Abstract
Background
The efficacy of interleukin-6 receptor antagonists in critically ill patients with coronavirus disease 2019 (Covid-19) is unclear.
Methods
We evaluated tocilizumab and sarilumab in an ongoing international, multifactorial, adaptive platform trial. Adult patients with Covid-19, within 24 hours after starting organ support in the intensive care unit (ICU), were randomly assigned to receive tocilizumab (8 mg per kilogram of body weight), sarilumab (400 mg), or standard care (control). The primary outcome was respiratory and cardiovascular organ support–free days, on an ordinal scale combining in-hospital death (assigned a value of −1) and days free of organ support to day 21. The trial uses a Bayesian statistical model with predefined criteria for superiority, efficacy, equivalence, or futility. An odds ratio greater than 1 represented improved survival, more organ support–free days, or both.
Results
Both tocilizumab and sarilumab met the predefined criteria for efficacy. At that time, 353 patients had been assigned to tocilizumab, 48 to sarilumab, and 402 to control. The median number of organ support–free days was 10 (interquartile range, −1 to 16) in the tocilizumab group, 11 (interquartile range, 0 to 16) in the sarilumab group, and 0 (interquartile range, −1 to 15) in the control group. The median adjusted cumulative odds ratios were 1.64 (95% credible interval, 1.25 to 2.14) for tocilizumab and 1.76 (95% credible interval, 1.17 to 2.91) for sarilumab as compared with control, yielding posterior probabilities of superiority to control of more than 99.9% and of 99.5%, respectively. An analysis of 90-day survival showed improved survival in the pooled interleukin-6 receptor antagonist groups, yielding a hazard ratio for the comparison with the control group of 1.61 (95% credible interval, 1.25 to 2.08) and a posterior probability of superiority of more than 99.9%. All secondary analyses supported efficacy of these interleukin-6 receptor antagonists.
Conclusions
In critically ill patients with Covid-19 receiving organ support in ICUs, treatment with the interleukin-6 receptor antagonists tocilizumab and sarilumab improved outcomes, including survival. (REMAP-CAP ClinicalTrials.gov number, NCT02735707.)
Globally, more than 112 million cases of coronavirus disease 2019 (Covid-19) have been reported, with more than 2.49 million deaths.1 Only glucocorticoids are known to improve survival among severely ill patients.2 The benefit from glucocorticoids in critically ill patients supports the concept that an excessive host inflammatory response is responsible for much of the serious illness and death from Covid-19.
Interleukin-6 is released in response to infection and stimulates inflammatory pathways as part of the acute-phase response. Tocilizumab and sarilumab are monoclonal antibodies that inhibit both membrane-bound and soluble interleukin-6 receptors and are used to treat inflammatory conditions, such as rheumatoid arthritis, as well as cytokine release syndrome after chimeric antigen receptor (CAR) T-cell therapy (tocilizumab). Their clinical use has been described in Covid-193-5; however, randomized, controlled trials to date have largely been negative, with the most positive study showing a decreased risk of mechanical ventilation but no effect on mortality.6-11 We investigated the effectiveness of tocilizumab and sarilumab on survival and organ support in critically ill patients with Covid-19 in the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
Methods
Trial Design and Oversight
REMAP-CAP is an international, adaptive platform trial designed to determine effective treatment strategies for patients with severe pneumonia in both pandemic and nonpandemic settings. The design of REMAP-CAP and its first results, regarding glucocorticoids in patients with Covid-19, were published previously.12,13
Patients eligible for the platform are assessed for eligibility to potentially undergo randomization to multiple interventions across multiple domains. A “domain” covers a common therapeutic area (e.g., antiviral therapy) and contains two or more interventions (including control; e.g., “no antiviral”). Patients are randomly assigned to one intervention in each domain for which they are eligible. REMAP-CAP is defined by a master (“core”) protocol with individual appendixes for each domain, regional governance, and adaptations for a declared pandemic (see the protocol, available with the full text of this article at NEJM.org).
The trial was designed and managed by an international trial steering committee whose members were unaware of the trial group assignments and an independent data and safety monitoring board whose members were aware of the trial group assignments. The trial is approved by relevant regional ethics committees and is conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Written or verbal informed consent, in accordance with regional legislation, is obtained from all the patients or their surrogates.
The trial has multiple international funders. Roche Products and Sanofi supported the trial through provision of tocilizumab and sarilumab in the United Kingdom. The funders as well as Roche and Sanofi had no role in designing the trial, analyzing the data, writing the manuscript, or making the decision to submit the manuscript for publication. All the authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol and statistical analysis plan.
Patients
Critically ill patients, 18 years of age or older, with either clinically suspected or microbiologically confirmed Covid-19 who were admitted to an intensive care unit (ICU) and receiving respiratory or cardiovascular organ support were classified as having a severe disease state and were eligible for enrollment in the Covid-19 Immune Modulation Therapy domain. Respiratory organ support was defined as invasive or noninvasive mechanical ventilation, including through high-flow nasal cannulae if the flow rate was more than 30 liters per minute and the fraction of inspired oxygen was more than 0.4. Cardiovascular organ support was defined as the intravenous infusion of any vasopressor or inotrope. Patients were excluded if there was a presumption that death was imminent with a lack of commitment to full support or if they had previously participated in REMAP-CAP within 90 days. Patients had to be enrolled within 24 hours after starting organ support in the ICU. Additional exclusion criteria, specific to the Immune Modulation Therapy domain, are listed in the Supplementary Appendix, available at NEJM.org.
