comparison of clinical characteristics on admission between the COVID-19 patients with virus negative and re-positive after discharge.
| Variables* | All cases (n = 145) | Negative (n = 132) | Re-positive (n = 13) | p value |
|---|---|---|---|---|
| Gender (male/female) | 77/68 | 70/62 | 7/6 | 0.955 |
| Age (median, range) | 47 (10–86) | 47 (10–86) | 54 (31–67) | 0.343 |
| Body Mass Index (mean ± SD) | 24.2 ± 2.94 | 24.2 ± 2.96 | 24.8 ± 2.85 | 0.453 |
| Body temperature (mean ± SD) | 37.1 ± 0.66 | 37.0 ± 0.64 | 37.4 ± 0.73 | 0.086 |
| Respiratory rate (median, range) | 19 (12–26) | 19 (12–26) | 19 (18–20) | 0.749 |
| Heart rate (median, range) | 82 (57–147) | 82 (57–147) | 89 (77–105) | 0.061 |
| Onset to discharge (days) | 25 (6–49) | 25 (6–49) | 24 (12–43) | 0.863 |
| Onset to re-positivity (days) | / | / | 38 (26–65) | / |
| Hospital stay (days) | 20 (5–43) | 20 (5–43) | 23 (7–40) | 0.181 |
| PaO2/FiO2 (mmHg) | 422 ± 119 | 430 ± 123 | 423 ± 81 | 0.809 |
| Corticosteroid used (yes/no) | 47 (32.4%) | 41 (31.1%) | 6 (46.2%) | 0.267 |
| Severe cases (yes/no) | 40 (27.6%) | 35 (26.5%) | 5 (38.5%) | 0.358 |
| Symptoms (yes/no) | ||||
| fever | 104 (71.7%) | 95 (72.0%) | 9 (69.2%) | 0.834 |
| dry cough | 43 (29.7%) | 38 (28.8%) | 5 (38.5%) | 0.466 |
| fatigue | 37 (25.5%) | 36 (27.3%) | 1 (7.7%) | 0.122 |
| chills | 27 (18.6%) | 26 (19.7%) | 1 (7.7%) | 0.289 |
| sore throat | 18 (12.4%) | 17 (12.9%) | 1 (7.7%) | 0.588 |
| runny nose | 11 (7.6%) | 9 (6.8%) | 2 (15.4%) | 0.266 |
| sputum production | 47 (32.4%) | 44 (33.3%) | 3 (23.1%) | 0.451 |
| headache | 21 (14.5%) | 19 (14.4%) | 2 (15.4%) | 0.923 |
| nausea or vomiting | 5 (3.4%) | 5 (3.8%) | 0 | 0.475 |
| myalgia | 6 (4.1%) | 6 (4.5%) | 0 | 0.432 |
| poor appetite | 47 (32.4%) | 45 (34.1%) | 2 (15.4%) | 0.169 |
| diarrhea | 14 (9.7%) | 13 (9.8%) | 1 (7.7%) | 0.802 |
| others | 41 (28.3%) | 35 (26.5%) | 6 (46.2%) | 0.134 |
| Pre-existing disorders (yes/no) | 86 (59.3%) | 76 (57.6) | 10 (76.9%) | 0.175 |
| chronic heart disease | 1 (0.7%) | 1 (0.8%) | 0 | 0.753 |
| diabetes | 18 (12.4%) | 17 (12.9%) | 1 (7.7%) | 0.588 |
| hypertension | 23 (15.9%) | 21 (15.9) | 2 (15.4%) | 0.431 |
| chronic renal disease | 2 (1.4%) | 1 (0.8%) | 1 (7.7%) | 0.041 |
| cancer | 3 (2.1%) | 3 (2.3%) | 0 | 0.583 |
| chronic liver disease | 10 (6.9%) | 9 (6.8%) | 1 (7.7%) | 0.906 |
| smoke | 15 (10.3%) | 12 (9.1%) | 3 (23.1%) | 0.084 |
| drink | 11 (7.6%) | 10 (7.6%) | 1 (7.7%) | 0.988 |
| chronic lung disease | 8 (5.5%) | 8 (6.1%) | 0 | 0.361 |
| others | 44 (30.3%) | 37 (28.0%) | 7 (58.3%) | 0.059 |
| Viral positivity duration (days)** | 12.0 (3.0–45.0) | 12.0 (3.0–42.0) | 17.0 (5.0–45.0) | 0.011 |
Values of the variables were record on the time of admission.
