Abstract
Background
To investigate the prognostic value of a novel immune-inflammatory index, the interleukin-6-to-lymphocyte ratio (IL-6/LY), with the clinical outcomes of severe coronavirus disease 2019 (COVID-19) cases.
Methods
A cohort study of COVID-19 patients in Tongji Hospital, from January 2020 to February 2020, was evaluated. Kaplan–Meier method and the log-rank test was performed to analyze survival data. Univariate and multivariate analyses were performed with COX proportional hazard regression model. The primary and secondary outcomes were in-hospital mortality and multiple organ dysfunction syndrome (MODS), respectively.
Results
Total 320 adult patients were enrolled in our analyses. Patients were divided into low IL-6/LY group and high IL-6/LY group based on the cutoff value with 2.50. The Kaplan-Meier survival curves showed that high-value group (IL-6/LY ≥ 2.50) had a greater risk of poor prognosis (P < 0.001, respectively). Multivariate analysis indicated that IL-6/LY was the independent risk predictor for in-hospital mortality (hazard ratio [HR], 3.404; 95% confidence interval [CI], 1.090–10.633, P = 0.035) and MODS development (HR, 4.143; 95%CI, 1.321–12.986, P = 0.015). Meanwhile, IL-6/LY was positively correlated with the MuLBSTA score (r = 0.137, P = 0.031), suggesting that IL-6/LY was associated with long-term mortality (90-day). Furthermore, kinetic analysis revealed that the dynamic changes of inflammatory immune indexes were related to the severity of the disease.
Conclusions
The elevated IL-6/LY was related with the increased risk of poor prognosis. Not only that, IL-6/LY could be used for risk stratification and early clinical identification of high-risk patients.
Keywords: Coronavirus disease 2019, Immune-inflammatory index, Interleukin-6, Lymphocyte, Prognosis
1. Introduction
In December 2019, the outbreak of coronavirus disease 2019 (COVID-19), caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly throughout the world [1]. Considering the present epidemic situation, the World Health Organisation (WHO) declared a global pandemic on March 11, 2020 [2]. The raging infectious disease has caused a serious threat to human health. This brings the global cumulative numbers to 110.7 million cases and over 2.4 million deaths since the start of the pandemic [3].
SARS-CoV-2 is a beta-coronavirus, similar to other two known virus: severe acute respiratory syndrome‐CoV (SARS‐CoV) and Middle East Respiratory Syndrome‐CoV (MERS‐CoV), which have caused potentially fatal infections over the last 20 years [4]. The whole genome sequencing indicated that SARS-CoV-2 was very closely related to SARS-CoV [5]. Although SARS-CoV-2 is less lethal than MERS‐CoV and SARS‐CoV, it has stronger interpersonal transmission ability [6], [7]. Most patients with COVID-19 were asymptomatic or presented mild to moderate symptoms, however, about 10–20% of cases developed severe symptoms, characterized by the rapid development of acute respiratory distress syndrome (ARDS), sepsis and/or multiple organ failure [2]. In particular, the elderly patients and those with comorbidities tended to develop severe symptoms [8], which might be due to their weaker immune function. Till now, the pathogenicity of COVID-19 has not been completely understood, and the underlying mechanism leading from mild to severe cases remained unclear.
Recently, it has been speculated that the cytokine storm and immune dysfunction was closely related to the rapid disease progression [9]. Researchers found that the levels of infection-related biomarkers played vital roles in severe cases of COVID-19 [10]. Moreover, previous studies showed that lower lymphocyte counts, especially decreased levels of T lymphocyte subsets were linked to severe cases, accompanied with elevated levels of pro-inflammatory cytokines like interleukin-6 (IL-6) [11], [12]. These suggested that immune dysfunction and cytokine dysregulation might be the key factors in the progression of the disease.
In the current situation, the identification of disease progression of COVID-19 mainly depends on the clinical manifestation, while effective biomarkers have not been proposed. It is important to find sensitive biomarkers to identify critically ill patients in a timely and effective manner. As mentioned above, elevated IL-6 levels and lymphopenia were correlated with severity of disease [11], [12]. The IL-6-to-lymphocyte ratio (IL-6/LY) based on the two factors may reflect the imbalance of inflammation response and immune dysfunction in the body more comprehensively. In our study, we investigated the predictive effect of the new immune-inflammatory complex index on the prognosis of COVID-19, in order to provide positive help for clinical risk assessment.
2. Materials and methods
2.1. Study population
A total of 320 adult patients with severe COVID-19 pneumonia who were hospitalized in Sino-French New City Branch of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, from January 2020 to February 2020, were enrolled in our retrospective study. Diagnosis of confirmed COVID-19 was based on the interim guidance issued by WHO [13]. The definitions of severe illness associated with COVID-19 were as follow: fever or suspected respiratory infection; plus one of the following: (1) respiratory rate > 30 breaths/min; (2) severe respiratory distress; (3) or SPO2 ≤ 93% on room air. Moreover, patients with suspected COVID-19, patients whose age was below 18 years old, or the patients died within 24 h of admission were excluded.
The ethics Committee of Tongji Hospital approved this study (TJ-IRB20200362) and waived the requirement for written informed consent due to the massive contagion outbreak of this infectious disease.
2.2. Clinical date collection
We reviewed the electronic medical records system in hospital and collected the clinical information of all participants, including age, sex, smoking history, past medical histories (such as coronary artery disease, hypertension and diabetes). In addition, the data of initial common symptoms were gathered at the time of admission. The treatments during hospitalization (like oxygen therapy, pharmacotherapy and invasive therapies) were included in our data as well. What’s more, the complications were destined to record.
Venous blood samples were routinely collected from all patients during the time of hospitalization. The data were checked by two trained physicians independently. Laboratory findings included complete blood counts, coagulation function (prothrombin time, activated partial thromboplastin time, D-dimer), cardiac function (high sensitivity cardiac troponin I, N-terminal pro-brain natriuretic peptide), liver function (aminopherase, creatine kinase, lactate dehydrogenase), kidney function (urea nitrogen, creatinine), lipid profile and immuno-inflammatory indices.
