Abstract
Introduction.
Daily combustible cigarette use is common among cannabis users, and dual use of cigarettes and cannabis is associated with detrimental outcomes. This study addresses gaps in the literature by examining data from the prenatal and adolescent phases of a prospective, longitudinal study to predict adult daily dual use.
Methods.
Young adult offspring (M age = 22.8 years, 53% female) from a prenatal cohort reported on combustible cigarette, and cannabis use (N = 500, 58% Black, 42% White). Pathways to daily dual use were modeled using variables from the gestational and adolescent phases of the study including prenatal tobacco, alcohol, and cannabis exposures; ages at initiation of cigarettes and cannabis; and adolescent learning/memory, impulsivity, and behavior problems.
Results.
Prenatal cannabis and tobacco use were not directly linked to adult daily dual use of cannabis and tobacco. However, structural equation modeling revealed three significant indirect pathways from prenatal cigarette and cannabis exposures to adult daily dual use of cigarettes and cannabis via early cigarette initiation, early cannabis initiation, and adolescent behavior problems.
Conclusions.
This study identified pathways from prenatal cannabis and tobacco exposure to adult daily dual use, in addition to clarifying adolescent outcomes that may be part of the pathways. In a climate of growing acceptance of cannabis use and increasing legalization of recreational use, these findings serve as a warning that early exposure to cannabis may have an important role in shaping long-term dual use of tobacco and cannabis.
Keywords: prenatal, cannabis, tobacco, marijuana, adolescent, smoking
1. INTRODUCTION
Most people who use cannabis also smoke combustible cigarettes1–3. Further, dual use of tobacco cigarettes and cannabis is becoming more prevalent as cigarette smokers become increasingly likely to use cannabis4–6. Daily cannabis use has increased among both daily and non-daily cigarette smokers7 and among the countries in the International Tobacco Control (ITC) survey, Americans had the highest rates of daily dual use of cigarettes and cannabis8. Daily dual use exposes individuals to a double dose of toxicants9 that may place them at risk for physical health problems such as respiratory disease10. There is also evidence that dual users are more likely to become tobacco and cannabis dependent2, 10–15, although findings are equivocal16–17. Among adolescents, dual use of cigarettes and cannabis has been linked to lower grades, illicit drug use, and mental health problems3,18, poorer physical health, more delinquency, less educational attainment, and lower life satisfaction by young adulthood14, 19–20. Research on adults has demonstrated that dual use is correlated with mental health problems and other drug use6, 14, 21–22. However, prior research on dual use of cannabis and cigarettes has been primarily cross-sectional and has not focused on adult daily dual use.
There is also a dearth of prospective, longitudinal studies examining early predictors of adult dual use. Most studies of dual use have either been cross-sectional or have examined outcomes across two waves of data collection spanning adolescence to young adulthood. However, prior research on the long-term effects of gestational exposures support the hypothesis that individuals exposed to prenatal tobacco or cannabis are at greater risk of becoming dual users of these substances. Maternal combustible cigarette and cannabis use during pregnancy are robust predictors of adult offspring cigarette and cannabis use23–27. Prenatal exposure to maternal cigarette use predicts adolescent risk for tobacco dependence, even after controlling for maternal nicotine dependence24. Gestational exposures to cigarettes and cannabis each predict earlier initiation of these substances28–29, and individuals who use them at an earlier age are more likely to continue using cigarettes and cannabis as adults30–31. Thus, it is likely that there are pathways from prenatal exposure to cigarettes and prenatal exposure to cannabis to adult daily dual use of cigarettes and cannabis via earlier initiation and dependence on these substances. Other promising candidates for pathways linking gestational exposures to adult smoking behavior include cognitive deficits32–33 and behavioral problems34–35 associated with prenatal exposures to cigarettes and cannabis.
In previous studies, dual use of cigarettes and cannabis has often been conceptualized as any cigarette and cannabis use in the past 30 days or any use reported for the past year. Consequently, prior work cannot distinguish between individuals who used cigarettes and cannabis daily and those who used them much less frequently, even though daily use of these substances is more closely associated with risk for dependence and other negative outcomes. In addition, there have been few longitudinal studies of adult dual use, and none have examined the role of risk factors prior to adolescence, including prenatal exposures to cigarettes and cannabis. The goal of this study was to address these gaps in the literature on dual use of combustible cigarettes and cannabis by using prospective, longitudinal data from a well-characterized prenatal cohort to identify early predictors of daily dual use in young adulthood. We hypothesized that gestational exposures to cigarettes and cannabis would predict adult daily dual use of these substances indirectly, and that there would be pathways from prenatal exposure to adult dual use via risk factors measured during adolescence.
