Abstract
Objective
A previous study revealed a preliminary trend towards higher in hospital mortality in patients admitted as an emergency with acute stroke during the COVID-19 pandemic in Germany. The current study aimed to further examine the possible impact of a confirmed SARS-CoV-2 infection on in hospital mortality.
Methods
This was a retrospective analysis of health insurance claims data from the second largest insurance fund in Germany, BARMER. Patients hospitalised for ST elevation (STEMI) and non-ST elevation (NSTEMI) myocardial infarction, acute limb ischaemia (ALI), aortic rupture, acute stroke, or transient ischaemic attack (TIA) between 1 January 2017, and 31 October 2020, were included. Admission rates per 10 000 insured and mortality were compared between March − June 2017 – 2019 (pre-COVID) and March − June 2020 (COVID). Mortality rates were determined by the occurrence of a confirmed SARS-CoV-2 infection.
Results
A total of 316 718 hospitalisations were included (48.7% female, mean 72.5 years), and 21 191 (6.7%, 95% CI 6.6% – 6.8%) deaths occurred. In hospital mortality increased during the COVID-19 pandemic when compared with the three previous years for patients with acute stroke from 8.3% (95% CI 8.0 – 8.5) to 9.6% (95% CI 9.1 – 10.2), while no statistically significant changes were observed for STEMI, NSTEMI, ALI, aortic rupture, and TIA. When comparing patients with confirmed SARS-CoV-2 infection (2.4%, 95% CI 2.3 – 2.5) vs. non-infected patients, a higher in hospital mortality was observed for acute stroke (12.4% vs. 9.0%), ALI (14.3% vs. 5.0%), and TIA (2.7% vs. 0.3%), while no statistically significant differences were observed for STEMI, NSTEMI, and aortic rupture.
Conclusion
This retrospective analysis of claims data has provided hints of an association between the COVID-19 pandemic and increased in hospital mortality in patients with acute stroke. Furthermore, confirmed SARS-CoV-2 infection was associated with increased mortality in patients with stroke, TIA, and ALI. Future studies are urgently needed to better understand the underlying mechanism and relationship between the new coronavirus and acute stroke.
Keywords: COVID-19, Emergencies, Health services research, Myocardial infarction, Pandemics, Stroke
What this paper adds.
This large scale analysis of nationwide and unselected health insurance claims data covering 316 718 hospitalisations for cardiovascular and cerebrovascular emergencies between January 2017 and October 2020 in Germany revealed an association between the COVID-19 pandemic and acute stroke and confirmed SARS-CoV-2 infection (for acute stroke, acute limb ischaemia, transient ischaemic attack) led to increased in hospital mortality in patients admitted with cardiovascular and cerebrovascular emergencies. These striking results add to the extremely limited knowledge base concerning a possible relationship between COVID-19 and stroke. Future studies are necessary to determine underlying causal factors.
Introduction
The rapid spread of a new coronavirus (SARS-CoV-2) threw the global healthcare system into turmoil.1 Beginning with the implementation of infection control measures during the first wave of the pandemic, the scientific community aimed to better understand possible collateral damage in patients with emergency conditions.2 In a previous study using health insurance claims data, the present authors’ observed declining admission rates for cardiovascular and cerebrovascular emergencies in Germany.3 These findings confirmed the growing knowledge base arising of real world data across the globe.4, 5, 6, 7, 8 Available outcome data in that previous study indicated a trend towards increasing in hospital mortality for acute stroke.3 This observation was in line with previous reports suggesting a particular impact of the pandemic on patients suffering from neurovascular conditions.4 , 9, 10, 11, 12, 13, 14
Only a few months later, the world faced a second pandemic wave, and numerous questions remained unanswered ahead of this challenge.15 , 16 Because of the complex multimorbidity of patients with cardiovascular and cerebrovascular emergencies, these patients are known to be at particular risk of a SARS-CoV-2 infection and unfavourable outcomes.17
The current study used large, updated health insurance claims data and additional information on confirmed SARS-CoV-2 infection status to further illuminate the impact of COVID-19 on in hospital mortality. The research hypothesis was that patients with cardiovascular and cerebrovascular emergencies experience worse direct and indirect mortality during the COVID-19 pandemic.