Defined by the interval from the day on which SARS-CoV-2 results were confirmed to be positive to the first day on which SARS-CoV-2 results returned to negative (at least 2 consecutive RT-PCR negative results) during the period of hospitalization [12].
3.2. Comparison of clinical characteristics between the patients with SARS-CoV-2 negative and re-positivity after discharge
Among these discharged COVID-19 patients, patients with SARS-CoV-2 re-positivity were found to be higher body temperature (37.4℃ vs. 37.0℃, p = 0.086) and heart rate (89/min vs. 82/min, p = 0.061) on admission, and to be elder (54 years vs. 47 years, p = 0.343) and longer hospital stays (23 days vs. 20 days, p = 0.181) than those with SARS-CoV-2 remain negative after discharge. Moreover, no statistical difference was observed between the two groups in terms of corticosteroid treatment status. However, SARS-CoV-2 re-positive cases had a significantly longer virus shedding duration (17 days vs. 12 days; p = 0.011; Table 1).
3.3. Comparison of laboratory data between patients with SARS-CoV-2 negative and re-positivity after discharge
On the day of admission, data revealed that discharged COVID-19 patients with SARS-CoV-2 re-positivity were with significantly lower levels of TNF-α (0.69 pg/mL vs. 1.25 pg/mL; p = 0.051), IgG (10.73 g/L vs. 12.72 g/L; p = 0.020), and obviously lower counts of CD8 + T cells (195.3/μL vs. 255.7/μL; p = 0.151), CD19 + B cells (89.7/μL vs. 136.6/μL; p = 0.186) and IgA (2.12 g/L vs. 2.37 g/L; p = 0.194). To be noted, other laboratory findings were not significantly different between the two groups. Furthermore, no statistical difference was also observed for these variables on the day of discharge between the two groups (Table 2 ).
Table 2.
Comparison of laboratory data between virus negative and re-positive COVID-19 patients after discharge.
| Variables | Normal reference | Day on admission |
Day on discharge |
||||
|---|---|---|---|---|---|---|---|
| negative (n = 132) | re-positive (n = 13) | p value | negative (n = 119) | re-positive (n = 13) | p value | ||
| WBC (109/L) | 3.5–9.5 | 5.4 (2.6–23.6) | 5.7 (3.5–10.0) | 0.626 | 5.8 (3.1–14.4) | 4.9 (3.7–8.1) | 0.375 |
| Neutrophil (109/L) | 1.8–6.3 | 3.5 (1.2–22.2) | 3.6 (1.8–7.5) | 0.468 | 3.3 (1.5–11.6) | 3.1 (2.5–5.8) | 0.745 |
| Lymphocyte (109/L) | 1.1–3.2 | 1.2 (0.3–3.0) | 1.3 (0.3–2.4) | 0.770 | 1.5 (0.6–3.5) | 1.4 (0.7–2.7) | 0.154 |
| CD3 + T cell (per μL) | 770–2041 | 620.1 (136.9–2012.3) | 554.9 (110.9–1249.3) | 0.316 | 1007.4 (377.2–2081.5) | 1181.7 (696.2–1313.7) | 0.568 |
| CD4 + T cell (per μL) | 414–1123 | 344.0 (85.54–1236.4) | 354.5 (68.3–828.1) | 0.437 | 521.3 (199.3–1348.5) | 609.8 (386.6–779.5) | 0.485 |
| CD8 + T cell (per μL) | 238–874 | 255.7 (41.2–805.83) | 195.3 (42.5–426.4) | 0.151 | 433.6 (150.6–982.6) | 421.6 (290.8–572.5) | 0.933 |
| CD19 + B cell (per μL) | 90–560 | 136.6 (28.45–551.85) | 89.7 (28.5–267.7) | 0.186 | 160.0 (48.1- −495.0) | 132.2 (44.7–291.2) | 0.672 |
| CD56 + NK cell (per μL) | 150–1100 | 199.8 (43.57–770.82) | 166.8 (62.7–351.9) | 0.356 | 223.1 (68.1–655.6) | 249.6 (165.9–438.1) | 0.553 |
