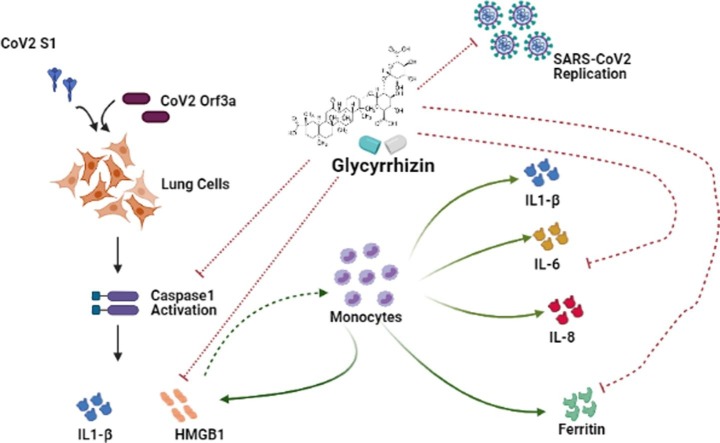
Graphical abstract
Keywords: SARS-CoV-2, COVID-19, HMGB1, Cytokine, Ferritin, Macrophages
Abstract
Efforts to understand host factors critical for COVID-19 pathogenesis have identified high mobility group box 1 (HMGB1) to be crucial for regulating susceptibility to SARS-CoV-2. COVID-19 disease severity is correlated with heightened inflammatory responses, and HMGB1 is an important extracellular mediator in inflammation processes. In this study, we evaluated the effect of HMGB1 inhibitor Glycyrrhizin on the cellular perturbations in lung cells expressing SARS-CoV-2 viral proteins. Pyroptosis in lung cells transfected with SARS-CoV-2 S-RBD and Orf3a, was accompanied by elevation of IL-1β and extracellular HMGB1 levels. Glycyrrhizin mitigated viral proteins-induced lung cell pyroptosis and activation of macrophages. Heightened release of proinflammatory cytokines IL-1β, IL-6 and IL-8, as well as ferritin from macrophages cultured in conditioned media from lung cells expressing SARS-CoV-2 S-RBD and Orf3a was attenuated by glycyrrhizin. Importantly, Glycyrrhizin inhibited SARS-CoV-2 replication in Vero E6 cells without exhibiting cytotoxicity at high doses. The dual ability of Glycyrrhizin to concomitantly halt virus replication and dampen proinflammatory mediators might constitute a viable therapeutic option in patients with SARS-CoV-2 infection.
1. Introduction
In the most critical COVID-19 patients, disease severity is correlated with increased levels of proinflammatory cytokines suggestive of a cytokine storm [1]. It has been suggested that it is the uncontrolled immune response-related damage rather than viral virulence that contributes to the disease severity [2]. As hyperinflammatory responses in patients with severe COVID-19 is associated with acute lung injury and disease severity, suppression of this cytokine storm to improve mortality is being considered as a viable strategy [3]. The immunopathology of respiratory viral diseases encompassing virus clearance and resolution of infection involves a complex interplay of viral factors and immune cells in the lungs [4]. Given the importance of exaggerated host response to viral proteins manifested as hyper-inflammation in contributing to disease severity; we investigated whether the cytopathic effects of SARS-CoV-2 viral proteins on lung cells could disrupt macrophage homeostasis leading to heightened inflammation.
HMGB1, a well-recognized damage-associated molecular pattern (DAMP) protein, is involved in the pathogenesis of many inflammatory diseases of infectious or sterile origin [5]. Extracellular HMGB1 promotes release of proinflammatory cytokines [6], and the efficacy of anti‐HMGB1‐based therapeutic strategies in inflammatory diseases is known [7]. Elevation in serum HMGB1 levels in severe COVID-19 patients has been reported [8]. SARS-CoV-2 enters cells by binding its S (spike) protein to the human angiotensin-converting enzyme 2 (hACE2) [9], and HMGB1 regulates ACE2 expression essential for SARS-CoV-2 entry [10]. DAMPs activate the immune system by interacting with the advanced glycosylation end product specific receptor (AGER) [11], and inhibition of the HMGB1-AGER pathway blocks ACE2 expression in SARS-CoV-2 infection [8]. Given its importance in SARS-CoV-2 infection, targeting HMGB1 proinflammatory activities has been suggested as an effective therapeutic strategy for the treatment of COVID-19 [12]
Glycyrrhizin, a natural anti-inflammatory and antiviral triterpene of licorice (Glycyrrhiza glabra) binds directly to HMGB1 to inhibit its cytokine activity [13]. Glycyrrhizin has been shown to interfere with H5N1 influenza A virus replication and H5N1-induced proinflammatory gene expression [14]. Also, Glycyrrhizin mediated inhibition of HMGB1 upregulation in Respiratory Syncytial Virus (RSV)-infected human bronchial epithelial cells is associated with reduction of viral replication [15]. Importantly, glycyrrhizin has been reported to interfere with replication of SARS-associated virus [16]. Glycyrrhizin being an appealing therapeutic target in several HMGB1-related inflammatory pathologies [17], we assessed its antiviral and anti-inflammatory properties against SARS-CoV-2.
2. Results
2.1. SARS-CoV-2 viral proteins induce death in lung cells
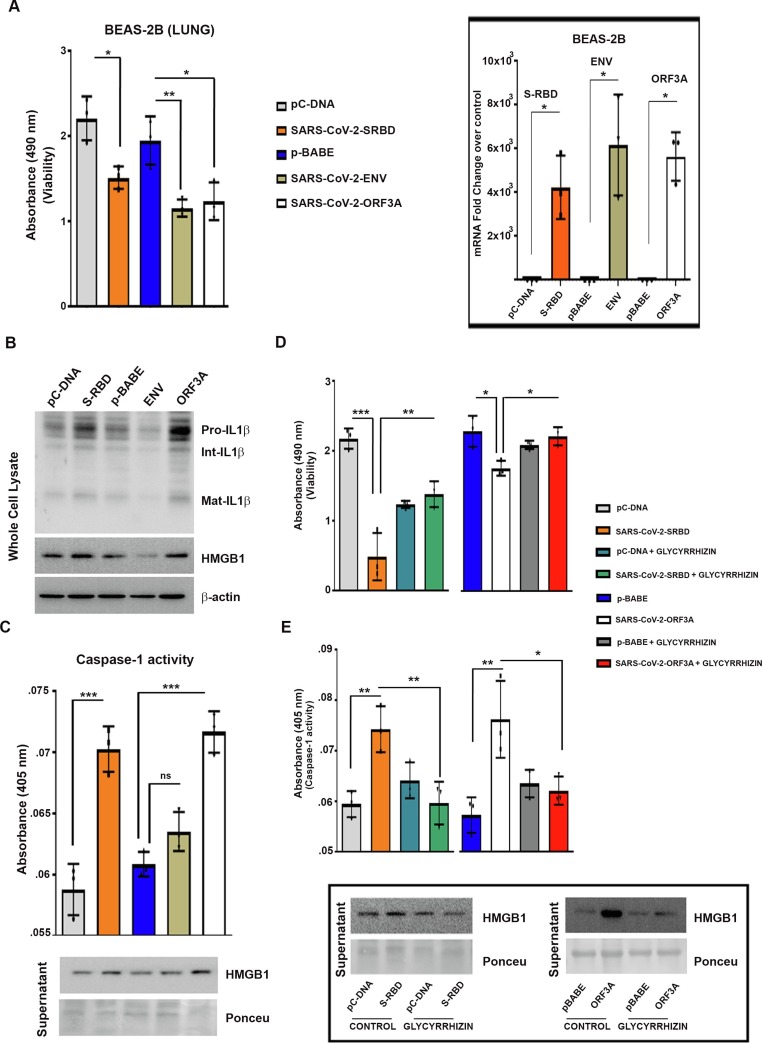
The receptor binding domain (RBD) in the S1 subunit of SARS-CoV-2 spike protein is important for binding to ACE2 receptor. Of the other SARS-CoV-encoded viroporins, only envelope (E) and Orf3a proteins are required for maximal SARS-CoV replication and virulence [18]. As the lung cells represent the physiological site of SARS-CoV-2 infection, non-neoplastic human bronchial epithelial cell line (BEAS-2B) and human malignant tumor respiratory epithelial cells (A549, H1299) were transfected to overexpress SARS-CoV-2 S1 (RBD), Env and Orf3a proteins to study the host response elicited by these viral components. Transfection with S-RBD, Env and Orf3a induced significant death of BEAS-2B cells as determined by MTS assay (Fig. 1a ). Transfections were confirmed by qRT-PCR using primers for SARS-CoV-2 S-RBD, Env and Orf3a (Fig. 1a). None of the viral proteins induced death in the malignant lung tumor cell lines. This could have stemmed from the aberrant expression of genes/signalling pathways typical of tumorigenesis that confer survival advantage.