Methods
Study design
This was a retrospective analysis of routinely collected health insurance claims data. The details of the current analysis have been published previously.3 In short, based on their primary diagnosis, all patients having inpatient treatment for cardiovascular and cerebrovascular emergencies between 1 January 2017 and 30 October 2020 were included. These included 1A) ST segment elevation myocardial infarction (STEMI), 1B) non-ST segment elevation myocardial infarction (NSTEMI), 2) acute limb ischaemia (ALI),18 3) acute aortic rupture, 4A) acute stroke, and 4B) transient ischaemic attack (TIA) (for detailed coding, see Supplementary Table S1).
The available study data were compared between the three previous years (1 January 2017 until 31 December 2019) and the time after the first disease outbreak news from the World Health Organization (WHO) had been issued (1 January 2020 until 31 October 2020). To address seasonal effects in terms of in hospital mortality, data collected during the peak of the pandemic (COVID: March through June 2020) were additionally compared with a control period in the previous three years (pre-COVID: March through June 2017 – 2019). For the comparison of in hospital mortality by SARS-CoV-2 infection status, corresponding data concerning confirmed infection status from 1 March 2020 and 31 October 2020 were available. The primary study endpoint was in hospital mortality among the patients admitted with cardiovascular and cerebrovascular emergencies by study period and confirmed SARS-CoV-2 infection status.
Sample and database
The longitudinal data of Germany’s second largest insurance fund, BARMER, includes the outpatient and inpatient medical care provided to up to 9 million (from 2008 to 2020) German citizens (10.8% of Germany’s population) involving more than 24 million hospitalisations between 1 January 2008 and 30 June 2020. Details concerning the database have been described in various previous studies.3 , 19, 20, 21
Study variables
The diagnoses and comorbidities routinely collected in health insurance claims data follow the commonly accepted international standard for reporting diseases and health conditions using World Health Organization (WHO) International Classification of Diseases in its 10th revision of the German Modification (ICD-10-GM) and Operations and Procedures Codes (OPS) as a German adaptation of the International Classification of Procedures in Medicine (ICPM) by WHO. The code U07.1 was used to identify patients with confirmed (laboratory test, e.g. with polymerase chain reaction or similar test) SARS-CoV-2 infection as per request of the WHO since March 2020.
Ethical considerations
The present study complied with the Helsinki Declaration of 2013. Several review boards and consensus guidelines determined that use of anonymised data from claims or national statistics retrospectively is not human subject research as de-identified datasets are used.22 , 23
Statistical analysis
Baseline characteristics of the patients were summarised with means and standard deviation for age and with percentages for discrete variables. A comparison of 95% confidence intervals (CI) and two proportion z test was used to test for differences. Rates per 10 000 insured were calculated on a monthly base. As sensitivity analysis, both complete data from 2020 and data concerning only the four months March − June 2017 – 2019 vs. 2020 were used.
The in hospital percentage mortality was calculated as the proportion of deceased patients among all patients with an in hospital stay during the study period. The admission rate was calculated as number of admissions per 10 000 insured patients during the same time period.
Data processing was performed with software SAS version 9.04 (SAS Institute, NC, USA) and SPSS version 25 (IBM Corporation, NY, USA). Visualisation was performed with software Adobe Illustrator version 24.1.2 (Adobe, CA, USA).
Results
Between 1 January 2017 and 31 October 2020, 316 718 hospitalisations were identified (mean age 72.5 years, 48.7% females, 95% CI 48.6% – 48.9%) for cardiovascular or cerebrovascular emergencies (monthly mean 6 885 patients). Among all hospitalisations, a total of 21 191 (6.7%, 95% CI 6.6% – 6.8%) deaths occurred. Between March and June 2017 – 2019, 84 106 hospitalisations were registered, and 25 024 hospitalisations were registered between March and June 2020.
Using data for the entire study period, the monthly hospital admission rate for cardiovascular or cerebrovascular emergencies was an average of 1.25 per 10 000 and decreased from 1.27 per 10 000 before 2020 to 1.15 per 10 000 in 2020.