2. METHODS
2.1. Study Design
The current study is a secondary data analysis of longitudinal data from a combined sample of individuals from two prenatal cohorts born at a university teaching hospital in Pittsburgh, PA. Pregnant adult women (≥ 18 years of age) were recruited by their fourth or fifth month of pregnancy for studies on the long-term effects of prenatal alcohol or cannabis exposure. The women selected for the studies were seen again at 7 months and at delivery, when the infants were also assessed. Offspring physical and mental health, behavior, and cognitive development were assessed at multiple time points from childhood to young adulthood (see [Anonymous]36 for more details about the parent studies). The current analysis focuses on data collected during the prenatal period (1st trimester), adolescence (age 14), and young adulthood (age 22). These specific periods were chosen because they represent important periods for exposure to tobacco and cannabis and the initiation and progression of tobacco and cannabis use to daily dual use.
Participants for this study were offspring from two prenatal cohorts in which mothers were recruited from 1982–1985 (combined birth cohort N = 763). One cohort was designed to examine the effects of prenatal cannabis exposure (half the women used cannabis at least twice a month in early pregnancy and half used less or no cannabis) and the other cohort was designed to examine the effects of prenatal alcohol exposure (half the women drank three or more drinks in early pregnancy and the remainder drank less often or abstained). Mothers could be in either or both cohorts, study protocols and assessment staff were identical for these cohorts, and data were combined into a single sample for analysis. Offspring were seen again with their mothers at multiple time points during infancy, childhood, adolescence, and most recently, during young adulthood. Analyses are based on the 500 young adult offspring with complete assessments at ages 14 and 22.
2.2. Measures
2.2.1. Prenatal exposures to maternal cigarette, alcohol, and cannabis use
In the parent study, pregnant women were asked about the quantity and frequency of their tobacco, alcohol, and cannabis use during the first trimester of pregnancy37. A calendar was used to ascertain pregnancy recognition and women’s cannabis and cigarette use prior to that date, and this was used to weight estimates of first trimester exposures. This approach is more rigorous and reliable than relying on retrospective self-report or medical chart data on prenatal substance use. Data on quantity and frequency of substance use were used to compute a daily average for cigarettes, alcoholic drinks, and joints for each trimester. Continuous measures of prenatal cannabis exposure (average joints per day) and prenatal tobacco exposure (average cigarettes per day) during the first trimester were used in these analyses.
2.2.2. Early initiation of substance use
At ages 14, 16, and 22, the offspring were asked if they had ever used cigarettes (more than a puff) or cannabis and, if yes, at what age they had initiated either substance. To avoid recall bias, the earliest age of initiation reported across phases was used in the current analysis. A cut-off of age 13 was chosen for early initiation of cigarette use. In nationally representative data of similarly aged youth from this period, less than half had smoked more than a puff of a cigarette by the 8th grade38. A cut-off of age 14 was used to create an early cannabis use variable. Less than 25% of American youth from this period had initiated cannabis by age 1439.
2.2.3. Other adolescent risk factors
Several variables from the age 14 assessment were chosen to examine possible pathways from prenatal exposures to adult dual use of cannabis and cigarettes, based on previous findings from studies of children prenatally exposed to cigarettes and cannabis. The Wide Range Assessment of Memory and Learning (WRAML) Memory Screening Index is a time-efficient assessment of learning and memory for children ages 5–1740. The Conners Continuous Performance Task (CPT)41 is a computerized test of sustained attention and response inhibition that is commonly used to assess problems with impulsivity and other symptoms of Attention Deficit/Hyperactivity Disorder (ADHD). Behavior problems were measured using the Child Behavior Checklist – maternal report (CBCL)42, a questionnaire validated for adolescents to report on internalizing problems such as depression and anxiety and externalizing problems such as aggressive behavior and delinquency.
2.2.4. Young adult outcomes
At age 22, the offspring were interviewed about the quantity and frequency of substance use in the past year. These data were used to create a dichotomous variable (yes/no) for daily dual use of tobacco cigarettes and cannabis that served as the primary outcome variable for this study. Other young adult outcomes were examined for the purposes of sample description including demographic variables and dependence on tobacco and other substances. Young adults completed the Fagerström Test of Nicotine Dependence (FTND)43 and scores ≥ 5 were used to indicate dependence on cigarettes. Binge-drinking was defined as 5 or more alcoholic drinks per occasion in the past year. Cannabis Use Disorder (CUD) and Alcohol Use Disorder (AUD) were determined using the Diagnostic Interview Schedule for the 4th Diagnostic and Statistical Manual (DIS-IV)44. This is a standardized assessment of mental health administered by trained and reliable interviewers. Participants also reported on social roles and adult functioning including educational attainment, employment, parenthood, and history of arrest.
