Abstract
Background
Selenium and peroxynitrite are known to support the growth and activity of immune cells, including T cells, B cells and macrophages. However, the role of these factors in the immune function of human immature dendritic cells (imDCs) is not clear.
Material/Methods
Monocytes from a mixture of blood samples were isolated using Ficoll density gradient centrifugation and purified with immunomagnetic beads before being induced into imDCs. Cells then either received no treatment (control group), or treatment with sodium selenite (Na2SeO3, Se), 3-morpholinosydnonimine (SIN1, which decomposes into peroxynitrite), or Se+SIN1. Cell viability, migration, and antiphagocytic abilities, oxidative stress, and protein expression of extracellular signal-regulated kinases (ERK) and MMP2 were assessed using a CCK8 assay, cell counter and flow cytometry, microplate spectrophotometer, and Western blot analysis, respectively.
Results
Viability of imDCs was unaffected by 0.1 μmol/L of Na2SeO3, although 1 mmol/L of SIN1 decreased it significantly (P<0.05). Chemotactic migration and antiphagocytic abilities were inhibited and enhanced, respectively, by treatment with Na2SeO3 and SIN1 (P<0.05). Activities of superoxide dismutase and glutathione peroxidase were increased by Na2SeO3 and Se+SIN1 (P<0.001). Glutathione content decreased with exposure to Na2SeO3 and SIN1 (P<0.05), but increased after treatment with Se+SIN1 (P<0.05). Levels of reactive oxygen species only increased with SIN1 treatment (P<0.05). Treatment with Na2SeO3, SIN1 and Se+SIN1 increased ERK phosphorylation and decreased MMP2 protein expression (P<0.05).
Conclusions
Selenium and peroxynitrite can influence immune function in imDCs by regulating levels of reactive oxygen species or glutathione to activate ERK and promote antigen phagocytosis, as well as by decreasing MMP2 expression to inhibit chemotactic migration.
Keywords: Cell Migration Assays, Dendritic Cells, Oxidative Stress, Peroxynitrous Acid, Selenium
Background
Dendritic cells (DCs) serve as potent messengers between the innate and adaptive immune systems and can be classified as immature (imDCs) or mature (mDCs), depending on their stage of differentiation. Specifically, the former captures and processes antigens from the environment, while the latter presents them to T cells in the lymph nodes [1–3].
To date, studies have indicated that both mitochondrial reactive oxygen species (ROS) and superoxide anions (O2−) contribute to the development of DCs. For example, investigations in mice have reported that ROS activates DCs and promotes interaction between DCs and T cells during antigen presentation [4], as well as induces apoptosis or necrosis of DCs via Acinetobacter baumannii outer membrane protein A [5]. Among humans, ROS stimulates differentiation of mononuclear cells into DCs [6], and O2− assists in the maturation of imDCs into mDCs [7]. Importantly, the reaction product of O2− and nitric oxide (peroxynitrite) is required for cytokine production in human monocytes [8], as nitric oxide synthase inhibitors have been linked to a reduction in peroxynitrite formation and partial inhibition of O2− induced maturation of DCs [7]. Previous studies showed that a high concentration of nitrite was found in LPS-activated mouse DCs [9]; however, the effect of nitrite in the tumor microenvironment [10] on DCs function remains unknown.
Interestingly, in recent years numerous investigations have also discovered that selenium exerts a substantial influence on the growth and function of immune cells by regulating their redox state through selenoproteins K, T, R, S, and 15 [11–18]. For mice, selenium can regulate the numbers of B cells and antibody production in their spleens [19–21], as well as the transformation of macrophages into different cell types [22]. Moreover, low levels of selenium (0.08 ppm) have been found to induce differentiation of T cells into Th2 cells, while high levels (1.0 ppm) trigger T cell differentiation into Th1 cells [19]. The effect of sodium selenite on the immune function of chicken DCs was recently studied [23,24]. In humans, however, studies have yet to clarify how physiological levels of selenium impact imDCs [4,23,24], especially under peroxynitrite conditions.