2.3. Clinical outcomes
The primary endpoint of our study was in-hospital mortality, which was identified as the all-cause death happened during hospitalization. The secondary end point was incidence of multiple organ dysfunction syndrome (MODS), which was identified as acute dysfunction or failure happened in more than one system simultaneously or sequentially because of the severe diseases (such as serious infection). Clinically, MODS was common in lung, kidney, liver, heart, central nervous system, immune system and hematologic system [14].
2.4. Statistical analysis
The IL-6/LY was calculated as follow: IL-6/LY = IL-6/lymphocyte counts. The neutrophil to lymphocyte ratio (NLR) was calculated as follow: N/L = neutrophil counts/lymphocyte counts. Continuous variables were described as mean ± standard error and tested for normal distribution by the Kolmogorov-Smirnov test. Using independent samples t test or Mann-Whitney U test to compare the continuous variables between groups. Categorical variables were described as frequency rates and percentages and compared with the chi-square test. The correlation between IL-6/LY and MuLBSTA score was tested by using Spearman correlation coefficient. The optimal cut-off point for IL-6/LY was calculated by receiver operating characteristic (ROC) curve. Kaplan–Meier method and the log-rank test was performed to analyze survival data. Univariate and multivariate analyses were conducted with COX proportional hazard regression model. Any variables examined in the univariate analysis for which the P value was <0.10 or several established risk factors were contained in the multivariate model. A P value <0.05 was considered statistically significant. All data were analyzed using SPSS version 22.0 and Graphpad 6.0 software.
3. Results
3.1. Baseline characteristics of COVID-19 patients
Totally, 320 severe patients with COVID-19 were enrolled in our study and they all met the diagnostic criteria of severe COVID-19 patients as described above. In accordance with the cut-off value of IL-6/LY, the whole population was divided into low IL-6/LY group (207 patients, 100 males) and high IL-6/LY group (113 patients, 40 males).
The demographics and clinical characteristics of study population were shown in Table 1 . The patients in high IL-6/LY group were older, smoking more and with more comorbidities of coronary heart disease, diabetes and chronic lung disease (P < 0.050, respectively). Additionally, more patients in the high IL-6/LY group experienced moderate to high-grade fever (P < 0.050), with longer days from onset to hospitalization (P = 0.023), more complications (P < 0.001), and higher occupancy ratio of oxygen therapy and invasive treatment applications (P < 0.050, respectively). Conversely, patients in the low IL-6/LY group stayed longer in hospital (P < 0.001). The main clinical manifestations were dyspnea and mild-grade fever, and more patients in low IL-6/LY group received pharmacotherapy in hospital (P < 0.050).
Table 1.
. Demographics and clinical characteristics of study population.
| All patients (n = 320) | Low IL-6/LY group (n = 207) | High IL-6/LY group (n = 113) | P | |
|---|---|---|---|---|
| Gender, (male), n (%) | 140 (43.75) | 100 (48.31) | 40 (35.40) | 0.026 |
| Age, year | 61.37 ± 0.86 | 57.87 ± 1.10 | 67.77 ± 1.15 | <0.001 |
| Smoking, n (%) | 41 (12.81) | 16 (7.73) | 25 (22.12) | <0.001 |
| Comorbidity, n (%) | ||||
| CHD | 46 (14.38) | 23 (11.11) | 23 (20.35) | 0.024 |
| Hypertension | 113 (35.31) | 65 (31.40) | 48 (42.48) | 0.048 |
| Diabetes | 109 (34.06) | 53 (25.60) | 56 (49.56) | <0.001 |
| Chronic lung disease | 26 (8.13) | 12 (5.80) | 14 (12.39) | 0.039 |
| Chronic kidney disease | 8 (2.5) | 6 (2.90) | 2 (1.77) | 0.537 |
| Chronic liver disease | 14 (4.38) | 7 (3.38) | 7 (6.19) | 0.240 |
| Autoimmune diseases | 8 (2.5) | 7 (3.38) | 1 (0.88) | 0.172 |
| Tumor | 19 (5.94) | 12 (5.80) | 7 (6.19) | 0.886 |
| Cerebrovascular disease | 16 (5.00) | 7 (3.38) | 9 (7.96) | 0.072 |
| Gastrointestinal disease | 16 (5.00) | 12 (5.80) | 4 (3.54) | 0.376 |
| Time of illness onset, days | 10.83 ± 0.34 | 10.06 ± 0.38 | 12.22 ± 0.64 | 0.023 |
| Hospital stays, days | 14.49 ± 0.42 | 15.89 ± 0.48 | 11.93 ± 0.75 | <0.001 |
| Initial common symptoms, n (%) | ||||
| Fever | 281 (87.81) | 179 (86.47) | 102 (90.27) | 0.322 |
| Dyspnea | 189 (59.06) | 107 (51.69) | 82 (72.57) | <0.001 |
