Abstract
Background.
Whether to repair non-severe tricuspid regurgitation (TR) during surgery for ischemic mitral regurgitation (IMR) remains uncertain.
Objectives.
To investigate incidence, predictors and clinical significance of TR progression and presence of ≥moderate TR after IMR surgery.
Methods.
Patients (n=492) with untreated non-severe TR within two prospectively randomized IMR trials were included. Key outcomes were TR progression (either progression by ≥2 grades, surgery for TR, or severe TR at 2 years) and presence of ≥moderate TR at 2 years.
Results.
Mean age was 66±10 years (67% male), and TR distribution was 60% ≤trace, 31% mild and 9% moderate. Among 2-year survivors, TR progression occurred in 20/325 (6%). Baseline tricuspid annular diameter (TAD) was not predictive of TR progression. At 2 years, 37/323 (11%) patients had ≥moderate TR. Baseline TR grade, indexed TAD and surgical ablation for atrial fibrillation were independent predictors of ≥moderate TR. However, TAD alone had poor discrimination (AUC≤0.65). Presence of ≥moderate TR at 2 years was higher in patients with MR recurrence (20% vs 9%;p=0.02) and permanent pacemaker/defibrillator (19% vs 9%;p=0.01). Clinical event rates (composite of ≥1 NYHA class increase, HF hospitalization, mitral valve surgery, stroke) were higher in patients with TR progression (55% vs 23%;p=0.003) and ≥moderate TR at 2 years (38% vs 22%;p=0.04).
Conclusions.
After IMR surgery, progression of unrepaired non-severe TR is uncommon. Baseline TAD is not predictive of TR progression, and is poorly discriminative of ≥moderate TR at 2 years. TR progression and presence of ≥moderate TR are associated with clinical events.
Keywords: tricuspid valve regurgitation, mitral valve regurgitation, ischemic heart disease mitral valve surgery, tricuspid annular dilation
Condensed Abstract
Progression of tricuspid regurgitation (TR) was evaluated in 492 patients (66±10 years, 67% male) with unrepaired non-severe TR during cardiac surgery for ischemic mitral regurgitation in two prospectively randomized trials. Among 2-year survivors, TR progression was uncommon (20/325, 6%) and not predicted by baseline tricuspid annular diameter. At 2 years, 37/323 (11%) patients had ≥moderate TR. Baseline TR grade, indexed annular diameter and surgical ablation for atrial fibrillation were independent predictors of ≥moderate TR. Postoperative MR recurrence and permanent pacemaker/defibrillator were also associated with ≥moderate TR at 2 years. Both TR progression and ≥moderate TR were associated with clinical events.
Introduction
Progression of tricuspid regurgitation (TR) after mitral valve (MV) surgery is associated with significant morbidity, yet the optimal indications for treating TR at time of MV surgery remain unclear(1-4). Current guidelines recommend concomitant tricuspid valve repair in cases of moderate or more preoperative TR, or in cases of preoperative tricuspid annular dilation (>40mm or 21mm/m2) in patients with only mild TR (class IIa)(5,6). A prospectively randomized trial investigating this strategy in patients with primary mitral regurgitation (MR) is ongoing (NCT02675244). However, in patients with ischemic mitral valve regurgitation (IMR), data on TR progression after mitral valve (MV) surgery is limited(7-9). The reported incidence of significant (≥moderate) TR after IMR surgery in retrospective series is as high as 50% at 1-3 years(7). Prospective confirmation of this high incidence is lacking. Given the important pathophysiological differences, results from the ongoing trial in primary MR cannot be extrapolated to surgery for ischemic MR. Moreover, the value of the preoperative tricuspid annular dimension to predict TR progression in an ischemic heart disease population is unclear.
In two prospective National Heart, Lung, and Blood Institute (NHLBI)-supported Cardiothoracic Surgical Trials Network (CTSN) trials investigating surgery for moderate or severe IMR a selective approach towards concomitant tricuspid valve repair was adopted and left to the discretion of the surgeon(10,11). Tricuspid valve surgery was performed in less than 8% of the patients undergoing surgery for IMR, while 92% of patients had no concomitant intervention at the level of the tricuspid valve. Within these trials, serial echocardiography including dedicated tricuspid valve and right ventricular (RV) assessment was performed at baseline, 6 months, 1 year and 2 years and analyzed by an independent and central core laboratory.
The purpose of the present analysis is to assess the rate of TR progression from pre-operative baseline and the presence of ≥moderate TR at 2 years after IMR surgery in patients with untreated non-severe secondary TR within the CTSN IMR trials. Clinical, echocardiographic and procedural predictors of TR progression and ≥moderate TR are evaluated, as well as the clinical impact of TR progression and ≥moderate TR after IMR surgery.