Previous studies suggested that mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinases (ERK), are involved in the maturation of DCs after various stimuli [24–28], and redox balance or oxidative stress may be the upstream signals of MAPKs [28,29]. It also demonstrated that matrix metalloproteinases (MMPs) play important roles in the migration of DCs [30]. However, the role of ERK and MMP2 in the function of human imDCs affected by selenium and peroxynitrite was unclear.
Against this background, the present study aimed to uncover the underlying mechanism by which selenium and peroxynitrite modulate the immune function of human imDCs, as well as to assess the migration and phagocytic capabilities, redox balance, and ERK and MMP2 signaling in these cells. We hypothesized that selenium and peroxynitrite would affect the activity of imDCs by oxidative stress, ERK, and MMP2.
Material and Methods
Preparation of imDCs from Human Peripheral Blood
Healthy individuals from Guizhou, China were enrolled and gave informed consent to participate. This study was approved by the Ethics Committee of Guizhou Medical University (Approval No. 2018 (15)). For each experiment, a mixture of blood from 10 donors was collected. Monocytes were first isolated from the fresh peripheral blood of our participants using Ficoll density gradient centrifugation and later purified with immunomagnetic beads. They were then cultured in an RPMI 1640 medium containing 10% fetal bovine serum (FBS) (Hyclone, USA), 1% antibiotics (Amersco, USA), 800 U/mL recombinant human granulocyte-macrophage colony-stimulating factor (PeproTech, Germany), and 500 U/mL recombinant human interleukin-4 (PeproTech, Germany) at 37°C in 5% CO2 for 5 days, after which imDCs were obtained [31].
Cell Treatment and CCK8 Assay
imDCs were seeded into 96-well plates at a density of 1×106 cells/mL. Next, they were treated for 12 h in an RPMI 1640 medium supplemented with cytokines and 0.0001, 0.001, 0.01, 0.1, or 1 μmol/L of sodium selenite (Na2SeO3, Se, Sinopharm, China), 0.2, 0.5, or 1 mmol/L of 3-morpholinosydnonimine (SIN1, Sigma, USA – this spontaneously decomposes to yield O2− and nitric oxide before forming peroxynitrite). Cell viability was then assayed using a CCK8 kit (Beyotime, China), according to the manufacturer’s protocol. In subsequent experiments, imDCs were divided into 4 groups: control group (C, no treatment), Se group (treatment with Na2SeO3), SIN1 group (treatment with SIN1), and Se+SIN1 group (pretreatment with Se and challenge with SIN1).
Antiphagocytic Ability of imDCs
After imDCs were treated with Se (0.1 μmol/L) or SIN1 (1 mmol/L), imDCs at a density of 1×106 cells/mL were resuspended in phosphate-buffered saline (PBS), incubated with FITC-dextrans (40KD, Sigma) at 37°C for 1.5 h, and then fixed with 3.7% paraformaldehyde at 4°C for 20 min. Next, they were washed twice with PBS and resuspended in it, and then 1×104 imDCs were subjected to a flow cytometry assay (NovoCyte, ACEA biosciences). The fluorescence intensity was analyzed and normalized to that of the control [32].
Migration Ability of imDCs
Cells at a density of 2.5×106 cells/mL were seeded into an upper transwell chamber with a 5 μm pore size (Millipore, Germany) containing an RPMI 1640 medium without FBS, and the lower chamber containing an RPMI 1640 medium with 10% FBS and either 100 ng/mL CCL2 (PeproTech, Germany) or not. After cells were incubated at 37°C for 36 h, they were counted by cell counter (Cellometer mini, Nexcelom, USA) and their migration rate was normalized to that of the control [33].
Measurement of Oxidative Stress
To assess superoxide dismutase (SOD) activity, imDCs at a density of 1×106 cells/mL were washed twice with cold PBS, homogenized in ice-cold PBS, subjected to a total SOD assay with WST-8 (Beyotime, China) at 37°C for 30 min, and then had their absorbance read by a microplate spectrophotometer (Cytation5, Biotek) at 450 nm. To evaluate glutathione peroxidase (GPx) activity, imDCs at a density of 1×106 cells/mL were homogenized, subjected to a cellular glutathione peroxidase assay (Beyotime, China), and then had their absorbance read by a microplate spectrophotometer at 340 nm. To measure ROS levels, imDCs at a density of 1×106 cells/mL were washed twice with PBS, resuspended in PBS, incubated with 2′, 7′-dichlorodihydro-fluorescein diacetate (Solarbio, China) for 20 min at 37oC, and then had their activity determined by flow cytometry assay [34]. To determine glutathione (GSH) content, imDCs at a density of 1×106 cells/mL were washed with PBS, repeatedly frozen and thawed with a protein removal reagent, and then had their absorbance read by a microplate spectrophotometer [35].