Monthly hospital admissions for cardiovascular and cerebrovascular emergencies between 2017 – 2019 and 2020
When comparing the time period between 2017 – 2019 (pre-COVID-19) and 2020 (COVID-19), the hospital admission rates per 10 000 insured people were 0.72 – 0.66/10 000 (−9%) for STEMI, 1.59 – 1.48/10 000 (−8%) for NSTEMI, 0.49 – 0.45/10 000 (−9%) for ALI, 0.05 – 0.04/10 000 (−17%) for aortic rupture, 3.46 – 3.24/10 000 (−5%) for acute stroke, and 1.32 – 1.18/10 000 (−13%) for TIA (Table 1, Table 2 ).
Table 1.
Baseline characteristics of the cohort admitted to a legally endorsed German hospital between January 2017 and October 2020 for peripheral vascular emergencies
| ALI |
Aortic rupture |
Acute stroke |
TIA |
|||||
|---|---|---|---|---|---|---|---|---|
| 2017–2019 n = 16 312 | Jan–Oct 2020 n = 4 070 | 2017–2019 n = 1 662 | Jan–Oct 2020 n = 397 | 2017–2019 n = 114 831 | Jan–Oct 2020 n = 28 996 | 2017–2019 n = 43 758 | Jan–Oct 2020 n = 10 537 | |
| Mean no. of hospital cases per month | 453 | 407 | 46 | 40 | 3 190 | 2 900 | 1 216 | 1 054 |
| Females | 8 726 (53.5) | 2 193 (53.9) | 609 (36.7) | 151 (38.2) | 63 501 (55.3) | 16 092 (55.5) | 26 079 (59.6) | 6 248 (59.3) |
| Age – y | 72.6 ± 0.6 | 72.4 ± 0.7 | 74.3 ± 1.6 | 72.8 ± 2.9 | 74.8 ± 0.5 | 74.9 ± 0.7 | 72.9 ± 0.7 | 73.5 ± 0.6 |
| In hospital mortality | 969 (5.9) [5.6–6.3] | 209 (5.1) [4.5–5.9] | 711 (42.8) [40.4–45.2] | 154 (38.8) [34.0–43.8] | 9 738 (8.5) [8.3–8.6] | 2 665 (9.6)∗ [9.1–10.2] | 148 (0.3) [0.3–0.4] | 42 (0.4) [0.3–0.5] |
| Monthly mean admission rate per 10 000 insured | 0.49 | 0.45 | 0.05 | 0.04 | 3.46 | 3.24 | 1.32 | 1.18 |
| Hospital cases with SARS-CoV-2 test | N/A | 3 323 | N/A | 314 | N/A | 23 997 | N/A | 8 515 |
| Thereof confirmed SARS-CoV-2 infection | N/A | 63 (1.9) [1.5–2.4] | N/A | 7 (2.2) [0.9–4.5] | N/A | 588 (2.5) [2.3–2.7] | N/A | 150 (1.8) [1.5–2.1] |
Data are presented as n (%) [95% confidence interval] or mean ± standard deviation, unless stated otherwise. TIA = transient ischaemic attack; ALI = acute limb ischaemia; N/A = not available.
∗p < .001 for comparison of 95% confidence interval and two-proportion z test.
Table 2.
Baseline characteristics of the cohort admitted to a legally endorsed German hospital between January 2017 and October 2020 for cardiac emergencies
| STEMI |
NSTEMI |
|||
|---|---|---|---|---|
| 2017–2019 n = 24 078 | Jan–Oct 2020 n = 5 931 | 2017–2019 n = 52 910 | Jan–Oct 2020 n = 13 236 | |
| Mean no. of hospital cases per month | 669 | 593 | 1 470 | 1 324 |
| Females | 8 957 (37.2) | 2 212 (37.3) | 21 957 (41.5) | 5 598 (42.3) |
| Age – y | 67.8 ± 0.6 | 67.8 ± 0.8 | 73.1 ± 0.6 | 72.9 ± 0.6 |
| In hospital mortality | 2 996 (12.4) [12.0–12.9] | 723 (12.1) [11.4–13.1] | 3 195 (6.0) [5.8–6.2] | 728 (5.5) [5.1–5.9] |
| Monthly mean admission rate per 10 000 insured | 0.72 | 0.66 | 1.59 | 1.48 |
| Hospital cases with SARS-CoV-2 test - n | N/A | 4 882 | N/A | 10 712 |
| Thereof confirmed SARS-CoV-2 infection | N/A | 117 (2.4) [2.0–2.9] | N/A | 318 (3.0) [2.7–3.3] |
Data are presented as n (%) [95% confidence interval] or mean ± standard deviation, unless stated otherwise. STEMI = ST-elevation myocardial infarction; NSTEMI = non-ST-elevation myocardial infarction; N/A = not available.