Methods
Patient population
The patient population originates from two randomized surgical trials in IMR patients conducted by the CTSN, as previously described(10-13). Briefly, a total of 301 patients with moderate IMR were randomized to coronary artery bypass grafting (CABG) alone versus CABG + MV repair, while 251 patients with severe IMR were randomized to MV repair ± CABG versus MV replacement ± CABG. The trials were conducted in 26 and 22 centers, respectively, with a coordinating center, an independent clinical events committee adjudicating mortality and adverse events, and a data and safety monitoring board that oversaw trial progress. Participating centers’ institutional review board approved the protocol and all patients signed a written informed consent. Complete inclusion and exclusion criteria have been previously reported(10-13).
In the present analysis, all patients who had moderate or less TR without concomitant tricuspid intervention at the time of IMR surgery within both trials were included for analysis. Figure 1 shows the study population.
Figure 1 – Study flow chart.
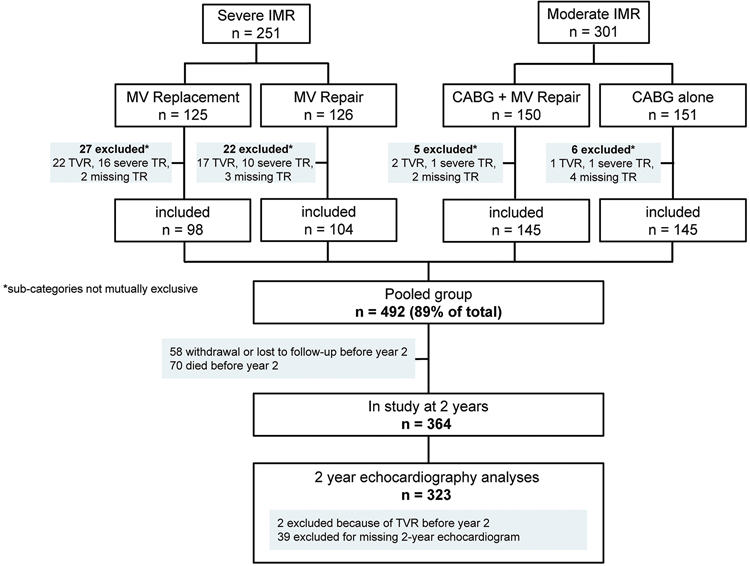
CABG, coronary artery bypass graft; IMR, ischemic mitral regurgitation; MV, mitral valve; TR, Tricuspid Regurgitation; TVR, Tricuspid Valve Repair.
Echocardiography
All echocardiographic exams were reviewed and analyzed by an independent central core laboratory. Measures of left ventricular function and MR were assessed according to international recommendations(14-16). TR was graded as none/trace, mild, moderate or severe using an integrative approach(15,16). Parameters used to grade TR included (1) the vena contracta width, (2) the radius of proximal flow conversion, and (3) qualitative assessment of the color flow TR jet, the density and shape of the continuous wave TR signal, and, when available, the hepatic vein flow signal.
The tricuspid annular diameter was measured in late diastole in a standard apical 4-chamber view, as recommended(17). Tricuspid annular dilation was defined as an annular dimension ≥40mm or 21mm/m2. In addition, measures of right ventricular size and function were obtained in a focused RV view(18).
Definition of TR progression
Progression of TR was defined as the composite of (1) presence of severe TR at 2 years post randomization, (2) re-operation for TR within 2 years post randomization, or (3) TR progression from baseline by two grades at 2 years post randomization, similar to the endpoint definition in the ongoing randomized trial in primary MV surgery (NCT02675244). In addition, presence of ≥moderate TR at 2 years was evaluated, irrespective of the TR grade at baseline.
Patients who died before the 2-year visit (n=70) or were missing the two-year echo endpoints (n=97), were excluded from outcome analyses (Figure 1). Details on TR status at baseline and at last follow-up in the patients who died are enclosed in the Supplementary Appendix (Online Tables I and II).
Clinical outcome
Major adverse clinical events (MACE) were defined as the composite of (1) increase of ≥1 New York Heart Association (NYHA) class, (2) hospitalization for heart failure, (3) redo mitral valve surgery, and (4) stroke (10,11). The association between either TR progression or ≥moderate TR at 2 years post-randomization with the incidence of MACE (or any of its components) within 2-years post-randomization was assessed.