| Stomachache | 31 (9.69) | 22 (10.63) | 9 (7.96) | 0.441 |
| Diarrhea | 100 (31.25) | 66 (31.88) | 34 (30.09) | 0.740 |
| Chest distress | 80 (25.00) | 45 (21.74) | 35 (30.97) | 0.068 |
| Chest pain | 24 (7.5) | 13 (6.28) | 11 (9.73) | 0.262 |
| Palpitation | 32 (10.00) | 17 (8.21) | 15 (13.27) | 0.149 |
| Dizziness | 32 (10.00) | 22 (10.63) | 10 (8.85) | 0.612 |
| Headache | 60 (18.75) | 36 (17.39) | 24 (21.24) | 0.399 |
| Temperature, ℃ | ||||
| High fever (39.1–41.0) | 9 (2.81) | 3 (1.45) | 6 (5.31) | 0.046 |
| Moderate fever (38.1–39.0) | 68 (21.25) | 31 (14.98) | 37 (32.74) | <0.001 |
| Mild fever (37.3–38.0) | 68 (21.25) | 40 (19.32) | 28 (24.78) | 0.254 |
| Pharmacotherapy in hospital, n (%) | ||||
| Antibiotic therapy | 295 (92.19) | 186 (89.86) | 109 (96.46) | 0.035 |
| Antiviral therapy | 213 (66.56) | 146 (70.53) | 67 (59.29) | 0.042 |
| GCS | 219 (68.44) | 126 (60.87) | 93 (82.30) | <0.001 |
| Oxygen therapy in hospital, n (%) | ||||
| NMV | 153 (47.81) | 68 (32.85) | 85 (75.22) | <0.001 |
| IMV | 85 (26.56) | 26 (12.56) | 59 (52.21) | <0.001 |
| Other treatment in hospital, n (%) | ||||
| CRRT | 19 (5.94) | 4 (1.93) | 15 (13.27) | <0.001 |
| ECMO | 5 (1.56) | 1 (0.48) | 4 (3.54) | 0.035 |
| Complications, n (%) | ||||
| Death | 145 (45.31) | 55 (26.57) | 90 (79.65) | <0.001 |
| MODS | 137 (42.81) | 51 (24.64) | 86 (76.11) | <0.001 |
| ARDS | 114 (35.63) | 44 (21.26) | 70 (61.95) | <0.001 |
| DIC | 75 (23.44) | 19 (9.18) | 56 (49.56) | <0.001 |
| Secondary infection | 42 (13.13) | 14 (6.76) | 28 (24.78) | <0.001 |
| MACE | 80 (25.00) | 29 (14.01) | 51 (45.13) | <0.001 |
| AKI | 53 (16.56) | 11 (5.31) | 42 (37.17) | <0.001 |
| Spesis shock | 52 (16.25) | 20 (9.66) | 32 (28.32) | <0.001 |
| Myelosuppression | 79 (24.69) | 24 (11.59) | 55 (48.67) | <0.001 |
| GIB | 14 (4.38) | 2 (0.97) | 12 (10.62) | <0.001 |
| Liver function impairment | 80 (25.00) | 35 (16.91) | 45 (39.82) | <0.001 |
The laboratory characteristics of the patients were summarized in Table 2 . Higher levels of white blood cells, neutrophil counts, monocyte counts, prothrombin time, D-dimer, hypersensitive cardiac troponin I, NT-proBNP, ALT, AST, globulin, triglyceride, creatine kinase, LDH, urea, creatinine, high-sensitivity C-reactive protein (Hs-CRP), ESR, ferritin, procalcitonin, IgA, IgG, IL-2R, IL-6, IL-8, IL-10, TNF-α and MuLBSTA score were found in high IL-6/LY group (P < 0.050, respectively), comparing to those in low IL-6/LY group. However, the value of lymphocyte, platelet, albumin, total cholesterol, HDL-C, LDL-C, C3 and C4 was lower in high IL-6/LY group compared with that in low IL-6/LY group (P < 0.050, respectively).
Table 2.
. Laboratory characteristics of study population.
| All patients (n = 320) | Low IL-6/LY group (n = 207) | High IL-6/LY group (n = 113) | P | |
|---|---|---|---|---|
| Blood routine index | ||||
| WBC, 109/L | 7.56 ± 0.27 | 5.99 ± 0.25 | 10.44 ± 0.53 | <0.001 |
| Neutrophil%, % | 75.95 ± 0.84 | 70.31 ± 1.04 | 86.30 ± 0.74 | <0.001 |
| Neutrophil, 109/L | 6.22 ± 0.28 | 4.55 ± 0.25 | 9.28 ± 0.52 | <0.001 |
| Lymphocyte%, % | 16.12 ± 0.64 | 20.44 ± 0.79 | 8.21 ± 0.56 | <0.001 |
| Lymphocyte, 109/L | 0.90 ± 0.04 | 1.04 ± 0.06 | 0.65 ± 0.03 | <0.001 |
| Monocyte%, % | 6.88 ± 0.22 | 7.83 ± 0.29 | 5.14 ± 0.29 | 0.252 |
| Monocyte, 109/L | 0.45 ± 0.02 | 0.43 ± 0.02 | 0.47 ± 0.03 | <0.001 |
| Hemoglobin, mg/dL | 124.73 ± 1.34 | 124.91 ± 1.59 | 124.41 ± 2.46 | 0.931 |
| Platelet, 109/L | 193.25 ± 5.09 | 204.74 ± 6.33 | 172.22 ± 8.23 | <0.001 |
| Coagulation function | ||||
| PT, sec | 15.34 ± 0.34 | 14.48 ± 0.21 | 16.95 ± 0.86 | <0.001 |
| APTT, sec | 42.29 ± 0.76 | 41.43 ± 0.60 | 43.57 ± 1.65 | 0.133 |
| D-dimer, ug/mL FEU | 4.85 ± 0.42 | 2.98 ± 0.40 | 8.39 ± 0.84 | <0.001 |
| Biochemical indexes | ||||
| CTnI, pg/ml | 616.36 ± 199.58 | 168.54 ± 97.51 | 1331.94 ± 487.36 | <0.001 |
| NT-proBNP, pg/ml | 2467.98 ± 521.93 | 1449.30 ± 531.33 | 4022.81 ± 1023.28 | <0.001 |
| ALT, u/L | 37.37 ± 3.56 | 31.75 ± 3.78 | 47.57 ± 7.21 | <0.001 |
| AST (u/L) | 48.30 ± 5.41 | 33.43 ± 1.91 | 75.27 ± 14.53 | <0.001 |
| Albumin, g/L | 33.30 ± 0.30 | 34.83 ± 0.33 | 30.51 ± 0.50 | <0.001 |
| Globulin, g/L | 34.17 ± 0.36 | 33.41 ± 0.45 | 35.56 ± 0.58 | <0.001 |
| TC, mmol/l | 3.57 ± 0.05 | 3.66 ± 0.06 | 3.42 ± 0.08 | 0.008 |
| TG, mmol/L | 1.64 ± 0.07 | 1.51 ± 0.09 | 1.80 ± 0.12 | 0.001 |
| HDL-C, mmol/l | 0.85 ± 0.02 | 0.95 ± 0.03 | 0.75 ± 0.03 | <0.001 |