Comparison of in hospital mortality in patients admitted with cardiovascular and cerebrovascular emergencies between 2017 and 2020
When comparing the pooled four months (March – June) between 2017 and 2020, the in hospital mortality of patients admitted with acute stroke increased during the COVID-19 pandemic when compared with the last three years from 8.3% (95% CI 8.0% – 8.5%) to 9.6% (95% CI 9.1% – 10.2%). No statistically significant differences were observed in patients admitted with STEMI, NSTEMI, ALI, aortic rupture, and TIA (Table 3 ). The in hospital mortality of patients admitted with acute stroke was negatively associated with hospital admission rate. In patients admitted with STEMI, NSTEMI, ALI, aortic rupture, and TIA, no association between in hospital mortality and hospital admission rate was observed.
Table 3.
In hospital mortality for patients admitted for cardiovascular and cerebrovascular emergencies in March, April, May, and June before (2017–2019) vs. during (2020) the COVID pandemic
| Pre-COVID March to June 2017–2019 | During COVID March to June 2020 | Change – % | |
|---|---|---|---|
| STEMI | 11.8 [11.1–12.6] | 11.6 [10.3–12.9] | −0.2 |
| NSTEMI | 5.7 [5.4–6.1] | 5.4 [4.8–6.0] | −0.3 |
| ALI | 5.9 [5.3–6.6] | 5.5 [4.4–6.7] | −0.4 |
| Aortic rupture | 43.0 [38.9–47.1] | 38.1 [30.4–46.2] | −4.9 |
| Acute stroke | 8.3 [8.0–8.5] | 9.6 [9.1–10.2] | +1.3 ∗ |
| TIA | 0.3 [0.2–0.4] | 0.3 [0.2–0.5] | ±0 |
Data are presented as % [95% confidence interval]. STEMI = ST elevation myocardial infarction; NSTEMI = non-ST elevation myocardial infarction; ALI = acute limb ischaemia; TIA = transient ischaemic attack.
p <.001 for two proportion z test.
Comparison of in hospital mortality in patients admitted with cardiovascular and cerebrovascular emergencies by confirmed SARS-CoV-2 infection status
Since the submission of a positive SARS-CoV-2 test result was requested by the WHO and German authorities in March 2020, a total of 1 243 patients (2.4%, 95% CI 2.3% – 2.5%) were treated with confirmed infection among the cohort. The infection rate varied between 1.8% (95% CI 1.5% – 2.1%) in patients admitted with TIA and 3.0% (95% CI 2.7% – 3.3%) in patients admitted with NSTEMI (Table 1, Table 2).