Fig. 1.
SARS-CoV-2 S-RBD and Orf3a induce pyroptosis and HMGB1 release in lung cells (a) Transfection with SARS-CoV-2 viral proteins induces BEAS-2B lung cell death (cell death was assessed after 24 h of transfection). Graph of qRT-PCR fold change values shows the efficacy of transfection. (b) Western blots demonstrating increased IL-1β and HMGB1 levels in BEAS-2B cells transfected with SARS-CoV-2 S-RBD and Orf3a. Blots are representative of data from three independent experiments with similar results. Blots were stripped and re-probed with β-actin to establish equivalent loading. (c) Increase in absorbance at 405 nm representing induced active caspase-1 in SARS-CoV-2 S-RBD and Orf3a-transfected BEAS-2B cells. Increase in caspase-1 activity is accompanied by HMGB1 release as demonstrated by Western blot using cell culture supernatant. (d) Treatment with 1 mM glycyrrhizin diminishes SARS-CoV-2 S-RBD and Orf3a-induced caspase-1 activity. Inset shows the efficacy of glycyrrhizin in inhibiting HMGB1 release. Ponceau S staining of the blots was used to establish equivalent loading in case of supernatants. (e) Increase in absorbance representing rescue of cell death by HMGB1 inhibitor Glycyrrhizin. (cell death was assessed 48 h post transfection) One-way ANOVA (with Tukey’s multiple comparison post-hoc test) was used for statistical analysis. p < 0.05*, p < 0.01**, p < 0.001***
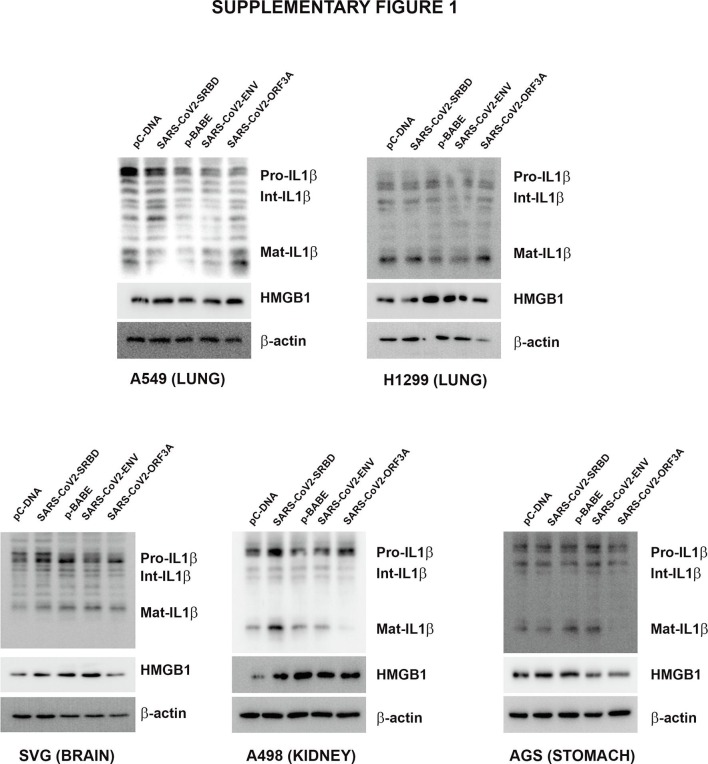
2.2. SARS-CoV-2 S-RBD- and Orf3a-mediated induction of IL-1β and HMGB1 is lung cell-specific
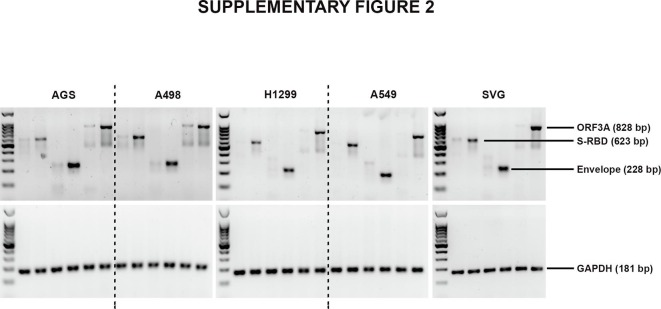
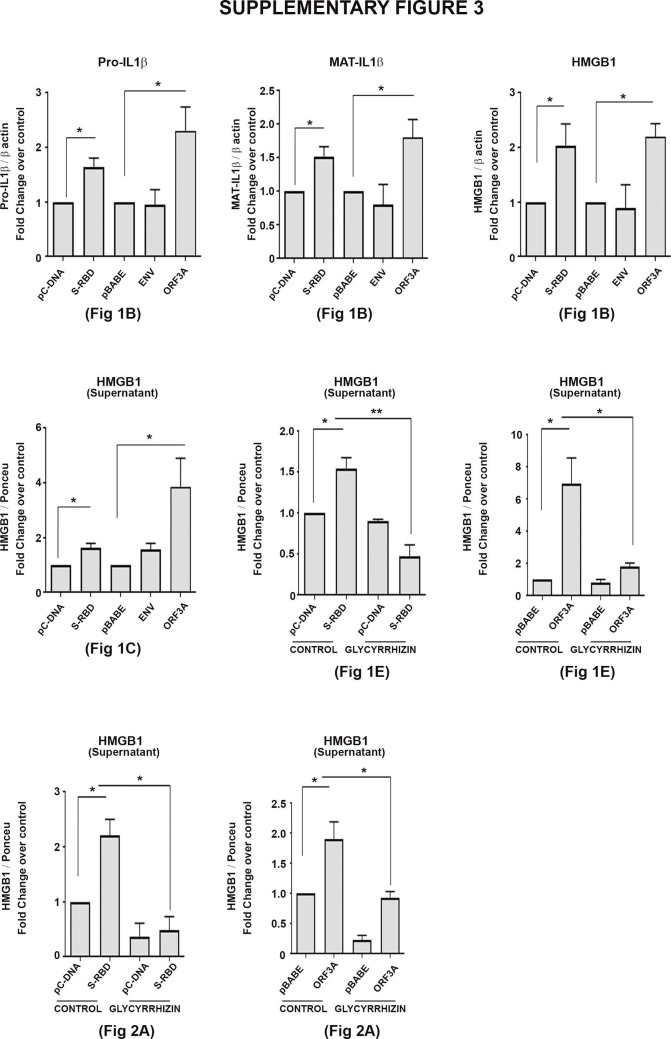
Pyroptosis, an inflammatory form of programmed cell death induced by cellular damage or infection [19], is characterised by the release of proinflammatory intracellular contents. As proinflammatory cytokine IL-1β undergoes caspase-1-dependent activation and secretion during pyroptosis [20], we determined whether cell death induced by viral proteins in BEAS-2B cells is accompanied by alterations in IL-1β levels. Elevated levels of pro- and mature IL-1β were observed in S-RBD- and Orf3a-transfected BEAS-2B cells (Fig. 1b, Supplementary Fig. 3). Env had no effect on either pro- or mature IL-1β levels (Fig. 1b, Supplementary Fig. 3). Although S-RBD, Env and Orf3a failed to induce death in malignant lung cells, an increase in mature IL-1β levels were observed in all the lung cells, malignant or non-malignant, upon transfection with S-RBD and Orf3a (Supplementary Fig. 1). Transfections were confirmed by PCR (Supplementary Fig. 2.
IL-1β is known to induce HMGB1 expression [21], and HMGB1 associates with IL-1β to enhance the proinflammatory properties of the latter [22]. An increase in HMGB1 expression concomitant with elevated IL-1β levels was observed in all lung cell lines transfected with S-RBD and Orf3a (Fig. 1b, Supplementary figure 1, Supplementary figure 3). The organotropism of SARS-CoV-2 that extends beyond the respiratory tract, to the kidneys, liver, and brain likely influences the course of disease [23]. While the lung cells represent the physiological site of infection; kidney carcinoma cell line (A498), gastric adenocarcinoma cell line (AGS) and astrocytic cell line (SVG p12) were also used to determine the relevance of COVID-19 infection