Abstract
Background
Coronavirus disease 2019 (Covid-19) is associated with immune dysregulation and hyperinflammation, including elevated interleukin-6 levels. The use of tocilizumab, a monoclonal antibody against the interleukin-6 receptor, has resulted in better outcomes in patients with severe Covid-19 pneumonia in case reports and retrospective observational cohort studies. Data are needed from randomized, placebo-controlled trials.
Methods
In this phase 3 trial, we randomly assigned patients who were hospitalized with severe Covid-19 pneumonia in a 2:1 ratio receive a single intravenous infusion of tocilizumab (at a dose of 8 mg per kilogram of body weight) or placebo. Approximately one quarter of the participants received a second dose of tocilizumab or placebo 8 to 24 hours after the first dose. The primary outcome was clinical status at day 28 on an ordinal scale ranging from 1 (discharged or ready for discharge) to 7 (death) in the modified intention-to-treat population, which included all the patients who had received at least one dose of tocilizumab or placebo.
Results
Of the 452 patients who underwent randomization, 438 (294 in the tocilizumab group and 144 in the placebo group) were included in the primary and secondary analyses. The median value for clinical status on the ordinal scale at day 28 was 1.0 (95% confidence interval [CI], 1.0 to 1.0) in the tocilizumab group and 2.0 (non-ICU hospitalization without supplemental oxygen) (95% CI, 1.0 to 4.0) in the placebo group (between-group difference, −1.0; 95% CI, −2.5 to 0; P=0.31 by the van Elteren test). In the safety population, serious adverse events occurred in 103 of 295 patients (34.9%) in the tocilizumab group and in 55 of 143 patients (38.5%) in the placebo group. Mortality at day 28 was 19.7% in the tocilizumab group and 19.4% in the placebo group (weighted difference, 0.3 percentage points (95% CI, –7.6 to 8.2; nominal P=0.94).
Conclusions
In this randomized trial involving hospitalized patients with severe Covid-19 pneumonia, the use of tocilizumab did not result in significantly better clinical status or lower mortality than placebo at 28 days. (Funded by F. Hoffmann–La Roche and the Department of Health and Human Services; COVACTA ClinicalTrials.gov number, NCT04320615.)
Coronavirus disease 2019 (COVID-19) has rapidly developed into a global health threat since emerging in China in late 2019.1 Severe Covid-19 pneumonia, which occurs in approximately 15% of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is associated with high mortality and places extensive burden on intensive care units (ICUs) to provide mechanical ventilation and other advanced forms of life support.2,3 As was observed in patients with Middle East respiratory syndrome and SARS-CoV-1,4 Covid-19 can begin with an initial phase of high viral replication that is followed by a second phase that may be driven by the host immune response.5 This progression can lead to a rapid increase in proinflammatory cytokines, an uncontrolled inflammatory response, acute respiratory distress syndrome (ARDS), and multiple organ failure.4,6
Levels of interleukin-6 correlate with Covid-19 severity,7,8 which suggests that immune dysregulation and ARDS may be influenced by interleukin-6.6,9 The accumulation of lymphocytes and inflammatory monocytes, endotheliitis, apoptosis, thrombosis, and angiogenesis in the pulmonary vasculature in patients with Covid-19 suggests that vascular inflammation and dysfunction contribute to the pathophysiological features of severe Covid-19 pneumonia.10,11 Since interleukin-6 promotes endothelial dysfunction and the development of vascular permeability, this cytokine may play a role in the vascular dysfunction associated with this disease.12
The potential role of interleukin-6 in Covid-19 pneumonia6,9 provides a rationale for the investigation of interleukin-6 signaling inhibitors. Tocilizumab is a monoclonal antibody against interleukin-6 receptor-alpha that is used to treat certain inflammatory diseases.13 Better outcomes in patients with severe Covid-19 pneumonia who received tocilizumab have been observed in case reports14-16 and supported by retrospective observational cohort studies that showed a rapid reduction in fever, a reduced use of oxygen support and mechanical ventilation, and a reduction in lung manifestations.17-23 We conducted COVACTA, a phase 3, international, randomized, double-blind, placebo-controlled trial, to assess the efficacy and safety of tocilizumab in hospitalized patients with severe Covid-19 pneumonia.
Methods
Trial Design and Randomization
In this trial, which was conducted at 62 hospitals in nine countries in Europe and North America (Canada, Denmark, France, Germany, Italy, the Netherlands, Spain, the United Kingdom, and the United States), we enrolled adults (≥18 years of age) with severe Covid-19 pneumonia, as confirmed by positive polymerase-chain-reaction (PCR) assay of any body fluid and evidenced by bilateral chest infiltrates on chest radiography or computed tomography. Eligible patients had a blood oxygen saturation of 93% or less or a ratio of the partial pressure of oxygen to the fraction of inspired oxygen of less than 300 mm Hg. Patients were excluded if the treating physician determined that death was imminent and inevitable within 24 hours or if they had active tuberculosis or a bacterial, fungal, or viral infection other than SARS-CoV-2. Standard care according to local practice (antiviral treatment, low-dose glucocorticoids, convalescent plasma, and supportive care) was provided. However, concomitant treatment with another investigational agent (except antiviral drugs) or any immunomodulatory agent was prohibited. Written informed consent was obtained from all the patients or, if written consent could not be provided, the patient’s legally authorized representative could provide oral consent with appropriate documentation by the investigator.