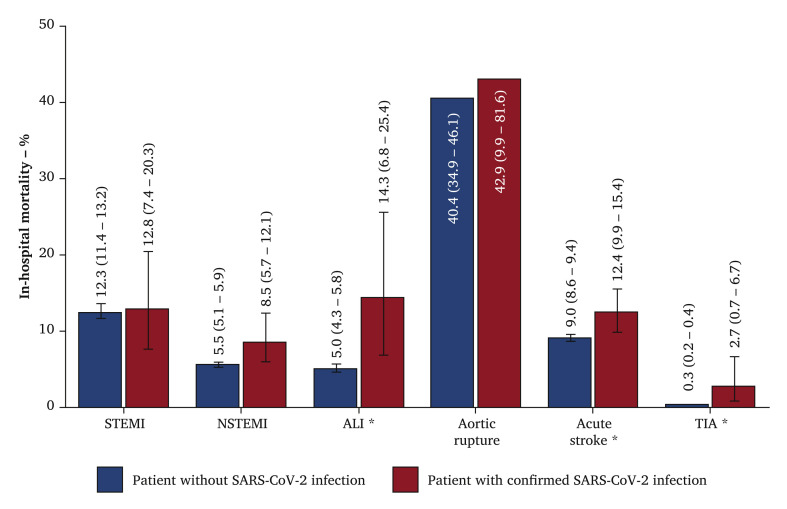
The in hospital mortality of SARS-CoV-2 positive patients admitted with cardiovascular and cerebrovascular emergencies was statistically significantly higher when compared with non-infected patients for acute stroke (12.4% vs. 9.0%), ALI (14.1% vs. 4.9%), and TIA (2.7% vs. 0.3%). No statistically significant differences were observed in mortality for patients admitted with STEMI, NSTEMI, or aortic rupture (Fig. 1 ).
Figure 1.
In hospital mortality as % and 95% confidence interval of patients treated for cardiovascular (ST elevation myocardial infarction [STEMI] or non-ST elevation myocardial infarction [NSTEMI]) and cerebrovascular (acute stroke or transient ischaemic attack [TIA]) emergencies between March and October 2020 by the occurrence of SARS-CoV-2 infection of no submitted infection (blue) or confirmed SARS-CoV-2 positive infection (red). ALI = acute limb ischaemia. ∗p < .001 for two proportion z test.
Discussion
In this large scale retrospective analysis of health insurance claims from Germany, the results suggested an association between the COVID-19 pandemic and increased in hospital mortality in patients admitted as an emergency with acute stroke. This association was apparent when comparing those treated during the pandemic vs. previous years, while mortality rates were not statistically significantly different during the pandemic in all other included emergency conditions. Thereby, increasing mortality rates in acute stroke patients were associated with a declining hospital admission rate. At patient level, confirmed SARS-CoV-2 infection status was associated with higher in hospital mortality in acute stroke, as well as in ALI and TIA. These striking results confirm previous international reports using real world data from imaging systems or registries.4 , 9, 10, 11, 12
Kansagra et al. used preliminary data from imaging software to analyse 231 753 patients treated at 856 hospitals in the USA from July to April 2020. The authors observed a distinct decrease in the numbers of patients who underwent acute stroke imaging by 39% during the COVID-19 pandemic.4 While the present authors previous study on emergency admissions in Germany confirmed these declining presentations of patients with acute stroke, unfortunately neither study provided reliable outcome data to further illuminate a possible relationship between COVID-19 and short term mortality. The current study provided complementary data on mortality by SARS-CoV-2 infection status.
Interestingly, Mao et al. reported a case series of 214 patients with new coronavirus disease treated in a known COVID-19 hotspot in Wuhan, China.24 Neurological manifestations were common among infected patients, suggesting an association between COVID-19 and acute stroke symptoms.24 There is growing evidence for a neuro-invasive potential of SARS-CoV-2, but it remains challenging to interpret the direction of the interaction between acute stroke and SARS-CoV-2 infection using data from clinical and administrative registries.13 , 25 , 26 There is also evidence for an increased rate of cryptogenic strokes possibly related to an acquired hypercoagulability and mortality, emphasising the complex confounding in available studies.10 Having said this, future studies should further illuminate the possible neurological impact of a confirmed SARS-CoV-2 infection.
To answer that question, Ntaios et al. recently initiated a global COVID-19 stroke registry. The authors used data on patients with acute ischaemic stroke in 28 sites in 16 countries to perform a propensity score matched comparison of COVID-19 positive vs. negative patients (n = 336). An association between COVID-19 and severe stroke was observed, further emphasising the need for studies to uncover their underlying relationship.12 Notably, in another registry analysis using data on almost 800 endovascular thrombectomies in Germany, the authors found no changes in workflow time intervals, efficacy, and functional outcomes during the COVID-19 pandemic when compared with 2019. However, only data from experienced stroke centres were included while the nationwide reality also includes patients admitted to low volume hospitals, as also discussed in a survey among stroke units in Germany.27 Emphasising a potential difference between cohorts, Tiedt et al. included fewer female patients with a slightly higher age when compared with the current study. Furthermore, the previous study was limited to endovascular thrombectomies while this study included all comers independent of their treatment modality.28 Another recent cross sectional study of patients from a large New York healthcare registry found that patients diagnosed with COVID-19 had only 25% the odds of stroke compared with other patients, but these stroke patients had a nine fold increase in mortality.29 Again, the current study could confirm these findings.