affecting other tissues in addition to the virus’s typical environment-the lung cells. While transfection with S-RBD increased the level of mature IL-1β in kidney, stomach and astrocytic cells, Env and Orf3a had no effect on IL-1β and HMGB1 levels (Supplementary Fig. 1). Transfections were confirmed by PCR (Supplementary Fig. 2). Since viral protein-induced cell death, and IL-1β and HMGB1 release were restricted to BEAS-2B cells; subsequent experiments were conducted on this bronchial epithelial cell line which is widely used in studies associated with respiratory diseases.
2.3. Glycyrrhizin prevents S-RBD- and Orf3a-mediated death and caspase-1 activation
Caspase-1-dependent cleavage of inflammatory cytokine IL-1β into its mature form is a defining feature of pyroptosis [19]. An increase in caspase-1 activation concomitant with increased IL-1β and HMGB1 levels was observed in BEAS-2B cells transfected with S-RBD and Orf3a. (Fig. 1c, Supplementary Fig. 3). No significant change in caspase-1 activity was observed in cells transfected with Env (Fig. 1c). It is possible that Env-mediated death involves mechanisms independent of inflammation. Since disruption of HMGB1 protects cells from SARS-CoV-2-induced cell death [10], we next investigated whether Glycyrrhizin affects SARS-CoV-2 S-RBD- and Orf3a-mediated HMGB1 release and cell death in lung cells. Treatment with glycyrrhizin not only reversed S-RBD- and Orf3a-induced extracellular HMGB1 release but also abrogated caspase-1 activation and rescued cell death ( Fig. 1d and 1e, Supplementary Fig. 3).
2.4. Glycyrrhizin rescues macrophage activation induced by viral proteins
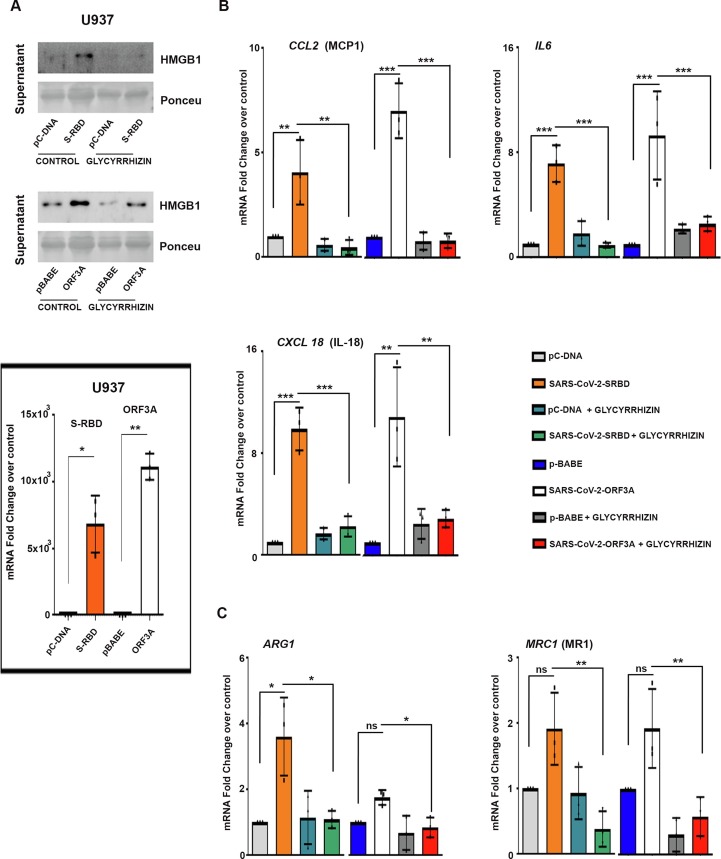
HMGB1 acts upstream of the proinflammatory cytokine cascade [6] and serves as key player in macrophage activation and reprogramming [24]. Activated macrophages exhibit plasticity and diverse functions as classical (M1) or alternative (M2) activation (or polarization) phenotypes. Pathology-associated changes in macrophage activation require M1 cells for initiating and sustaining inflammation, while M2 cells are linked with resolution of chronic inflammation [25]. Dampening of the inflammatory monocyte response facilitates effective viral clearance and limits inflammatory and immune-mediated damage to the lungs [4]. While viral proteins had no effect on monocyte survival, an effect on differentiation was noted. Elevation in extracellular HMGB1 in S-RBD- and Orf3a-transfected U937 cells upon phorbol 12-myristate 13-acetate (PMA)-mediated differentiation (Fig. 2a , Supplementary Fig. 3), was accompanied by increased expression of M1-specific markers (CCL2, IL6, and CXCL18) (Fig. 2a and 2b, Supplementary Fig. 3). Transfection was confirmed by qRT-PCR (Fig. 2a). No significant change in the expression of markers ARG1 and MRC1 (mannose receptor 1), associated with alternatively activated M2 macrophages, was observed in Orf3a-transfected U937 cells. While S-RBD-transfected U937 cells showed significant increase in ARG1 expression, no change in MRC1 was observed. The ability of Glycyrrhizin to reduce viral protein-induced expression of markers associated with both M1 (Fig. 2a and 2b) and M2 phenotype suggested its effectiveness in dampening overall macrophage activation (Fig. 2c).