| Mononuclear (109/L) | 0.1–0.6 | 0.4 (0.1–1.2) | 0.5 (0.2–0.8) | 0.100 | 0.50 (0.20–1.30) | 0.50 (0.20–0.80) | 0.797 |
| Eosinophil (109/L) | 0.02–0.52 | 0.01 (0.0–0.34) | 0.01 (0.0–0.09) | 0.795 | 0.10 (0.0–1.43) | 0.08 (0.04–0.20) | 0.533 |
| Basophil (109/L) | 0.00–0.06 | 0.01 (0.0–0.07) | 0.02 (0.0–0.06) | 0.451 | 0.02 (0.0–0.09) | 0.02 (0.01–0.04) | 0.889 |
| IL-2 (pg/mL) | 1.1–9.8 | 1.35 (0.19–3.27) | 1.07 (0.50–10.31) | 0.519 | 1.36(0.29–2.81) | 1.23 (0.49–2.10) | 0.442 |
| IL-4 (pg/mL) | 0.1–3.0 | 1.52 (0.10–8.53) | 1.26 (0.50–3.12) | 0.226 | 1.62 (0.13–11.71) | 1.87 (0.30–3.09) | 0.387 |
| IL-6 (pg/mL) | 1.7–16.6 | 8.03 (0.78–413.63) | 12.48 (1.36–22.16) | 0.963 | 3.35 (1.08–347.81) | 3.73 (1.85–23.1) | 0.135 |
| IL-10 (pg/mL) | 2.6–4.9 | 3.57 (0.61–39.53) | 3.71 (0.19–24.13) | 0.883 | 2.64 (0.57–7.19) | 3.30 (1.00–5.68) | 0.181 |
| TNF-α (pg/mL) | 0.1–5.2 | 1.25 (0.09–5.32) | 0.69 (0.0–2.55) | 0.051 | 1.00 (0.22–4.97) | 0.920 (0.015–1.64) | 0.191 |
| IFN-γ (pg/mL) | 1.6–17.3 | 1.95 (0.18–178.86) | 1.39 (0.30–12.88) | 0.535 | 1.59 (0.18–178.66) | 1.35 (0.15–2.66) | 0.406 |
| IgG (g/L) | 7.0–16.0 | 12.72 (7.73–28.51) | 10.73 (7.80–18.67) | 0.020 | 12.29 (9.50–15.47) | / | / |
| IgA (g/L) | 0.70–4.0 | 2.37 (0.57–5.26) | 2.12 (0.79–3.61) | 0.194 | 2.34 (1.12–5.31) | / | / |
| IgM (g/L) | 0.40–2.30 | 1.02 (0.38–4.41) | 0.95 (0.33–1.71) | 0.531 | 1.12 (0.74–2.30) | / | / |
| CRP (mg/L) | <0.5 | 10.20 (0.10–185.0) | 5.10 (0.20–65.10) | 0.243 | 1.90 (0.20–66.6) | / | / |
| LDH | 80–285 | 207.0 (110.0–927.0) | 186.0 (137.0–627.0) | 0.958 | 162.0 (110.0–310.0) | 150.0 (136.0–158.0) | 0.299 |
| D-dimer | 0.00–0.55 | 0.24 (0.04–4.38) | 0.33 (0.15–12.98) | 0.680 | 0.54 (0.17–2.07) | / | |
| Alanine aminotransferase (U/L) | 7–40 | 20.0 (5.0–152.0) | 22.0 (10.0–106.0) | 0.890 | 26.0 (6.0–121.0) | 18.5 (11.0–85.0) | 0.548 |
| Aspartate aminotransferase (U/L) | 13–35 | 24.0 (11.0–77.0) | 26.0 (13.0–115.0) | 0.878 | 22.0 (12.0–68.0) | 21.5 (13.0–47.0) | 0.996 |
| Alkaline phosphatase (U/L) | 35–100 | 71.0 (35.0–376.0) | 69.0 (40.0–100.0) | 0.560 | 74.0 (40.0–356.0) | 68.5 (40.0–93.0) | 0.275 |
| gamma-glutamyltransferase (U/L) | 7–45 | 24.0 (10.0–132.0) | 19.0 (11.0–76.0) | 0.409 | 33.0 (12.0–293.0) | 25.0 (11.0–57.0) | 0.075 |
| Total bilirubin (mmol/L) | 5.0–21.0 | 12.75 (3.4–36.5) | 11.9 (5.30–32.7) | 0.906 | 11.4 (4.0–88.0) | 13.7 (4.30–52.7) | 0.126 |
3.4. Risk factors for SARS-CoV-2 re-positivity after discharge
Covariate binary logistic regression analysis with forward conditional method (both clinical and laboratory variables on admission with p < 0.200 were included) were carried out to evaluate the power of risk factors for SARS-CoV-2 re-positivity after discharge. Results revealed that longer virus shedding duration [odd ratio (OR) = 1.280, 95% confidence interval (CI):1.052–1.558; p = 0.013], and lower IgG levels (OR = 0.690, 95% CI: 0.528–0.901; p = 0.007] are independent risk factors for SARS-CoV-2 return positive after discharge (Table 3 ).