Western Blotting
Cell lysates were prepared using an RIPA buffer containing a protease inhibitor cocktail, and then antibodies purchased from Affinity for ERK (AF0155), pERK (AF1015), MMP2 (AF0577), and GAPDH (AF0911) were used for western blot analyses as previously described [36].
Statistical Analysis
Analysis of variance was applied to determine differences between groups, followed by Tukey’s test for multiple comparisons; P values <0.05 indicated statistical significance. The data are presented as mean±standard deviation (SD) of at least 3 independent experiments, and all analyses were performed using SPSS software (version 17.0, IBM, USA).
Results
Cell Viability of imDCs
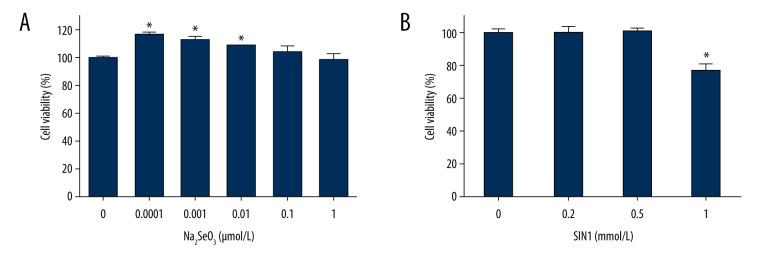
Treatment with Na2SeO3 significantly increased viability of imDCs at concentrations of 0.0001, 0.001, and 0.01 μmol/L (P<0.05), but not at concentrations of 0.1 and 1 μmol/L (P>0.05) (Figure 1A). Treatment with SIN1 significantly decreased cell viability at a concentration of 1 mmol/L (P<0.05) (Figure 1B).
Figure 1.
Effect of sodium selenite and SIN1 on viability of imDCs. The viability of imDCs treated with sodium selenite (A) and SIN1 (B) was determined by a CCK8 assay. Data are presented as mean±SD (n=6). * P<0.05, compared with the control group.
Regulation of the Migration and Antiphagocytic Ability of imDCs
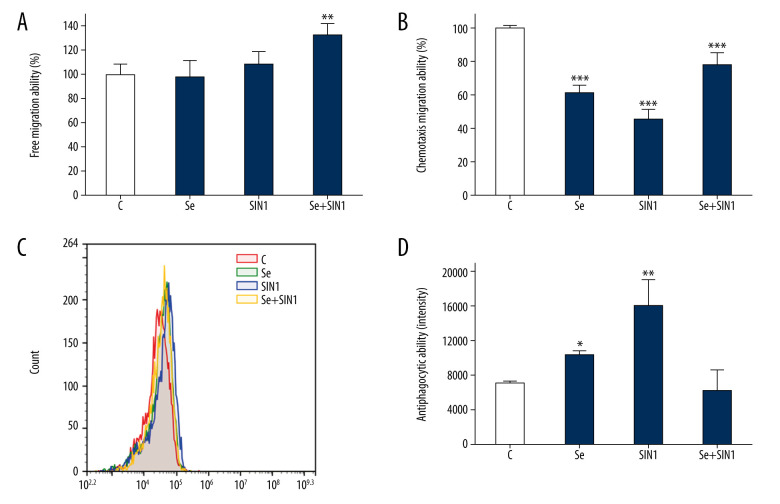
Treatment with Se+SIN1 significantly increased the free migration ability of imDCs (P<0.01) (Figure 2A). The treatments of Se, SIN1, and Se+SIN1 all significantly decreased the chemotactic migration ability of imDCs (P<0.001), although exposure to SIN1 was found to partially restore chemotactic migration ability in imDCs first treated with Se (P<0.001) (Figure 2B). Antiphagocytic ability of imDCs significantly increased following treatment with Se and SIN1 (P<0.05) (Figure 2C, 2D).