2.3. Statistical analysis
To address the study hypothesis, adult daily dual use of cigarettes and cannabis was regressed on gestational and adolescent risk factors. The results of this stepwise regression were used to create the most parsimonious structural equation model (SEM) of early risk factors for young adult dual use of cigarettes and cannabis with an alpha of 0.05 the threshold for variable inclusion. Prenatal exposure to alcohol was not associated with adult dual use in the regression model. The CPT measure (adolescent impulsivity) was not significantly related to either adult daily dual use or prenatal exposure after controlling for other risk factors in regression analysis, and subsequently was not included in the path model. MacKinnon’s z’ test was used to test indirect effects, modeling pathways from cannabis and tobacco gestational exposures to adolescent risk factors and young adult daily dual use of cigarettes and cannabis.
3. RESULTS
Prenatal exposures, demographic characteristics, young adult substance use, and social roles and functioning are presented in Table 1. The young adult sample was roughly half female, 58% Black, and 42% White. As mothers had been oversampled for prenatal alcohol and cannabis use, it is not surprising that there were high rates of prenatal exposure to cigarettes, alcohol, and cannabis. Similarly, the prevalence of substance use and arrests among the young adult offspring were higher than would be expected in the general population.
Table 1.
Sample characteristics (N = 500)
| Percent or M ± SD (range) | |
|---|---|
| A. Prenatal Exposures to Maternal Substance Use | |
| 1st trimester cigarette exposure (yes/no) | 53% |
| 1st trimester alcohol exposure (yes/no) | 64% |
| 1st trimester cannabis exposure (yes/no) | 42% |
| B. Adolescent Characteristics (age 14) | |
| Tobacco cigarette use by age 13 | 37% |
| Cannabis use by age 14 | 35% |
| Impulsivity (CPT errors of commission) | 14.7 (7) |
| Learning and memory (WRAML composite score) | 89.1 (14) |
| Externalizing behavior problems (CBCL) | 53.2 (11) |
| C. Young Adult Demographic Characteristics (age 22) | |
| Mean age (years) | 22.7± 0.6 (21–26) |
| Sex – Female | 53% |
| Male | 47% |
| Race – Black | 58% |
| White | 42% |
| D. Young Adult Substance Use (age 22) | |
| Current cigarette smoker (yes/no) | 43% |
| Nicotine dependence (FTND ≥5)a | 10% |
| Current cannabis user (yes/no) | 50% |
| Lifetime Cannabis Use Disorder (DIS)b | 13% |
| Binge-drinking | 62% |
| Lifetime Alcohol Use Disorder (DIS)b | 17% |
| E. Young Adult Roles and Functioning (age 22) | |
| Education (years) | 12.8 ± 1.6 (8–18) |
| Currently employed (yes/no) | 60% |
| Currently in school/training (yes/no) | 25% |
| Military service (yes/no) | 4% |
| Ever had a child (yes/no) | 36% |
| Ever arrested (yes/no) | 36% |
Notes.
Nicotine dependence was assessed with the Fagerström Test of Nicotine Dependence (FTND).
AUD and CUD were assessed with the Diagnostic Interview Schedule (DIS) for DSM-IV
Comparisons between offspring who were daily dual users of combustible cigarettes and cannabis and those who were not daily dual users are presented in Table 2. Daily dual users were significantly different from participants who did not use cannabis and cigarettes every day in many respects. They were more likely to be male, non-White, and prenatally exposed to cannabis and alcohol. During adolescence, dual users of cigarettes and cannabis were more likely to have initiated cannabis by age 14, were more impulsive, had lower learning and memory scores, and more externalizing behavior problems. As young adults, dual users were more likely to have completed fewer than 12 years of education, have had a child, been arrested, engaged in binge-drinking, have a history of AUD or CUD, be unemployed, and be tobacco dependent. However, adult daily dual users of tobacco cigarettes and cannabis were not more likely to have been prenatally exposed to tobacco or to use combustible cigarettes by age 13.
Table 2.