| LDL-C, mmol/l | 2.18 ± 0.06 | 2.30 ± 0.07 | 2.05 ± 0.11 | 0.014 |
| CK, u/L | 254.18 ± 37.96 | 147.14 ± 18.86 | 382.39 ± 78.34 | <0.001 |
| LDH, u/L | 427.50 ± 16.44 | 334.58 ± 13.65 | 597.58 ± 33.86 | <0.001 |
| Urea, mmol/L | 7.72 ± 0.45 | 5.62 ± 0.31 | 11.55 ± 1.04 | <0.001 |
| Creatinine, mmol/L | 95.75 ± 5.04 | 89.05 ± 6.76 | 107.96 ± 6.98 | <0.001 |
| Immuno-inflammatory indices | ||||
| Hs-CRP, mg/L | 76.95 ± 4.01 | 52.85 ± 3.93 | 119.18 ± 7.05 | <0.001 |
| ESR, mm/H | 38.93 ± 1.58 | 35.39 ± 1.85 | 45.02 ± 2.78 | 0.003 |
| Ferritin, ug/L | 1483.87 ± 188.08 | 854.89 ± 87.86 | 2366.85 ± 421.14 | <0.001 |
| PCT, ng/mL | 1.41 ± 0.48 | 0.23 ± 0.05 | 3.44 ± 1.28 | <0.001 |
| Ig A, g/L | 2.40 ± 0.09 | 2.20 ± 0.10 | 2.61 ± 0.14 | 0.017 |
| Ig G, g/L | 12.43 ± 0.29 | 11.71 ± 0.35 | 13.21 ± 0.47 | 0.010 |
| Ig M, g/L | 1.06 ± 0.04 | 1.09 ± 0.06 | 1.03 ± 0.07 | 0.214 |
| Alexin C3, g/L | 0.83 ± 0.02 | 0.87 ± 0.02 | 0.79 ± 0.03 | 0.007 |
| Alexin C4, g/L | 0.24 ± 0.01 | 0.25 ± 0.01 | 0.22 ± 0.01 | 0.014 |
| IL-1β, pg/ml | 5.93 ± 0.26 | 5.67 ± 0.23 | 6.25 ± 0.51 | 0.890 |
| IL-2R, pg/ml | 1008.15 ± 51.85 | 716.46 ± 59.66 | 1356.64 ± 77.04 | <0.001 |
| IL-6, pg/ml | 113.32 ± 31.35 | 51.18 ± 36.95 | 187.55 ± 52.10 | <0.001 |
| IL-8, pg/ml | 68.94 ± 30.36 | 21.64 ± 3.83 | 125.45 ± 66.25 | <0.001 |
| IL-10, pg/ml | 12.44 ± 1.25 | 7.92 ± 0.67 | 17.83 ± 2.55 | <0.001 |
| TNF-α, pg/ml | 11.70 ± 0.63 | 9.13 ± 0.63 | 14.76 ± 1.10 | <0.001 |
| MuLBSTA score | 7.88 ± 0.21 | 7.46 ± 0.25 | 8.65 ± 0.37 | 0.024 |
Mean ± SEM and n (%) are reported for continuous and categorical variables, respectively. Abbreviations in Table 1: standard error of the mean (SEM), not available (NA),interleukin-6 to lymphocyte ratio (IL-6/LY), coronary heart disease (CHD), glucocorticoids (GCS), noninvasive mechanical ventilation (NMV), invasive mechanical ventilation (IMV), continuous renal replacement therapy (CRRT), extracorporeal membrane oxygenation (ECMO), multiple organ dysfunction syndrome (MODS), acute respiratory distress syndrome (ARDS), disseminated intravascular coagulation (DIC), major adverse cardiac events (MACE), acute kidney injury (AKI), gastrointestinal bleeding (GIB).
Abbreviations in Table 2: white blood cells count (WBC), prothrombin time (PT), activated partial thromboplastin time (APTT), Hypersensitive cardiac troponin I (CTnI), N-terminal pro brain natriuretic peptide (NT-proBNP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), creatine kinase (CK), lactate dehydrogenase (LDH), high-sensitivity C-reactive protein (Hs-CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT), immunoglobulin A (Ig A), immunoglobulin G (Ig G), immunoglobulin M (Ig M), interleukin-1β (IL-1β), interleukin-2 receptor (IL-2R), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), tumor necrosis factor α(TNF-α).
3.2. Prognostic factors for clinical outcomes
The following categorical variables were entered in a forward stepwise COX regression analysis: age, sex, smoking history, coronary heart disease, hypertension, diabetes, the days of illness onset, blood routine index, coagulation dysfunction indicators, serum biochemical indices, inflammatory markers and cytokines. Elevated IL-6/LY (hazard ratio [HR], 3.404; 95% confidence interval [CI], 1.090–10.633, P = 0.035) along with older age (HR, 1.034; 95%CI, 1.004–1.064, P = 0.025), lower LY% (HR, 0.897; 95%CI, 0.846–0.952, P < 0.001), lower hemoglobin (HR, 0.980; 95%CI, 0.968–0.992, P = 0.002) and higher ferritin (HR, 1.000; 95%CI, 0.999–1.001, P = 0.007) was associated with greater risk of in-hospital mortality according to multivariate COX regression analysis. In addition, compared with other inflammatory markers (including NLR, Hs-CRP, procalcitonin and interleukins; all P > 0.05), IL-6/LY showed more significant predictability on in-hospital mortality (Table 3 ). Similarly, diabetes (HR, 3.353; 95%CI, 1.640–6.856, P = 0.001), lower hemoglobin (HR, 0.984; 95%CI, 0.972–0.997, P = 0.016), prolonged prothrombin time (HR, 1.050; 95%CI, 1.022–1.079, P < 0.001), higher NT-ProBNP level (HR, 2.424; 95%CI, 1.660–3.541, P < 0.001) and elevated IL-6/LY (HR,4.143; 95%CI, 1.321–12.986, P = 0.015) was associated with higher likelihood of MODS development. As a matter of fact, among the numerous inflammatory factors considered in the computational model, IL-6/LY had the best predictive ability (Table 4 ).
Table 3.