Figure 2.
Effect of sodium selenite and SIN1 on the migration and antiphagocytic abilities of imDCs. The free and chemotaxis migration ability of imDCs after treatment with sodium selenite and SIN1 were assayed using transwell assay (A, B), and the antiphagocytic ability was detected by flow cytometry (C, D). Data are presented as mean±SD of 3 independent experiments. * P<0.05, ** P<0.01, *** P<0.001, compared with the control group.
Redox Balance of imDCs
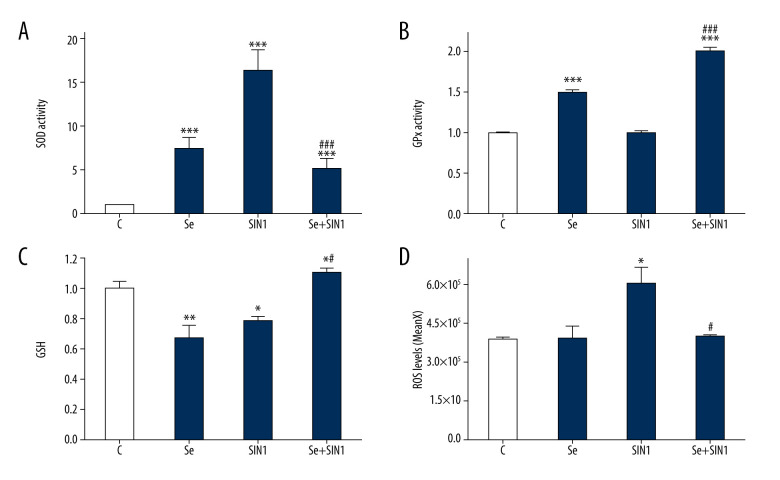
SOD activity in imDCs was significantly increased by treatment with Se, SIN1, and Se+SIN1 in imDCs (P<0.001), although treatment with Se+SIN1 significantly decreased activity compared to that of SIN1 (P<0.001) (Figure 3A). GPx activity was significantly increased by treatment with Se and Se+SIN1 (P<0.001) (Figure 3B). GSH content significantly decreased following treatment with Se and SIN1 (P<0.05), but significantly increased with Se+SIN1 treatment compared to that of the control and SIN1 groups (P<0.05) (Figure 3C). ROS levels significantly increased upon treatment with SIN1 (P<0.05), but decreased after treatment with Se+SIN1 compared to that of SIN1 (P<0.05) (Figure 3D).
Figure 3.
Effect of sodium selenite and SIN1 on the redox balance of imDCs. The activity of superoxide dismutase (A) and glutathione peroxidase (B) and content of glutathione (C) were assayed by their respective kits and read using a spectrophotometer, and the level of reactive oxygen species was detected by flow cytometry (D). Data are presented as mean±SD of 3 independent experiments. * P<0.05, ** P<0.01, *** P<0.001, compared with the control group. # P<0.05, ### P<0.001, compared with the SIN1 group.
The Potential role of ERK and MMP2 in imDCs
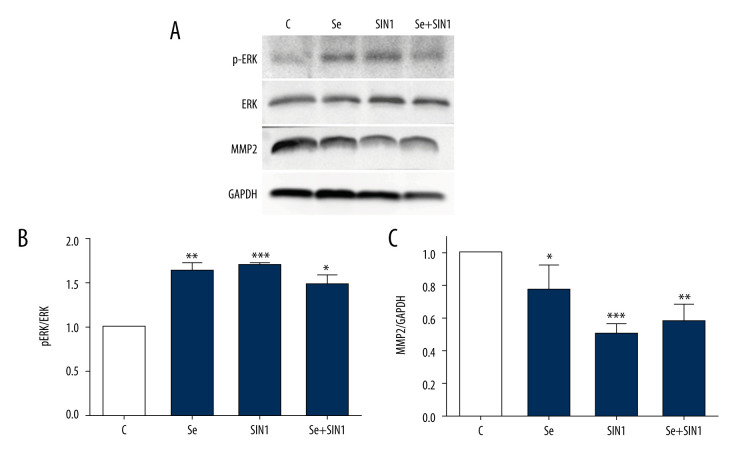
Treatment with Se, SIN1, and Se+SIN1 significantly increased ERK phosphorylation in imDCs (P<0.05) (Figure 4A, 4B), but significantly decreased MMP2 levels (P<0.05) (Figure 4A, 4C).