Table 3.
Covariate regression analysis between SARS-CoV-2 remian negative and re-positivity after discharge.
| Variables | Negative (n = 132) | Re-positive (n = 13) | Covariate regression |
||
|---|---|---|---|---|---|
| p | OR (95% CI) | p | |||
| Body temperature (mean ± SD) | 37.0 ± 0.64 | 37.4 ± 0.73 | 0.086 | / | 0.080 |
| Heart rate (median, range) | 82 (57–147) | 89 (77–105) | 0.061 | / | 0.063 |
| Hospital stay (days) | 20 (5–43) | 23 (7–40) | 0.181 | / | 0.804 |
| CD8 + T cell (per μL) | 256 (41–806) | 195 (43–426.) | 0.151 | / | 0.961 |
| CD19 + B cell (per μL) | 137 (28–552) | 90 (29–268) | 0.186 | / | 0.391 |
| Mononuclear (109/L) | 0.4 (0.1–1.2) | 0.5 (0.2–0.8) | 0.100 | / | 0.159 |
| TNF-α (pg/mL) | 1.25 (0.09–5.32) | 0.69 (0.0–2.55) | 0.051 | / | 0.065 |
| IgG (g/L) | 12.72 (7.73–28.51) | 10.73 (7.80–18.67) | 0.020 | 0.690 (0.528–0.901) | 0.007 |
| IgA (g/L) | 2.37 (0.57–5.26) | 2.12 (0.79–3.61) | 0.194 | / | 0.375 |
| Viral shedding duration (days) | 12.0 (3.0–42.0) | 17.0 (5.0–45.0) | 0.006 | 1.280 (1.052–1.558) | 0.013 |
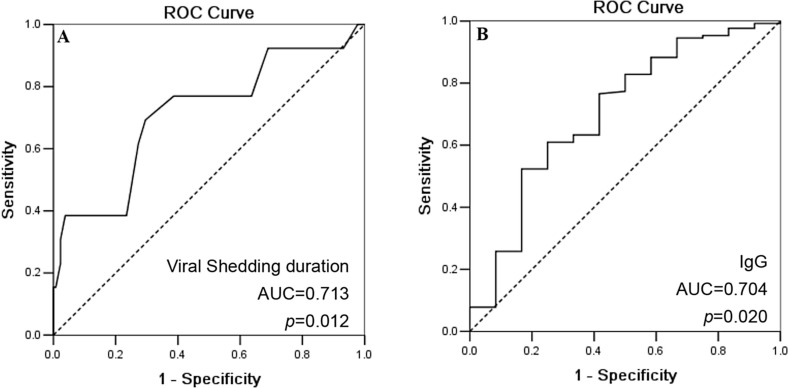
ROC analysis results showed that the area under curve (AUC) for viral shedding duration is 0.713 (95% CI: 0.549–0.876; p = 0.012; Fig. 1 A), with an optimal cut-off of 15.5 days (sensitivity: 0.714; specificity: 0.710). AUC for IgG levels is 0.704 (95% CI: 0.538–0.870; p = 0.020), with an optimal cut-off of 12.1 g/L (sensitivity: 0.609; specificity: 0.750; Fig. 1B).
Fig. 1.
ROC curve for viral shedding duration and IgG levels to distinguish COVID-19 patients with virus remain negative and re-positivity after discharge.
