Abstract
Liver injury is one of the nonpulmonary manifestations described in coronavirus disease 2019 (COVID-19). Post–COVID-19 cholangiopathy is a special entity of liver injury that has been suggested as a variant of secondary sclerosing cholangitis in critically ill patients (SSC-CIP). In the general population, the outcome of SSC-CIP has been reported to be poor without orthotopic liver transplantation (OLT). However, the role of OLT for post–COVID-19 cholangiopathy is unknown. We present a case report of a 47-year-old man who recovered from acute respiratory distress syndrome from COVID-19 and subsequently developed end-stage liver disease from post–COVID-19 cholangiopathy. The patient underwent OLT and is doing well with normal liver tests for 7 months. To our knowledge, this is the first case report of a patient who underwent successful liver transplantation for post–COVID-19 cholangiopathy.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in December 2020 in Wuhan City, China and was declared a global pandemic by the World Health Organization on March 11, 2020. The disease is termed coronavirus disease 2019 (COVID-19). COVID-19 is typically characterized by fever, fatigue, dry cough, anosmia, and headache that may evolve to respiratory failure [1]. Liver test abnormalities have been identified as one of a growing spectrum of nonpulmonary manifestations described in COVID-19 that may be potentially attributable to hepatic expression of the main viral entry receptor of the RNA virus, angiotensin-converting enzyme II (ACE2) [2,3].
The incidence of abnormal liver tests in patients with COVID-19 ranges from 14% to 76%, and most of them are aspartate aminotransferase (AST) and alanine aminotransferase (ALT). The aminotransferases are mildly elevated on admission in most cases (less than 2 times the upper limit of normal), and total bilirubin levels can modestly increase early in the disease process. Although most liver damage in COVID-19 infection is the hepatocellular type, severe cholestasis can also occur and 12% of patients showed total bilirubin levels elevated to more than 3 times the upper limit of normal [4,5]. A recent study revealed an association between abnormal liver tests and severe COVID-19, including intensive care unit (ICU) admission, mechanical ventilation, and death [6].
The unusual entity of secondary sclerosing cholangitis in critically ill patients (SSC-CIP) has been recognized as a novel entity in patients after COVID-19 infection [7] and recently named post–COVID-19 cholangiopathy [8]. It is characterized by marked cholestasis associated with ongoing jaundice that persists long after pulmonary and renal recovery. We report a case of a patient who developed post–COVID-19 cholangiopathy requiring a liver transplant. To our knowledge, this is the first case report of a patient developing post–COVID-19 cholangiopathy who underwent successful liver transplantation. This case is being reported to provide reference and guidance for possible long-term complications after the COVID-19 pandemic, including liver-related consequences with liver transplantation as a viable treatment option.
Case Presentation
Clinical Presentation and Diagnostic Tests
A 47-year-old man with class 3 (severe) obesity, body mass index of 51, obstructive sleep apnea, hypertension, and hyperlipidemia with no history of liver disease presented to an outside facility with respiratory symptoms of dyspnea, cough, and fever. Chest x-ray showed diffuse patchy airspace opacities compatible with multifocal pneumonia, and he was subsequently found to be positive for SARS-CoV-2 infection. The patient was treated with hydroxychloroquine (Plaquenil), azithromycin, and high-dose vitamin C. Laboratory findings were remarkable for elevated AST of 79 U/L and ALT 52 U/L, with a total bilirubin of 0.3 mg/dL at the time of presentation. The patient experienced rapid clinical decline that included acute respiratory distress syndrome and acute kidney injury. He required prolonged mechanical ventilation (29 days) and continuous venovenous hemofiltration. Although his pulmonary function subsequently improved and he was weaned off mechanical ventilation, his acute kidney injury persisted and required regular hemodialysis.