Figure 4.
Expression of ERK and MMP2 in imDCs. Protein levels of phosphorylated ERK, ERK, and MMP2 were detected by western blot (A) and statistically analyzed with Image J software (B, C). Data are presented as mean±SD of 3 independent experiments. * P<0.05, ** P<0.01, *** P<0.001, compared with the control group.
Discussion
Previous studies have indicated that selenium influences production of B cells and their resident antibodies, as well as the differentiation of T cells and macrophages across multiple species [19–22]. It has been suggested that the differentiation and immune function of chicken DCs can also be influenced by selenium [23,24]; moreover, high levels of organoselenium drugs like ebselen have been shown to inhibit DC-induced proliferation of T cells and secretion of cytokines [4]. In this study, human imDCs were exposed to different dosages of Na2SeO3 and findings revealed that those below 0.1 μmol/L were capable of promoting proliferation. These results partially agree with data reported by Zhang et al [32], who stated that stromal cells enhanced the proliferation of mDCs and differentiation of mDCs into regulatory DCs (in a fibronectin-dependent manner). Since it is unknown why our imDCs proliferated, evaluation of the intake and baseline levels of selenium by our donors is necessary.
Several lines of research have also demonstrated that peroxynitrite is essential for immune function, including the release of proinflammatory cytokines from monocytes at a low concentration [8], and the regulation of inflammation in immune cells [37,38]. A study showed that there were more than 20 μmol/L of nitrites in the LPS-activated mouse DCs [9], and higher nitrites levels (51.2 μmol/L) in tumor tissue were observed [10]. Here, treatment with 1 mmol/L of SIN1 reduced the viability and chemotactic migration of imDCs, but augmented antigen phagocytosis. In addition, administration of Na2SeO3 prior to SIN1 had no effect on the latter phenomenon. From this, we might predict that high levels of peroxynitrite produced in the tumor microenvironment will damage imDCs, but stimulate them to partake in antigen phagocytosis, and 0.1 μmol/L of selenium might be detrimental to the antigen uptake capacity of imDCs.
Redox balance is well known to play an important role in immunity, with research showing that selenium participates in the production of ROS in immune cells [18,28,29,39], peroxynitrite inhibits GPx activity in the absence of GSH [40], and GSH assists in the maturation of DCs [29]. The present experiments showed that treatment with Se, SIN1, and Se+SIN1 significantly increased SOD activity, with the highest amounts of ROS observed in imDCs treated with SIN1. Additionally, exposure to Se+SIN1 lowered ROS levels and SOD activity in imDCs when compared to those given SIN1, but increased GSH content and GPx activity. Among imDCs treated only with SIN1, no change in GPx activity was observed and it is likely this was due to the reduced amount of GSH. Lastly, administration of Se and SIN1 decreased levels of GSH in imDCs and this corresponded to enhanced antigen phagocytosis, although an increase in GSH content had no effect. Such outcomes may be attributed to the formation of superoxide anions [7] and peroxynitrite [8,37,38,41], and thus warrant further investigation.
Various stimuli have been found to contribute to the maturation of DCs by triggering phosphorylation of ERK [24–26]. In our study, results showed that treatment with Se and SIN1 increased ERK phosphorylation and led to enhanced phagocytosis, whereas exposure to Se+SIN1 inhibited ERK phosphorylation and resulted in decreased antigen phagocytosis when compared to imDCs given SIN1. Emerging evidence has demonstrated that ERK signaling is regulated by levels of ROS and GSH, as ERK phosphorylation induced by 2,4-dinitrofluorobenzene is inhibited by GSH [28,29]. Here, an inverse relationship was detected between the amount of ERK phosphorylation and GSH content, which is consistent with the aforementioned findings. In addition, given that phosphorylation levels of ERK in imDCs administered Se+SIN1 were higher than those in the control group and that phagocytosis was unaffected, it is possible that ROS and GSH were responsible.