Statistical analysis
Continuous data were expressed as mean ± SD and compared using t-tests, Wilcoxon tests, or analysis of variance (ANOVA) as appropriate. Categorical data were expressed as percentages and compared using χ2 or Fisher exact tests as appropriate. Univariable and multivariable logistic regression analyses were performed to determine whether any pre-specified clinically relevant baseline measures, including age, sex, history of atrial fibrillation, history of permanent pacemaker or implantable cardioverter/defibrillator (ICD), MR effective regurgitant orifice area, severity of TR, tricuspid annular dimension, tricuspid annular plane systolic excursion, RV fractional area change, tricuspid regurgitation peak velocity, type of mitral valve intervention, and concomitant surgical ablation for atrial fibrillation, were associated with ≥moderate TR at 2 years post randomization. Variables with a p-value <0.15 in univariable analyses were considered for inclusion in a multivariable logistic regression model. The final model was selected using backwards selection. Results were reported as odds ratios (ORs) with 95% confidence intervals (95% CIs). Receiver operating characteristic (ROC) curve analysis was used to evaluate the discrimination of baseline annular dimension for predicting TR progression and ≥moderate TR at 2 years. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Patient characteristics
A total of 492 patients were included in the study, of which 202 patients were enrolled in the severe IMR trial and 290 were enrolled in the moderate IMR trial. Table 1 summarizes the baseline characteristics of the study population. TR at baseline was none or trace in 60.4%, mild in 31.1% and moderate in 8.5%, with a higher proportion of moderate TR in the severe IMR group (26/202 (12.9%) vs. 16/290 (5.5%)).
Table 1.
Baseline characteristics of the patient cohort
| Variables | Total cohort (N=492) |
Severe IMR group (N=202) |
Moderate IMR group (N=290) |
P-value |
|---|---|---|---|---|
| Mean ±SD or No. (%) |
Mean ±SD or No. (%) |
Mean ±SD or No. (%) |
||
| Age, years | 66.3 ±10.4 | 68.4 ±9.8 | 64.8 ±10.5 | 0.0001 |
| Male gender | 328 (66.7) | 127 (62.9) | 201 (69.3) | 0.14 |
| Body surface area, m2 | 1.9 ±0.3 | 1.9 ±0.2 | 2.0 ±0.3 | 0.03 |
| Medical and surgical history | ||||
| Diabetes | 205 (41.8) | 71 (35.3) | 134 (46.2) | 0.02 |
| Hypertension | 405 (82.3) | 162 (80.2) | 243 (83.8) | 0.30 |
| Renal insufficiency | 103 (21) | 54 (26.7) | 49 (17) | 0.009 |
| Myocardial infarction | 343 (69.7) | 150 (74.3) | 193 (66.6) | 0.07 |
| Heart Failure | 293 (59.6) | 141 (69.8) | 152 (52.4) | 0.0001 |
| Atrial fibrillation | 108 (22) | 58 (28.7) | 50 (17.4) | 0.003 |
| Permanent Pacemaker or ICD | 60 (12.2) | 36 (17.8) | 24 (8.3) | 0.002 |
| Echocardiography data | ||||
| LV ejection fraction, % | 40.4 ±11.3 | 40.8 ±11.6 | 40.2 ±11.2 | 0.53 |
| LVESVI, mL/m2 | 60.3 ± 26.0 | 64.2 ±26.3 | 57.6 ±25.5 | 0.006 |
| MR effective regurgitant orifice area, cm2 | 0.29 ±0.14 | 0.39 ±0.15 | 0.23 ±0.09 | <.0001 |
| Tricuspid regurgitation | <.0001 | |||
| None/Trace | 297 (60.4) | 97 (48) | 200 (69.0) | |
| Mild | 153 (31.1) | 79 (39.1) | 74 (25.5) | |
| Moderate | 42 (8.5) | 26 (12.9) | 16 (5.5) | |
| Tricuspid annular diameter, mm | 38.3 ±5.2 | 38.2 ±5.5 | 38.3 ±5.1 | 0.88 |
| Tricuspid annular Index (mm/m2 BSA) | 20.3 ±3 | 20.5 ±3 | 20.1 ±2.9 | 0.09 |
| TAPSE, mm | 16.8 ±3.8 | 16.4 ±3.7 | 17 ±3.9 | 0.11 |
| RV fractional area change, % | 42.4 ±8.5 | 42.2 ±7.8 | 42.6 ±9 | 0.63 |
| Tricuspid regurgitation peak velocity, cm/s | 293.1 ±54.5 | 305 ±49 | 283.7 ±56.8 | 0.0002 |
| Operative data | ||||
| Cardiopulmonary bypass time, min | 136.5 ±54.6 | 139.8 ±47.3 | 134.3 ±59.2 | 0.25 |
| Aortic cross-clamp time, min | 98.2 ±40.8 | 101.2 ±39.6 | 96.1 ±41.6 | 0.17 |
| MV Repair | 246 (50) | 97 (48) | 149 (51.4) | 0.46 |
| MV Replacement | 106 (21.5) | 105 (52) | 1 (0.3) | <.0001 |
| CABG | 448 (91.1) | 158 (78.2) | 290 (100) | <.0001 |
| Surgical AF ablation | 42 (8.5) | 22 (10.9) | 20 (6.9) | 0.12 |
AF, atrial fibrillation; BSA, body surface area; CABG, coronary artery bypass grafting; ICD, implantable cardioverter-defibrillator; IMR, ischemic mitral regurgitation; LV, left ventricle; MR, mitral regurgitation; MV, mitral valve; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion.