Fig. 2.
Glycyrrhizin rescues SARS-CoV-2 S-RBD and Orf3a-induced macrophage activation (a) Transfection with SARS-CoV-2 viral proteins induces HMGB1 release in S-RBD- and Orf3a-transfected U937 cells upon PMA-mediated differentiation. Graph of qRT-PCR fold change values shows the efficacy of transfection. Ponceau S staining of the blots was used to establish equivalent loading. (b) qRT-PCR analysis shows that glycyrrhizin rescues the expression of SARS-CoV-2 S-RBD- and Orf3a-induced M1 macrophage markers CCL2, IL6, and CXCL18 in S-RBD- and Orf3a-transfected U937 cells. The graphs represent average fold change values from three independent experiments. (c) Expression of M2 phenotype markers ARG1 and MRC1 in S-RBD- and Orf3a-transfected U937 cells in the presence and absence of 1 mM glycyrrhizin, as indicated by qRT-PCR analysis. The graphs represent average fold change values from three independent experiments. One-way ANOVA (with Tukey’s multiple comparison post-hoc test) was used for statistical analysis. p < 0.05*, p < 0.01**, p < 0.001***
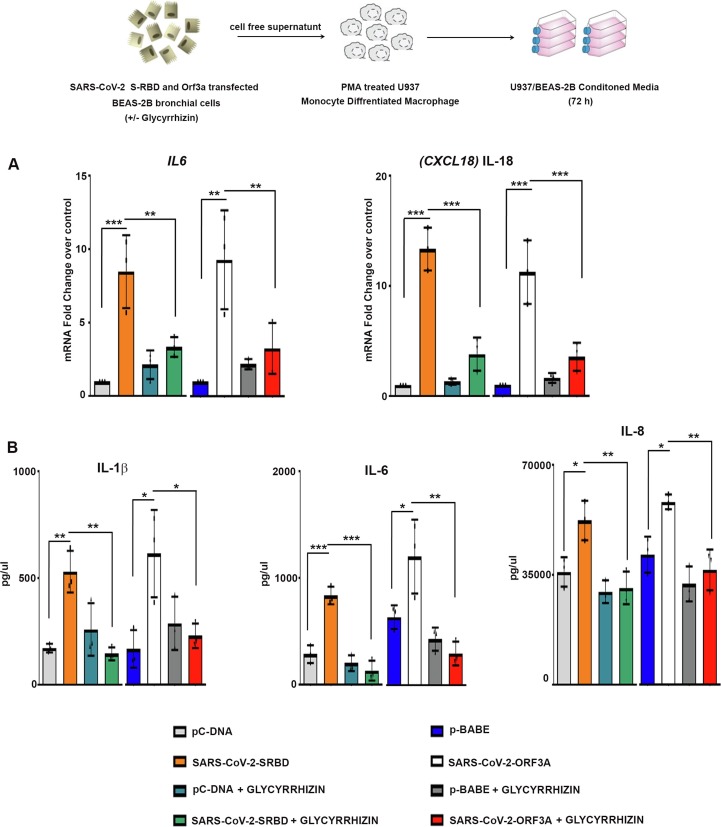
2.5. Glycyrrhizin prevents cytokine release from macrophages cultivated in BEAS-2B conditioned medium
Macrophages are potent producers of cytokines which serve as mediators of immunopathology. As macrophages play a crucial role in modulating immune response following respiratory viral infection [4], the effect of mediators released from lung cells overexpressing viral proteins on macrophages was studied. This was achieved by cultivating PMA-differentiated U937 in cell-free culture supernatant from BEAS-2B cells expressing viral proteins. A significant increase in the expression of proinflammatory cytokines IL6 and CXCL18 was observed in macrophages cultured in conditioned media from S-RBD- and Orf3a-transfected lung cells (Fig. 3a ). COVID-19 mortality is associated with enhanced pro-inflammatory IL-6 levels [1], and SARS-CoV-2-infection induces release of IL-6 in human primary monocytes [26]. Glycyrrhizin attenuated S-RBD and Orf3a conditioned media-induced expression of proinflammatory cytokines IL6 and CXCL18 in macrophages (Fig. 3a). Cytometric bead array also revealed glycyrrhizin-mediated inhibition of IL-6, IL-1β, and IL-8 release from uninfected macrophages cultivated with S-RBD and Orf3a conditioned media (Fig. 3b).
Fig. 3.