Similar to ERK, increased MMP2 expression is critical for proper immune function, including being required for the migration of skin DCs [28], activation of DCs [42], and differentiation of monocytes to DCs [43]. In our analyses, low levels of MMP2 in imDCs corresponded to poor chemotactic migration. Curiously, however, we did not see activation of ERK heighten production of MMP2, as reported by others [44], so it is likely that the underlying mechanism is due to p38 [45,46] or other signaling pathways.
Conclusions
In summary, the results of this study suggest that selenium and peroxynitrite enhance levels of ROS or GSH to activate ERK and promote antigen phagocytosis in imDCs, as well as decrease MMP2 to inhibit chemotactic migration independent of ERK.
Footnotes
Conflict of Interest
None.
Source of support: This research was funded by the National Natural Science Foundation of China (No. 21561006 and No. 21867007), the Guizhou Provincial Natural Science Foundation (No. [2019]1258 and No. LH[2016]7372), and the Opening Fund of Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica (No. BCMM202002)
References
- 1.Qian C, Cao X. Dendritic cells in the regulation of immunity and inflammation. Semin Immunol. 2018;35:3–11. doi: 10.1016/j.smim.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Worbs T, Hammerschmidt SI, Förster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17(1):30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 3.Menager MM, Littman DR. Actin dynamics regulates dendritic cell-mediated transfer of HIV-1 to T cells. Cell. 2016;164(4):695–709. doi: 10.1016/j.cell.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HJ, Park JS, Yoo HJ, et al. The Selenoprotein MsrB1 instructs dendritic cells to induce T-helper 1 immune responses. Antioxidants (Basel) 2020;9(10):1021. doi: 10.3390/antiox9101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JS, Choi CH, Kim JW, et al. Acinetobacter baumannii outer membrane protein A induces dendritic cell death through mitochondrial targeting. J Microbiol. 2010;48(3):387–92. doi: 10.1007/s12275-010-0155-1. [DOI] [PubMed] [Google Scholar]
- 6.Zaccagnino P, Saltarella M, Maiorano S, et al. An active mitochondrial biogenesis occurs during dendritic cell differentiation. Int J Biochem Cell Biol. 2012;44:1962–69. doi: 10.1016/j.biocel.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Wang X, Saredy J, et al. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020;37:101759. doi: 10.1016/j.redox.2020.101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matata BM, Galinanes M. Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-κB DNA binding activity. J Biol Chem. 2002;277(3):2330–35. doi: 10.1074/jbc.M106393200. [DOI] [PubMed] [Google Scholar]
- 9.Fraszczak J, Trad M, Janikashvili N, et al. Peroxynitrite-dependent killing of cancer cells and presentation of released tumor antigens by activated dendritic cells. J Immunol. 2010;184(4):1876–84. doi: 10.4049/jimmunol.0900831. [DOI] [PubMed] [Google Scholar]
- 10.Szaleczky E, Pronai L, Nakazawa H, et al. Evidence of in vivo peroxynitrite formation in patients with colorectal carcinoma, higher plasma nitrate/nitrite levels, and lower protection against oxygen free radicals. J Clin Gastroenterol. 2000;30:47–51. doi: 10.1097/00004836-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Verma S, Hoffmann FKW, Kumar M, et al. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol. 2011;186(4):2127–37. doi: 10.4049/jimmunol.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z, Hoffmann FKW, Norton RL, et al. Selenoprotein K is a novel target of m-calpain, and cleavage is regulated by Toll-like receptor-induced calpastatin in macrophages. J Biol Chem. 2011;286(40):34830–38. doi: 10.1074/jbc.M111.265520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Li R, Huang Y, et al. Selenoprotein K modulate intracellular free Ca2+ by regulating expression of calcium homoeostasis endoplasmic reticulum protein. Biochem Biophys Res Commun. 2017;484(4):734–39. doi: 10.1016/j.bbrc.2017.01.117. [DOI] [PubMed] [Google Scholar]