Of the patients that were excluded from this analysis (Figure 1), a total of 50 patients had baseline severe TR and/or received concomitant tricuspid valve surgery at the discretion of the surgeon during the index IMR surgery. These patients were 68.1 ± 8.8 years old, 46% were female and the baseline tricuspid annular dimension was 43.2 ± 6.4mm (indexed value of 23.1 ± 3.9mm/m2). TR severity at baseline was severe in 20 (48%), moderate in 11 (26%), mild in 8 (19%), trace in 2 (5%) and unreported in 1 (2%) of the patients that received concomitant tricuspid valve surgery.
TR progression after IMR surgery
Postoperative TR at each study visit is shown in Figure 2.A. At 2 years after surgery, there was evidence of TR progression in 6.2% of the evaluated patients (20/325). Of these, 8 patients had severe TR (baseline TR grade was none/trace in 2 patients, mild in 4 patients and moderate in 2 patients, respectively), 2 patients had received tricuspid valve surgery during follow-up (baseline TR grade none/trace in 1 patient and mild in 1 patient) and 10 patients had progressed from none/trace to moderate TR.
Figure 2 – Tricuspid regurgitation at baseline and at different stages of follow-up.
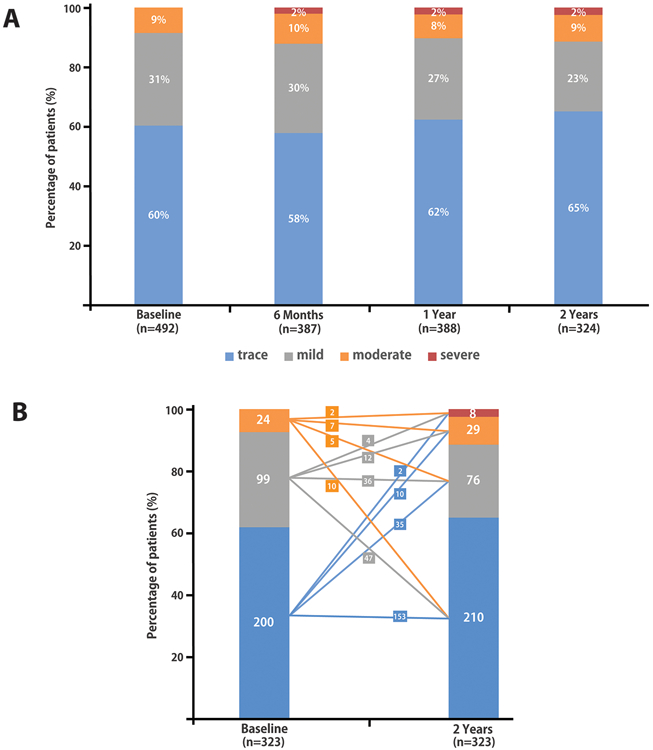
(A) Tricuspid regurgitation grade in the study cohort at baseline, and six months, 1 year and 2 years after the index surgery. (B) Tricuspid regurgitation grade at baseline and at 2 years after the index surgery for the patients with 2-year follow-up available (n=323, excluding one patient with 2-year echo follow-up that underwent interim tricuspid valve surgery). Changes in TR from baseline to 2 years (absolute numbers of patients) are presented.
Moderate or severe TR at 2 years was observed in 11.5% (37/323) of patients, of whom 8 had severe TR and 29 had moderate TR. Figure 2.B shows the difference between the baseline TR grade and the TR grade at 2 years in patients with available 2-year follow-up data. The two patients who underwent tricuspid valve surgery during follow-up were excluded from this analysis at 2 years. Of the 42 patients with moderate TR at baseline, 15 patients (36%) died during the 2-year follow-up, and 3 patients were missing the 2-year echocardiogram. Among the 24 patients with moderate TR at baseline and whom had two-year follow-up data, 5 had mild TR and 10 only had none/trace TR at the 2-year follow-up visit.