Eligible patients were randomly assigned in a 2:1 ratio to receive a single intravenous infusion of tocilizumab (at a dose of 8 mg per kilogram of body weight, with a maximum dose of 800 mg) or placebo plus standard care by means of an interactive voice or Web-based response system and permuted-block randomization. Randomization was stratified according to geographic region (North America or Europe) and the use of mechanical ventilation (yes or no). If clinical signs or symptoms did not improve or worsened (defined as sustained fever or worsened clinical status on an ordinal scale), a second infusion of tocilizumab or placebo could be administered 8 to 24 hours after the first dose. The primary analysis was performed at day 28, and the final trial visit occurred at day 60. Additional details regarding the trial design are provided in the protocol document (which includes the statistical analysis plan), available with the full text of this article at NEJM.org.
Evaluations
For the evaluation of patients in this trial, baseline was defined as the last observation before the administration of tocilizumab or placebo on day 1. The patients’ clinical status was assessed on an ordinal scale according to the following categories: 1, discharged or ready for discharge; 2, hospitalization in a non–intensive care unit (ICU) without supplemental oxygen; 3, non–ICU hospitalization with supplemental oxygen; 4, ICU or non–ICU hospitalization with noninvasive ventilation or high-flow oxygen; 5, ICU hospitalization with intubation and mechanical ventilation; 6, ICU hospitalization with extracorporeal membrane oxygenation or mechanical ventilation and additional organ support; and 7, death. Clinical status was recorded at baseline and every day during hospitalization.
Patients were also evaluated according to the level of clinical severity on the National Early Warning Score 2, which is a standardized assessment for identifying acutely ill patients on the basis of respiration rate, oxygen saturation, systolic blood pressure, pulse rate, level of consciousness, and temperature; values on this instrument range from 0 to 20, with higher scores indicating greater clinical risk.
Outcome Measures
The primary efficacy outcome was clinical status at day 28, as assessed on the seven-category ordinal scale. Key secondary efficacy outcomes were clinical status at day 14 on the ordinal scale, mortality at day 28, number of ventilator-free days by day 28, the time to improvement from baseline by at least two categories on the ordinal scale, and the time to hospital discharge or readiness for discharge; the latter was defined as a normal body temperature and respiratory rate and stable oxygen saturation while breathing ambient air or 2 liters or less of supplemental oxygen. Other secondary outcomes were the time until clinical failure, which was defined as death, discontinuation from trial participation during hospitalization, initiation of mechanical ventilation, or ICU transfer or a 1-category worsening of clinical status in patients who were receiving mechanical ventilation or who were in the ICU at baseline; the initiation of mechanical ventilation among patients who were not receiving mechanical ventilation at randomization; the incidence of ICU transfer among patients who were not in an ICU at baseline; and the duration of ICU stay. Adverse events were recorded according to the system organ class and preferred terms in the Medical Dictionary for Regulatory Activities, version 23.0.
Trial Oversight
The trial was conducted in accordance with the Good Clinical Practice guidelines of the International Council for Harmonisation E6 and the principles of the Declaration of Helsinki or local regulations, whichever afforded greater patient protection. The protocol was reviewed by the institutional review board or ethics committee at each site.
The first draft of the manuscript was written by the penultimate author, with writing support provided by ApotheCom and funded by the sponsor, F. Hoffmann–La Roche. The data were analyzed by the sponsor. The authors had access to all the data for the patients who were enrolled at their trial site. All the authors made the decision to submit the manuscript for publication and vouch for the completeness and accuracy of the data and for the adherence of the trial to the protocol.
Statistical Analysis
We performed efficacy assessments of the primary and secondary outcomes in the modified intention-to-treat population, which included all the patients who had undergone randomization and received a dose of tocilizumab or placebo. We calculated that a sample size of 450 patients would provide a power of 90% to determine a between-group difference in the primary outcome (clinical status at day 28), assuming a distribution on the ordinal scale that corresponded to an odds ratio of 2.0. If significance was met, we tested mortality at day 28 at the 5% level using a hierarchical approach, but no other adjustment for multiple comparisons was planned. In the statistical analysis plan, up to three interim efficacy analyses were specified but were not performed because of rapid enrollment.
The analyses were stratified according to region and mechanical-ventilation status at randomization, except for some subgroup analyses, as prespecified. For the primary outcome of clinical status at day 28, we compared the distribution on the ordinal scale using a nonparametric van Elteren test. We used a proportional-odds model to calculate odds ratios and 95% confidence intervals to determine the odds of being in a better clinical-status category in the tocilizumab group than in the placebo group. A multiple-imputation approach was used to handle missing data and was implemented by means of bootstrapping. This approach assumed that data were missing at random within strata and trial group. (Details regarding these methods are provided in the Methods section in the Supplementary Appendix, available at NEJM.org.)
We used the Cochran–Mantel–Haenszel test to analyze differences in mortality and incidence of mechanical ventilation and ICU transfer, the van Elteren test to assess differences in the number of ventilator-free days, and a log-rank test and Kaplan–Meier plots to assess secondary outcomes in time-to-event analyses. Data regarding deaths were censored at day 28 for all time-to-event analyses involving clinical improvement. Patients who had died by day 28 were considered to have had no ventilator-free days.24 Patients who had died or discontinued participation in the trial before discharge by day 28 were assumed to have required mechanical ventilation or ICU transfer for the respective incidence analyses. Cumulative incidence plots were generated with the use of the nonparametric Aalen–Johansen estimator, in which death is a competing risk, and additional cause-specific Cox regression was performed.