- 14.You L, Liu C, Yang ZJ, et al. Prediction of selenoprotein T structure and its response to selenium deficiency in chicken immune organs. Biol Trace Elem Res. 2014;160(2):222–31. doi: 10.1007/s12011-014-0049-x. [DOI] [PubMed] [Google Scholar]
- 15.Pan T, Liu T, Tan S, et al. Lower selenoprotein T expression and immune response in the immune organs of broilers with exudative diathesis due to selenium deficiency. Biol Trace Elem Res. 2018;182(2):364–72. doi: 10.1007/s12011-017-1110-3. [DOI] [PubMed] [Google Scholar]
- 16.Youssef A, Lihrmann I, Falluel-Morel A, et al. Selenoprotein T is a key player in ER proteostasis, endocrine homoeostasis and neuroprotection. Free Radic Biol Med. 2018;127:145–52. doi: 10.1016/j.freeradbiomed.2018.05.076. [DOI] [PubMed] [Google Scholar]
- 17.Lee BC, Lee SG, Choo MK, et al. Selenoprotein MsrB1 promotes anti-inflammatory cytokine gene expression in macrophages and controls immune response in vivo. Sci Rep. 2017;7(1):5119. doi: 10.1038/s41598-017-05230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid Redox Sign. 2012;16(7):705–43. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairweather-Tait SJ, Bao Y, Broadley MR, et al. Selenium in human health and disease. Antioxid Redox Sign. 2011;14(7):1337–83. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 20.Avery JC, Hoffmann PR. Selenium, selenoproteins, and immunity. Nutrients. 2018;10(9):1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vega L, Rodriguez-Sosa M, Garcia-Montalvo EA, et al. Non-optimal levels of dietary selenomethionine alter splenocyte response and modify oxidative stress markers in female mice. Food Chem Toxicol. 2007;45:1147–53. doi: 10.1016/j.fct.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Nelson SM, Lei X, Prabhu KS. Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages. J Nutr. 2011;141(9):1754–61. doi: 10.3945/jn.111.141176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Z, Liu C, Pan T, et al. Selenium accelerates chicken dendritic cells differentiation and affects selenoproteins expression. Dev Comp Immunol. 2017;77:30–37. doi: 10.1016/j.dci.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Sun Z, Xu Z, Wang D, et al. Selenium deficiency inhibits dendritic cells differentiation and immune function, imbalance the Th1/Th2 of dendritic cells. Metallomics. 2018;10(5):759–67. doi: 10.1039/c8mt00039e. [DOI] [PubMed] [Google Scholar]
- 25.Soukup K, Halfmann A, Le Bras M, et al. The MAPK-activated kinase MK2 attenuates dendritic cell-mediated Th1 differentiation and autoimmune encephalomyelitis. J Immunol. 2015;195(2):541–52. doi: 10.4049/jimmunol.1401663. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Wang Y, Wu Y, et al. Echinacea purpurea extracts promote murine dendritic cell maturation by activation of JNK, p38 MAPK and NF-κB pathways. Dev Comp Immunol. 2017;73:21–26. doi: 10.1016/j.dci.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Lim HX, Jung HJ, Lee A, et al. Lysyl-transfer RNA synthetase induces the maturation of dendritic cells through MAPK and NF-κB pathways, strongly contributing to enhanced Th1 cell responses. J Immunol. 2018;201(9):2832–41. doi: 10.4049/jimmunol.1800386. [DOI] [PubMed] [Google Scholar]
- 28.Matos TJ, Duarte CB, Gonçalo M, et al. Role of oxidative stress in ERK and p38 MAPK activation induced by the chemical sensitizer DNFB in a fetal skin dendritic cell line. Immunol Cell Biol. 2005;83(6):607–14. doi: 10.1111/j.1440-1711.2005.01378.x. [DOI] [PubMed] [Google Scholar]
- 29.Mizuashi M, Ohtani T, Nakagawa S, et al. Redox imbalance induced by contact sensitizers triggers the maturation of dendritic cells. J Invest Dermatol. 2005;124(3):579–86. doi: 10.1111/j.0022-202X.2005.23624.x. [DOI] [PubMed] [Google Scholar]