Baseline predictors of postoperative TR
In patients with versus those without TR progression, there were no significant differences in baseline TR grade, tricuspid annular dimension or procedural characteristics, yet the number of patients with TR progression was low. In patients with versus those without ≥moderate TR at 2 years, the most relevant baseline and procedural characteristics are summarized in Table 2. In univariable analysis, the baseline TR grade, tricuspid annular dimension, atrial fibrillation, surgical ablation of atrial fibrillation and male gender were different between these two patient groups. In multivariable analysis, baseline TR grade, concomitant surgical ablation of atrial fibrillation and indexed tricuspid annular dimension were independently predictive of ≥moderate postoperative TR (Table 3).
Table 2.
Baseline and operative characteristics in patients with versus without ≥moderate TR at 2-year follow-up.
| Variables | All (N=323) |
≥Moderate TR at 2 Years (N=37) |
<Moderate TR at 2 Years (N=286) |
P-value |
|---|---|---|---|---|
| Mean ±SD or No. (%) |
Mean ±SD or No. (%) |
Mean ±SD or No. (%) |
||
| Age, years | 65.6 ±10.1 | 68.5 ±10.7 | 65.3 ±10 | 0.07 |
| Male gender | 220 (68.1) | 20 (54.1) | 200 (69.9) | 0.05 |
| Body surface area, m2 | 1.9 ±0.3 | 1.9 ±0.3 | 1.9 ±0.3 | 0.27 |
| Medical and surgical history | ||||
| Atrial fibrillation | 66 (20.6) | 17 (45.9) | 49 (17.3) | <.0001 |
| Permanent Pacemaker or ICD (at baseline) | 31 (9.6) | 6 (16.2) | 25 (8.7) | 0.15 |
| Severe IMR Trial | 129 (39.9) | 19 (51.4) | 110 (38.5) | 0.13 |
| Echocardiography data | ||||
| LVESVI, mL/m2 | 60.2 ±24.6 | 53.0 ±22.1 | 61.1 ±24.8 | 0.06 |
| MR effective regurgitant orifice area, cm2 | 0.29 ±0.15 | 0.30 ±0.12 | 0.29 ±0.15 | 0.81 |
| Tricuspid regurgitation | <.0001 | |||
| None/Trace | 200 (61.9) | 12 (32.4) | 188 (65.7) | |
| Mild | 99 (30.7) | 16 (43.2) | 83 (29) | |
| Moderate | 24 (7.4) | 9 (24.3) | 15 (5.2) | |
| Tricuspid annular diameter, mm | 38 ±5.2 | 39.6 ±5.1 | 37.8 ±5.1 | 0.04 |
| Tricuspid annular Index (mm/m2 BSA) | 20.1 ±2.9 | 21.6 ±2.8 | 19.9 ±2.9 | 0.0008 |
| TAPSE, mm | 16.8 ±4 | 16.7 ±3.8 | 16.9 ±4 | 0.85 |
| RV fractional area change, % | 42.5 ±8.4 | 43.1 ±6.9 | 42.4 ±8.5 | 0.67 |
| Tricuspid regurgitation peak velocity, cm/s | 290 ±51.4 | 301.8 ±51.6 | 288 ±51.2 | 0.15 |
| Operative data | ||||
| Cardiopulmonary bypass time, min | 136.5 ±53 | 136.3 ±46.7 | 136.5 ±53.8 | 0.98 |
| Aortic cross-clamp time, min | 99.7 ±37.9 | 101.5 ±39.6 | 99.5 ±37.8 | 0.76 |
| MV Repair | 170 (52.6) | 21 (56.8) | 149 (52.1) | 0.59 |
| MV Replacement | 66 (20.4) | 7 (18.9) | 59 (20.6) | 0.81 |
| CABG | 300 (92.9) | 34 (91.9) | 266 (93) | 0.74 |
| Surgical AF ablation | 23 (7.1) | 7 (18.9) | 16 (5.6) | 0.01 |
AF, atrial fibrillation; BSA, body surface area; CABG, coronary artery bypass grafting; ICD, implantable cardioverter-defibrillator; IMR, ischemic mitral regurgitation; LV, left ventricle; MR, mitral regurgitation; MV, mitral valve; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Table 3.
Independent baseline predictors of ≥moderate TR at 2 years
| Baseline predictors | OR (95% CI) | P-value | |
|---|---|---|---|
| Baseline TR | Mild vs None/Trace | 2.51 (1.11, 5.66) | 0.002 |
| Moderate vs None/Trace | 6.66 (2.31, 19.23) | ||
| Tricuspid annular Index (mm/m2 BSA) | 1.17 (1.03, 1.34) | 0.02 | |
| Surgical AF ablation | 3.44 (1.20, 9.84) | 0.02 | |
AF, atrial fibrillation; BSA, body surface area; TR, tricuspid regurgitation.