Glycyrrhizin rescues elevated pro inflammatory cytokine release from macrophages cultivated in BEAS-2B conditioned media (a) Treatment with 1 mM glycyrrhizin rescues the heightened expression of IL6 and CXCL18 in macrophages cultivated in conditioned media from SARS-CoV-2 S-RBD- and Orf3a-transfected BEAS-2B cells, as indicated by qRT-PCR analysis. The graphs represent average fold change values from three independent experiments. (b) Cytometric bead array demonstrating release of proinflammatory cytokines IL-1β, IL-6 and IL-8 from macrophages cultivated in the conditioned media of SARS-CoV-2 S-RBD- and Orf3a-transfected BEAS-2B cells, in the presence and absence of 1 mM Glycyrrhizin. The graphs represent average fold change values from three independent experiments. One-way ANOVA (with Tukey’s multiple comparison post-hoc test) was used for statistical analysis. p < 0.05*, p < 0.01**, p < 0.001***
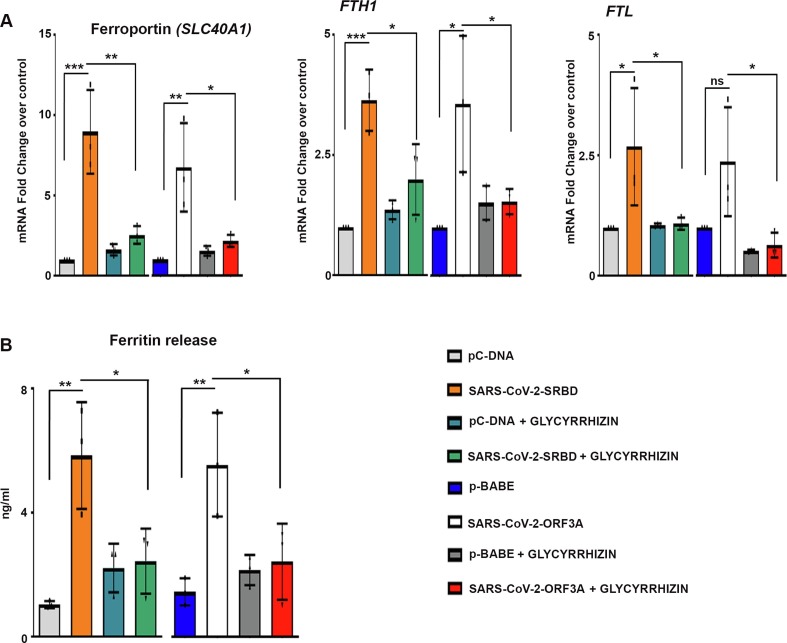
2.6. Glycyrrhizin diminishes ferritin release in macrophages cultivated in conditioned media from BEAS-2B cells transfected with S-RBD and Orf3a
The synthesis of serum ferritin, a protein that stores iron in a soluble form is regulated by inflammatory cytokines. Composed of heavy and light chain, ferritin - a major intracellular iron storage protein mediates immune dysregulation especially in hyperferritinemia through its immune-suppressive and proinflammatory effects [27]. H-ferritin induces expression of different inflammatory mediators, including IL-1β, which is known to induce ferritin gene expression [28]. Elevated serum ferritin levels are concomitant with hyper-inflammation in severe COVID-19 patients [1]. Since ferritin can be actively secreted by macrophages [29], we investigated whether elevated proinflammatory cytokine expression in macrophages was accompanied by elevated ferritin levels. The increased expression of SLC40A1 (ferroportin), FTH1 (ferritin heavy chain) and FTL (ferritin light chain) mRNA levels (Fig. 4a ), as well as ferritin release (Fig. 4b) from macrophages cultured in conditioned media from BEAS-2B cells transfected with S-RBD and Orf3a was rescued by Glycyrhizzin. Thus, in addition to dampening SARS-CoV-2-induced HMGB1 and cytokine release, Glycyrhizzin also reduced ferritin release from macrophages.
Fig. 4.
Glycyrrhizin decreases ferritin release from macrophages cultivated in BEAS-2B conditioned media (a) qRT-PCR analysis depicts the effect of Glycyrrhizin on expression of SLC40A1, FTH1 and FTL in macrophages cultivated in conditioned media from SARS-CoV-2 S-RBD- and Orf3a-transfected BEAS-2B cells. The graphs represent average fold change values from three independent experiments. (b) Human ferritin ELISA demonstrating increased release of ferritin from macrophages cultivated in conditioned media of SARS-CoV-2 S-RBD- and Orf3a-transfected BEAS-2B cells. Treatment with 1 mM Glycyrrhizin significantly reduces the release of ferritin. The graphs represent average fold change values from three independent experiments. One-way ANOVA (with Tukey’s multiple comparison post-hoc test) was used for statistical analysis. p < 0.05*, p < 0.01**, p < 0.001***
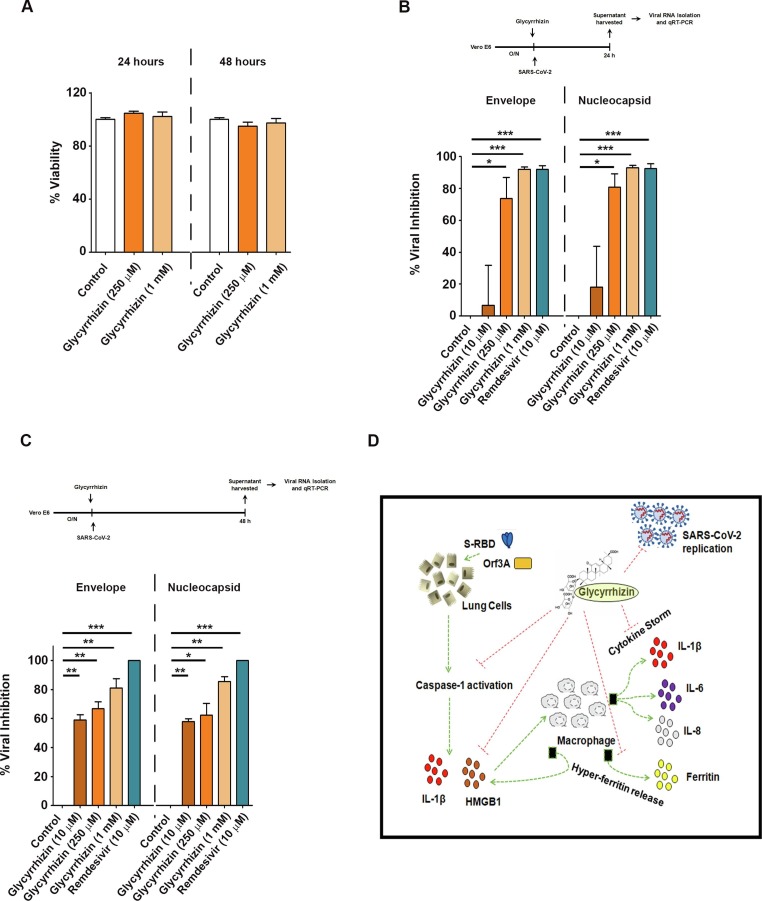
2.7. Glycyrhizzin inhibits SAR-CoV-2 replication in Vero E6 cells
Glycyrrhizin has been shown to be effective against SARS-CoV at 1 mM and 4 mM concentrations [16]. To determine if Glycyrrhizin could protect cells from SARS-CoV-2 infection and to evaluate its toxicity, the antiviral effectiveness of increasing concentrations of Glycyrrhizin was measured in Vero E6 cells infected with a clinical isolate of SARS-CoV-2. Cytotoxicity assay revealed that Glycyrrhizin had no significant effect on viability of Vero E6 cells (Fig. 5a ). qRT-PCR for SARS-CoV-2 E (envelope) and N (nucleocapsid) gene sequences after treatment with Glycyrrhizin showed significant inhibition of SARS-CoV-2 replication in a dose-dependent manner (Fig. 5b and 5c). By demonstrating the antiviral activity of Glycyrrhizin in Vero E6 cells which do not recapitulate the host cell inflammatory pathways, our findings indicate an alternative pathway that addresses the utility of Glycyrrhizin beyond amelioration of the inflammatory response. These findings suggest that in addition to (i) mitigating viral protein induced lung cell pyroptosis, and (ii) attenuating cytokine and ferritin release from macrophages, Glycyrrhizin also strongly inhibits viral replication without exhibiting cytotoxicity (as shown in Vero E6 cells) (Fig. 5d).
Fig. 5.