Randomization
The Immune Modulation Therapy domain included five interventions — two interleukin-6 receptor antagonists, tocilizumab and sarilumab; an interleukin-1 receptor antagonist, anakinra; interferon beta-1a; and control (no immune modulation). Investigators at each site prespecified at least two interventions, one of which had to be control, to which patients would be randomly assigned. Participants were assigned by means of a centralized computer program to each intervention, starting with balanced assignment for tocilizumab, sarilumab, or control, with actual proportions dependent on the number of interventions available at each site.
Tocilizumab, at a dose of 8 mg per kilogram of actual body weight (up to a maximum of 800 mg), was administered as an intravenous infusion over a period of 1 hour; this dose could be repeated 12 to 24 hours later at the discretion of the treating clinician if clinical improvement was judged insufficient. Sarilumab, at a dose of 400 mg, was administered as an intravenous infusion once only. All investigational drugs were dispensed by local pharmacies and were open label.
Procedures
Other aspects of patient care were provided according to the standard of care at each site. In addition to receiving assignments in this domain, participants could be randomly assigned to other interventions within other domains, depending on domains active at the site, patient eligibility, and consent (see the protocol and www.remapcap.org). Randomization to the Corticosteroid domain for Covid-19 closed on June 17, 2020.13 Thereafter, glucocorticoids were allowed according to the recommended standard of care. Although clinical staff were aware of the intervention assignment of individual patients, neither they nor the members of the international trial steering committee were provided any information about aggregate patient outcomes.
Outcome Measures
The primary outcome was the number of respiratory and cardiovascular organ support–free days up to day 21. The definitions of respiratory and cardiovascular organ support were the same as in the inclusion criteria. In this composite ordinal outcome, all deaths within the hospital are assigned the worst outcome (–1). Among survivors, days free of respiratory and cardiovascular organ support are calculated up to day 21, such that a higher number represents faster recovery. In a previous Food and Drug Administration–approved trial, 1.5 days was considered to be a minimal clinically important difference.14 Secondary outcomes were all prespecified, and details are provided in the Supplementary Appendix.
Statistical Analysis
REMAP-CAP uses a Bayesian design with no maximum sample size. Regular, interim analyses are performed and randomization continues, potentially with response-adaptive randomization with preferential assignment to the interventions that appear most favorable, until predefined statistical criteria are met.
The primary analysis was generated from a Bayesian cumulative logistic model, which calculated posterior probability distributions of organ support–free days to day 21 (primary outcome) on the basis of evidence accumulated in the trial and the prior probability distribution (the assumed previous knowledge). Prior distributions for individual treatment effects were neutral.
The primary model was adjusted for location (site, nested within country), age (categorized into six groups), sex, and time period (2-week calendar epochs) to account for rapid changes in clinical care and outcomes over time during the pandemic. The model contained treatment effects for each intervention within each domain and prespecified treatment-by-treatment interactions across domains. The treatment effects for tocilizumab and sarilumab were “nested” in the model with a hierarchical prior distribution sharing the same neutral prior distribution in an overall “interleukin-6 receptor antagonist effect,” but distinct intervention-specific effects were estimated. When consistent effects are observed for tocilizumab and sarilumab, the posterior distribution for each intervention effect is shrunk toward the overall estimate of the interleukin-6 receptor antagonist effect.15
The primary analysis was conducted by the statistical analysis committee in all the patients with severe disease randomly assigned to any domain up to November 19, 2020 (and with complete follow-up). The inclusion of additional patients who were enrolled outside the Immune Modulation Therapy domain allows incorporation of all information, which provides robust estimation of the coefficients of all covariates, according to the principle of the REMAP-CAP design.12,13 Not all patients were eligible for all domains or for all interventions (dependent on active domains and interventions at the site, eligibility criteria, and patient or surrogate consent). Therefore, the model included covariate terms reflecting each patient’s domain eligibility, such that the estimate of the effectiveness of an intervention, relative to any other intervention within that domain, was generated from patients who might have been eligible to be randomly assigned to those interventions within the domain.
The cumulative log odds for the primary outcome were modeled such that a value greater than 0 reflects an increase in the cumulative log odds for the outcome of organ support–free days, implying benefit. Missing outcomes were not imputed. The model was fit with the use of a Markov chain Monte Carlo algorithm that drew iteratively (10,000 draws) from the joint posterior distribution, which allowed calculation of posterior odds ratios with their 95% credible intervals and the probability that each intervention (including control) was optimal (i.e., better than all other treatments tested) in the domain, that an intervention was superior to control (efficacy), that two noncontrol interventions were equivalent, or that an intervention was futile as compared with control. An odds ratio greater than 1 represents improved survival, more organ support–free days, or both. The predefined statistical criteria for determining trial conclusions and for triggering the disclosure of results were as follows: a posterior probability of greater than 99% that an intervention was more effective than all other interventions; an inferiority conclusion if the posterior probability that an intervention was more effective than all other interventions was less than 0.25%; intervention efficacy if the posterior probability that the odds ratio was greater than 1.0 as compared with control was greater than 99%; intervention futility if the posterior probability that the odds ratio was greater than 1.2 as compared with control was less than 5%; and equivalence if the probability that the odds ratio was between 0.83 and 1.2 for two noncontrol interventions was greater than 90%.