Safety was assessed in the population that included all the patients who had received a dose of tocilizumab or placebo, according to the trial agent that was first received. Patients who received either tocilizumab or placebo in error were included in the safety analysis.
Results
Patients
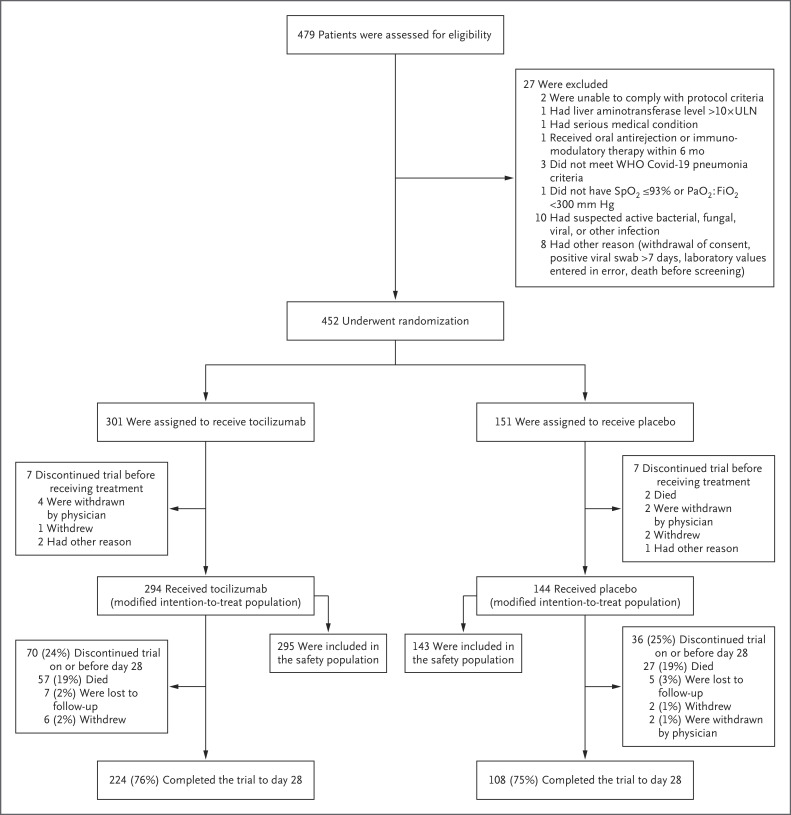
From April 3, 2020, through May 28, 2020, a total of 479 patients underwent screening. Of these patients, 452 patients underwent randomization, and 438 were included in the modified intention-to-treat population (294 in the tocilizumab group and 144 in the placebo group) (Figure 1). The number of patients who underwent randomization at each of the 62 trial sites ranged from 1 to 25. The safety population included 295 and 143 patients, respectively, because 1patient who was assigned to receive placebo received tocilizumab. The 28-day follow-up evaluation was completed in 224 of 294 patients (76.2%) in the tocilizumab group and in 108 of 144 patients (75.0%) in the placebo group. In addition to the patients who died, trial discontinuation before day 28 occurred in 13 patients (4.4%) in the tocilizumab group and in 9 (6.3%) in the placebo group. None of the patients discontinued participation because of safety reasons.
Figure 1. Enrollment and Outcomes.
One patient who was assigned to the placebo group received tocilizumab; this patient was included in the placebo group for analyses performed in the modified intention-to-treat population (mITT) and in the tocilizumab group for analyses performed in the safety population. One patient in each trial group died after they had withdrawn from the trial; therefore, for these patients, death is not listed as the reason for discontinuation. Pao2:Fio2 denotes the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen, Spo2 oxygen saturation, ULN upper limit of the normal range, and WHO World Health Organization.
Baseline demographic and disease characteristics were generally well balanced in the two trial groups. Approximately 70% of the patients in each group were men; in the tocilizumab group, 176 patients (59.9%) were White and 40 (13.6%) were Black, as compared with 76 (52.8%) and 26 (18.1%), respectively, in the placebo group. The mean (±SD) age was 60.9±14.6 years in the tocilizumab group and 60.6±13.7 years in the placebo group.
A lower percentage of patients received glucocorticoids in the tocilizumab group than in the placebo group both at baseline (57 [19.4%] vs. 41 [28.5%]) (Table 1) and during the trial (99 [33.7%] vs. 75 [52.1%]). The percentages of patients who received antiviral treatment were similar in the tocilizumab group and the placebo group, both at baseline (71 [24.1%] vs. 42 [29.2%]) and during the trial (68 [23.1%] vs. 35 [24.3%]). Convalescent plasma was administered during the trial to 10 patients (3.4%) in the tocilizumab group, including 5 (1.7%) at baseline, and in 6 patients (4.2%) in the placebo group, including 1 (0.7%) at baseline. A second dose of tocilizumab or placebo was administered to 65 patients (22.1%) in the tocilizumab group and 43 patients (29.9%) in the placebo group.