In light of these previous studies, the current study findings suggest that the COVID-19 pandemic has a negative impact on short term outcomes in acute stroke patients in Germany, although there are various confounders and a selection bias probably interfering with the COVID-19 and mortality relationship. It has been discussed that patients with severe neurological symptoms will still be hospitalised while less affected patients avoid emergency services.
Another interesting finding of the current study was related to the admission and treatment of patients suffering from ALI.18 In contrast to previous reports suggesting an increasing rate of this emergency during the COVID-19 pandemic, the current study observed rather stable admission rates.30, 31, 32 However, the pronounced association between confirmed SARS-CoV-2 infection and in hospital mortality in patients with ALI confirms that there is an underlying relationship between the two conditions. From this study, it must be highlighted that the German healthcare system with its distinct peculiarities in terms of hospital beds and intensive care infrastructure was less affected than other healthcare systems such as in Italy or Spain.
This study has limitations. The retrospective observational design of the current study allows only derivation of associations and generation of hypotheses valid for Germany, which should be addressed by prospective randomised studies in the future. There are probably various confounders with possible impact on the relationship between COVID-19 and in hospital mortality in the affected target population. Besides the direct impact of a SARS-CoV-2 infection on mortality, it appears also possible that changed staffing or infrastructure led to worsened accessibility to care. It remains unknown whether a patient first got infected with SARS-CoV-2 and then developed stroke, or the other way around. Moreover, data on the severity of the disease for the admitted patients was not available. False positives in reverse transcription PCR testing might additionally interfere with the results of the current study. Unfortunately, the nature of the data and timeliness of the research question made adjusted analyses impossible. Having said that, a statistically significant selection with impact on central conclusions cannot be ruled out. However, the striking observations made in the current study and the circumstances during the ongoing health crisis emphasise the importance of searching for hypotheses in the available data. Furthermore, because of the topicality of the current study data, it may be possible that missing outcome data introduce a possible selection bias. As sensitivity analysis, both complete data from 2020 and data concerning only the four months March to June were used, where appropriate completeness was secured. Because of the ecological study design, it cannot be quantified to what extent the declining hospital admission rate potentially explains higher acute stroke mortality. Yet, the fact that mean age and sex ratio were largely stable over time in this patient group provides little evidence for this hypothesis. Further, in hospital mortality and COVID-19 time period were unrelated in all other conditions included in this study. And finally, the direct impact of SARS-CoV-2 infection on acute stroke mortality was visible at patient level. A statistically significant selection bias in the current study cannot be ruled out as only inpatients admitted to legally endorsed hospitals were included. It appears possible that there is a subgroup not covered because they decided not to use the emergency system.
The cardiovascular community should further support ongoing efforts to collect data to protect patients from avoidable collateral damage.33 With growing evidence for an impact of COVID-19 on patients with acute stroke derived from real world data, particular attention should be paid to this vulnerable group. Increased awareness and public recommendations to use emergency services may be reasonable.
Conclusions
This retrospective analysis of claims data provided hints for an association between the COVID-19 pandemic and increased in hospital mortality in patients with acute stroke. Furthermore, confirmed SARS-CoV-2 infection was associated with increased mortality in patients with stroke, TIA, and ALI. Future studies are urgently needed to better understand the underlying mechanism and relationship between the new coronavirus and acute stroke.