At day 58 from his initial presentation, his pertinent laboratory blood tests included AST of 384 U/L, ALT of 175 U/L, alkaline phosphatase of 1644 U/L, and total bilirubin of 19.0 mg/dL. Initial abdominal ultrasound showed severe fatty liver and innumerable gallstones throughout the gallbladder without biliary dilation or gallbladder wall thickening. Computed tomography (CT) of the abdomen and pelvis without contrast showed normal liver size and contour without focal hepatic lesions or evidence of biliary ductal dilatation. At day 73, his follow-up blood tests were AST of 236 U/L, ALT of 121 U/L, alkaline phosphatase 970 U/L, and serum total bilirubin of 10.9 mg/dL. A liver biopsy demonstrated mechanical bile duct obstruction presumably related to sepsis, with drug-induced liver injury less likely. Endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy was performed and a small pigment stone retrieved. Noteworthy is the finding of diffuse intrahepatic biliary strictures or cholangiopathy (Fig 1 ).
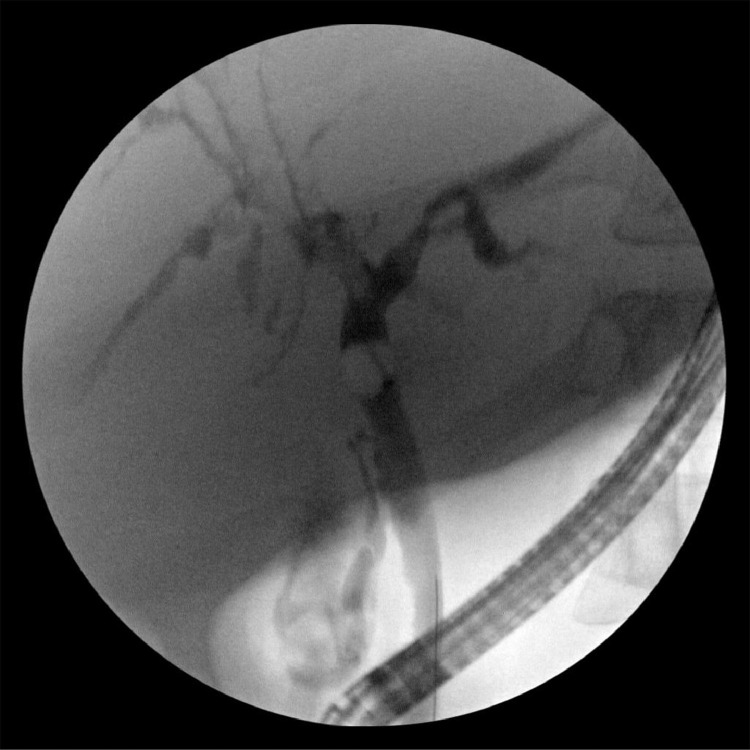
Fig 1.
Endoscopic retrograde cholangiography showing a normal common bile duct with diffuse stricturing of the intrahepatic ducts.
On day 81 from his initial presentation, the patient was hospitalized for hypotension during hemodialysis. Pertinent laboratory tests were ALT of 130 U/L, AST of 491 U/L, alkaline phosphatase of 2730 U/L, and marked hyperbilirubinemia with total bilirubin of 19 mg/dL. Abdominal ultrasound showed cholelithiasis without evidence of acute cholecystitis, and magnetic resonance cholangiopancreatography demonstrated mild intrahepatic biliary ductal dilatation with multifocal strictures or beading without extrahepatic biliary dilatation. ERCP confirmed findings of secondary sclerosing cholangitis: short segments of strictures and dilatations of the intrahepatic ducts, with no pathologic findings in the common hepatic and common bile ducts.
Preoperative Treatment Planning and Management
The patient was evaluated and placed on the list for liver transplantation with a Model for End-Stage Liver Disease score of 37. Although the patient met criteria for a combined liver and kidney transplantation, the multidisciplinary liver and kidney transplantation team pursued a single-organ liver transplantation as a life-saving treatment. Waitlisitng for renal transplantation was deferred because of comorbidity that included morbid obesity and post–COVID-19 infection pulmonary dysfunction: restrictive ventilatory defect with low-expiratory volume and reduced diffusion capacity and evidence of postinflammatory state with mild interstitial edema. Therefore, staged renal transplantation is planned once the patient has recovered from his severe illness.