Analysis of the primary outcome was then repeated in a second model that used only data from patients enrolled in domains that had stopped and were unblinded at the time of analysis with no adjustment for assignment in other ongoing domains. The secondary outcomes were also analyzed in this second model. One subgroup analysis, based on terciles of serum C-reactive protein levels at enrollment, was prespecified. Further details of all analyses are provided in the Supplementary Appendix. Prespecified analyses are listed in Section 15 of the statistical analysis plan, available with the protocol. Data management and summaries were created with the use of R software, version 3.6.0; the primary analysis was computed with R software, version 4.0.0, with the use of the rstan package, version 2.21.1. Additional data management and analyses were performed with SQL Server 2016; SPSS software, version 26; and Stata software, version 14.2.
Results
Enrollment and Randomization
The first patient with Covid-19 was enrolled in REMAP-CAP on March 9, 2020, and the first patient underwent randomization in the Immune Modulation Therapy domain on April 19 as tocilizumab became available. Sarilumab only became available on June 20. On the basis of an interim analysis as of October 28, the independent data and safety monitoring board reported that tocilizumab had met the statistical criteria for efficacy (posterior probability, 99.75%; odds ratio, 1.87; 95% credible interval, 1.20 to 2.76). According to the protocol, further assignment to control closed on November 19, with randomization continuing between different active immune modulation interventions. At that time, 2046 patients who had severe disease had undergone randomization in at least one REMAP-CAP domain and 895 had undergone randomization in the Immune Modulation Therapy domain (366 were assigned to tocilizumab, 48 to sarilumab, 412 to control, and 69 to other interventions within the domain) at 113 sites across six countries (Figure 1). Thirty patients subsequently withdrew consent, and 11 patients had missing primary outcomes. After a subsequent interim analysis, the data and safety monitoring board reported that sarilumab had also met the statistical criteria for efficacy, so these results are also reported.
Figure 1. Screening, Enrollment, Randomization, and Inclusion in Analysis.
Patients who were ineligible for the platform or the Immune Modulation Therapy domain could meet more than one ineligibility criterion; full details are provided in the Supplementary Appendix. Contraindications to agents in the Immune Modulation Therapy domain include hypersensitivity, elevated levels of alanine aminotransferase or aspartate aminotransferase, thrombocytopenia, and pregnancy. Among patients who underwent randomization to an Immune Modulation Therapy domain intervention, the group assigned to receive no immune modulation only included patients when tocilizumab or sarilumab was a randomization option (i.e., direct concurrent controls). Other interventions included anakinra, interferon beta-1a, and no immune modulation when tocilizumab or sarilumab was not available as a randomization option (i.e., nondirect controls). The primary analysis of alternative interventions within the Immune Modulation Therapy domain is estimated from a model that adjusts for patient factors and for assignment to interventions in other domains. To obtain the most reliable estimation of the effect of these patient factors and of other interventions on the primary outcome, all the patients who were enrolled in the severe coronavirus disease 2019 (Covid-19) cohort (for whom there is consent and follow-up) are included. However, the model also factors eligibility for the Immune Modulation Therapy domain and its interventions, such that the final estimate of the effectiveness of an Immune Modulation Therapy domain intervention relative to any other within that domain is generated from the patients who might have been eligible to undergo randomization to those interventions within the domain. ICU denotes intensive care unit, and REMAP-CAP Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia.
Patients
Baseline characteristics were balanced across intervention groups and typical of a critically ill population with Covid-19 (Table 1 and Table S1 in the Supplementary Appendix). All but 3 patients were receiving respiratory support at the time of randomization, including oxygen through high-flow nasal cannulae (29%), noninvasive ventilation (42%), and invasive mechanical ventilation (29%). The majority of patients (707) were enrolled after June 17 and the announcement of the dexamethasone result from the RECOVERY trial16,17; of these patients, 93% (610 of 654) were treated with glucocorticoids at enrollment or within the following 48 hours (Table S2). Of the 158 patients recruited before June 17, a total of 107 underwent randomization in the Corticosteroid domain within REMAP-CAP, with 41 assigned to a 7-day course of hydrocortisone, 39 to shock-dependent hydrocortisone, and 27 to no hydrocortisone.13 Remdesivir use was recorded in 265 of 807 patients (33%).