- 30.Adema GJ, de Vries IJM, Punt CJ, et al. Migration of dendritic cell-based cancer vaccines: In vivo veritas? Curr Opin Immunol. 2005;17(2):170–74. doi: 10.1016/j.coi.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Liu X, Long J, et al. Interleukin-10 reorganizes the cytoskeleton of mature dendritic cells leading to their impaired biophysical properties and motilities. PLoS One. 2017;12(2):e0172523. doi: 10.1371/journal.pone.0172523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Tang H, Guo Z, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5(11):1124–33. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Z, Yao W, Xu X, et al. Hepatocellular carcinoma cells deteriorate the biophysical properties of dendritic cells. Cell Biochem Biophys. 2009;55(1):33–43. doi: 10.1007/s12013-009-9055-6. [DOI] [PubMed] [Google Scholar]
- 34.Jia Y, Li Y, Du S, et al. Involvement of MsrB1 in the regulation of redox balance and inhibition of peroxynitrite-induced apoptosis in human lens epithelial cells. Exp Eye Res. 2012;100:7–16. doi: 10.1016/j.exer.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann FW, Hashimoto AC, Shafer LA, et al. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr. 2010;140(6):1155–61. doi: 10.3945/jn.109.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia Y, Zhou J, Liu H, et al. Effect of methionine sulfoxide reductase B1 (SelR) gene silencing on peroxynitrite-induced F-actin disruption in human lens epithelial cells. Biochem Biophys Res Commun. 2014;443(3):876–81. doi: 10.1016/j.bbrc.2013.12.055. [DOI] [PubMed] [Google Scholar]
- 37.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6(8):662–80. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 38.Wu D, Ryu JC, Chung YW, et al. A far-red-emitting fluorescence probe for sensitive and selective detection of peroxynitrite in live cells and tissues. Anal Chem. 2017;89(20):10924–31. doi: 10.1021/acs.analchem.7b02707. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Wang S, Zhang Q, et al. Selenomethionine alleviates LPS-induced chicken myocardial inflammation by regulating the miR-128-3p-p38 MAPK axis and oxidative stress. Metallomics. 2020;12:54–64. doi: 10.1039/c9mt00216b. [DOI] [PubMed] [Google Scholar]
- 40.Benhar M. Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radic Biol Med. 2018;127:160–64. doi: 10.1016/j.freeradbiomed.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 41.Chen AY, Lü JM, Yao Q, et al. Entacapone is an antioxidant more potent than vitamin C and vitamin E for scavenging of hypochlorous acid and peroxynitrite, and the inhibition of oxidative stress-induced cell death. Med Sci Monit. 2016;22:687–96. doi: 10.12659/MSM.896462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godefroy E, Gallois A, Idoyaga J, et al. Activation of toll-like receptor-2 by endogenous matrix metalloproteinase-2 modulates dendritic-cell-mediated inflammatory responses. Cell Rep. 2014;9(5):1856–70. doi: 10.1016/j.celrep.2014.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kis-Toth K, Bacskai I, Gogolak P, et al. Monocyte-derived dendritic cell subpopulations use different types of matrix metalloproteinases inhibited by GM6001. Immunobiology. 2013;218(11):1361–69. doi: 10.1016/j.imbio.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Sun X, Xu Y, Wang Y, et al. Synergistic inhibition of thalidomide and icotinib on human non-small cell lung carcinomas through ERK and AKT signaling. Med Sci Monit. 2018;24:3193–203. doi: 10.12659/MSM.909977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim ES, Kim MS, Moon A. TGF-β-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol. 2004;25(5):1375–82. [PubMed] [Google Scholar]
- 46.Wu Z, He D, Zhao S, et al. IL-17A/IL-17RA promotes invasion and activates MMP-2 and MMP-9 expression via p38 MAPK signaling pathway in non-small cell lung cancer. Mol Cell Biochem. 2019;455(1–2):195–206. doi: 10.1007/s11010-018-3483-9. [DOI] [PubMed] [Google Scholar]