Antiviral activity of Glycyrrhizin against SARS-CoV-2 (a) Effect of Glycyrrhizin on viability of Vero E6 cells. (b-c) Percentage inhibition in replication of SARS-CoV-2 in Vero E6 cells in the presence of increasing concentrations of Glycyrrhizin or 10 μM remdesivir. The graphs represent average values from three independent experiments. Two-tailed paired t-test was used for statistical analysis. p < 0.05 *, p < 0.01 **, p < 0.001 *** (d) Schematic depiction of the multifaceted potential of Glycyrrhizin to inhibit viral replication in Vero E6 cells as well as to ameliorate host-inflammatory responses.
3. Discussion
Severe COVID-19, characterized by acute respiratory distress syndrome (ARDS), is associated with uncontrolled inflammatory responses leading to cytokine storm linked with multiple organ failure [30]. HMGB1 has been suggested to be a biomarker and therapeutic target for severe COVID-19 [8]. HMGB1-specific antagonists have been shown to ameliorate inflammatory conditions associated with elevated extracellular HMGB1 levels in viral infection-mediated pulmonary inflammation [31], [32]. The small-molecule inhibitor of HMGB1 - Glycyrrhizin, has been investigated in a wide number of diseases involving HMGB1 [17]. Given the documented antiviral activity of Glycyrrhizin against respiratory viruses including SARS-CoV [16] and RSV [15], this study was undertaken to generate proof of concept of antiviral and anti-inflammatory effectiveness of Glycyrrhizin in COVID-19.
Proinflammatory cell death in lung epithelial cells upon respiratory viral infections is known, with inflammatory cell death pyroptosis underpining the innate immune response against pathogens. Given the crucial role of pyroptosis in regulating inflammation, targeting pyroptosis and downstream cytokines has been suggested as a promising therapy against severe COVID-19-associated disease [33]. Our findings suggest that S-RBD, Env and Orf3a induce death in normal lung cells to different extents while sparing malignant lung, stomach and kidney cell lines. The tissue specific difference in responsiveness to viral proteins highlights the complexities of host tissue-SARS-CoV-2 interactions in determining the inflammatory outburst. It is likely that pyroptosis induced by viral proteins in lung cells could trigger lung pathology through enhanced death and release of IL-1β and HMGB1. High levels of HMGB1 induce caspase-1-mediated endothelial pyroptotic cell death in Kawasaki disease [34], and Kawasaki-like disease, characterised by multi-system inflammation, has been reported in children with COVID-19 [35].
Ferritin acts as a pro-inflammatory cytokine [27], and IL-1β enhances expression of FTH1 [36]. Ferritin-reinforced feedforward inflammatory loop [27] could play a role in sustaining hyperinflammation in pathological setting of COVID-19 characterized by elevated serum ferritin and cytokine storm. Association of HMGB1 with IL-1β enhances the proinflammatory properties of IL-1β and contributes to macrophage activation [22]. It is tempting to speculate that HMGB1 upholds the proinflammatory milieu through a feed-forward loop that stimulates production of IL-1β and ferritin crucial for macrophage activation necessary to sustain the hyper-inflammatory state in SARS-CoV-2 infection. By dampening HMGB1 release from macrophages, Glycyrrhizin diminishes both HMGB1-IL-1β and Ferritin-IL-1β crosstalk involved in driving macrophage activation. As both uninfected and infected monocytes, resident or infiltrating, can coexist in SARS-CoV-2 infected lungs and share similar responsiveness towards mediators released from lungs; the ability of Glycyrrhizin to target macrophages together with infected lung cells holds promise. Glycyrrhizin, an active component of Yashtimadhu, is used to treat respiratory problems in Indian traditional medicine Ayurveda. A randomised controlled trial has been initiated in India to study the efficacy of herbal extracts containing Yashtimadhu to restore respiratory health in COVID-19 positive patients [37]. Besides, Glycyrrhizin used as clinically approved preparation SNMC (Stronger Neo-Minophagen C) with its very mild side‐effects is approved in Japan for the treatment of chronic hepatic diseases [38]. In the light of its known safety profile [38], and its ability to reduce (i) SARS-CoV-2 replication without displaying any cytotoxicity even at high concentrations, and (ii) viral protein-induced mortality of lung cells and proinflammatory mediator levels; the potential therapeutic effects of Glycyrrhizin merit further exploration in COVID-19. Taken together, the efficacy of Glycyrrhizin in resolving hyper-inflammatory processes and viral replication provide a rationale for clinical evaluation in the therapy of COVID-19.
4. Materials and methods
4.1. Gateway cloning
The pcDNA3-SARS-CoV-2-S-RBD-Fc (#141183), pDONR207 SARS-CoV-2 E (#141273) and pDONR207 SARS-CoV-2 Orf3A (SARS-CoV-2 isolate Wuhan-Hu-1) constructs were purchased from Addgene (#149319). Expression clones were generated by recombining the entry clones with the destination vector pBABEpuro-gateway (#51070, Addgene) using the Gateway LR Clonase II enzyme mix (#11791020, Invitrogen) according to the manufacturer's directions.
4.2. Cell culture
Human lung cell carcinoma line, A549 (CCL-185, ATCC) was grown in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) (#12400–024, Gibco). Human lung cancer cell line NCI-H1299 (CRL-5803, ATCC) was grown in RPMI-1640medium (R6504, Sigma-Aldrich). The human bronchial epithelial cell line BEAS-2B (a kind gift from Dr. Munia Ganguli, Institute of Genomics and Integrative Biology, New Delhi, India) was cultured in Ham’s Nutrient Mixture F12 (51651C, SAFC). Human gastric adenocarcinoma cell line, AGS, and kidney carcinoma cell line, A498 were obtained from National Centre for Cell Science (Pune, India) and were maintained in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) and Minimum Essential Medium Eagle (MEM) (M0644, Sigma Aldrich) respectively. Human astroglial cell line SVGp12 was cultured in Minimum Essential Medium Eagle (MEM). Human monocytic cell line U937 was grown in RPMI-1640 medium. All culture media were supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS) (#16140, Gibco) and penicillin (100 U/mL)- streptomycin (100 μg/mL) (#15140, Gibco).
4.3. Treatment and transfection
Transfections were done as described previously [39]. Briefly, cells were grown in their respective culture media and upon reaching 70% confluence the media was replaced with OptiMEM (31985, Gibco) 2 hours prior to transfection. The cells were transfected with pcDNA SARS-CoV-2-S-RBD, pBABE SARS-CoV-2 E, pBABE SARS-CoV-2 Orf3A, or their corresponding empty vectors by magnetofection using PolyMag Neo transfection reagent (PG61000, OZ Biosciences) (for BEAS-2B and U937 cells), or using Lipofectamine 2000 transfection reagent (#11668027, Invitrogen) (for A549, NCI-H1299, AGS, A498 and SVGp12 cells). Transfected cells were either harvested for further processing or serum starved for 6 hours, after which they were treated with 1 mM glycyrrhizic acid sodium salt (GA) (G2137, Sigma) for 24 hours.