Table 1. Baseline Characteristics of the Patients in the Immune Modulation Therapy Domain.*.
| Characteristic | Tocilizumab (N=353) |
Sarilumab (N=48) |
Control (N=402)† |
All Patients (N=865)‡ |
|---|---|---|---|---|
| Age — yr | 61.5±12.5 | 63.4±13.4 | 61.1±12.8 | 61.4±12.7 |
| Male sex — no. (%) | 261 (74) | 39 (81) | 283 (70) | 629 (73) |
| Race or ethnic group — no./total no. (%)§ | ||||
| White | 160/228 (70) | 29/39 (74) | 206/279 (74) | 420/580 (72) |
| Asian | 41/228 (18) | 8/39 (21) | 47/279 (17) | 99/580 (17) |
| Black | 12/228 (5) | 1/39 (3) | 9/279 (3) | 23/580 (4) |
| Mixed | 2/228 (1) | 0/39 | 5/279 (2) | 7/580 (1) |
| Other | 13/228 (6) | 1/39 (3) | 12/279 (4) | 31/580 (5) |
| Body-mass index¶ | ||||
| Patients evaluated | 342 | 39 | 377 | 815 |
| Median (IQR) | 30.5 (26.9–34.9) | 29.2 (26.0–33.8) | 30.9 (27.1–34.9) | 30.5 (26.8–34.9) |
| APACHE II score‖ | ||||
| Patients evaluated | 337 | 42 | 381 | 820 |
| Median (IQR) | 13 (8–19) | 10 (7–16) | 12 (8–18) | 12 (8–19) |
| Confirmed SARS-CoV-2 infection — no./total no. (%)** | 284/345 (82) | 44/47 (94) | 334/394 (85) | 715/847 (84) |
| Median time to enrollment (IQR) | ||||
| From hospital admission — days | 1.2 (0.8–2.8) | 1.4 (0.9–2.8) | 1.2 (0.8–2.8) | 1.2 (0.8–2.8) |
| From ICU admission — hr | 13.1 (6.6–19.0) | 16.0 (11.4–20.8) | 14.0 (6.8–19.5) | 13.6 (6.6–19.4) |
| Acute respiratory support — no./total no. (%) | ||||
| None or supplemental oxygen only | 1/353 (<1) | 0/48 | 2/402 (<1) | 3/865 (<1) |
| High-flow nasal cannulae | 101/353 (29) | 17/48 (35) | 110/402 (27) | 249/865 (29) |
| Noninvasive ventilation only | 147/353 (42) | 23/48 (48) | 169/402 (42) | 359/865 (42) |
| Invasive mechanical ventilation | 104/353 (29) | 8/48 (17) | 121/402 (30) | 254/865 (29) |
| Vasopressor support — no./total no. (%) | 63/353 (18) | 4/48 (8) | 79/402 (20) | 163/865 (19) |
| Pao2:Fio2 | ||||
| Patients evaluated | 335 | 35 | 354 | 780 |
| Median (IQR) | 115 (89–162) | 126 (99–157) | 118 (89–169) | 116.5 (89–165) |
| Laboratory values†† | ||||
| C-reactive protein | ||||
| Patients evaluated | 207 | 37 | 244 | 533 |
| Median (IQR) — μg/ml | 150 (85–221) | 136 (105–204) | 130 (71–208) | 136 (79–208) |
| d-dimer | ||||
| Patients evaluated | 159 | 20 | 172 | 385 |
| Median (IQR) — ng/ml | 832 (461–1763) | 828 (355–1435) | 1010 (500–2115) | 910 (480–1916) |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. Fio2 denotes the fraction of inspired oxygen, ICU intensive care unit, IQR interquartile range, and Pao2 the partial pressure of arterial oxygen.
Control patients include all the patients randomly assigned to standard care who were also eligible to be randomly assigned to tocilizumab or sarilumab (i.e., direct concurrent controls).
All patient includes those who underwent randomization in the Immune Modulation Therapy domain, with assignment to control (including where tocilizumab and sarilumab were not randomization options; i.e., nondirect controls), tocilizumab, sarilumab, anakinra, or interferon beta-1a.
Race and ethnic group were reported by the patients. Data collection was not approved in Canada and continental Europe. “Other” includes “declined” and “multiple.”
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Scores on the Acute Physiology and Chronic Health Evaluation (APACHE) II range from 0 to 71, with higher scores indicating greater severity of illness.
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was confirmed by a respiratory tract polymerase-chain-reaction test.
Values were from the sample collected closest to randomization, up to 8 hours before randomization. If no samples were collected up to 8 hours before the time of randomization, the sample collected closest to the time of randomization up to 2 hours after randomization was used (other than for Pao2:Fio2, which was a prerandomization value only). Laboratory values were only added to the case-report form on August 6, 2020.
In the tocilizumab group, 92% of the patients received at least one dose, and 29% received a second dose at the discretion of the treating clinician. In the sarilumab group, 90% of the patients received the assigned drug. In the control group, 2% of the patients were given one of the immune-modulating drugs outside the trial protocol.