4. Discussion
While the pandemic COVID-19 is still threatening the public health worldwide, a certain proportion of SARS-CoV-2 nucleic acid returned to be positive in discharged COVID-19 patients during their convalescent stage also deserves our attention [13]. The percentage of SARS-CoV-2 re-positivity in discharged COVID-19 patients varies markedly depending on the different cohorts studied, which ranges from 2.4% to 69.2%, while most patients with SARS-CoV-2 re-positivity after discharge are asymptomatic or have mild symptoms according to previous reports [6], [7], [14]. A recent systematic meta‑analysis included 14 studies (13 conducted in China and one in Brunei) published between 17th Mar 2020 and 29th May 2020, 318 out of 2568 COVID-19 patients were experienced SARS-CoV-2 re-positivity with the pooled estimated incidence with 14.8% [15]. Authors also reported that the estimated interval between disease onset to the SARS-CoV-2 re-positivity is 35.4 days. In our study, the median interval between disease onset to the SARS-CoV-2 re-positivity is 38.0 days (range: 26.0 days–65.0 days).
Though the re-positivity of SARS-CoV-2 among discharged patients with COVID-19 is a common phenomenon, detail cause for this is unclear. In this context, several explanation for the re-positivity of SARS-CoV-2 have been addressed, which could be caused by the false RT-PCR test or environmental contamination [16], SARS-CoV-2 reactivation [2], re-infected with the same or another SARS-CoV-2 strain [17], [18], intermittent virus shedding or prolonged viral nucleic acid conversion [19], [20].
Fortunately, no case has been reported due to the contact with SARS-CoV-2 re-positive patients to date. However, few COVID-19 patients with re-positivity of SARS-CoV-2 do have clinical consequences. In a case report with SARS-CoV-2 re-positivity, authors supposed that a weak humoral immune response with very low anti-SARS-CoV-2 antibody may account for the virus reactivation [17]. However, a cohort with 11 out of 150 COVID-19 patients (7.3%) confirmed to be re-positive of SARS-CoV-2, no remarkable difference was observed for both prevalence and levels of IgM or IgG to SARS-CoV-2 between patients with SARS-CoV-2 re-positive and those not, indicating that humoral immune response could not be a main factor for the SARS-CoV-2 re-positivity [21]. SARS-CoV-2 re-positivity by re-infection with another SARS-CoV-2 strain also has been described. To et al. [18] revealed that a COVID-19 patient re-infected with another SARS-CoV-2 strain and became SARS-CoV-2 re-positivity after 142 days. As human-to-human transmission of SARS-CoV-2 from asymptomatic patients was emphasized in previous studies [9], [22]. However, being lack the evidence of virus isolation, whether virus replicated or reactivated, or only the ‘dead virus’ genetic materials remain to be investigated, the potential of infectivity can’t be ignored among discharged COVID-19 patients with SARS-CoV-2 re-positivity. Thus, early predictive risk factors for re-positivity of SARS-CoV-2 after discharge could be of significance in prevention and management of the disease. Indeed, more and more studies focusing on risk factors for the prediction of SARS-CoV-2 re-positivity have been released, however, conclusions remain controversial. The discrepancy across studies could be caused by heterogeneity of the cohorts, such as difference in cohort size, patient gender, age, treatment regime and physical or laboratory index included to analysis. In this context, data revealed that COVID-19 patients with younger, mild and moderate severity and higher lymphocyte counts were prone to be SARS-CoV-2 re-positive after discharge [15], [23], while cases with elder age above 60 years, severe and higher higher lymphocyte counts were found to be SARS-CoV-2 re-positive in other studies [19], [24], [25], [26]. In our study, data showed that patients with longer viral shedding duration and lower serum IgG levels are significantly related to the recurrence of SARS-CoV-2 after discharge, but not with patient age, severity and the corticosteroid treatment. The underlying mechanisms for delayed SARS-CoV-2 shedding remain elusive. We previously reported that lower CD8 + T cells is an predictive factor for the delayed viral shedding [12], which echoes findings that immune suppression such as excessive usage of corticosteroid for or COVID-19 treatment or immunocompromised patients whose adaptive immune function including T and B cells were impaired have been related to the prolonged viral shedding [27], [28]. In addition to laboratory findings, other clinical data such as chronic rhinosinusitis and atopy have been found to be related to an increased risk for delayed viral shedding [29]. However, no relationship was observed between the viral shedding duration and symptoms or pre-existing disorders in this study (data not shown).