Comparing Daily Dual Users to Other Offspring
| Daily Dual Use | |||
|---|---|---|---|
| Yes (n = 62) | No (n = 438) | p | |
| Age (yrs) (mean) | 22.7 | 22.7 | N.S. |
| Male (%) | 64.5 | 44.1 | < 0.01 |
| White (%) | 25.8 | 44.1 | < 0.01 |
| Prenatal cigarette exposure (%) | 53.2 | 53.2 | N.S. |
| Early cigarette use (%) (≤ 13 years old) | 33.9 | 37.9 | N.S. |
| Prenatal cannabis exposure (%) | 54.8 | 40.4 | < 0.05 |
| Early cannabis use (%) (≤ 14 years) | 59.7 | 31.3 | < 0.001 |
| Prenatal alcohol exposure (%) | 16.5 | 14.4 | < 0.05 |
| Impulsivity (age 14) | |||
| Learning and memory scores (age 14) | 84.0 | 89.9 | < 0.01 |
| Externalizing behavior problems (age 14) | 57.0 | 52.6 | < 0.01 |
| Had a child by age 22(%) | 50.0 | 34.0 | < 0.01 |
| Unemployed at age 22 (%) | 59.7 | 36.8 | < 0.001 |
| Ever arrested by age 22 (%) | 75.8 | 30.6 | < 0.001 |
| Current binge-drinking, age 22 (5+ drinks/occasion) | 74.2 | 60.0 | < 0.05 |
| Alcohol Use Disorder (AUD)a by age 22 (%) | 36.2 | 14.6 | < 0.001 |
| Cannabis Use Disorder (CUD)a by age 22 (%) | 31.0 | 10.7 | < 0.001 |
| Tobacco dependent at age 22 (FTND > 4)b (%) | 17.7 | 8.7 | < 0.05 |
Notes.
AUD and CUD were assessed with the Diagnostic Interview Schedule (DIS) for DSM-IV.
Nicotine dependence was assessed with the Fagerström Test of Nicotine Dependence (FTND).
A stepwise logistic regression was applied to examine the direct effects of prenatal tobacco and cannabis exposure and the adolescent risk factors (impulsivity, learning and memory, externalizing behavior problems) on adult daily dual use while adjusting for gender and race. At each step, we examined which variables remained significant. Prenatal cannabis exposure, impulsivity, and learning and memory were significantly related to daily dual use in bivariate analyses (Table 2). However, after adjusting for race and gender, only externalizing behavior problems remained a significant predictor of daily dual use in the final regression model (Table 3).
Table 3.
Predictors that were significant in the logistic regression model on daily dual use of cigarettes and cannabis
| Variables | Coefficient | Adjusted OR (95% CI) | Coef./SE | Significance |
|---|---|---|---|---|
| Race | −1.10 | 0.33 (0.17–0.63) | −3.3 | < 0.001 |
| Sex (male) | 1.01 | 2.74 (1.53–4.91) | 3.4 | < 0.001 |
| Externalizing Behavior | 0.05 | 1.05 (1.02–1.08) | 3.4 | < 0.001 |
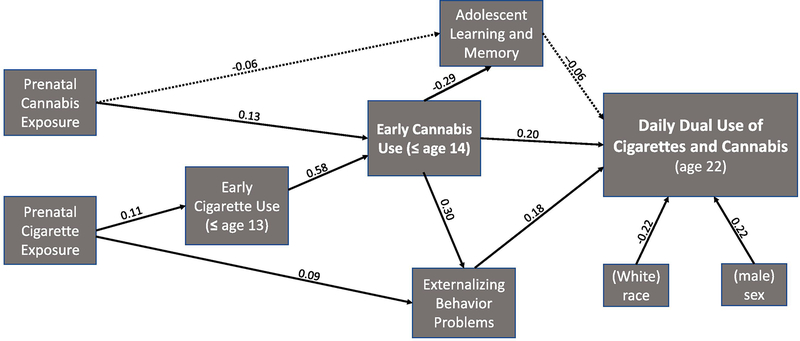
The results of the Structural Equation Model (SEM) on the indirect effects of prenatal exposures to cannabis and tobacco to adult offspring daily dual use is presented in Figure 1. Standardized coefficients are provided to demonstrate the relative significance of the paths. Early cannabis use and adolescent externalizing behavior problems directly predicted young adult daily dual use of cannabis and cigarettes (Figure 1). The total indirect effect from prenatal cannabis exposure to adult daily dual use of cigarettes and cannabis was significant (z’= 2.1, p <0.05). There were significant individual indirect effects of prenatal cannabis on adult daily dual use of cigarettes and cannabis via early cannabis use (z’=1.6, p <0.05) and adolescent externalizing behavior problems (z’=1.7, p <0.05). The total indirect effect from prenatal tobacco exposure to daily dual use was also significant (z’ =2.1, p <0.05) with significant individual indirect effects via early cigarette and cannabis use (z’=1.4, p <0.05), and externalizing behavior problems (z’=1.4, p <0.05). The indirect effects of prenatal cannabis and tobacco exposure on adult daily dual use via adolescent learning and memory problems were not significant.