Primary Outcome
The median number of organ support–free days was 10 (interquartile range, −1 to 16) in the tocilizumab group, 11 (interquartile range, 0 to 16) in the sarilumab group, and 0 (interquartile range, −1 to 15) in the control group (Table 2 and Figure 2). The median adjusted odds ratios (primary model) were 1.64 (95% credible interval, 1.25 to 2.14) for tocilizumab and 1.76 (95% credible interval, 1.17 to 2.91) for sarilumab as compared with control, yielding posterior probabilities of superiority of more than 99.9% and of 99.5%, respectively. The in-hospital mortality in the pooled interleukin-6 receptor antagonist groups was 27% (108 of 395 patients), as compared with 36% (142 of 397 patients) in the control group. The median adjusted odds ratios for in-hospital survival were 1.64 (95% credible interval, 1.14 to 2.35) for tocilizumab and 2.01 (95% credible interval, 1.18 to 4.71) for sarilumab as compared with control, yielding posterior probabilities of superiority of 99.6% and 99.5%, respectively. The results of the sensitivity analyses were consistent with those of the primary analysis (Table 2, Tables S3 and S4, and the analysis reports by the statistical analysis committee and the international trial steering committee in the Supplementary Appendix). The estimates of the treatment effect in patients treated with either tocilizumab or sarilumab and glucocorticoids in combination were greater than the estimates for any intervention on its own, and the estimated interaction between interleukin-6 receptor antagonists and glucocorticoids was additive and slightly in the direction of synergistic, but with substantial variability in the estimate (Tables S5 and S6).
Table 2. Primary and Secondary Outcomes.*.
| Outcome or Analysis | Tocilizumab (N=353) |
Sarilumab (N=48) |
Control (N=402) |
|---|---|---|---|
| Primary outcome | |||
| Organ support–free days | |||
| Median (IQR) | 10 (−1 to 16) | 11 (0 to 16) | 0 (−1 to 15) |
| Adjusted odds ratio | |||
| Mean | 1.65±0.23 | 1.83±0.44 | 1 |
| Median (95% credible interval) | 1.64 (1.25 to 2.14) | 1.76 (1.17 to 2.91) | 1 |
| Probability of superiority to control — % | >99.9 | 99.5 | — |
| Subcomponents of organ support–free days | |||
| In-hospital death — no./total no. (%) | 98/350 (28) | 10/45 (22) | 142/397 (36) |
| Concurrent with tocilizumab randomization | — | — | 127/355 (36)† |
| Concurrent with sarilumab randomization | — | — | 19/63 (30)† |
| Median no. of days free of organ support in survivors (IQR) | 14 (7 to 17) | 15 (6 to 17) | 13 (4 to 17) |
| Primary in-hospital survival | |||
| Adjusted odds ratio | |||
| Mean | 1.66±0.31 | 2.25±0.96 | 1 |
| Median (95% credible interval) | 1.64 (1.14 to 2.35) | 2.01 (1.18 to 4.71) | 1 |
| Probability of superiority to control — % | 99.6 | 99.5 | — |
| Secondary analysis of primary outcome | |||
| Adjusted odds ratio | |||
| Mean | 1.68±0.24 | 1.84±0.44 | 1 |
| Median (95% credible interval) | 1.66 (1.26 to 2.18) | 1.77 (1.18 to 2.90) | 1 |
| Probability of superiority to control — % | >99.9 | 99.6 | — |
| Secondary analysis of primary in-hospital survival | |||
| Adjusted odds ratio | |||
| Mean | 1.67±0.31 | 2.24±0.94 | 1 |
| Median (95% credible interval) | 1.65 (1.15 to 2.34) | 2.00 (1.17 to 4.69) | 1 |
| Probability of superiority to control — % | 99.6 | 99.4 | — |
Plus–minus values are means ±SD. The primary analysis of organ support–free days and in-hospital death used data from all the patients enrolled in the trial who met coronavirus disease 2019 (Covid-19) severe state criteria and who underwent randomization within at least one domain (1928 patients), with adjustment for age, sex, time period, site, region, domain, intervention eligibility, and intervention assignment. Secondary analyses were restricted to patients enrolled in the Immune Modulation Therapy domain and any domains that have ceased recruitment (Corticosteroid and Covid-19 Antiviral domains) (1293 patients), with adjustment for age, sex, time period, site, region, domain, intervention eligibility, and intervention assignment. Definitions of outcomes are provided in the trial protocol. All models are structured such that a higher odds ratio is favorable.
The numbers of patients do not sum to the numbers in the entire control group because at some sites, both tocilizumab and sarilumab were concurrently available as potential randomization assignments alongside control.
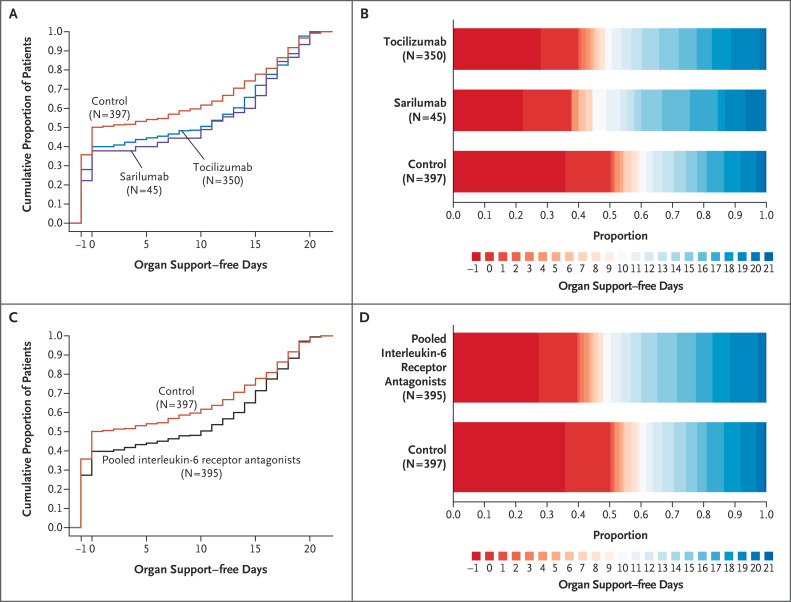
Figure 2. Distributions of Organ Support–free Days.