In summary, our findings revealed that longer viral shedding duration and lower IgG levels on admission are risk factors for re-positivity of SARS-CoV-2. Obviously, our study have several limitations. First, the major limitation is that our study is based on a rather small cohort from our single medical center, and only 13 patients confirmed to be SARS-CoV-2 re-positive. The statistical power is far from sufficient to draw a definite conclusion. Larger cohort and multi-center studies are extremely necessary to identify generalized findings and further evaluate the significance of early risk factors for SARS-CoV-2 re-positive after discharge. Second, only limited clinical features and laboratory variables in a very short follow-up were included, other variables such as imaging and biochemical index with extended follow-up needs to be conducted. Third, only nasopharyngeal swab samples were tested which may increases the risk of false negative results. Thus, underestimate the proportion of SARS-CoV-2 re-positive patients.
Compliance with Ethical Standards
This study was approved by the Ethics committee of the Taizhou Hospital of Zhejiang Province (#K20200111). Written informed consent was obtained from all patients or guardian.
Funding Information
This work was supported by grants from Science and Technology Bureau of Taizhou (1901ky01; 1901ky04).
CRediT author statement
Lu-Xiao Hong: Data curation, Investigation, Resources,Validation. Lian Liu: Data curation, Investigation, Resources,Validation. Aifen Lin: Data curation, Funding acquisition, Validation, Writing - review & editing. Wei-Hua Yan: Conceptualization, Formal analysis, Funding acquisition, Methodology, Validation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hong L.-X., Lin A., He Z.-B., Zhao H.-H., Zhang J.-G., Zhang C., Ying L.-J., Ge Z.-M., Zhang X., Han Q.-Y., Chen Q.-Y., Ye Y.-H., Zhu J.-S., Chen H.-X., Yan W.-H. Mask wearing in pre-symptomatic patients prevents SARS-CoV-2 transmission: An epidemiological analysis. Travel Med. Infect. Dis. 2020;36:101803. doi: 10.1016/j.tmaid.2020.101803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye G., Pan Z., Pan Y., Deng Q., Chen L., Li J., Li Y., Wang X. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J. Infect. 2020;80(5):e14–e17. doi: 10.1016/j.jinf.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dao T.L., Hoang V.T., Gautret P. Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: a narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40(1):13–25. doi: 10.1007/s10096-020-04088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y., Xu H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020;323(15):1502. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Z., Xu Y., Guo Y.e., Xu D., Zhang L.i., Wang X.u., Sun C., Qiu S., Ma K. A systematic review of re-detectable positive virus nucleic acid among COVID-19 patients in recovery phase. Infect. Genet. Evol. 2020;85:104494. doi: 10.1016/j.meegid.2020.104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du H.-W., Chen J.-N., Pan X.-B., Chen X.-L., Yixian-Zhang, Fang S.-F., Li X.-Q., Xia P.-C., Gao L., Lin H.-L., Chen L.-M., Liu N. Fujian Medical Team Support Wuhan for COVID-19. Prevalence and outcomes of re-positive nucleic acid tests in discharged COVID-19 patients. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40(2):413–417. doi: 10.1007/s10096-020-04024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habibzadeh P., Sajadi M.M., Emami A., et al. Rate of re-positive RT-PCR test among patients recovered from COVID-19. Biochem. Med (Zagreb). 2020;30(3) doi: 10.11613/BM.2020.030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang Y.J. South Korea's COVID-19 Infection Status: From the Perspective of Re-positive Test Results After Viral Clearance Evidenced by Negative Test Results. Disaster Med. Public Health Prep. 2020:1–3. doi: 10.1017/dmp.2020.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C., Ji F., Wang L., Wang L., Hao J., Dai M., Liu Y., Pan X., Fu J., Li L.i., Yang G., Yang J., Yan X., Gu B. Asymptomatic and Human-to-Human Transmission of SARS-CoV-2 in a 2-Family Cluster, Xuzhou, China. Emerg. Infect. Dis. 2020;26(7):1626–1628. doi: 10.3201/eid2607.200718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenyon C. The prominence of asymptomatic superspreaders in transmission mean universal face masking should be part of COVID-19 de-escalation strategies. Int. J. Infect. Dis. 2020;S1201–9712(20):30409–30414. doi: 10.1016/j.ijid.2020.05.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Health Commission (NHC), People’s Republic of China. Diagnosis and Treatment Guidance of Corona Virus Diseases 2019 (Tentative 7th Edition), http://www.gov.cn/zhengce/zhengceku/2020-03/04/content_5486705.htm.