Fig. 1.
Pathways to Daily Dual Use of Cigarettes and Cannabis.
Note. Solid lines represent paths that are statistically significant (p < .05, one-tail). Dotted lines represent paths that are non-significant (p > .05). Standardized coefficients are provided for all paths tested in the model.
4. CONCLUSIONS
In contrast to prior research examining correlates of any dual use of combustible cigarettes and cannabis in adolescence or adulthood, this investigation models pathways from early risk factors to daily dual use of cigarettes and cannabis during young adulthood. Using three waves of prospective data from a unique, well-characterized prenatal cohort, we were able to tease apart the gestational and adolescent risk factors for adult daily dual use. Early cannabis use (but not early cigarette use) and adolescent externalizing behavior problems independently predicted daily dual adult use of cigarettes and cannabis, controlling for the effects of race, sex, prenatal exposures to cigarettes and cannabis, and early cigarette use. One of the implications of these results is that individuals at-risk for daily dual use of cigarettes and cannabis can be identified as early as age 14 by their early initiation of cannabis. Of even greater concern, given the rising rates of cannabis use, is that prenatal cannabis exposure has long-lasting effects on behavior, including risk for daily cigarette and cannabis use.
These findings add to a converging body of literature on the role of early cannabis initiation as a risk factor for daily adult cigarette use, providing evidence that early cannabis use is also associated with risk for adult daily dual use of cigarettes and cannabis, controlling for other well-known risk factors for smoking. Early initiation of cannabis has been operationalized in different ways previously, and prior studies varied in their inclusion/exclusion of adolescents who had previously initiated cigarette use, yet findings from several other cohort studies point to a heightened risk of initiation and progression of cigarette use among adolescents who use cannabis at a younger age. For example, weekly cannabis use among non-smoking adolescents ages 14–15 predicted initiation of tobacco use by young adulthood in an Australian cohort study45 and cannabis use by age 17 was associated with greater risk of tobacco dependence in an Australian twin study46. In the National Longitudinal Survey of Adolescent to Adult Health, earlier age of cannabis initiation predicted daily smoking and nicotine dependence by ages 23–2747. Similarly, Seaman and colleagues6 found that individuals in the National Health and Examination survey who engaged in any dual use in the past 30 days had an earlier age of cannabis initiation than those who had only used cannabis in the past 30 days. Taken together, the results of these studies suggest that earlier cannabis initiation is a risk factor for adult cigarette use, with implications for tobacco cessation efforts in a generation that is increasingly likely to use cannabis before cigarettes.
The results of this study also contribute to the literature on the effects of prenatal exposure to cigarettes and cannabis by illustrating several indirect pathways from these gestational exposures to daily dual use of cigarettes and cannabis in young adult offspring. Prior research has established that prenatal tobacco exposure contributes to the risk of early cigarette use23, 27, nicotine dependence24–25, and adult smoking behavior26, 48. Similarly, prenatal cannabis exposure has been related to early initiation of27, 36 and adult use of cannabis49. However, this is the first study to examine pathways from these gestational exposures to daily dual use of combustible cigarettes and cannabis use, testing several adolescent outcomes of prenatal exposures as possible mediators to daily dual use. These findings suggest that the effects of gestational exposures to cigarettes and cannabis on adolescent behavior problems and early cannabis initiation may predispose prenatally exposed individuals to adult daily dual use of cigarettes and cannabis.
The results of this prospective study of adult daily dual use of cigarette and cannabis use should be interpreted with caution, given several important limitations. Young adult data for the study were collected from 2006–2009 in Western Pennsylvania and may not reflect more current patterns of cannabis and cigarette use in other geographic areas. The demographics of the current study reflected the local teaching hospital where participants were born. The population was half Black and half White. Few Hispanic and Asian American women gave birth there in the mid-1980s. The parent study oversampled for exposure to prenatal alcohol and cannabis use and there were only data available on adult offspring’s concurrent use of cigarettes and cannabis in the past year. Future studies should investigate the temporality of dual use because adults who co-administer or use these products sequentially may use more of both substances and engage in risky behavior14. This study was not powered for multiple group SEM of daily dual use, but sex differences in pathways to daily dual use should also be investigated in future research, as girls prenatally exposed to tobacco may be more likely to progress from initiation to daily cigarette use26 and become nicotine dependent50.