. Predictors of in-hospital mortality in univariable and multivariable Cox regression analyses.
| Variables | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.046 | 1.033–1.060 | <0.001 | 1.034 | 1.004–1.064 | 0.025 |
| Gender (male) | 1.617 | 1.148–2.279 | 0.006 | |||
| Smoking | 2.445 | 1.669–3.583 | <0.001 | |||
| Comorbidity | ||||||
| CHD | 1.771 | 1.171–2.679 | 0.007 | |||
| Hypertension | 1.999 | 1.440–2.776 | <0.001 | |||
| Diabetes | 2.620 | 1.889–3.634 | <0.001 | |||
| Time of illness onset | 1.039 | 1.012–1.066 | 0.004 | |||
| Blood routine index | ||||||
| WBC | 1.133 | 1.108–1.159 | <0.001 | |||
| Neutrophil | 1.138 | 1.114–1.164 | <0.001 | |||
| Neutrophil% | 1.095 | 1.075–1.115 | <0.001 | |||
| Lymphocyte | 0.121 | 0.070–0.207 | <0.001 | |||
| Lymphocyte% | 0.881 | 0.859–0.904 | <0.001 | 0.897 | 0.846–0.952 | <0.001 |
| Monocyte | 1.538 | 1.042–2.270 | 0.030 | |||
| Monocyte% | 0.826 | 0.783–0.871 | <0.001 | |||
| Hemoglobin | 0.993 | 0.987–1.000 | 0.038 | 0.980 | 0.968–0.992 | 0.002 |
| Platelet | 0.994 | 0.992–0.996 | <0.001 | |||
| Coagulation function | ||||||
| PT | 1.036 | 1.024–1.048 | <0.001 | |||
| D-Dimmer | 1.088 | 1.068–1.108 | <0.001 | |||
| Biochemical indexes | ||||||
| CTnI | 1.369 | 1.164–1.611 | <0.001 | |||
| NT-proBNP | 2.319 | 1.964–2.739 | <0.001 | |||
| ALT | 1.003 | 1.001–1.005 | <0.001 | |||
| AST | 1.002 | 1.001–1.003 | <0.001 | |||
| Albumin | 0.873 | 0.846–0.900 | <0.001 | |||
| Globulin | 1.018 | 0.998–1.039 | 0.079 | |||
| HDL-C | 0.087 | 0.037–0.208 | <0.001 | |||
| LDL-C | 0.571 | 0.419–0.778 | <0.001 | |||
| CK | 1.000 | 1.000–1.001 | <0.001 | |||
| LDH | 1.002 | 1.001–1.002 | <0.001 | |||
| Creatinine | 1.002 | 1.001–1.003 | <0.001 | |||
| Immuno-inflammatory indices | ||||||
| IL-6/LY | 8.889 | 5.282–14.961 | <0.001 | 3.404 | 1.090–10.633 | 0.035 |
| NLR | 1.041 | 1.034–1.048 | <0.001 | |||
| Hs-CRP | 1.009 | 1.007–1.011 | <0.001 | |||
| ESR | 1.006 | 0.999–1.013 | 0.122 | |||
| Ferritin | 5.521 | 3.588–8.497 | <0.001 | 1.000 | 0.999–1.001 | 0.007 |
| PCT | 1.028 | 1.015–1.040 | <0.001 | |||
| Alexin C3 | 0.067 | 0.023–0.190 | <0.001 | |||
| Alexin C4 | 0.003 | 0.001–0.049 | <0.001 | |||
| IL-2R | 1.000 | 1.000–1.001 | <0.001 | |||
| IL-6 | 1.001 | 1.000–1.001 | <0.001 | |||
| IL-8 | 1.000 | 1.000–1.001 | 0.006 | |||
| IL-10 | 1.012 | 1.008–1.017 | <0.001 | |||
| TNF-α | 1.044 | 1.032–1.056 | <0.001 | |||
Hazard ratio (HR), confidence interval (CI), coronary heart disease (CHD), white blood cell count (WBC), prothrombin time (PT), Hypersensitive cardiac troponin I (CTnI), N-terminal pro-brain natriuretic peptide (NT-proBNP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), lactate dehydrogenase (LDH), creatine kinase (CK), IL-6 to lymphocyte ratio (IL-6/LY), neutrophil to lymphocyte ratio (NLR), high-sensitivity C-reactive protein (Hs-CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT), interleukin-2 receptor (IL-2R), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), tumor necrosis factor α(TNF-α).
Table 4.
. Predictors of MODS development in univariable and multivariable Cox regression analyses.
| Variables | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.047 | 1.033–1.062 | <0.001 | |||
| Gender (male) | 1.640 | 1.152–2.335 | 0.006 | |||
| Smoking | 2.630 | 1.788–3.867 | <0.001 | |||
| Comorbidity | ||||||
| CHD | 1.752 | 1.141–2.691 | 0.010 | |||
| Hypertension | 1.990 | 1.420–2.790 | <0.001 | |||
| Diabetes | 2.816 | 2.010–3.945 | <0.001 | 3.353 | 1.640–6.856 | 0.001 |
| Time of illness onset | 1.033 | 1.005–1.062 | 0.020 | |||
| Blood routine index | ||||||
| WBC | 1.135 | 1.109–1.161 | <0.001 | |||
| Neutrophil | 1.140 | 1.115–1.167 | <0.001 | |||
| Neutrophil% | 1.096 | 1.076–1.116 | <0.001 | |||
| Lymphocyte | 0.103 | 0.058–0.183 | <0.001 | |||
| Lymphocyte% | 0.879 | 0.856–0.902 | <0.001 | |||
| Monocyte | 1.615 | 1.101–2.369 | 0.014 | |||
| Monocyte% | 0.830 | 0.786–0.876 | <0.001 | |||
| Hemoglobin | 0.993 | 0.987–1.000 | 0.058 | 0.984 | 0.972–0.997 | 0.016 |
| Platelet | 0.994 | 0.992–0.996 | <0.001 | |||
| Coagulation function | ||||||
| PT | 1.037 | 1.024–1.049 | <0.001 | 1.050 | 1.022–1.079 | <0.001 |
| D-Dimmer | 1.087 | 1.067–1.108 | <0.001 | |||
| Biochemical indexes | ||||||
| CTnI | 1.425 | 1.204–1.688 | <0.001 | |||
| NT-proBNP | 2.421 | 2.035–2.881 | <0.001 | 2.424 | 1.660–3.541 | <0.001 |
| ALT | 1.003 | 1.002–1.005 | <0.001 | |||
| AST | 1.002 | 1.001–1.003 | <0.001 | |||
| Albumin | 0.876 | 0.848–0.905 | <0.001 | |||
| Globulin | 1.016 | 0.994–1.038 | 0.153 | |||
| HDL-C | 0.091 | 0.037–0.221 | <0.001 | |||
| LDL-C | 0.535 | 0.388–0.738 | <0.001 | |||
| CK | 1.000 | 1.000–1.001 | <0.001 | |||
| LDH | 1.002 | 1.002–1.003 | <0.001 | |||
| Creatinine | 1.002 | 1.001–1.003 | <0.001 | |||
| Immuno-inflammatory indices | ||||||
| IL-6/LY | 8.487 | 5.032–14.314 | <0.001 | 4.143 | 1.321–12.986 | 0.015 |
| NLR | 1.042 | 1.035–1.050 | <0.001 | |||
| Hs-CRP | 1.009 | 1.007–1.011 | <0.001 | |||
| ESR | 1.002 | 0.995–1.010 | 0.514 | |||
| Ferritin | 5.733 | 3.674–8.946 | <0.001 | |||
| PCT | 1.029 | 1.016–1.041 | <0.001 | |||
| Alexin C3 | 0.074 | 0.025–0.221 | <0.001 | |||
| Alexin C4 | 0.004 | 0.001–0.073 | <0.001 | |||
| IL-2R | 1.000 | 1.000–1.001 | <0.001 | |||
| IL-6 | 1.001 | 1.000–1.001 | <0.001 | |||
| IL-8 | 1.000 | 1.000–1.001 | 0.005 | |||
| IL-10 | 1.013 | 1.008–1.017 | <0.001 | |||
| TNF-α | 1.046 | 1.034–1.058 | <0.001 | |||
Hazard ratio (HR), confidence interval (CI), multiple organ dysfunction syndrome (MODS), coronary heart disease (CHD), white blood cell count (WBC), prothrombin time (PT), Hypersensitive cardiac troponin I (CTnI), N-terminal pro brain natriuretic peptide (NT-proBNP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), lactate dehydrogenase (LDH), creatine kinase (CK), IL-6 to lymphocyte ratio (IL-6/LY), neutrophil to lymphocyte ratio (NLR), high-sensitivity C-reactive protein (Hs-CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT), interleukin-2 receptor (IL-2R), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), tumor necrosis factor α(TNF-α).