4.4. Caspase-1 activity assay
Levels of active caspase-1 were analyzed using caspase-1 activity assay kit (218790, Merck Millipore). Briefly, ~2–4 × 106 BEAS-2B cells expressing SARS-CoV-2 proteins in the presence or absence GA were pelleted and resuspended in chilled lysis buffer followed by centrifugation at 10,000g for 1 min. Protein content in the cell lysates was quantified and the lysates were incubated with 2× reaction buffer and substrate (200 µMYVAD-pNA) at 37 °C for 2 hours. Absorbance at 405 nm gave the measure of caspase-1 activity.
4.5. Culture of macrophages in lung cell conditioned media
To obtain BEAS-2B conditioned media, cell-free supernatants were removed from BEAS-2B cells transfected with SARS-CoV-2 viral components with or without treatment with HMGB1 inhibitor, Glycyrrhizin. PMA-derived macrophages were grown in a media containing 50% BEAS-2B conditioned media and 50% RPMI-1640. All cultures were cultivated for 72 hours, after which cell-free supernatants were collected, aliquoted and stored at −80 °C until further processing [40].
4.6. Determination of cell viability
The viability of BEAS-2B cells transfected with SARS-CoV-2 viral components in the presence or absence of Glycyrrhizin was assessed using MTS assay (G3582, Promega) as previously described [41].
4.7. Western blot analysis
Proteins isolated from the cells were electrophoresed on polyacrylamide gel and Western blot was performed as described previously [41]. The blots were probed using antibodies against IL-1β (#AF-201-NA, R&D Systems), HMGB1 (ab79823, Abcam). Secondary antibodies were purchased from Vector Laboratories Inc. After addition of enhanced chemiluminescent HRP substrate (WBKLS0500, Millipore), blots were imaged in Syngene G: Box XX8 system (Syngene) using GeneSys software (Syngene). The blots were stripped and re-probed with peroxidase conjugated anti-β-actin (A3854, Sigma-Aldrich) to determine equivalent loading. Ponceau S staining of the blots was used to establish equivalent loading in case of supernatants.
4.8. Quantitative real time PCR
RNA was isolated using RNeasy Mini kit (74104, Qiagen), and cDNA was synthesised using HighCapacity cDNA Reverse Transcription kit (4368814, Thermo Fisher Scientific) on ProFlex PCR system (Applied Biosystems) as per manufacturer’s instructions. Real time PCR was performed as described previously [39] using QuantStudio5 Real Time thermocycler (Applied Biosystems Inc.) and results were plotted as delta Ct values or fold change over control for each mRNA transcript. 18S rRNA was used as internal control. The qPCR primers used are listed in Table 1 .
Table 1.
Sequences of primers used in the study.
| Transcript | Primer | |
|---|---|---|
| 1. | Spike protein (RBD) | Forward: TTCGGTGAGGTTTTTAACGC Reverse: TTTGTCGGATCCCTTTTTGG |
| 2. | Envelope protein | Forward: ATGTACTCTTTCGTGAGCGA Reverse: TTACACCAGCAGGTCAGGCA |
| 3. | ORF3A | Forward: ATGGACCTGTTCATGAGAAT Reverse: TTACAGTGGCACGGAGGTGG |
| 4. | IL6 | Forward: GCCTTCGGTCCAGTTGCCTT Reverse: AGTGCCTCTTTGCTGCTTTCA |
| 5. | CXCL18 | Forward: TCTATACCTCCTGGCAGATTC Reverse: TTTCTGGACCCACTTCTTATTG |
| 6. | CCL2 | Forward: ACTCTCGCCTCCAGCATGAA Reverse: TGATTGCATCTGGCTGAGC |
| 7. | MRC1 | Forward: GCCAAATGACGAATTGTGGA Reverse: CACGAAGCCATTTGGTAAACG |
| 8. | ARG1 | Forward: GTGGACAGACTAGGAATTGGC Reverse: TCCAGTCCGTCAACATCAAAAC |
| 9. | FTL | Forward: AGCGTCTCCTGAAGATGCAA Reverse: CAGCTGGCTTCTTGATGTCC |
| 10. | FTH1 | Forward: GCCAGAACTACCACCAGGAC Reverse: CATCATCGCGGTCAAAGTAG |
| 11. | SLC40A1 | Forward: CACAACCGCCAGAGAGGATG Reverse: ACCAGAAACACAGACACCGC |
| 12. | 18S rRNA | Forward: GAGGGAGCCTGAGAAACGG Reverse: GTCGGGAGTGGGTAATTTGC |
4.9. Cytometric bead array for inflammatory cytokines
IL-6, IL-8 and IL-1β levels were measured in cell-free supernatants using Cytometric Bead Array (CBA) of human inflammatory cytokines (#551811; BD Biosciences) according to manufacturer’s protocol as described previously [21]. Data was acquired using BD FACSVerse (BD Biosciences).
4.10. Human ferritin ELISA
Ferritin levels were measured in cell-free supernatants using Human Ferritin ELISA kit (RAB0197; Sigma) according to manufacturer’s protocol. Absorbance at 450 nm gave the measure of ferritin levels.
4.11. Cytotoxicity assay
104 Vero E6 cells were seeded in each well of a 96 well plate and allowed to grow overnight at 37 °C. The cells were then incubated with 10 μM, 250 μM and 1 mM GA for 24 and 48 hours. Cells without GA treatment were used as control. The treatments were done in triplicates for each time period. After incubation, the cells were stained with Hoechst 33342 as a marker for nuclei and Sytox orange dye as an indicator of cell death. Cells were imaged using ImageXpress Microconfocal (Molecular Devices) at 10X magnification. 16 images were taken per well. Cytotoxicity of GA was determined by counting the total number of cells and Sytox-positive cells. This assay was performed as a service provide by the Advanced Technology Platform Centre (ATPC) at Regional Centre for Biotechnology, Faridabad, (Under the aegis of Dept. of Biotechnology, Govt. of India).
4.12. Antiviral screening
104 VeroE6 cells were seeded in each well of a 96 well plate and allowed to grow overnight at 37 °C. The cells were then incubated with 250 μM and 1 mM GA. Cells without GA treatment were used as control. This was followed by infection of the cells with SARS-CoV-2 [42] at an MOI (multiplicity of infection) of 0.01. After 24 and 48 hours, viral RNA was isolated from the cell culture supernatant. qRT-PCR was used to determine the Ct values for the viral N and E genes. The fold changes in Ct values with respect to control were used to determine the inhibition of viral replication by GA. Remdesivir was used as a positive control for inhibition of viral replication. This assay was performed as a service provided by ATPC at Regional Centre for Biotechnology, Faridabad, India.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We acknowledge Dr. Anirban Basu, National Brain Research Centre, Manesar, India for his valuable suggestions and Meenakshi Bhaskar for her technical inputs with CBA. The help of Dr. S. Chandru and Dr. Nirpendra Singh of ATPC, RCB in performing the antiviral screening is acknowledged. Graphical abstract was created with BioRender.com.
Authors contribution
Conceptualization and visualization: Pruthvi Gowda and Ellora Sen.
Data curation and investigation: Pruthvi Gowda, Shruti Patrick, Shanker Dutt Joshi and Rajesh Kumar Kumawat.