Table 1. Demographic and Disease Characteristics of the Patients at Baseline.*.
| Characteristic | Tocilizumab (N=294) |
Placebo (N=144) |
|---|---|---|
| Male sex — no. (%) | 205 (69.7) | 101 (70.1) |
| Age | ||
| Mean — yr | 60.9±14.6 | 60.6±13.7 |
| Distribution — no. (%) | ||
| 18–64 yr | 163 (55.4) | 81 (56.2) |
| 65–84 yr | 117 (39.8) | 60 (41.7) |
| ≥85 yr | 14 (4.8) | 3 (2.1) |
| Weight — kg | 88.9±23.6 | 88.1±24.3 |
| Race or ethnic group — no. (%)† | ||
| American Indian or Alaska Native | 8 (2.7) | 5 (3.5) |
| Asian | 28 (9.5) | 10 (6.9) |
| Black | 40 (13.6) | 26 (18.1) |
| Native Hawaiian or other Pacific Islander | 3 (1.0) | 5 (3.5) |
| White | 176 (59.9) | 76 (52.8) |
| Multiple | 0 | 1 (0.7) |
| Unknown | 39 (13.3) | 21 (14.6) |
| Region — no. (%) | ||
| Europe | 120 (40.8) | 59 (41.0) |
| North America | 174 (59.2) | 85 (59.0) |
| Illness severity on National Early Warning Score 2‡ | 7.1±3.0 | 7.0±3.0 |
| Ordinal scale for clinical status — no. (%)§ | ||
| 2 | 9 (3.1) | 6 (4.2) |
| 3 | 78 (26.5) | 44 (30.6) |
| 4 | 94 (32.0) | 39 (27.1) |
| 5 | 45 (15.3) | 15 (10.4) |
| 6 | 68 (23.1) | 40 (27.8)¶ |
| Interleukin-6 — ng/liter‖ | ||
| No. of patients with data | 233 | 100 |
| Mean value | 201.9±418.4 | 195.4±368.2 |
| Median (range) | 88.1 (3.1–4020) | 71.2 (3.1–2810) |
| C-reactive protein — mg/liter | ||
| No. of patients with data | 237 | 125 |
| Mean value | 168.4±101.4 | 172.6±114.0 |
| Median (range) | 157.2 (1.1–446.6) | 150.3 (1.6–499.6) |
| Ferritin — pmol/ml | ||
| No. of patients with data | 241 | 128 |
| Mean value | 6891±106,736 | 4027±45,431 |
| Median (range) | 2.3 (0.0–1,657,000) | 2.2 (0.1–514,000) |
| Mechanical ventilation | ||
| Patients — no. (%) | 111 (37.8) | 54 (37.5) |
| No. of days between initiation and randomization** | ||
| No. of patients with data | 107 | 51 |
| Mean no. | 5.1±5.5 | 4.3±4.5 |
| Median (range) | 3.0 (0.0–28.0) | 3.0 (0.0–20.0) |
| Symptoms at diagnosis — no. (%) | ||
| Fever | 193 (65.6) | 98 (68.1) |
| Cough | 216 (73.5) | 102 (70.8) |
| Shortness of breath | 213 (72.4) | 93 (64.6) |
| Gastrointestinal | 96 (32.7) | 41 (28.5) |
| Headache | 37 (12.6) | 21 (14.6) |
| Fatigue | 91 (31.0) | 44 (30.6) |
| Coexisting illness — no. (%)†† | ||
| ≥1 Diagnosis | 231 (78.6) | 124 (86.1) |
| Obesity | 63 (21.4) | 27 (18.8) |
| Diabetes | 105 (35.7) | 62 (43.1) |
| Cardiovascular impairment | 88 (29.9) | 35 (24.3) |
| Hypertension | 178 (60.5) | 94 (65.3) |
| Hepatic impairment | 6 (2.0) | 2 (1.4) |
| Chronic lung disease | 49 (16.7) | 22 (15.3) |
| No. of days from onset of Covid-19 symptoms | ||
| No. of patients with data | 291 | 143 |
| Mean no. | 12.1±6.6 | 11.4±6.9 |
| Median (range) | 11.0 (1.0–49.0) | 10.0 (2.0–50.0) |
| Other treatments — no. (%)‡‡ | ||
| Glucocorticoids | 57 (19.4) | 41 (28.5) |
| Antiviral drugs | 71 (24.1) | 42 (29.2) |
| Convalescent plasma | 5 (1.7) | 1 (0.7) |
Plus–minus values are means ±SD. For the evaluation of patients, baseline was defined as the last observation before the administration of tocilizumab or placebo on day 1.
Race or ethnic group was reported by the patients.
Scores for illness severity on the National Early Warning Score 2 evaluation range from 0 to 20, with higher scores indicating greater clinical risk.
The patients’ clinical status was assessed on an ordinal scale as follows: 1, discharged or ready for discharge; 2, hospitalization in a non–intensive care unit (ICU) without supplemental oxygen; 3, non–ICU hospitalization with supplemental oxygen; 4, ICU or non–ICU hospitalization with noninvasive ventilation or high-flow oxygen; 5, ICU hospitalization with mechanical ventilation; 6, ICU hospitalization with extracorporeal membrane oxygenation or mechanical ventilation and additional organ support; and 7, death. No patients were in categories 1 or 7 at baseline.
Included in this category is a patient who died on trial day 1 (ordinal category 7) but was listed in category 6 on day 1 before death.
Values below the lower limit of quantitation of 3.12 ng per liter were set at this value.
The number of days that patients were receiving mechanical ventilation before baseline were counted from the recorded initiation of intubation to the day before trial day 1. The earliest start date was used if multiple procedures were recorded. Patients who were first intubated on trial day 1 were categorized as having received mechanical ventilation for 0 days before baseline.