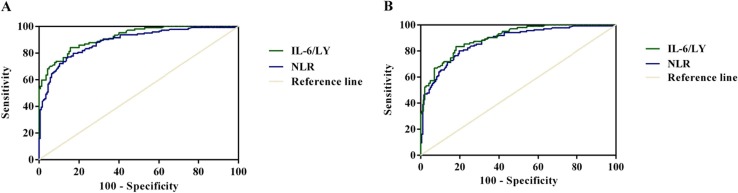
As shown in Fig. 1 A, compared with the ROC analysis of NLR (AUC = 0.882, 95% CI, 0.844–0.920, P < 0.001; sensitivity = 76.60%, specificity = 85.70%), IL-6/LY had better performance with in-hospital mortality (AUC = 0.919, 95% CI, 0.887–0.951, P < 0.001; sensitivity = 84.10%, specificity = 84.30%). As illustrated in Fig. 1B, such comparisons with NLR (AUC = 0.869, 95% CI, 0.829–0.909, P < 0.001; sensitivity = 79.60%, specificity = 80.30%) suggested that the IL-6/LY had better prediction power of MODS development (AUC = 0.900, 95% CI, 0.863–0.937, P < 0.001; sensitivity = 83.50%, specificity = 81.90%).
Fig. 1.
The effectiveness of IL-6/LY and NLR predictor for poor prognosis of COVID-19 by ROC curves. (A) ROC curves of IL-6/LY and NLR for in-hospital mortality (AUC = 0.919, 95% CI, 0.887–0.951, P < 0.001; AUC = 0.882, 95% CI, 0.844–0.920, P < 0.001). (B) ROC curves of IL-6/LY and NLR for MODS development (AUC = 0.900, 0.863–0.937, P < 0.001; AUC = 0.869, 95% CI, 0.829–0.909, P < 0.001). IL-6/LY: interleukin-6 to lymphocyte ratio; NLR: neutrophil to lymphocyte ratio; COVID-19: coronavirus disease 2019; ROC: receiver operating characteristic curve; AUC: area under the curve; CI: confidence interval; MODS: multiple organ dysfunction syndrome.
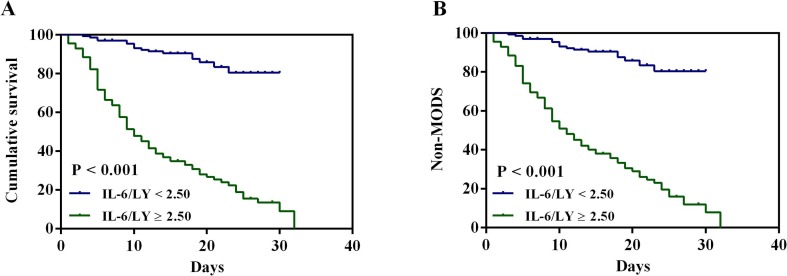
The Kaplan-Meier survival curves for high-value and low-value groups were shown in Fig. 2 . It was suggested that the high-value group (IL-6/LY ≥ 2.50) has a greater risk in poor prognosis (P < 0.001, respectively).
Fig. 2.
Kaplan-Meier cumulative survival curves for COVID-19 patients with in-hospital mortality and developed MODS according to the cutoff value of IL-6/LY. (A) Kaplan-Meier survival curves of in-hospital mortality (log-rank test: P < 0.001). (B) Kaplan-Meier survival curves of developed MODS (log-rank test: P < 0.001). COVID-19: coronavirus disease 2019; MODS: multiple organ dysfunction syndrome; IL-6/LY: interleukin-6 to lymphocyte ratio.
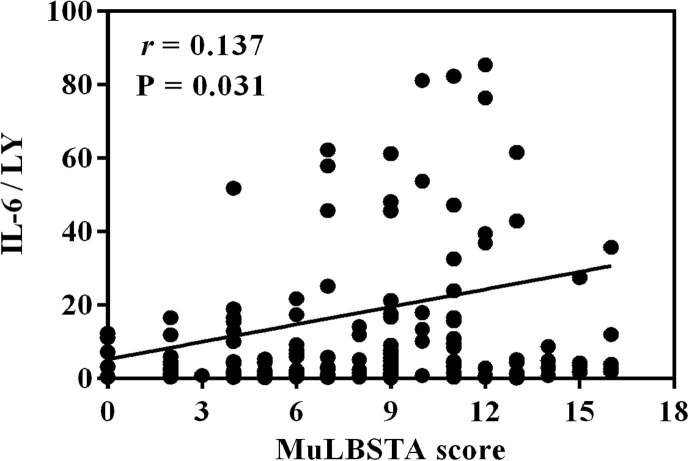
Interestingly, we also found that IL-6/LY was positively correlated with the MuLBSTA score which was used to predict the mortality risk of viral pneumonia (r = 0.137, P = 0.031; Fig. 3 ).