Panel A shows the cumulative proportion of patients for each intervention group according to day, with death shown first. Curves that rise more gradually indicate a more favorable distribution in the number of days alive and free of organ support. The height of each curve at “−1” indicates the in-hospital mortality for each intervention. The height of each curve at any time point indicates the proportion of patients who had that number of organ support–free days or fewer (e.g., the height at day 10 indicates the proportion of patients with ≤10 organ support–free days). The difference in the height of the curves at any point represents the difference in the percentile in the distribution of organ support–free days associated with that number of days alive and free of organ support. Panel B shows organ support–free days as horizontally stacked proportions according to intervention group. Red represents worse outcomes, and blue represents better outcomes. The median adjusted odds ratios from the primary analysis, which used a Bayesian cumulative logistic model, were 1.64 (95% credible interval, 1.25 to 2.14) and 1.76 (95% credible interval, 1.17 to 2.91) for the tocilizumab and sarilumab groups, respectively, as compared with control, yielding probabilities of superiority to control of more than 99.9% and of 99.5%, respectively. Panels C and D are similar to Panels A and B but with the tocilizumab and sarilumab groups pooled together. The median adjusted odds ratio was 1.65 (95% credible interval, 1.27 to 2.14), yielding a probability of superiority to control of more than 99.9%.
Secondary Outcomes
The secondary outcomes are listed in Figure 3 and Table S7. Tocilizumab and sarilumab were effective across all secondary outcomes, including 90-day survival, time to ICU and hospital discharge, and improvement in the World Health Organization ordinal scale at day 14.18 Similar effects were seen across subgroups (Tables S8 and S9).
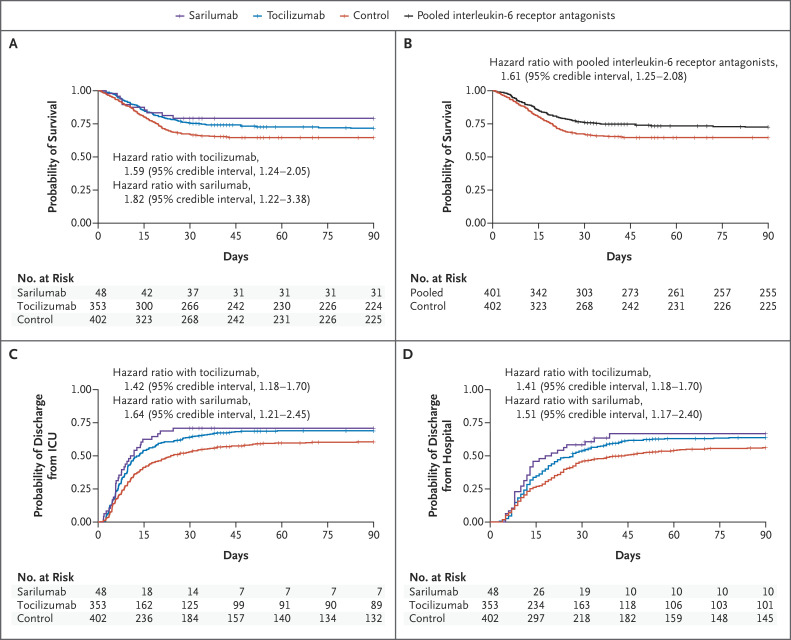
Figure 3. Time-to-Event Analyses.
Shown are Kaplan–Meier curves for survival according to individual intervention group (Panel A) and survival with the tocilizumab and sarilumab groups pooled together (Panel B). There were 109 deaths in the pooled intervention group (99 with tocilizumab and 10 with sarilumab) and 142 in the control group. This resulted in a hazard ratio of 1.61 (95% CI, 1.25 to 2.08), yielding a more than 99.9% posterior probability of superiority of the interleukin-6 receptor antagonists to control. Also shown are the time to ICU discharge according to individual intervention group (Panel C) and the time to hospital discharge according to individual intervention group (Panel D). All hazard ratios are for the comparison with control.
Nine serious adverse events were reported in the tocilizumab group, including one secondary bacterial infection, five bleeding events, two cardiac events, and one deterioration in vision. Eleven serious adverse events were reported in the control group, including four bleeding events and seven thromboses. No serious adverse events were reported in the sarilumab group.
Discussion
We found that in critically ill patients with Covid-19, the interleukin-6 receptor antagonists tocilizumab and sarilumab were both effective as compared with the current standard of care, which included glucocorticoids in the majority of patients (>80%). The benefit was consistent across primary and secondary outcomes and across subgroups and secondary analyses.