MR recurrence and pacemaker/ICD leads
Among survivors at 2 years with known TR progression status, postoperative recurrence of ≥moderate MR was observed in 60/311 (19.3%) of patients at 1 year and in 65/323 (20.1%) at 2 years. The rate of TR progression and ≥moderate TR at 2 years was 13.3% (8/60) and 20.7% (12/58) respectively in patients that had MR recurrence at 1 year. This was lower in those patients without MR recurrence, in which TR progression and ≥moderate TR at 2 years was observed in only 4.4% (11/251) and 8.8% (22/251) respectively (Table 4).
Table 4.
TR progression and ≥moderate TR at 2 years by recurrent mitral regurgitation and presence of pacemaker/ICD lead at 1- and 2-years follow-up.
| 2 Year Outcome |
≥Moderate MR at 1 Year | P- value |
≥Moderate MR at 2 Years* | P- value |
||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| TR Progression | 8/60 (13.3) | 11/251 (4.4) | 0.02 | 6/65 (9.2)* | 12/258 (4.7) | 0.22 |
| ≥Moderate TR | 12/58 (20.7) | 22/251 (8.8) | 0.009 | 13/65 (20) | 24/258 (9.3) | 0.02 |
| 2 Year Outcome |
Pacemaker or ICD by 1 Year | P- value |
Pacemaker or ICD by 2 Years | P- value |
||
| Yes | No | Yes | No | |||
| TR Progression | 6/79 (7.6) | 14/246 (5.7) | 0.59 | 7/87 (8.0) | 13/238 (5.5) | 0.39 |
| ≥Moderate TR | 15/78 (19.2) | 22/245 (9.0) | 0.01 | 16/85 (18.8) | 21/238 (8.8) | 0.01 |
2 patients that had MV surgery in between year 1 and 2 are excluded from the denominator.
ICD, implantable cardioverter-defibrillator; MR, mitral regurgitation; TR, tricuspid regurgitation.
Across all 492 patients included in this study, during the 2-year follow-up period, a total of 69 patients underwent a permanent pacemaker or ICD implantation, in addition to the 60 patients with a history of a pacemaker or ICD lead at baseline. The proportion of patients with ≥moderate TR at 2 years was higher in patients with a permanent pacemaker or ICD lead by 2 years post-randomization (Table 4).
Tricuspid annular dilation
At baseline the tricuspid annulus in the study population measured 38.3 ± 5.2mm (or 20.3 ±3mm/m2 indexed for body surface area). A higher TR grade at baseline was associated with a larger annular size (37.6mm ± 5.0mm in none/trace TR, 39.0 ± 5.6mm for mild TR, and 40 ± 5.2mm in moderate TR patients respectively, p= 0.003, Online Figure 2).
Tricuspid annular dilation according to the guideline definition (≥40mm or 21mm/m2) was present in 269 of the 491 patients (54.8%). By baseline TR grade, 145/296 patients with none/trace TR (49.0%), 91/153 patients with mild TR (59.5%), and 33/42 patients with moderate TR (78.6%) had annular dilation. The value of tricuspid annular dilation at baseline for predicting either TR progression or ≥moderate TR at 2 years is visually displayed in Figure 3 (additional details in Online Table III). ROC curve analysis to assess the value of non-indexed and indexed annular dimensions for TR progression at 2 years (AUC 0.58 and 0.56, respectively), or for prediction of presence of ≥moderate TR at 2 years (AUC 0.60 and 0.65, respectively) yielded no annular dimension cut-offs with sensitivity and specificity >60%.
Figure 3 – Discriminative value of baseline tricuspid annular dilation for TR progression and >=moderate TR after surgery.
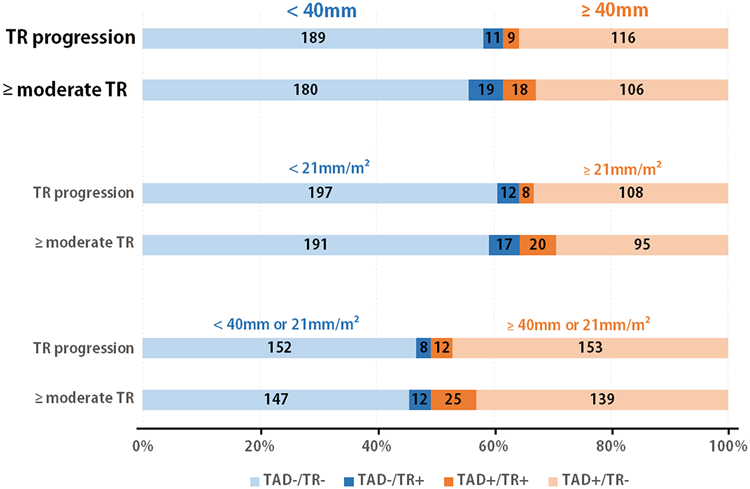
Tricuspid regurgitation (TR) at 2 years according to tricuspid annular dilation (TAD) at baseline, applying the non-indexed, indexed and combined dilation cut-off respectively. Patients without annular dilation based on the cut-off are displayed in blue, and patients with annular dilation are displayed in orange. The number of patients that had either TR progression (n=20) or ≥moderate TR (n=37) are highlighted in dark colors, whereas the patients without TR progression or <moderate TR are displayed in light colors.