Fig. 3.
The correlation between IL-6/LY and MuLBSTA score (r = 0.137, P = 0.031). IL-6/LY: interleukin-6 to lymphocyte ratio.
3.3. Dynamic changes of LY%, LY, IL-6 and IL-6/LY
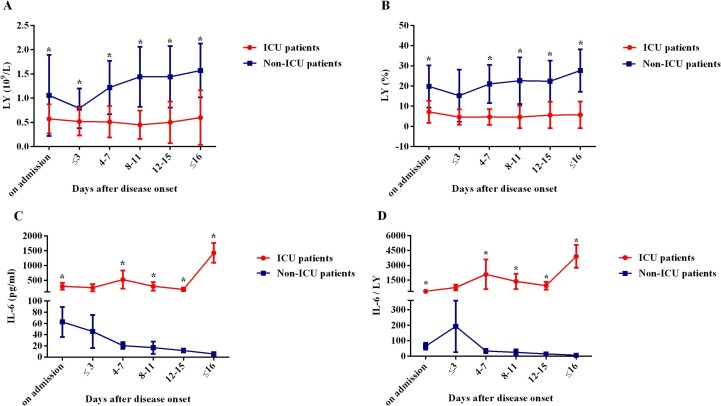
Among our population, there were 105 intensive care patients and 215 non-intensive care patients. As the Fig. 4 A–B showed, the blood LY% and LY were much lower in intensive care unit (ICU) group than those in non-ICU group on admission, and the difference was statistically significant (P < 0.001, respectively). Besides, the LY% and LY gradually declined during the first three days of hospitalization in non-ICU group, while since then, they increasingly rose to normal levels upon discharge (P < 0.001, respectively). In contrast, the above trend did not appear in ICU patients and both the LY% and LY still remained at a low level until discharge.
Fig. 4.
Kinetic analysis of LY, LY%, IL-6 and IL-6/LY in COVID-19 patients. The counts of LY (A), LY% (B), IL-6 (C), and IL-6/LY (D) in the peripheral blood of non-ICU COVID-19 patients (blue line) and ICU COVID-19 patients (red line) were analyzed at different time points during the time of hospitalization. Error bars, mean ± SE; *P < 0.050. LY: lymphocyte; LY%: lymphocyte percentage; IL-6: interleukin-6; IL-6/LY: interleukin-6 to lymphocyte ratio; ICU: intensive care unit; COVID-19: coronavirus disease 2019.
In addition, significantly increased in IL-6 level and IL-6/LY were observed in ICU group compared with those in non-ICU group at several time points except for the first three days in hospital (the day of at admission, four to seven days after admission, eight to eleven days after admission, twelve to fifteen days after admission, before discharge, all P < 0.001). The difference between ICU group and non-ICU group was significant at the time point of 4–7 days and became even greater before discharge (Fig. 4C–D).
4. Discussion
In current study, we found that patients with elevated IL-6/LY (≥2.50) was the independent risk factor for in-hospital mortality and the development of MODS in severe COVID-19 patients. Besides, our results showed that the age along with LY% were also the predictors of in-hospital mortality, and the diabetes, prothrombin time, and NT-ProBNP were significantly associated with higher likelihood of MODS development. Meanwhile, the dynamic changes of the inflammatory biomarkers could reflect the clinical severity in patients with COVID-19.
A series of evidences showed that the uncontrolled inflammatory response and immunity dysregulation were the prominent feature of critical ill COVID-19 patients [2], [15]. In addition, several other researches also proved that some seriously ill COVID-19 patients had increased cytokine profile similar to cytokine storm in severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [16], [17], [18]. Cytokine storm stems from the coronavirus infected place and then spread throughout the organism by mass of inflammatory cells infiltration which in turn lead to acute lung injury, ARDS and death [17], [18]. It was reported that higher levels of inflammatory parameters, especially IL-6 could be shown in severe COVID-19 patients, which was consistent with previous studies [8], [19], [20]. IL-6 has multiple effects in regulating inflammation. Cifaldi et al. has reported that elevated IL-6 level was linked to impaired cytolytic function by overstimulating the immune system and finally might result in multiple organ failure [21].
Furthermore, significantly reduced counts of T cells have been found in severe COVID-19 patients in recent studies [22], [23]. Previous body biopsies reported that the COVID-19 patients’ secondary lymphoid tissues had been destroyedvery and very few lymphocytes infiltrated in the alveolar of damaged lung tissue [10], [16], [24]. A similar decrease in lymphocyte counts and the subset of T cells could be seen in SARS patients according to previous investigations [25], [26]. However, the exact mechanism of lymphopenia in severe COVID-19 patients remains still unclear. Zhang et al. speculated that lymphocytes were directly invaded by virus infection or indirectly damaged by cytokine storm which induced by immune response [16]. And a substantial decrease in lymphocytes revealed that the immune cells may be consumed by the viruses and the body’s cellular immune function may be restrained [27], [28]. It suggested that COVID-19 infection can lead to immune dysfunction through affecting the subsets of T cells [29].
Through analyzing the immune cells and inflammatory cytokines in the severe COVID-19 patients, Zhou et al. noted that the Th1 cells (GM-CSF+IFN-γ+) and inflammatory monocytes (CD14+CD16+ with high expression of IL-6) existed particularly in ICU patients [30]. Therefore, lots of these pathogenic T cells and inflammatory monocytes may get into the pulmonary circulation and arouse inflammatory storm which probably prevents alveolar gas exchange and contributing to the high mortality of severe COVID-19 patients [31]. Given their weight during the course of COVID-19, our study developed a novel biomarker, named as IL-6/LY, in order to estimate condition, evaluate prognosis and conduct risk stratification. As we know, this was the first research for exploring the effect of IL-6/LY on predicting the clinical outcomes for COVID-19 cases.