Orthotopic Liver Transplantation and Explant Histopathology
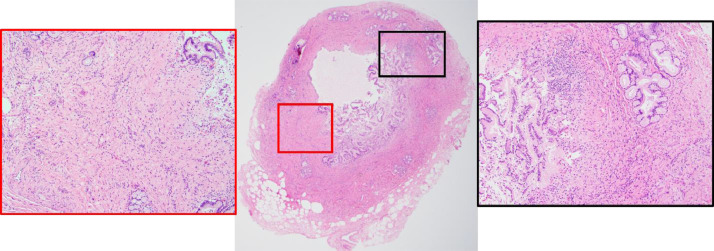
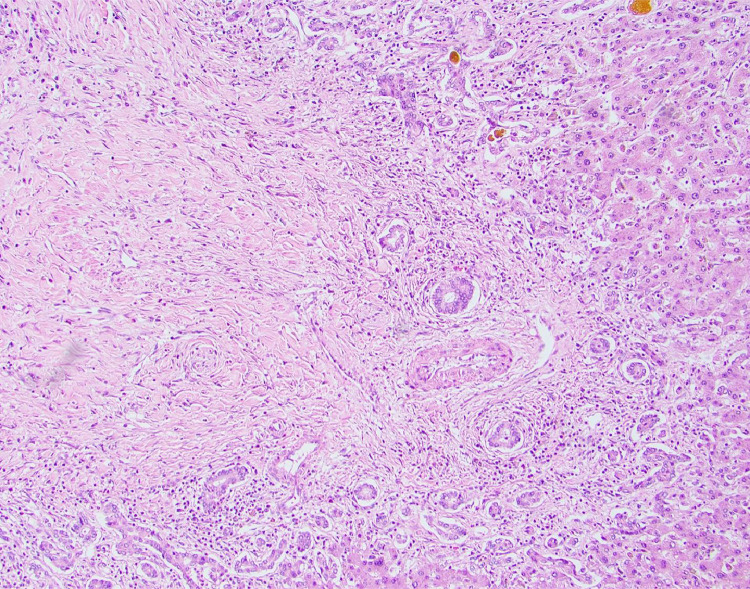
On day 108 from his initial presentation, the patient underwent an orthotopic liver transplantation (OLT) with a whole hepatic allograft from a deceased donor. OLT was performed with intraoperative renal replacement therapy and total peripheral and mesenteric venovenous bypass. A staged choledochocholedochostomy was performed the following day [9]. Noteworthy are findings on the native liver: 4000 g in weight with histologic findings of severe sclerosing cholangitis with hepatic abscesses (Fig. 2 -6 ). There was no histologic evidence of IgG4 or other causes of secondary sclerosing cholangitis.
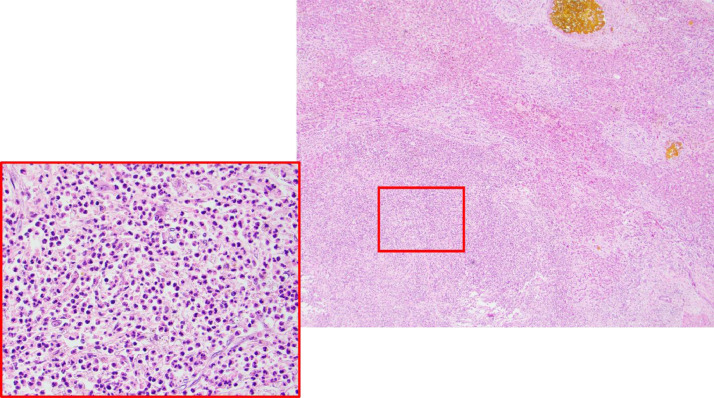
Fig 3.
Liver abscess occupying the bottom left field (40×, hematoxylin and eosin stain). One bile lake occupying top right field (insert, 400× hematoxylin and eosin stain, showing neutrophil-rich abscess contents).