Impact of trial and randomization arm on the TR progression
In the severe IMR trial, TR progression was observed in 6 out of 129 patients surviving at 2 years (4.7%) and ≥moderate TR was present in 19 out of 129 (14.7%). In the moderate IMR trial, TR progression occurred in 14 out of 196 patients surviving at 2 years (7.1 %) and ≥moderate TR was present in 18 out of 194 patients (9.3%). Differences between the trials were not significantly different (p=0.36 for TR progression; p=0.13 for difference in ≥moderate TR). Moreover, baseline MR severity (effective regurgitant orifice area) was not predictive of TR progression or ≥moderate TR at 2 years (Table 2).
Differences across randomization arms are summarized in Online Table IV. Briefly, in the severe IMR trial there was a tendency for more ≥moderate TR at 2 years after MV repair arm versus MV replacement (13/68 (19%) versus 6/61 (9.8%), p=0.14). In the moderate IMR trial, TR progression tended to be higher after isolated CABG versus after CABG + repair (TR progression in 9/94 (9.6%) versus 5/102 (4.9%), p=0.20).
Impact of right coronary artery (RCA) grafting
The RCA was revascularized in a total of 73 patients (14.9%), of which 53/290 patients were included in the moderate IMR trial and 20/201 in the severe IMR trial. RCA grafting occurred in 3/20 patients with TR progression at 2 years (15%), versus 47/304 patients without TR progression (15.5%, p>0.99). RCA grafting tended to be more common in patients with less than moderate TR at 2 years (47/285 patients, 16.5%) compared to patients with ≥moderate TR at 2 years (3/37 patients, 8.1%), although this was not statistically significant (p=0.19).
Clinical outcome
TR progression and ≥moderate TR at 2 years were associated with MACE during the 2-year follow-up period (Table 5). Specifically, a higher proportion of patients with postoperative TR had an increase of ≥1 NYHA class during follow-up, and/or a higher rate of hospitalizations for heart failure.
Table 5.
Clinical events in patients with versus without TR progression or ≥moderate TR
| Clinical events | No Progression (N=305) |
TR Progression (N=20) |
P- value |
<Moderate TR at 2 Years (N=286) |
≥Moderate TR at 2 Years (N=37) |
P- value |
|---|---|---|---|---|---|---|
| No./No. Observed (%) |
No./No. Observed (%) |
No./No. Observed (%) |
No./No. Observed (%) |
|||
| MACE | 69/305 (22.6) | 11/20 (55) | 0.003 | 64/286 (22.4) | 14/37 (37.8) | 0.04 |
| Increase of ≥1 NYHA Class | 23/299 (7.7) | 4/19 (21.1) | 0.07 | 20/280 (7.1) | 7/37 (18.9) | 0.03 |
| Rehospitalization for Heart Failure | 44/305 (14.4) | 9/20 (45) | 0.002 | 41/286 (14.3) | 10/37 (27.0) | 0.05 |
| Mitral-valve Surgery after Index Procedure | 4/305 (1.3) | 2/20 (10) | 0.05 | 3/286 (1) | 1/37 (2.7) | 0.39 |
| Stroke | 7/305 (2.3) | 0/20 (0) | >0.99 | 7/286 (2.4) | 0/37 (0) | >0.99 |
MACE, major adverse clinical events; NYHA, New York Heart Association; TR, tricuspid regurgitation.
Discussion
This analysis investigated the evolution of non-severe TR that was not corrected during surgery for IMR in two prospective randomized trials. Key findings are that: (1) the incidence of TR progression (6%) and the incidence of ≥moderate TR (11%) at 2 years after IMR surgery is lower than expected based on retrospective data; (2) baseline tricuspid annular dilation (40mm or 21mm/m2) is not predictive of TR progression, and is poorly discriminative of ≥moderate TR at 2 years; and (3) both TR progression and ≥moderate TR at 2 years are associated with postoperative MR recurrence and presence of a permanent pacemaker, as well as with a higher clinical event rate during follow-up.