Coexisting conditions were coded according to the terms used in the Medical Dictionary for Regulatory Activities, version 23.0.
Listed here are any treatments that were administered during the period from trial day −7 until the initiation of tocilizumab or placebo on day 1. The use of glucocorticoids includes only systemic use. Antiviral drugs included lopinavir–ritonavir, remdesivir, lopinavir, ritonavir, chloroquine, hydroxychloroquine, and hydroxychloroquine sulfate.
Primary Outcome
The median value for clinical status on the ordinal scale at day 28 was 1.0 (95% confidence interval [CI], 1.0 to 1.0) in the tocilizumab group and 2.0 (non-ICU hospitalization without supplemental oxygen) (95% CI, 1.0 to 4.0) in the placebo group (between-group difference, −1.0; 95% CI, −2.5 to 0; P=0.31 by the van Elteren test) (Table 2, and Fig. S1 in the Supplementary Appendix). The percentage of patients who had missing data for the primary outcome was 3.7% in the tocilizumab group and 2.1% in the placebo group. In a prespecified analysis of the primary data (last postbaseline observation carried forward for missing data), the results were similar to those with the multiple-imputation approach (P=0.36 by the van Elteren test) (Table S1).
Table 2. Primary and Secondary Efficacy Outcomes.*.
| Outcome | Tocilizumab (N=294) |
Placebo (N=144) |
Difference or Hazard Ratio (95% CI) |
P Value |
|---|---|---|---|---|
| Primary outcome | ||||
| Median value for clinical status on 7-category ordinal scale at day 28 (95% CI) | 1.0 (1.0 to 1.0) | 2.0 (1.0 to 4.0) | –1.0 (–2.5 to 0.0) | 0.31† |
| Secondary outcomes | ||||
| Median value for clinical status at day 14 on 7-category ordinal scale (95% CI)‡ | 3.0 (2.0 to 4.0) | 4.0 (3.0 to 5.0) | –1.0 (–2.0 to 0.5) | |
| Death at day 28 — no. (%) | 58 (19.7) | 28 (19.4) | 0.3 (–7.6 to 8.2)§ | 0.94 |
| Median no. of days until hospital discharge or readiness for discharge (95% CI) | 20.0 (17.0 to 27.0) | 28.0 (20.0 to NE) | 1.35 (1.02 to 1.79)¶ | |
| Median no. of days until improvement by ≥2 categories on 7-category ordinal scale in clinical status (95% CI) | 14.0 (12.0 to 17.0) | 18.0 (15.0 to 28.0) | 1.26 (0.97 to 1.64)¶ | |
| Median no. of days in ICU (95% CI) | 9.8 (7.0 to 15.7) | 15.5 (8.7 to 25.5) | –5.8 (–15.0 to 2.9) | |
| Incidence of ICU stay among patients not in ICU at baseline — no./total no. (%) | 27/127 (21.3) | 23/64 (35.9) | –14.8 (–28.6 to –1.0)‖ | |
| Median no. of ventilator-free days at day 28 (95% CI) | 22.0 (18.0 to 28.0) | 16.5 (11.0 to 26.0) | 5.5 (–2.8 to 13.0) | |
| Incidence of mechanical ventilation among patients not receiving mechanical ventilation at randomization — no./total no. (%) | 51/183 (27.9) | 33/90 (36.7) | –8.9% (–20.7 to 3.0)‖ | |
| Clinical failure among patients not receiving mechanical ventilation at randomization — no./total no. (%)** | 53/183 (29.0) | 38/90 (42.2) | 0.61 (0.40 to 0.94)†† |
Primary and secondary efficacy outcomes were analyzed in the modified intention-to-treat population. ICU denotes intensive care unit, and NE not evaluable.
This P value was calculated by means of the van Elteren test stratified according to region and the presence or absence of mechanical ventilation at randomization.
In this category, the last observation was carried forward for missing data.
This weighted difference in percentages was calculated with the use of the Cochran–Mantel–Haenszel test stratified according to region and the presence or absence of mechanical ventilation at randomization.
This value is a hazard ratio that was calculated by means of a Cox proportional-hazards model stratified according to region and the presence or absence of mechanical ventilation at randomization.
This weighted difference in percentages was calculated with the use of the Cochran–Mantel–Haenszel test stratified according to region at randomization.
Clinical failure was defined as death, withdrawal from the trial during hospitalization, transfer to the ICU, or the initiation of invasive mechanical ventilation by 28 days.
This value is a hazard ratio that was calculated by means of a Cox proportional-hazards model stratified according to region. This post hoc analysis was not prespecified.
The prespecified point estimate of the treatment effect for the primary outcome was an odds ratio; the assumption of proportional odds was not met for this analysis (P=0.0005 for trial group). Therefore, odds ratios should not be used for statistical comparisons. The odds ratios are provided in Table S2 for a complete representation of the planned analyses. Observed proportions and fitted data from the model are provided in Figure S2 and Table S3.