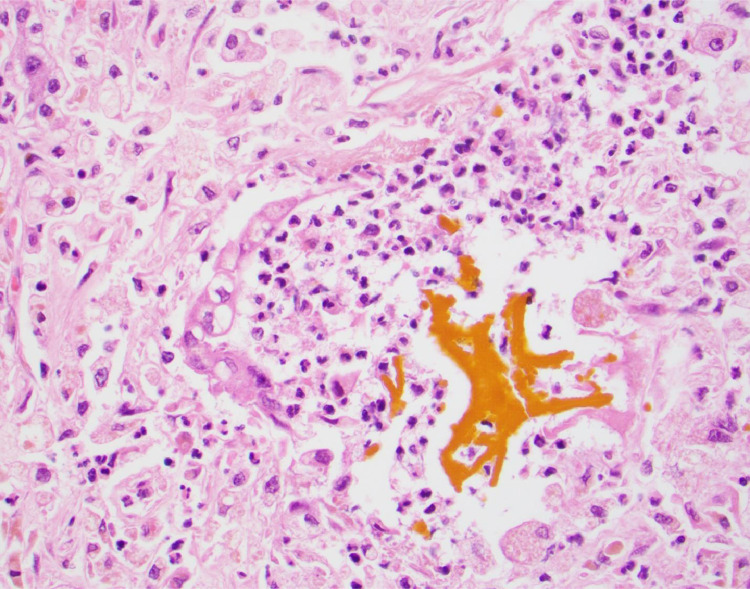
Fig 4.
Bile lake associated with bile duct injury with vacuolization and neutrophilia (400×, hematoxylin and eosin stain).
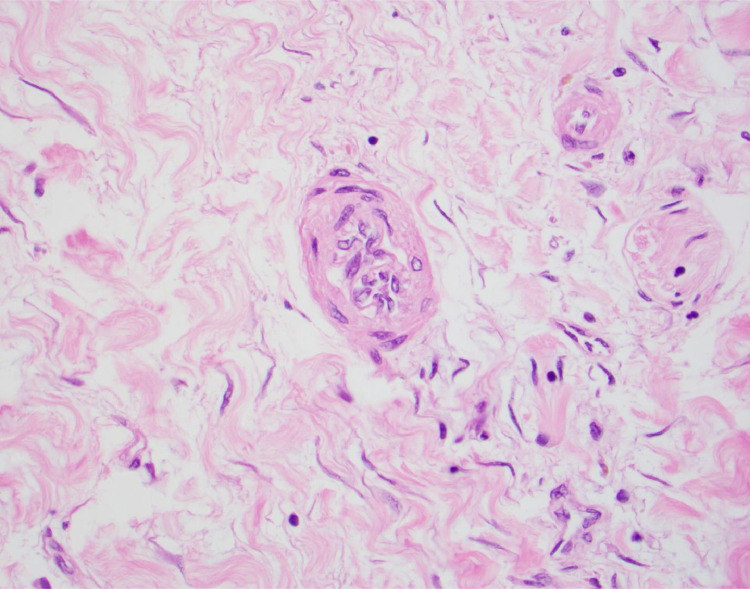
Fig 5.
Microarteriopathy with endothelial cell swelling and obliteration of the lumen (400×, hematoxylin and eosin stain).
Fig 2.
Hilar bile duct with findings of inflammation and fibrosis (20×, hematoxylin and eosin stain). Red and black inserts (100×) show increased collagen deposition with associated mononuclear inflammatory infiltration within the wall of the bile duct.
Fig 6.
Obliterative portal venopathy (100×, hematoxylin and eosin stain).
Postoperative Care
The induction immunosuppression regimen consisted of basiliximab (Simulect) on post-OLT days 0 and 4, solumedrol taper, and everolimus (Zortress) followed by maintenance therapy with tacrolimus and everolimus. His hepatic allograft function normalized within 8 days after OLT, and the patient was weaned off mechanical ventilation on post-OLT day 13. The patient continued his recuperation in the acute rehabilitation unit beginning post-OLT day 46 and was subsequently discharged home on the post-OLT day 55.
At 7 months after OLT, the patient's hepatic allograft function remained normal: ALT of 27 U/L, AST of 28 U/L, alkaline phosphatase of 123 U/L, and total bilirubin 0.2 mg/dL. He did not experience an episode of acute cellular- or antibody-mediated rejection during the post-OLT period. Regarding his renal function, he continues to require regular hemodialysis. After a successful weight loss of 50 kg in our weight loss management program, the patient is currently undergoing a comprehensive evaluation for the renal transplantation wait list.