Formal analysis and validation: Pruthvi Gowda and Shruti Patrick.
Writing - original draft, review & editing: Pruthvi Gowda, Shruti Patrick and Ellora Sen.
Supervision, project administration and funding acquisition: Ellora Sen.
Funding
This work was supported by NBRC (Department of Biotechnology, Government of India) core funds.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cyto.2021.155496.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
Supplementary figure 3.
References
- 1.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.S. Felsenstein, et al., COVID-19: Immunology and treatment options, Clin. Immunol. 215, 108448. [DOI] [PMC free article] [PubMed]
- 3.P. Mehta, et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet 395(10229), 1033–1034. [DOI] [PMC free article] [PubMed]
- 4.Newton A.H., Cardani A., Braciale T.J. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin. Immunopathol. 2016;38(4):471–482. doi: 10.1007/s00281-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U. Andersson, K.J. Tracey, HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29, 139–62. [DOI] [PMC free article] [PubMed]
- 6.Andersson U., et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000;192(4):565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H., et al. The cytokine activity of HMGB1. J. Leukoc. Biol. 2005;78(1):1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- 8.Chen R., et al. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon. 2020;6(12) doi: 10.1016/j.heliyon.2020.e05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.M. Hoffmann, et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell. 181(2), 271–280 e8. [DOI] [PMC free article] [PubMed]
- 10.Wei J., et al. Genome-wide CRISPR Screens reveal host factors critical for SARS-CoV-2 infection. Cell. 2021;184(1):76–91 e13. doi: 10.1016/j.cell.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchi M.E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 12.Andersson U., Ottestad W., Tracey K.J. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol. Med. 2020;26(1):42. doi: 10.1186/s10020-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mollica L., et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007;14(4):431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Michaelis M., et al. Glycyrrhizin exerts antioxidative effects in H5N1 influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS ONE. 2011;6(5) doi: 10.1371/journal.pone.0019705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manti S., et al. Induction of high-mobility group Box-1 in vitro and in vivo by respiratory syncytial virus. Pediatr. Res. 2018;83(5):1049–1056. doi: 10.1038/pr.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cinatl J., et al. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musumeci D., Roviello G.N., Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol. Ther. 2014;141(3):347–357. doi: 10.1016/j.pharmthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 18.C. Castano-Rodriguez, et al., Role of Severe acute respiratory syndrome coronavirus viroporins E, 3a, and 8a in replication and pathogenesis, mBio 9(3). [DOI] [PMC free article] [PubMed]
- 19.S.M. Man, R. Karki, T.D. Kanneganti, Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases, Immunol. Rev. 277(1), 61–75. [DOI] [PMC free article] [PubMed]
- 20.Brough D., Rothwell N.J. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J. Cell Sci. 2007;120(Pt 5):772–781. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- 21.Gupta P., et al. beta-defensin-3 negatively regulates TLR4-HMGB1 axis mediated HLA-G expression in IL-1beta treated glioma cells. Cell. Signal. 2013;25(3):682–689. doi: 10.1016/j.cellsig.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Sha Y., et al. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J. Immunol. 2008;180(4):2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 23.Puelles V.G., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaper F., et al. High mobility group box 1 skews macrophage polarization and negatively influences phagocytosis of apoptotic cells. Rheumatology (Oxford) 2016;55(12):2260–2270. doi: 10.1093/rheumatology/kew324. [DOI] [PubMed] [Google Scholar]
- 25.Martinez F.O., Helming L., Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 26.Fintelman-Rodrigues N., Atazanavir,, et al. Alone or in combination with ritonavir, inhibits SARS-CoV-2 replication and proinflammatory cytokine production. Antimicrob. Agents Chemother. 2020;64(10) doi: 10.1128/AAC.00825-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.K.F. Kernan, J.A. Carcillo, Hyperferritinemia and inflammation, Int. Immunol. 29(9), 401–409. [DOI] [PMC free article] [PubMed]
- 28.Rogers J.T., et al. Translational control during the acute phase response. Ferritin synthesis in response to interleukin-1. J. Biol. Chem. 1990;265(24):14572–14578. [PubMed] [Google Scholar]
- 29.L.A. Cohen, et al., Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway, Blood. 116(9), 1574–84. [DOI] [PubMed]
- 30.Chakraborty S., Basu A. The COVID-19 pandemic: catching up with the cataclysm. F1000Res. 2020;9 doi: 10.12688/f1000research.24963.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito Y., et al. Increased levels of cytokines and high-mobility group box 1 are associated with the development of severe pneumonia, but not acute encephalopathy, in 2009 H1N1 influenza-infected children. Cytokine. 2011;56(2):180–187. doi: 10.1016/j.cyto.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Rayavara K., et al. Proinflammatory effects of respiratory syncytial virus-induced epithelial HMGB1 on Human innate immune cell activation. J. Immunol. 2018;201(9):2753–2766. doi: 10.4049/jimmunol.1800558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.J.K.Y. Yap, M. Moriyama, A. Iwasaki, Inflammasomes and pyroptosis as therapeutic targets for COVID-19, J Immunol. [DOI] [PMC free article] [PubMed]
- 34.C. Jia, et al., Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation, Cell Death Dis. 10(10), 778. [DOI] [PMC free article] [PubMed]
- 35.R.M. Viner, E. Whittaker, Kawasaki-like disease: emerging complication during the COVID-19 pandemic, Lancet. 395(10239), 1741–1743. [DOI] [PMC free article] [PubMed]
- 36.Rogers J.T., et al. Translational enhancement of H-ferritin mRNA by interleukin-1 beta acts through 5' leader sequences distinct from the iron responsive element. Nucleic Acids Res. 1994;22(13):2678–2686. doi: 10.1093/nar/22.13.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rangnekar H., et al. Safety and efficacy of herbal extracts to restore respiratory health and improve innate immunity in COVID-19 positive patients with mild to moderate severity: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):943. doi: 10.1186/s13063-020-04906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyake K., et al. Efficacy of Stronger Neo-Minophagen C compared between two doses administered three times a week on patients with chronic viral hepatitis. J. Gastroenterol. Hepatol. 2002;17(11):1198–1204. doi: 10.1046/j.1440-1746.2002.02876.x. [DOI] [PubMed] [Google Scholar]
- 39.Gowda P., et al. Mutant Isocitrate dehydrogenase 1 disrupts PKM2-beta-catenin-BRG1 transcriptional network-driven CD47 expression. Mol. Cell. Biol. 2018;38(9) doi: 10.1128/MCB.00001-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller-Quernheim U.C., et al. Tumor-cell co-culture induced alternative activation of macrophages is modulated by interferons in vitro. J. Interferon Cytokine Res. 2012;32(4):169–177. doi: 10.1089/jir.2011.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta P., Dixit D., Sen E. Oncrasin targets the JNK-NF-kappaB axis to sensitize glioma cells to TNFalpha-induced apoptosis. Carcinogenesis. 2013;34(2):388–396. doi: 10.1093/carcin/bgs352. [DOI] [PubMed] [Google Scholar]
- 42.Wan Y., et al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]