Secondary Outcomes
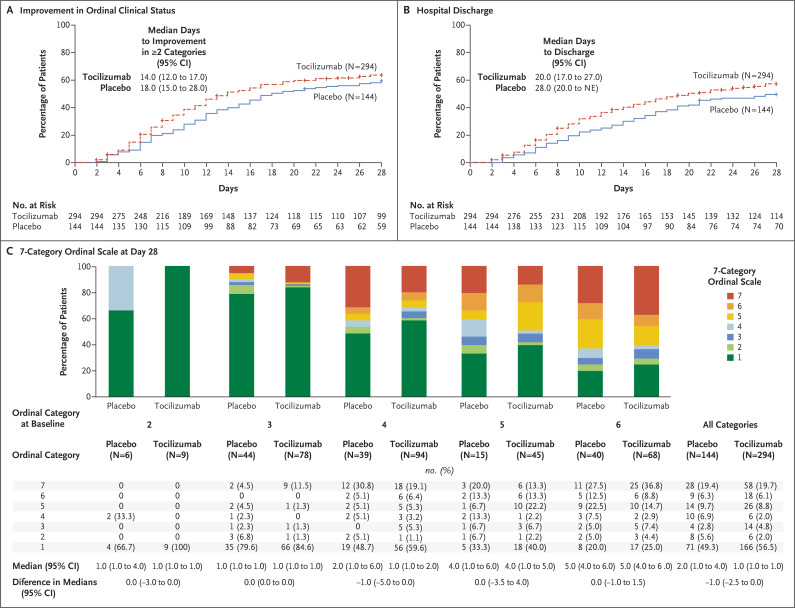
At day 14, the median value for clinical status on the seven-category ordinal scale was 3.0 (95% CI, 2.0 to 4.0) in the tocilizumab group and 4.0 (95% CI, 3.0 to 5.0) in the placebo group, for a between-group difference of −1.0 (95% CI, −2.0 to 0.5) (Table 2 and Fig. S3A). Death was reported by day 28 in 58 patients (19.7%) in the tocilizumab group and in 28 (19.4%) in the placebo group, for a weighted difference of 0.3 percentage points (95% CI, −7.6 to 8.2; P=0.94). The median number of ventilator-free days was 22.0 (95% CI, 18.0 to 28.0) with tocilizumab and 16.5 (95% CI, 11.0 to 26.0) with placebo, for a difference of 5.5 days (95% CI, −2.8 to 13.0) (Fig. S4 and Table S6). The median time from baseline until patients had an improvement by at least two categories on the seven-category ordinal scale was 14 days (95% CI, 12 to 17) in the tocilizumab group and 18 days (95% CI, 15 to 28) in the placebo group (Cox proportional-hazards ratio, 1.26; 95% CI, 0.97 to 1.64) (Figure 2A and Table S7). The median time until patients were discharged from the hospital or ready to be discharged was 20 days (95% CI, 17 to 27) in the tocilizumab group and 28 days (95% CI, 20 to not evaluable) in the placebo group (Cox proportional-hazards ratio, 1.35; 95% CI, 1.02 to 1.79) (Figure 2B and Table S7). The median duration of ICU stay was 9.8 days in the tocilizumab group and 15.5 days in the placebo group, for a difference of 5.8 days (95% CI, –15.0 to 2.9). Data regarding the time to improvement in clinical status, time to hospital discharge or readiness for discharge, and mortality are provided in Figure S5.
Figure 2. Changes in Clinical Status and Hospital Discharge.
Panel A shows the time until improvement from baseline by at least two categories on the seven-category ordinal scale that was used to assess the patients’ clinical status. Panel B shows the number of days until hospital discharge or readiness for discharge by day 28. Data in Panels A and B are plotted as 1 minus the Kaplan–Meier estimator. Data for patients who discontinued the trial or were lost to follow-up for any reason were censored (indicated by hatch marks) at the time of their last ordinal-scale assessment. Data for patients who died were censored at day 28. Panel C shows the patients’ clinical status as assessed on the seven-category ordinal scale at day 28, according to the category at baseline. Categories on the ordinal scale were as follows: 1, discharged or ready for discharge; 2, hospitalization in a non–intensive care unit (ICU) without supplemental oxygen; 3, non–ICU hospitalization with supplemental oxygen; 4, ICU or non–ICU hospitalization with noninvasive ventilation or high-flow oxygen; 5, ICU hospitalization with mechanical ventilation; 6, ICU hospitalization with extracorporeal membrane oxygenation or mechanical ventilation and additional organ support; and 7, death. Data in category 6 include a patient who died on trial day 1 but had been assigned to category 6 on that day before receiving placebo.
Subgroup Analyses
Among the patients who were receiving mechanical ventilation at randomization, the median value for clinical status on the ordinal scale at day 28 was 5.0 (95% CI, 3.0 to 5.0) in the tocilizumab group (in 111 patients) and 5.0 (95% CI, 4.0 to 6.0) in the placebo group (in 54 patients). Among the patients who were not receiving mechanical ventilation at randomization, the median value for clinical status at day 28 was 1.0 (95% CI, 1.0 to 1.0) in both the tocilizumab group (in 183 patients) and the placebo group (in 90 patients) (Fig. S6). Clinical status at day 28 and day 14 according to the ordinal-scale category at baseline are presented in Figure 2C and Figure S3B, respectively.
Among the patients who were not receiving mechanical ventilation at randomization, the initiation of the procedure during the trial occurred in 51 of 183 patients (27.9%) in the tocilizumab group and in 33 of 90 patients (36.7%) in the placebo group, for a weighted difference of –8.9 percentage points (95% CI, –20.7 to 3.0). Among the patients who were not being treated in the ICU at baseline, transfer to the ICU occurred in 27 of 127 patients (21.3%) in the tocilizumab group and in 23 of 64 patients (35.9%) in the placebo group, for a weighted difference of –14.8 percentage points (95% CI, –28.6 to –1.0). In a post hoc analysis, among the patients who were not receiving mechanical ventilation at baseline, clinical failure (as defined in the Methods section) occurred in 53 of 183 patients (29.0%) in the tocilizumab group and in 38 of 90 patients (42.2%) in the placebo group (hazard ratio, 0.61; 95% CI, 0.40 to 0.94).