Discussion
SSC-CIP is a recently recognized form of cholestatic liver disease occurring in patients without a history of hepatobiliary disease, after receiving treatment in the ICU in different settings, including cardiothoracic surgery, infection, trauma, and burns [10,11].
The pathophysiology of SSC-CIP is not completely understood. The presence of critical illness and its treatment in an ICU seem to play an important role in the development of the disease, but the main pathogenic mechanism seems to be a combination of bile duct ischemia, changes in bile composition, and biliary infection [12,13]. The diagnosis is made by ERCP or magnetic resonance cholangiopancreatography revealing diffuse strictures and dilatations of the intrahepatic bile ducts with filling defects caused by the presence of biliary casts [13,14].
Among the various pathophysiologic events associated with critical illness, biliary ischemia seems to play a major role in the cause of SSC-CIP. Whereas the hepatocytes receive a dual blood supply from the portal vein and the hepatic artery, the biliary epithelium receives its blood supply solely from the peribiliary plexus. Thus, the cholangiocytes are more susceptible to ischemia than the hepatocytes. When ischemia of the biliary epithelium takes place, blood supply to the bile ducts is reduced, resulting in necrosis and sloughing of the biliary epithelium and bile cast formation [12,15]. Deltenre et al [16] demonstrated that the degree of ischemic cholangiopathy was inversely proportional to the caliber of the supplying occluded artery.
Toxic bile may also play a role in the pathogenesis of SSC-CIP. Destruction of the protective mechanisms for the cholangiocytes, that is, secretion of phospholipids from the hepatocytes and biliary secretion of bicarbonate, will cause their lipid membrane to be susceptible to the detergent properties of the hydrophobic bile acids [12]. Biliary secretion of bicarbonate, via the transporter ion exchanger 2, forms a protective alkaline bicarbonate film on the apical cholangiocyte membrane as part of a defense strategy [17]. A disturbance in the fine balance between bile acids and protective mechanisms can lead to damage to the biliary epithelium leading to sclerosing cholangitis. A heightened inflammatory response through the release of proinflammatory cytokines will also add to the development of toxic bile and contribute to cholangiocyte necrosis [12].
Multimodal treatments for critical illness have been associated with the development of SSC-CIP. Prolonged hypotension and vasopressor administration are common in patients with SSC-CIP. All the patients experienced an episode of severe hemodynamic instability with a decrease in mean arterial blood pressure <65 mm Hg for at least 60 minutes and often longer [12,18]. Vasopressor administration is also very common before the development of SSC-CIP. Epinephrine, norepinephrine, dopamine, and dobutamine all increase systemic blood pressure but do not have the same effect on hepato-splanchnic blood flow. Dopamine has a positive effect on liver perfusion [19], contrary to epinephrine and norepinephrine, which are thought to have a negative effect on splanchnic blood flow [20].
Mechanical ventilation with high positive end-expiratory pressures >10 cm H2O also has been shown to contribute to microcirculatory ischemia within the hepato-splanchnic vascular plexus [21]. Additionally, excessive use of prone positioning of mechanically ventilated patients has been linked to the development of SSC-CIP [22]. SSC-CIP has a mortality of up to 50% in patients during an ICU admission. Adverse prognostic factors include associated renal failure, a high Model for End-Stage Liver Disease score, and rapid deterioration to liver cirrhosis [23]. In another study of SSC-CIP patients, 60% survived the ICU, 40% developed stable biliary cirrhosis, and 20% required a liver transplant [24]. Without liver transplant, the median survival in such patients is 12 to 44 months [25].
Post–COVID-19 cholangiopathy has been recently described [7,8] and refers to SSC-CIP in patients who recovered from severe COVID-19 infection. All the patients described in the case reports had no pre-existing liver disease, and all had a prolonged hospitalization because of acute hypoxemic respiratory failure requiring mechanical ventilation and additional complications from COVID-19. All the patients developed marked cholestasis with associated jaundice that persisted long after pulmonary and renal recovery. None of the imaging studies was suggestive of cirrhosis.