Multiple observational and ex vivo laboratory studies have shown that interleukin-6 is an important cytokine associated with disease severity and mortality.19-21 A recent genomic analysis in critically ill patients with Covid-19 showed that genetic variants in the interleukin-6 inflammatory pathway are associated with life-threatening disease.22 These observations support a therapeutic strategy of inhibiting interleukin-6 pathways in patients with severe Covid-19.
Our trial should be compared with other trials of interleukin-6 receptor antagonists in Covid-19. Many previously reported trials included less severely ill patients and excluded patients already receiving respiratory support.6-8 In those trials, no clear evidence suggested that tocilizumab was effective at preventing disease progression, and no benefit with respect to survival was seen. One trial was stopped early at an interim analysis owing to safety concerns, although it should be noted that mortality in the control group was remarkably low (2 of 64 patients, 3%) and the time to hospital discharge was shorter in the tocilizumab group.11 The EMPACTA (Evaluating Minority Patients with Actemra) trial showed that patients who received tocilizumab were less likely than those who received placebo to undergo mechanical ventilation or to die by day 28 (hazard ratio, 0.56; 95% confidence interval [CI], 0.33 to 0.97), although no substantial difference in overall mortality was noted (weighted difference, 2.0 percentage points; 95% CI, −5.2 to 7.8).9 The COVACTA trial, in which approximately 38% of the patients were mechanically ventilated, showed no significant difference between the tocilizumab and placebo groups with respect to clinical status or mortality at day 28, although the time to hospital discharge was shorter with tocilizumab (hazard ratio, 1.35; 95% CI, 1.02 to 1.79).10 The data for a trial of sarilumab are not yet available, but press releases indicated no benefit in the whole population but a trend toward reduced mortality in the critically ill group and a trend toward harm in a subgroup not mechanically ventilated.23,24
We saw both a shorter time to clinical improvement and lower mortality with tocilizumab and with sarilumab than with control. It is therefore possible that the maximum clinical benefit from interleukin-6 inhibition (i.e., improved survival) is seen in the most severely ill patients with Covid-19, who are at the highest risk for death. However, it is important to note that in our trial, patients had to be enrolled within 24 hours after starting organ support in the ICU. This may be an important factor to maximize effectiveness: treating critically ill patients early, while any developing organ dysfunction may be more reversible.
Investigators have proposed using C-reactive protein or other inflammatory markers to select patients with a hyperinflammatory state for treatment.6,8 We saw beneficial effects of interleukin-6 inhibition across all C-reactive protein subgroups in this critically ill population. Although Covid-19 has been described as producing a “cytokine storm,”25 recent studies have shown that systemic levels of cytokines may not be as high as seen with other causes of sepsis and acute respiratory distress syndrome.26 It may be that local inflammation, as evidenced by respiratory dysfunction, is a more useful indicator of which patients will benefit from interleukin-6 inhibition. Concern has been expressed about administering immune-modulating drugs, such as tocilizumab and sarilumab, to patients with critical illness due to infection with a novel virus. One consistent result across all trials to date, including our trial, is that no increased incidences of serious adverse events were reported.
The pragmatic, international design of REMAP-CAP means that our results are probably generalizable to the wider critically ill patient population with Covid-19, although the standard of care may vary in other ICUs and over time, and other populations may include different high-risk patients. The trial has other limitations. It uses an open-label design, but awareness of intervention assignment is unlikely to affect the mortality component of the primary outcome. Because this is an early, preliminary report, some data are missing, including 11 outcomes. Some patients remain in the hospital, so long-term outcomes may differ from the short-term outcomes presented here. Because the trial has a Bayesian design, the results depend on a complex statistical model that may be unfamiliar to many clinicians. The multifactorial design also allows multiple interventions to be evaluated simultaneously, providing more efficient results and accounting for potential treatment-by-treatment interactions. However, many of these interventions continue, and their effects and possible interactions are still to be reported.
In critically ill adult patients with Covid-19 receiving organ support in ICUs, treatment with the interleukin-6 receptor antagonists tocilizumab and sarilumab improved outcomes, including survival.