According to existing research results, we proposed potential mechanisms of high IL-6/LY was resulted from the increased IL-6 and the decreased lymphocyte counts. Recent studies' results would give some explanations. Wan S et al. found that patients with severe COVID-19 patients were more likely to have higher IL-6 levels than those mild COVID-19 cases [23]. It was also showed that lymphopenia was one of the important features of COVID-19 infection and which was also the common ground in most severe patients [1]. As shown in another study, IL-6 could suppress the T cell activation, which may explain the decrease of lymphocyte [32]. Furthermore, according to the study by Jing Liu et al., they revealed that the T cell counts were negatively associated with the examined cytokine levels (such as IL-6) [33]. Accordingly, the balance of IL-6 and lymphocyte played a crucial role in the immunoregulation of patients with COVID-19. When evaluating patients infected with COVID-19, inflammatory biomarkers might be useful to help clinicians start treatment and monitor closely. Compared to other biomarkers, people were often hesitant to use a inflammatory marker alone because it might be influenced by many factors. While IL-6/LY, consisted of two inflammatory components (IL-6 and lymphocyte), might reflect the immune dysregulation in COVID-19 patients more realistically and comprehensively. Still not only such, IL-6/LY could be analyzed rapidly and accurately in the light of the blood test at the time of admission. Our data suggested that IL-6/LY could serve as an indicator of poor prognosis of COVID-19. The dynamic changes of IL-6/LY in patients revealed that the magnitude of the immune response dysregulation was related to the severity of COVID-19 patients. Herby, the physicians could identify the specific subpopulations of COVID-19 patients who were at greater risk for unfavorable outcomes at an early stage.
What’s more, in our study, the relationship between other immunoinflammatory parameters and poor prognosis were also evaluated. We found that individual variable (such as WBC, Hs-CRP, procalcitonin, ferritin and interleukins) had an influence on the occurrence of clinical outcomes partly. However, after adjustment for potential confounders, the results statistically supported the conclusions that the incidence of high IL-6/LY was significantly correlated with the poor prognosis of the disease.
Not only that, older age and comorbidities were proved to be in connection with severe COVID-19 [34]. Weina Guo et al suggested that COVID-19 patients with diabetes had a poor prognosis and diabetes was proposed as a risk factor for the progression of COVID-19 cases [35], [36]. Clinically, infected by SARS-CoV-2 caused multiple system organ failure, such as the hematologic system [37], [38], [39]. Previous studies showed that prothrombin time was positively correlated with 28-day mortality [40]. Besides, a retrospective study showed that NT-proBNP was an independent risk factor for in-hospital death in patients with severe COVID-19 [41]. In fact, these results were in consistent with our findings.
We have to acknowledge that there were some limitations in this study. Firstly, the retrospective design of the study set a limit to the convincement of our study. Due to the nature of our study, the results must be explained with caution, given the possibility of confounders. Secondly, because of the objective conditions, the patients were not followed up outside of hospital. And we did not study the relationship between IL-6/LY and the long-term outcomes. Thirdly, our sample size was small. Therefore, prospective clinical studies with larger population are needed.
5. Conclusion
In conclusion, the elevated IL-6/LY was an independent risk factor for in-hospital mortality and the development of MODS in severe COVID-19 patients. The dynamic change of IL-6/LY was associated with the severity of the disease, which could predict the rapid progression and bad prognosis of COVID-19. Thus, more intensive attention should be paid to the immune dysregulation and systemic inflammatory response in severe cases with COVID-19.
6. Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Boyi Yang: Project administration, Writing - review & editing. Xiaoyan Chang: Methodology, Resources. Jiabao Huang: Investigation, Data curation. Wen Pan: Validation. Zhilong Si: Formal analysis, Software. Cuntai Zhang: Supervision. Hong Li: Conceptualization, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We acknowledge the volunteers who participant our study in Tongji hospital as well as those who participant in our research design, date collection, date analysis and manuscript writing or reviewing.
References
- 1.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarzi-Puttini P., et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38(2):337–342. [PubMed] [Google Scholar]
- 3.O. World Health, Coronavirus disease (COVID-19): Situation Report128. 2019:2020. [Google Scholar]
- 4.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan J.F.-W., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh M.D., et al. Viral load kinetics of MERS coronavirus infection. N. Engl. J. Med. 2016;375(13):1303–1305. doi: 10.1056/NEJMc1511695. [DOI] [PubMed] [Google Scholar]
- 8.Chen N., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin C., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020:ciaa248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X., et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 2020:ciaa449. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiappelli F., et al. COVID-19 Immunopathology and Immunotherapy. Bioinformation. 2020;16(3):219–222. doi: 10.6026/97320630016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O. World Health . World Health Organization; Geneva: 2020. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease is Suspected: Interim Guidance. [Google Scholar]
- 14.Ramirez M. Multiple organ dysfunction syndrome. Curr. Probl. Pediatr. Adolesc. Health Care. 2013;43(10):273–277. doi: 10.1016/j.cppeds.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Jamilloux Y., et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020:102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chousterman B.G., et al. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020:e200994. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cifaldi L., et al. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 2015;67(11):3037–3046. doi: 10.1002/art.39295. [DOI] [PubMed] [Google Scholar]
- 22.Wang F., et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.S. Wan, et al., Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP), medRxiv (2020) 2020.02.10.20021832.
- 24.Xu Z., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li T., et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J. Infect. Dis. 2004;189(4):648–651. doi: 10.1086/381535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cecere T.E., et al. Regulatory T cells in arterivirus and coronavirus infections: do they protect against disease or enhance it? Viruses. 2012;4(5):833–846. doi: 10.3390/v4050833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y.-H., et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J. Infect. 2020;80(4):394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J.J., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 29.Tufan A., et al. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020;50(SI-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yonggang Z., et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl. Sci. Rev. 2020 doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu B., et al. Why tocilizumab could be an effective treatment for severe COVID-19? J. Transl. Med. 2020;18(1):164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan W.J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busetto L., et al. Obesity and COVID-19: an Italian snapshot. Obesity (Silver Spring, Md.) 2020 doi: 10.1002/oby.22918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo W., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bikdeli B., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020;S0735-1097(20):35008–35017. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang L., et al. Coronavirus disease 2019: coronaviruses and blood safety. Transfus. Med. Rev. 2020 doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang N., et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao L., et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir. Res. 2020;21(1):83. doi: 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]