Patients with severe COVID-19 infection have several predisposing conditions for SSC-CIP, such as hypotension and administration of vasopressors. The presence of COVID-19–associated coagulopathy has a high risk for arterial and venous thromboembolism [26,27]. Mechanical ventilation with the use of positive end-expiratory pressure for prolonged periods because of the challenges of weaning [28] plus the use of prone positioning in such patients for up to 16 hours per day are relatively common [29]. Increased proinflammatory cytokines with the syndrome of uncontrolled immune activation leads to cytokine storm [30] and contributes to the development of toxic bile and then cholangiocyte necrosis [12].
The histologic picture of patients with post–COVID-19 cholangiopathy seems to differ from the histologic findings seen in patients with SSC-CIP of other causes [8]. All the biopsy samples exhibited extensive degenerative cholangiocyte injury with extreme cholangiocyte cytoplasmic vacuolization and regenerative change not previously described for SSC-CIP. The microvascular features of hepatic artery endothelial swelling, portal vein phlebitis, and sinusoidal obstruction syndrome are also unique, as is the intrahepatic microangiopathy affecting all 3 microvascular compartments, as noted in autopsy findings in patients succumbing to COVID-19 [31]. These histologic changes suggest direct hepatic injury from COVID-19 in patients with underlying SSC-CIP.
The patient we describe in this report had similar findings, including destruction of the biliary epithelium characterized by vacuolar degeneration with cytoarchitectural disarray, anisonucleosis, and even cholangiocyte necrosis. The extensive biliary injury was associated with marked cholestasis, ductular reaction, and ductulocentric fibrosis. Furthermore, our patient demonstrated obliterative portal venopathy and microarteriopathy characterized by endothelial cell swelling with obliteration of the arterial lumen. Many of these features have been previously described in post–COVID-19 cholangiopathy [8]. Therefore, post–COVID-19 cholangiopathy seems to be a variant of SSC-CIP and further investigation is needed in regard to the pathogenicity of COVID-19 in the biliary epithelium. A major concern for patients with post–COVID-19 cholangiopathy is that it may lead to progressive liver injury with the potential need for liver transplantation, as seen in our patient [8].
To our knowledge, this is the first report of a patient requiring liver transplantation because of fulminant post–COVID-19 cholangiopathy. Given the increased number of COVID-19 infections in intensive care medical management, it is important to develop a practical approach to screening and evaluation of patients who are likely to develop post–COVID-19 cholangiopathy, particularly those who will progress to a fulminant course and require an expedited OLT.
Acknowledgments
The authors gratefully acknowledge the Froedtert and the Medical College of Wisconsin liver transplantation coordinators and staff, transplant surgery physician assistants, nurse practitioners, and research nurse for their dedication to the highest level of medical care for all our patients.
Footnotes
This work was supported in part by The Kevin T. Cottrell Memorial Fund for Organ Transplantation Research and The Virginia Tronca Estate Gift for Transplantation Research.