Acknowledgments
We thank the staffs of the NIHR Clinical Research Network (United Kingdom), UPMC Health System Health Services Division (United States), and the Direction de la Recherche Clinique et de l’Innovation de l’Assistance Publique–Hôpitaux de Paris (France) for their support of participant recruitment.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
The members of the writing committee are as follows: Anthony C. Gordon, M.B., B.S., M.D., Paul R. Mouncey, M.Sc., Farah Al-Beidh, Ph.D., Kathryn M. Rowan, Ph.D., Alistair D. Nichol, M.D., Ph.D., Yaseen M. Arabi, M.D., Djillali Annane, M.D., Ph.D., Abi Beane, Ph.D., Wilma van Bentum-Puijk, M.Sc., Lindsay R. Berry, Ph.D., Zahra Bhimani, M.P.H., Marc J.M. Bonten, M.D., Ph.D., Charlotte A. Bradbury, M.B., Ch.B., Ph.D., Frank M. Brunkhorst, M.D., Ph.D., Adrian Buzgau, M.Sc., Allen C. Cheng, M.B., B.S., Ph.D., Michelle A. Detry, Ph.D., Eamon J. Duffy, B.Pharm., Lise J. Estcourt, M.B., B.Ch., Ph.D., Mark Fitzgerald, Ph.D., Herman Goossens, Ph.D., Rashan Haniffa, Ph.D., Alisa M. Higgins, Ph.D., Thomas E. Hills, Ph.D., Christopher M. Horvat, M.D., Francois Lamontagne, M.D., Patrick R. Lawler, M.D., M.P.H., Helen L. Leavis, M.D., Ph.D., Kelsey M. Linstrum, M.S., Edward Litton, M.D., Ph.D., Elizabeth Lorenzi, Ph.D., John C. Marshall, M.D., Florian B. Mayr, M.D., M.P.H., Daniel F. McAuley, M.D., Anna McGlothlin, Ph.D., Shay P. McGuinness, M.D., Bryan J. McVerry, M.D., Stephanie K. Montgomery, M.Sc., Susan C. Morpeth, M.D., Ph.D., Srinivas Murthy, M.D., Katrina Orr, B.Pharm., Rachael L. Parke, Ph.D., Jane C. Parker, B.N., Asad E. Patanwala, Pharm.D., M.P.H., Ville Pettilä, M.D., Emma Rademaker, M.D., Marlene S. Santos, M.D., M.S.H.S., Christina T. Saunders, Ph.D., Christopher W. Seymour, M.D., Manu Shankar-Hari, M.D., Ph.D., Wendy I. Sligl, M.D., Alexis F. Turgeon, M.D., Anne M. Turner, M.P.H., Frank L. van de Veerdonk, M.D., Ph.D., Ryan Zarychanski, M.D., Cameron Green, M.Sc., Roger J. Lewis, M.D., Ph.D., Derek C. Angus, M.D., M.P.H., Colin J. McArthur, M.D., Scott Berry, Ph.D., Steve A. Webb, M.D., Ph.D., and Lennie P.G. Derde, M.D., Ph.D.
The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research (NIHR), or the Department of Health and Social Care.
This article was published on February 25, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by the European Union — through FP7-HEALTH-2013-INNOVATION: the Platform for European Preparedness Against (Re-)emerging Epidemics (PREPARE) consortium (602525); and the Horizon 2020 research and innovation program: the Rapid European Covid-19 Emergency Research response (RECOVER) consortium (101003589) — and by the Australian National Health and Medical Research Council (APP1101719), the Health Research Council of New Zealand (16/631), a Canadian Institutes of Health Research Strategy for Patient-Oriented Research Innovative Clinical Trials Program Grant (158584), the U.K. NIHR and the NIHR Imperial Biomedical Research Centre, the Health Research Board of Ireland (CTN 2014-012), the UPMC Learning While Doing Program, the Breast Cancer Research Foundation, the French Ministry of Health (PHRC-20-0147), the Minderoo Foundation, Amgen, Eisai, the Global Coalition for Adaptive Research, and the Wellcome Trust Innovations Project (215522). Dr. Gordon is funded by an NIHR Research Professorship (RP-2015-06-18), and Dr. Shankar-Hari by an NIHR Clinician Scientist Fellowship (CS-2016-16-011).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.World Health Organization. Coronavirus disease (COVID-19) pandemic. 2021. (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). [PubMed]
- 2.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324:1330-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gremese E, Cingolani A, Bosello SL, et al. Sarilumab use in severe SARS-CoV-2 pneumonia. EClinicalMedicine 2020;27:100553-100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis 2020. July 11 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020;383:2333-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:24-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;384:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. DOI: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ 2021;372:n84-n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angus DC, Berry S, Lewis RJ, et al. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) study. Rationale and design. Ann Am Thorac Soc 2020;17:879-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA 2020;324:1317-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laterre P-F, Berry SM, Blemings A, et al. Effect of selepressin vs placebo on ventilator- and vasopressor-free days in patients with septic shock: the SEPSIS-ACT randomized clinical trial. JAMA 2019; 322:1476-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viele K, Berry S, Neuenschwander B, et al. Use of historical control data for assessing treatment effects in clinical trials. Pharm Stat 2014;13:41-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. Press release, June 16, 2020. (https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19.)
- 17.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020;20(8):e192-e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Fu B, Zheng X, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev 2020;7:998-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J, Pang J, Ji P, et al. Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis. J Med Virol 2021;93:35-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pairo-Castineira E, Clohisey S, Klaric L, et al. Genetic mechanisms of critical illness in Covid-19. Nature 2020. December 11 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 23.Sanofi provides update on Kevzara (sarilumab) phase 3 trial in severe and critically ill COVID-19 patients outside the U.S. Press release, September 1, 2020. (https://www.sanofi.com/en/media-room/press-releases/2020/2020-09-01-07-00-00.)
- 24.Sanofi and Regeneron provide update on Kevzara (sarilumab) phase 3 U.S. trial in COVID-19 patients. Press release, July 2, 2020. (https://www.sanofi.com/en/media-room/press-releases/2020/2020-07-02-22-30-00).
- 25.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 2020;383:2255-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha P, Calfee CS, Cherian S, et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med 2020;8:1209-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.