Acknowledgements
The authors are grateful and sincerely acknowledge the submission of research data from BARMER, Germany. The authors would like to thank Ursula Marschall (BARMER, Wuppertal, Germany) for her support and scientific contributions to this project. The authors would also like to thank Stefan Blankenberg, Fabian Brunner, and Marko Remmel (Department of Cardiology, University Heart and Vascular Centre UKE Hamburg, Hamburg, Germany) for their critical revisions and valuable input to the manuscript. We would further like to thank Sebastian Debus and Tilo Kölbel (Department of Vascular Medicine, University Heart and Vascular Centre UKE Hamburg, Hamburg, Germany) for their critical revisions to the manuscript. The authors would like to thank Frederik Peters (Research Group GermanVasc, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany) for his scientific and biostatistical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejvs.2021.03.006.
Conflict of interest
None.
Funding
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Coding criteria used for this retrospective observational study of health insurance claims.
References
- 1.Melissano G., Mascia D., Baccellieri D., Kahlberg A., Bertoglio L., Rinaldi E. Pattern of vascular disease in Lombardy, Italy, during the first month of the COVID-19 outbreak. J Vasc Surg. 2020;72:4–5. doi: 10.1016/j.jvs.2020.04.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Björck M., Boyle J.R., Dick F. The need of research initiatives amidst and after the Covid-19 pandemic: a message from the Editors of the EJVES. Eur J Vasc Endovasc Surg. 2020;59:695–696. doi: 10.1016/j.ejvs.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seiffert M., Brunner F.J., Remmel M., Thomalla G., Marschall U., L’Hoest H. Temporal trends in the presentation of cardiovascular and cerebrovascular emergencies during the COVID-19 pandemic in Germany: an analysis of health insurance claims. Clin Res Cardiol. 2020;109:1540–1548. doi: 10.1007/s00392-020-01723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kansagra A.P., Goyal M.S., Hamilton S., Albers G.W. Collateral rffect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383:400–401. doi: 10.1056/NEJMc2014816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Filippo O., D’Ascenzo F., Angelini F., Bocchino P.P., Conrotto F., Saglietto A. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in Northern Italy. N Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metzler B., Siostrzonek P., Binder R.K., Bauer A., Reinstadler S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enache B., Claessens Y.E., Boulay F., Dor V., Eker A., Civaia F. Reduction in cardiovascular emergency admissions in Monaco during the COVID-19 pandemic. Clin Res Cardiol. 2020;109:1577–1578. doi: 10.1007/s00392-020-01687-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt A.S., Moscone A., McElrath E.E., Varshney A.S., Claggett B.L., Bhatt D.L. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76:280–288. doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudilosso S., Laredo C., Vera V., Vargas M., Renú A., Llull L. Acute stroke care is at risk in the era of COVID-19: experience at a comprehensive stroke center in Barcelona. Stroke. 2020;51:1991–1995. doi: 10.1161/STROKEAHA.120.030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paolucci M., Biguzzi S., Cordici F., Lotti E.M., Morresi S., Romoli M. Impact of COVID-19 pandemic on acute stroke care: facing an epidemiological paradox with a paradigm shift. Neurol Sci. 2021;42:399–406. doi: 10.1007/s10072-020-04914-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ntaios G., Michel P., Georgiopoulos G., Guo Y., Li W., Xiong J. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke. Stroke. 2020;51:e254–e258. doi: 10.1161/STROKEAHA.120.031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz J.M., Libman R.B., Wang J.J., Sanelli P., Filippi C.G., Gribko M. Cerebrovascular complications of COVID-19. Stroke. 2020;51:e227–e231. doi: 10.1161/STROKEAHA.120.031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cagnazzo F., Piotin M., Escalard S., Maier B., Ribo M., Requena M. European multicenter study of ET-COVID-19. Stroke. 2021;52:31–39. doi: 10.1161/STROKEAHA.120.031514. [DOI] [PubMed] [Google Scholar]