Safety
In the safety population, adverse events were reported in 77.3% of 295 patients in the tocilizumab group and in 81.1% of 143 patients in the placebo group through day 28 (Table 3); serious adverse events were reported in 34.9% and 38.5%, respectively. Fatal events occurred in 58 patients (19.7%) in the tocilizumab group and in 28 (19.6%) in the placebo group through day 28. The most commonly reported cause of death was Covid-19 pneumonia. Adverse events of special interest with respect to tocilizumab were generally well balanced between the trial groups. No patients who received tocilizumab had anaphylaxis. By day 28, 76 serious infections were reported in 62 patients (21.0%) in the tocilizumab group and 49 serious infections in 37 patients (25.9%) in the placebo group. Similar percentages of patients in each trial group had adverse events and serious adverse events at the clinical cutoff date of June 24, 2020 (Tables S8 and S9).
Table 3. Adverse Events (Safety Population).
| Adverse Event | Tocilizumab (N=295) |
Placebo (N=143) |
|---|---|---|
| Any adverse event | ||
| Patients with ≥1 event — no. (%) | 228 (77.3) | 116 (81.1) |
| No. of events | 778 | 360 |
| Any serious adverse event — no. (%) | ||
| Patients with ≥1 event | 103 (34.9) | 55 (38.5) |
| No. of events | 160 | 101 |
| Death — no. (%) | 58 (19.7) | 28 (19.6) |
| Patients with adverse events of special interest — no. (%) | ||
| Infection | 113 (38.3) | 58 (40.6) |
| Serious | 62 (21.0) | 37 (25.9) |
| Opportunistic* | 1 (0.3) | 1 (0.7) |
| Medically confirmed cancer | 1 (0.3) | 0 |
| Hypersensitivity† | 19 (6.4) | 4 (2.8) |
| Anaphylaxis per Sampson criteria | 0 | 1 (0.7) |
| Hepatic event | 5 (1.7) | 3 (2.1) |
| Abnormal liver-function value‡ | 6 (2.0) | 6 (4.2) |
| Myocardial infarction | 3 (1.0) | 2 (1.4) |
| Stroke | 3 (1.0) | 2 (1.4) |
| Bleeding | ||
| Any | 45 (15.3) | 16 (11.2) |
| Serious | 13 (4.4) | 5 (3.5) |
| Serious infection reported in >1% of patients in either trial group§ | ||
| Covid-19 resulting in death | 39 (13.2) | 18 (12.6) |
| Septic shock | 7 (2.4) | 6 (4.2) |
| Pneumonia | ||
| Any source | 7 (2.4) | 4 (2.8) |
| Bacterial | 6 (2.0) | 2 (1.4) |
| Sepsis | 3 (1.0) | 4 (2.8) |
| Bacteremia | 2 (0.7) | 3 (2.1) |
Opportunistic infections were reported in one patient with candida sepsis in the tocilizumab group and in one patient with respiratory moniliasis in the placebo group.
Hypersensitivity reactions include all events that occurred during or within 24 hours after the infusion of tocilizumab or placebo and that were assessed by the investigator as being related to the infused agent, regardless of whether the episode was clinically consistent with hypersensitivity.
The determination of an abnormal liver-function value was based on the criteria of Hy’s law. Included among these abnormalities was an alanine or aspartate aminotransferase level of more than three times the upper limit of the normal range (ULN) with a bilirubin level of more than two times the ULN.
Listed are the preferred terms in the Medical Dictionary for Regulatory Activities, version 23.0.
Discussion
In this trial involving hospitalized patients with severe Covid-19 pneumonia, we found no significant difference in clinical status between the tocilizumab group and the placebo group at day 28. No mortality benefit was associated with the use of tocilizumab, although the trial was not powered for this outcome. No safety concerns associated with tocilizumab in this population were observed. The data suggested a possible benefit for tocilizumab in the time until hospital discharge (or readiness for discharge) and in the duration of ICU stay, both of which require additional study. Adverse events, including those of special interest for tocilizumab (bleeding, hepatic, and cardiac events), were generally well balanced in the two trial groups, and the incidences of infections or serious infections were lower in the tocilizumab group.
The design and conduct of clinical trials involving patients with Covid-19 present unique challenges and limitations. Our trial population was intentionally chosen to be heterogeneous with regard to demographic and clinical characteristics, previous or concurrent treatments, and disease severity to allow for an assessment of potential benefit across a broad range of patients and to reflect real-world practice in the expanding pandemic. This heterogeneity is reflected in the wide range of values for interleukin-6, C-reactive protein (CRP), and ferritin at baseline (Table 1). Median values were consistent with those in studies involving patients with severe or critical Covid-19 pneumonia.7,25,26 Despite this heterogeneity, the percentage of patients who were discharged or ready for discharge by day 28 was higher in the tocilizumab group than in the placebo group across the baseline ordinal scale of clinical-status categories, whereas no consistent pattern was observed for mortality.