The current study also has several strengths, including prospectively collected data on gestational exposures and adolescent risk factors from over 500 individuals from a prenatal cohort with excellent follow-up rates. Other studies reporting prenatal exposures have relied on retrospective reports or a notation in the medical record, rather than detailed maternal interviews of quantity and frequency of substance use conducted for each trimester of the pregnancy. In the current study, individuals were assessed again during adolescence, allowing us to determine (1) if prenatal exposures had a direct or indirect effect on adult daily dual use of combustible cigarettes and cannabis and (2) which adolescent factors might be part of a pathway from gestational exposures to daily adult usage. Rather than examining any past month or past year dual use, which combines experimental and occasional users with chronic users, we focused our analysis on daily dual use of tobacco cigarettes and cannabis. Overall, the present study leveraged a unique and rich dataset to answer research questions about the roles of prenatal cannabis exposure and early onset of cannabis use as risk factors for adult daily dual use of cigarettes and cannabis. This is timely as cannabis use is increasing among adults in general and in pregnant women specifically.
As cannabis use increases and daily cannabis use increases among cigarette smokers7, it is timely and necessary to investigate correlates and pathways to daily dual use of cigarettes and cannabis. The results of our investigation suggest that it may be possible to determine who is at higher risk of becoming a daily dual user of cigarettes and cannabis by mid-adolescence. Modeling data from gestation to young adulthood demonstrated independent pathways from early risk factors to adult daily dual use that included gestational exposures to cannabis and tobacco, early cannabis use, and adolescent externalizing behavior problems.
Highlights.
This is a prospective study of adult daily dual use of cigarettes and cannabis.
Prenatal cigarette and prenatal cannabis exposures predict adult daily dual use.
Prenatal and adolescent exposures to cannabis play a role in adult daily dual use.
Externalizing behavior problems link gestational exposures to adult dual use.
It is possible to identify children at-risk of adult daily dual use by age 14.
Acknowledgments
Role of Funding Sources
This work was supported by the National Institutes of Health (DA037209, PI: De Genna). The NIDA had no role in the study design, data collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Conflict of Interest
There are no conflicts of interest for any of the authors of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schauer GL, Berg CJ, Kegler MC, Donovan DM, & Windle M (2016). Differences in tobacco product use among past month adult marijuana users and nonusers: Findings from the 2003–2012 National Survey on Drug Use and Health. Nicotine Tob Res, 18(3), 281–288. [DOI] [PubMed] [Google Scholar]
- 2.Schauer GL, King BA, & McAfee TA (2017). Prevalence, correlates, and trends in tobacco use and cessation among current, former, and never adult marijuana users with a history of tobacco use, 2005–2014. Addict Behav, 73, 165–171. [DOI] [PubMed] [Google Scholar]
- 3.Smith DM, Miller C, O’Connor RJ, Kozlowski LT, Wadsworth E, Fix BV, et al. (2020. January). Modes of delivery in concurrent nicotine and cannabis use (“co-use”) among youth: Findings from the International Tobacco Control (ITC) Survey. Substance Abuse, 11, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauer GL, Berg CJ, Kegler MC, Donovan DM, & Windle M (2015). Assessing the overlap between tobacco and marijuana: Trends in patterns of co-use of tobacco and marijuana in adults from 2003–2012. [DOI] [PubMed]
- 5.Cohn AM, Abudayyeh H, Perreras L, & Peters EN (2019). Patterns and correlates of the co-use of marijuana with any tobacco and individual tobacco products in young adults from Wave 2 of the PATH Study. Addict Behav, 92, 122–127. [DOI] [PubMed] [Google Scholar]
- 6.Seaman EL, Green KM, Wang MQ, Quinn SC, & Fryer CS (2019). Examining prevalence and correlates of cigarette and marijuana co-use among young adults using ten years of NHANES data. Addict Behav, 96, 140–147. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin RD, Pacek LR, Copeland J, et al. (2018). Trends in daily cannabis use among cigarette smokers: United States, 2002–2014. Am J Public Health, 108(1), 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravely S, Driezen P, Smith DM, Borland R, Lindblom EN, Hammond D, et al. (2020. May). International differences in patterns of cannabis use among adult cigarette smokers: Findings from the 2018 ITC Four Country Smoking and Vaping Survey. International Journal of Drug Policy., 1(79), Article 102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier E, & Hatsukami DK (2016). A review of the additive health risk of cannabis and tobacco co-use. Drug Alcohol Depend, 166, 6–12. [DOI] [PubMed] [Google Scholar]