References
- 1.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai X, Lonfei H, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. Bio-Rxiv [Preprint] 2020 https://www.biorxiv.org/content/10.1101/2020.02.03.931766v1 [cited 2020 Apr 6]. Available from: [Google Scholar]
- 4.Cai Q, Huang D, Yu H, et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD Expert Panel consensus statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1,827 patients in a major U.S. hospital network. Hepatology. 2020;72:1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS-CoV-2 infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth NC, Kim A, Vitkovski T, et al. Post-COVID-19 cholangiopathy: a novel entity [e-pub ahead of print]. Am J Gastroenterol doi:10.14309/ajg.0000000000001154, accessed March 24, 2021. [DOI] [PubMed]
- 9.Pearson T, Zimmerman MA, Kim J, et al. Staged biliary reconstruction after orthotopic liver transplantation: a practical surgical strategy for high-acuity adult recipients. Transplant Direct. 2019;5:e482. doi: 10.1097/TXD.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheppach W, Druge G, Wittenberg G, et al. Sclerosing cholangitis and liver cirrhosis after extrabiliary infections: report on three cases. Crit Care Med. 2001;29:438–441. doi: 10.1097/00003246-200102000-00042. [DOI] [PubMed] [Google Scholar]
- 11.Esposito I, Kubisova A, Stiehl A, Kulaksiz H, Schirmacher P. Secondary sclerosing cholangitis after intensive care unit treatment: clues to the histopathological differential diagnosis. Virchows Arch. 2008;453:339–345. doi: 10.1007/s00428-008-0654-1. [DOI] [PubMed] [Google Scholar]
- 12.Leonhardt S, Veltzke-Schlieker W, Adler A, et al. Trigger mechanisms of secondary sclerosing cholangitis in critically ill patients. Crit Care. 2015;19:131. doi: 10.1186/s13054-015-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laurent L, Lemaitre C, Minello A, et al. Cholangiopathy incritically ill patients surviving beyond the intensive care period: a multicentre survey in liver units. Aliment Pharmacol Ther. 2017;46:1070–1076. doi: 10.1111/apt.14367. [DOI] [PubMed] [Google Scholar]
- 14.Benninger J, Grobholz R, Oeztuerk Y, et al. Sclerosing cholangitis following severe trauma: description of a remarkable disease entity with emphasis on possible pathophysiologic mechanisms. World J Gastroenterol. 2005;11:4199–4205. doi: 10.3748/wjg.v11.i27.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelbmann CM, Rummele P, Wimmer M, et al. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007;102:1221–1229. doi: 10.1111/j.1572-0241.2007.01118.x. [DOI] [PubMed] [Google Scholar]
- 16.Deltenre P, Valla DC. Ischemic cholangiopathy. J Hepatol. 2006;44:806–817. doi: 10.1016/j.jhep.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Beuers U, Hohenester S, de Buy Wenniger LJ, Kremer AE, Jansen PL, Elferink RP. The biliary HCO(3)(-) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology. 2010;52:1489–1496. doi: 10.1002/hep.23810. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Ari Z, Levingston D, Weitzman E, et al. Secondary sclerosing cholangitis following major burn. Ann Hepatol. 2015;14:695–701. [PubMed] [Google Scholar]
- 19.Hiltebrand LB, Krejci V, Sigurdsson GH. Effects of dopamine, dobutamine, and dopexamine on microcirculatory blood flow in the gastrointestinal tract during sepsis and anesthesia. Anesthesiology. 2004;100:1188–1197. doi: 10.1097/00000542-200405000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Meier-Hellmann A, Reinhart K, Bredle DL, Specht M, Spies CD, Hannemann L. Epinephrine impairs splanchnic perfusion in septic shock. Crit Care Med. 1997;25:399–404. doi: 10.1097/00003246-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Kirchner GI, Rummele P. Update on sclerosing cholangitis in critically ill patients. Viszeralmedizin. 2015;31:178–184. doi: 10.1159/000431031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weig T, Schubert MI, Gruener N, et al. Abdominal obesity and prolonged prone positioning increase risk of developing sclerosing cholangitis in critically ill patients with influenza A-associated ARDS. Eur J Med Res. 2012;17:30. doi: 10.1186/2047-783X-17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voigtlander T, Negm AA, Schneider AS, et al. Secondary sclerosing cholangitis in critically ill patients: model of end-stage liver disease score and renal function predict outcome. Endoscopy. 2012;44:1055–1058. doi: 10.1055/s-0032-1325733. [DOI] [PubMed] [Google Scholar]
- 24.Lin T, Qu K, Xu X, et al. Sclerosing cholangitis in critically ill patients: an important and easily ignored problem based on a German experience. Front Med. 2014;8:118–126. doi: 10.1007/s11684-014-0306-6. [DOI] [PubMed] [Google Scholar]
- 25.Kulaksiz H, Heuberger D, Engler S, Stiehl A. Poor outcome in progressive sclerosing cholangitis after septic shock. Endoscopy. 2008;40:214–218. doi: 10.1055/s-2007-967024. [DOI] [PubMed] [Google Scholar]
- 26.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan C, Chen L, Lu C, et al. Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. 2020;201:1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagana SM, Kudose S, Iuga AC, et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]