Clinical status on the ordinal scale was chosen as the primary outcome because it integrated several outcomes that are potentially important in the management of a pandemic illness, including the time until hospital discharge, escalating levels of inpatient care, and death. However, this outcome has important limitations, including sensitivity to differences in local clinical practice, lack of proportionality between categories, insensitivity to events before the time point of assessment, and lack of an established minimum clinically important difference for therapeutic effect. Different primary outcomes, including the time until recovery or hospital discharge, have been selected for other studies.27
The lack of standardized treatment across trial sites and countries is an important limitation of our trial, since there was extensive potential for interactions with antiviral drugs and glucocorticoids. More patients in the placebo group than in the tocilizumab group received concomitant glucocorticoids; however, this imbalance is unlikely to have obscured a significant treatment effect because mortality was similar in the two trial groups regardless of glucocorticoid use and was higher in patients who received glucocorticoids than in those who did not in each group (Table S10). In addition, it is possible that worsening clinical status among patients in the placebo group could have led to increased glucocorticoid use and more frequent administration of a second dose of placebo. Patients who received a second dose of either tocilizumab or placebo generally had worse outcomes (Fig. S7), which may reflect a selection bias, because only patients whose condition did not improve after the first dose could be considered for a second dose. The administration of tocilizumab typically results in decreased CRP levels and in increased interleukin-6 levels; therefore, to minimize the risk of unblinding among investigators, both levels were measured in central laboratories and not reported to the trial sites.
Among all the trial patients, the range of the time from the onset of symptoms to baseline was 1 to 50 days. Of note, there was no clear pattern of responses in patients who were treated earlier rather than later in the course of illness (Fig. S7), whereas other studies have shown different responses.28
Since the time when our trial was initiated, there has been substantial evolution in our understanding of what constitutes standard care and of the natural history of Covid-19 and its associated complications. On the basis of current knowledge, effective treatments and meaningful outcomes for clinical trials are likely to differ for different stages of disease. Future trials should be more narrowly focused or enroll a larger number of patients to allow for further stratification on the basis of disease severity and other baseline characteristics.
Results of this trial must be interpreted in the context of therapies for severe Covid-19 pneumonia. Among the treatments for hospitalized patients with Covid-19 that have been investigated in randomized, controlled trials, dexamethasone was found to reduce mortality only among patients who were receiving mechanical ventilation or supplemental oxygen at randomization.28 Remdesivir shortened the time until recovery but did not have a significant effect on 14-day mortality.27 Clinical trials are under way to investigate many potential treatments, including other antiviral and antiinflammatory drugs, other targeted immunomodulators (e.g., sarilumab, anakinra, baricitinib, and canakinumab), anticoagulants, and antifibrotics (tyrosine kinase inhibitors).29 However, the need for effective treatments for patients with severe Covid-19 pneumonia continues to be a major challenge at this point in the pandemic.
Acknowledgments
We thank Sara Duggan, Ph.D., of ApotheCom, for providing editorial support.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on February 25, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported F. Hoffmann–La Roche and by a grant (HHSO100201800036C) from the Biomedical Advanced Research and Development Authority of the Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services. Drs. Cooper and Youngstein were supported in part by the Biomedical Research Centre of the United Kingdom National Institute for Health Research. Dr. Malhotra is supported by the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.World Health Organization. Coronavirus disease (COVID-19) pandemic. 2020. (https://www.who.int/emergencies/diseases/novel-coronavirus-2019).
- 2.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity 2020;52:910-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV-1 and MERS-CoV viral load dynamics, duration of viral shedding and infectiousness — a living systematic review and meta-analysis. July 29, 2020. (https://www.medrxiv.org/content/10.1101/2020.07.25.20162107v2). preprint. [DOI] [PMC free article] [PubMed]
- 6.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020;27(6):992.e3-1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol 2020;92:2283-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Pang J, Ji P, et al. Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis. J Med Virol 2020. May 29 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care 2020;24:353-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubbert-Roth A, Furst DE, Nebesky JM, Jin A, Berber E. A review of recent advances using tocilizumab in the treatment of rheumatic diseases. Rheumatol Ther 2018;5:21-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cellina M, Orsi M, Bombaci F, Sala M, Marino P, Oliva G. Favorable changes of CT findings in a patient with COVID-19 pneumonia after treatment with tocilizumab. Diagn Interv Imaging 2020;101:323-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michot J-M, Albiges L, Chaput N, et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol 2020;31:961-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Song K, Tong F, et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv 2020;4:1307-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antwi-Amoabeng D, Kanji Z, Ford B, Beutler BD, Riddle MS, Siddiqui F. Clinical outcomes in COVID-19 patients treated with tocilizumab: an individual patient data systematic review. J Med Virol 2020;92:2516-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020;92:814-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mastroianni A, Greco S, Apuzzo G, et al. Subcutaneous tocilizumab treatment in patients with severe COVID-19-related cytokine release syndrome: an observational cohort study. EClinicalMedicine 2020;24:100410-100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaye A, Siegel R. The efficacy of IL-6 inhibitor tocilizumab in reducing severe COVID-19 mortality: a systematic review. September 3, 2020. (https://www.medrxiv.org/content/10.1101/2020.07.10.20150938v2). preprint. [DOI] [PMC free article] [PubMed]
- 22.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2020;2(8):e474-e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis 2020. July 11 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med 2019;200:828-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Valle DM, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020;26:1636-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 — preliminary report: reply. N Engl J Med 2020;383:994-994. [DOI] [PubMed] [Google Scholar]
- 28.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782-793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.