- 10.Strong DR, Myers MG, Pulvers K, Noble M, Brikmanis K, & Doran N (2018). Marijuana use among US tobacco users: Findings from wave 1 of the Population Assessment of Tobacco Health (PATH) study. Drug Alcohol Depend. 186, 16–22. [DOI] [PubMed] [Google Scholar]
- 11.Weinberger AH, Platt J, Copeland J, & Goodwin RD (2018). Is cannabis use associated with increased risk of initiation, persistence, and relapse to cigarette smoking? Longitudinal data from a representative sample of US adults. J Clin Psychiatry., 79(2), 17m1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger AH, Pacek LR, Wall MM, Gbedemah M, Lee J, & Goodwin RD (2019). Cigarette smoking quit ratios among adults in the USA with cannabis use and cannabis use disorders, 2002–2016. Tob Control, 29(1), 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel EA, Rubinstein ML, Prochaska JJ, & Ramo DE (2018). Associations between marijuana use and tobacco cessation outcomes in young adults. J Subst Abuse Treat, 94, 69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucker JS, Pedersen ER, Seelam R, Dunbar MS, Shih RA, & D’Amico EJ (2019). Types of cannabis and tobacco/nicotine co-use and associated outcomes in young adulthood. Psychol Addict Behav, 33(4), 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore BA, & Budney AJ (2001). Tobacco smoking in marijuana-dependent outpatients. J Subst Abuse, 13(4), 583–596. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery L (2015). Marijuana and tobacco use and co-use among African Americans: Results from the 2013, National Survey on Drug Use and Health. Addict Behav., 51, 18–23. [DOI] [PubMed] [Google Scholar]
- 17.Hendricks PS, Delucchi KL, Humfleet GL, & Hall SM (2012). Alcohol and marijuana use in the context of tobacco dependence treatment: Impact on outcome and mediation of effect. Nicotine Tob Res, 14(8), 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pulvers K, Ridenour C, Woodcock A, et al. (2018). Marijuana use among adolescent multiple tobacco product users and unique risks of dual tobacco and marijuana use. Drug Alcohol Depend, 189, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgiades K, & Boyle MH (2007). Adolescent tobacco and cannabis use: Young adult outcomes from the Ontario Child Health Study. J Child Psychol Psychiatry, 48(7), 724–731. [DOI] [PubMed] [Google Scholar]
- 20.Tucker JS, Rodriguez A, Dunbar MS, Pedersen ER, Davis JP, Shih RA, et al. (2019. November). Cannabis and tobacco use and co-use: Trajectories and correlates from early adolescence to emerging adulthood. Drug and alcohol dependence., 1(204), Article 107499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery L, Schiavon S, & Cropsey K (2019). Factors associated with marijuana use among treatment-seeking adult cigarette smokers in the criminal justice population. J Addict Med, 13(2), 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thrul J, Vijayaraghavan M, Kalkhoran S, & Satterfield JM (2020). Patterns of cigarette, e-cigarette, and cannabis use among adult smokers in primary care 2014–2015. Addictive behaviors., 100, 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niaura R, Bock B, Lloyd EE, Brown R, Lipsitt LP, & Buka S (2001). Maternal transmission of nicotine dependence: Psychiatric, neurocognitive and prenatal factors. Am J Addict, 10(1), 16–29. [DOI] [PubMed] [Google Scholar]
- 24.Oncken C, O’Malley S, Krishnan-Sarin S, McKee S, & Mazure C (2004). Gender effects of reported in utero tobacco exposure on smoking initiation, progression and nicotine dependence in adult offspring. Nicotine Tob Res, 6(5), 829–833. [DOI] [PubMed] [Google Scholar]
- 25.Porath AJ, & Fried PA (2005). Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol Teratol, 27(2), 267–277. [DOI] [PubMed] [Google Scholar]
- 26.Breslau N, & Peterson EL (1996). Smoking cessation in young adults: Age at initiation of cigarette smoking and other suspected influences. Am J Public Health, 86(2), 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chassin L, Presson CC, Sherman SJ, & Edwards DA (1990). The natural history of cigarette smoking: Predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol, 9(6), 701–716. [DOI] [PubMed] [Google Scholar]