- 12.Lin A., He Z.-B., Zhang S., Zhang J.-G., Zhang X., Yan W.-H. Early Risk Factors for the Duration of Severe Acute Respiratory Syndrome Coronavirus 2 Viral Positivity in Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2020;71(16):2061–2065. doi: 10.1093/cid/ciaa490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osman A.A., Al Daajani M.M., Alsahafi A.J. Re-positive coronavirus disease 2019 PCR test: could it be a reinfection? New Microbes New Infect. 2019;37(2020) doi: 10.1016/j.nmni.2020.100748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C., Gao G., Xu Y., et al. SARS-CoV-2-Positive Sputum and Feces After Conversion of Pharyngeal Samples in Patients With COVID-19. Ann. Intern Med. 2020;172(12):832–834. doi: 10.7326/M20-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azam M., Sulistiana R., Ratnawati M., et al. Recurrent SARS-CoV-2 RNA positivity after COVID-19: a systematic review and meta-analysis. Sci. Rep. 2020;10(1):20692. doi: 10.1038/s41598-020-77739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei H., Ye F., Liu X., et al. SARS-CoV-2 environmental contamination associated with persistently infected COVID-19 patients. Influenza Other Respir. Viruses. 2020;14(6):688–699. doi: 10.1111/irv.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X., Zhou J., Zhao J. Recurrent pneumonia in a patient with new coronavirus infection after discharge from hospital for insufficient antibody production: a case report. BMC Infect. Dis. 2020;20(1):500. doi: 10.1186/s12879-020-05231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.To K.K., Hung I.F., Ip J.D., et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1275. ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An J., Liao X., Xiao T., et al. Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test. Ann. Transl. Med. 2020;8(17):1084. doi: 10.21037/atm-20-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao A.T., Tong Y.X., Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: Rather than recurrence. J. Med. Virol. 2020;92(10):1755–1756. doi: 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T., Wu S., Zeng G., et al. Recurrent positive SARS-CoV-2: Immune certificate may not be valid. J. Med. Virol. 2020;92(11):2384–2386. doi: 10.1002/jmv.26074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R., Chen J., Hozumi Y., et al. Decoding Asymptomatic COVID-19 Infection and Transmission. J. Phys. Chem. Lett. 2020;11(23):10007–10015. doi: 10.1021/acs.jpclett.0c02765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J., Zheng L., Li Z., et al. Kinetics of SARS-CoV-2 positivity of infected and recovered patients from a single center. Sci. Rep. 2020;10(1):18629. doi: 10.1038/s41598-020-75629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong J., Koh W.C., Momin R.N., et al. Probable causes and risk factors for positive SARS-CoV-2 test in recovered patients: Evidence from Brunei Darussalam. J. Med. Virol. 2020;92(11):2847–2851. doi: 10.1002/jmv.26199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J., Xu X., Hu J., et al. Clinical course and risk factors for recurrence of positive SARS-CoV-2 RNA: a retrospective cohort study from Wuhan, China. Aging (Albany NY) 2020;12(17):16675–16689. doi: 10.18632/aging.103795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao C., Zhu L., Jin C.C., et al. Prevalence and impact factors of recurrent positive SARS-CoV-2 detection in 599 hospitalized COVID-19 patients. Clin. Microbiol. Infect. 2021;8:00055. doi: 10.1016/j.cmi.2021.01.028. S1198-743X(21)00055-0 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao H.R., Zhu X.Y., Zhou L., et al. Factors associated with delayed viral shedding in COVID-19 infected patients: A retrospective small-scale study. Respir. Med. 2021;178 doi: 10.1016/j.rmed.2021.106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarhini H., Recoing A., Bridier-Nahmias A., et al. Long term SARS-CoV-2 infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab075. jiab075 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Recalde-Zamacona B., Tomás-Velázquez A., Campo A., et al. Chronic rhinosinusitis is associated with prolonged SARS-CoV-2 RNA shedding in upper respiratory tract samples: A case-control study. J. Intern. Med. 2020 doi: 10.1111/joim.13237. Epub ahead of print. [DOI] [PubMed] [Google Scholar]