- 15.Chakfé N., Mertes P.-M., Lejay A. Learn from the first wave to surf the next one optimally. Eur J Vasc Endovasc Surg. 2021 2021;61:316. doi: 10.1016/j.ejvs.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pini R., Faggioli G., Vacirca A., Gallitto E., Mascoli C., Attard L. Is it possible to safely maintain a regular vascular practice during the COVID-19 pandemic? Eur J Vasc Endovasc Surg. 2020;60:127–134. doi: 10.1016/j.ejvs.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 18.Björck M., Earnshaw J.J., Acosta S., Bastos Gonçalves F., Cochennec F., Debus E.S. Editor’s Choice - European Society for Vascular Surgery (ESVS] 2020 Clinical Practice Guidelines on the Management of Acute Limb Ischaemia. Eur J Vasc Endovasc Surg. 2020;59:173–218. doi: 10.1016/j.ejvs.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Heidemann F., Peters F., Kuchenbecker J., Kreutzburg T., Sedrakyan A., Marschall U. Long term outcomes after revascularisations below the knee with paclitaxel coated devices: a propensity score matched cohort analysis. Eur J Vasc Endovasc Surg. 2020;60:549–558. doi: 10.1016/j.ejvs.2020.06.033. [DOI] [PubMed] [Google Scholar]
- 20.Kreutzburg T., Peters F., Riess H.C., Hischke S., Marschall U., Kriston L. Editor’s Choice - Comorbidity patterns among patients with peripheral arterial occlusive disease in Germany: a trend analysis of health insurance claims data. Eur J Vasc Endovasc Surg. 2020;59:59–66. doi: 10.1016/j.ejvs.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Peters F., Kreutzburg T., Riess H.C., Heidemann F., Marschall U., L’Hoest H. Optimal pharmacological treatment of symptomatic peripheral arterial occlusive disease and evidence of female patient disadvantage: an analysis of health insurance claims data. Eur J Vasc Endovasc Surg. 2020;60:421–429. doi: 10.1016/j.ejvs.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Swart E., Gothe H., Geyer S., Jaunzeme J., Maier B., Grobe T.G. [Good Practice of Secondary Data Analysis (GPS]: guidelines and recommendations] Gesundheitswesen. 2015;77:120–126. doi: 10.1055/s-0034-1396815. [DOI] [PubMed] [Google Scholar]
- 23.Peters F., Kreutzburg T., Kuchenbecker J., Marschall U., Remmel M., Dankhoff M. Quality of care in surgical/interventional vascular medicine: what can routinely collected data from the insurance companies achieve? Gefässchirurgie. 2020 doi: 10.1007/s00772–020–00664-x. [epub ahead of print] [DOI] [Google Scholar]
- 24.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19]: a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann-Haefelin T., Faiss J., Glahn J., Grau A., Häusler K.G., Thomalla G. Stroke care in Germany during the early phase of the COVID-19 pandemic: Results of a survey conducted by the Stroke Unit Commission of the German Stroke Society. DGNeurologie. 2020;1–5 [Google Scholar]
- 28.Tiedt S., Bode F.J., Uphaus T., Alegiani A., Gröschel K., Petzold G.C. Impact of the COVID-19-pandemic on thrombectomy services in Germany. Neurol Res Pract. 2020;2:44. doi: 10.1186/s42466-020-00090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bekelis K., Missios S., Ahmad J., Labropoulos N., Schirmer C.M., Calnan D.R. Ischemic stroke occurs less frequently in patients with COVID-19. Stroke. 2020;51:3570–3576. doi: 10.1161/STROKEAHA.120.031217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellosta R., Luzzani L., Natalini G., Pegorer M.A., Attisani L., Cossu L.G. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mestres G., Puigmacia R., Blanco C., Yugueros X., Esturrica M., Riambau V. Risk of peripheral arterial thrombosis in COVID-19. J Vasc Surg. 2020;72:756–757. doi: 10.1016/j.jvs.2020.04.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sena G., Gallelli G. An increased severity of peripheral arterial disease in the COVID-19 era. J Vasc Surg. 2020;72:758. doi: 10.1016/j.jvs.2020.04.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Oria M., Mills J.L., Sr, Cohnert T., Oderich G.S., Hultgren R., Lepidi S. The “Vascular Surgery COVID-19 Collaborative” (VASCC) Eur J Vasc Endovasc Surg. 2020;60:489–490. doi: 10.1016/j.ejvs.2020.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coding criteria used for this retrospective observational study of health insurance claims.