- 28.Cornelius MD, Goldschmidt L, De Genna NM, & Larkby C (2012). Long-term effects of prenatal cigarette smoke exposure on behavior dysregulation among 14- year-old offspring of teenage mothers. Maternal and Child Health Journal, 16(3), 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornelius MD, Leech SL, Goldschmidt L, & Day NL (2000). Prenatal tobacco exposure: Is it a risk factor for early tobacco experimentation? Nicotine & Tobacco Research, 2(1), 45–52. [DOI] [PubMed] [Google Scholar]
- 30.Cornelius MD, Ryan CM, Day NL, Goldschmidt L, & Willford JA (2001). Prenatal tobacco effects on neuropsychological outcomes among preadolescents. Journal of Developmental & Behavioral Pediatrics, 22(4), 217–225. [DOI] [PubMed] [Google Scholar]
- 31.Day NL, Goldschmidt L, & Thomas CA (2006. September). Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction, 101(9), 1313–1322. [DOI] [PubMed] [Google Scholar]
- 32.Day NL, & Robles N (1989). Methodological issues in the measurement of substance use. New York Academy of Sciences, 562(1), 8–13. [DOI] [PubMed] [Google Scholar]
- 33.De Genna NM, Goldschmidt L, Day NL, & Cornelius MD (2017). Prenatal tobacco exposure, maternal postnatal nicotine dependence and adolescent risk for nicotine dependence: Birth cohort study. Neurotoxicology and Teratology, 61, 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldschmidt L, Day NL, & Richardson GA (2000). Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicology and Teratology, 22 (3), 325–336. [DOI] [PubMed] [Google Scholar]
- 35.Goldschmidt L, Richardson GA, Willford J, & Day NL (2008). Prenatal marijuana exposure and intelligence test performance at age 6. Journal of the American Academy of Child & Adolescent Psychiatry, 47(3), 254–263. [DOI] [PubMed] [Google Scholar]
- 36.Klein H, Sterk CE, & Elifson KW (2013). Initial Smoking Experiences and Current Smoking Behaviors and Perceptions among Current Smokers. J Addict., 491797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CY, Storr CL, & Anthony JC (2009). Early-onset drug use and risk for drug dependence problems. Addictive Behav, 34(3), 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, Patrick M. Monitoring the Future national survey results on drug use, 1975–2018: Volume I, Secondary school students. Ann Arbor: Institute for Social Research, The University of Michigan, 2019.
- 39.Gfroerer JC, Wu LT, & Penne MA (2002). Initiation of marijuana use: Trends, patterns, and implications (Analytic Series: A-17, DHHS Publication No. SMA 02–3711). Rockville, MD: Substance Abuse and Mental Health Services Administration. Office of Applied Studies.
- 40.Sheslow D, & Adams W (1990). Wide Range Assessment for Memory and Learning. Wilmington, DE: Jastak Associates. [Google Scholar]
- 41.Conners’, C. CK. (2000). Continuous Performance Test II. CPT II. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- 42.Achenbach TM, & Edelbrock C (1991). Child Behavior Checklist. Burlington, VT., 7, 371–392. [Google Scholar]
- 43.Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- 44.Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, & Rourke KM (2000). Diagnostic Interview Schedule for DSM-IV. Department of Psychiatry, St. Louis, MO: Washington University School of Medicine. [Google Scholar]
- 45.Patton GC, Coffey C, Carlin JB, Sawyer SM, & Lynskey M (2005). Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction, 100(10), 1518–1525. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal A, Lynskey MT, Pergadia ML, et al. (2008). Early cannabis use and DSM-IV nicotine dependence: A twin study. Addiction, 103(11), 1896–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Timberlake DS, Haberstick BC, Hopfer CJ, et al. (2007). Progression from marijuana use to daily smoking and nicotine dependence in a national sample of US adolescents. Drug Alcohol Depend, 88(2–3), 272–281. [DOI] [PubMed] [Google Scholar]
- 48.Al Mamun A, O’Callaghan FV, Alati R, O’Callaghan M, Najman JM, Williams GM, et al. (2006). Does maternal smoking during pregnancy predict the smoking patterns of young adult offspring? A birth cohort study. Tobacco Control, 15 (6), 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonon KE, Richardson GA, Cornelius JR, Kim KH, & Day NL (2015). Prenatal marijuana exposure predicts marijuana use in young adulthood. Neurotoxicol Teratol, 47, 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rydell M, Cnattingius S, Granath F, Magnusson C, & Galanti MR (2012). Prenatal exposure to tobacco and future nicotine dependence: Population-based cohort study. Br J Psychiatry, 200(3), 202–209. [DOI] [PubMed] [Google Scholar]