Incidence of TR progression after IMR surgery
In contrast to the multitude of reports and the ongoing debate on TR progression after surgery for primary MV disease, data on TR progression after IMR surgery are scarce and limited to retrospective observations. In IMR patients, given the presence of clinical heart failure and ischemic heart disease (potentially even RV ischemia), the prevalence and significance of secondary TR is expected to be high and the threshold to intervene on the tricuspid valve during IMR surgery might be lower than for primary MR (19). Matsunaga and Duran observed ≥moderate TR in >50% of patients at 1- to 3-years after MV repair for IMR(7). Other groups report ≥moderate TR at 5 years post IMR surgery in 31% of patients with baseline trace or mild TR(8), or TR progression of at least two TR grades in 25% of patients approximately 7 years after surgery for secondary MR (66% ischemic etiology)(9).
TR severity, however, can be dynamic and load-dependent(20), and retrospective database analyses predispose towards capturing sicker patients in a decompensated state, when seeking medical advice or at hospital admission. In the present study, echo data were prospectively collected at predefined time points in patients receiving heart failure medication at the discretion of the investigators. This study adds important data to the current perception in that it demonstrates a rate of TR progression and ≥moderate TR after IMR surgery that is several times smaller than previously reported.
Tricuspid annular dilation
In this study, the baseline tricuspid annular dimension correlated with baseline TR severity, corroborating the value of the tricuspid annular dimension as surrogate marker of TR severity(1). Nonetheless, the performance of the parameter in predicting TR progression or ≥moderate TR was poor, even when assessing for alternative (i.e. higher) cut-offs in ROC analysis. Measurement of the tricuspid annulus in two-dimensional echocardiography has well-known limitations given the non-circular three-dimensional (3D) annular shape(21,22). Although 3D annular sizing has been advocated, a strategy based on a 3D annular measurement could not improve the predictive value for TR progression after primary MR surgery(23). Reverse RV remodeling after CABG in patients with preoperative RV ischemia and annular dilation might play a role specifically in an IMR population (35% of patients with moderate TR at baseline showed improvement in TR after surgery). In addition, the association between postoperative TR and time-dependent postoperative MR recurrence and implantation of pacemaker leads, both prevalent in IMR population, likely interfere with the predictive value of baseline tricuspid annular size.
Clinical implications
Despite the lower than anticipated incidence of TR progression and degree of ≥moderate TR at 2 years, the clinical impact of TR progression after IMR surgery is confirmed by this study. Increasing epidemiologic evidence suggests that both TR and the progression of TR is associated with clinical events and impaired long term outcome(24-27). Efforts to reduce and/or avoid postoperative TR in IMR patients remain warranted. The poor discriminative value of the tricuspid annular dimension in this analysis however does not support the routine application of concomitant tricuspid valve repair based on tricuspid annular dimension alone. Other predictors of ≥moderate TR were TR at baseline and concomitant surgical ablation of atrial fibrillation. Atrial fibrillation is a known predictor of TR progression in patients with LV dysfunction(27) and post MV repair(28), with ongoing bi-atrial remodeling and dilation causing progressive TR. Patients that underwent a surgical ablation of atrial fibrillation in the trials likely represented a subgroup of patients with therapy-refractory or persistent atrial fibrillation that are at higher risk of developing ongoing atrial remodeling and TR. Whether in this subgroup non-severe TR should be treated during IMR surgery remains to be determined.
Study limitations
This is a post-hoc secondary analysis using data from two prospective randomized controlled trials. Follow-up duration is limited to 2 years. Further follow-up of this patient population will be important to determine further progression of TR, predictors of progression and its clinical impact. There is a potential survival bias by this cross-sectional analysis at 2 years, as well as potential ascertainment bias due to patients that were lost to follow-up. However, when including the available echo data at last visit of the patients that died or were lost to follow-up, the overall rate of TR progression remains similar (Online Tables I and II). This study includes both moderate IMR and severe IMR patients that were treated with either MV replacement, MV repair or isolated CABG. This however did not interfere with the findings of the study, as demonstrated in the comparison between trials and randomization arms (Online Table IV). Finally, medical therapies during follow-up were not monitored, nor does this analysis account for the impact of post-operative occurrence of atrial fibrillation, pulmonary hypertension or interim myocardial infarction on the post-operative progression of TR.
Conclusions
After IMR surgery, progression of unrepaired non-severe TR is uncommon. Baseline tricuspid annular dilation is not predictive of TR progression, and although associated, only poorly discriminative of ≥moderate TR at 2 years. Both TR progression and the presence of ≥moderate TR at 2 years after IMR surgery are associated with high clinical event rates.
Supplementary Material
Central Illustration – Progression of TR after Cardiac Surgery for Ischemic Mitral Regurgitation.
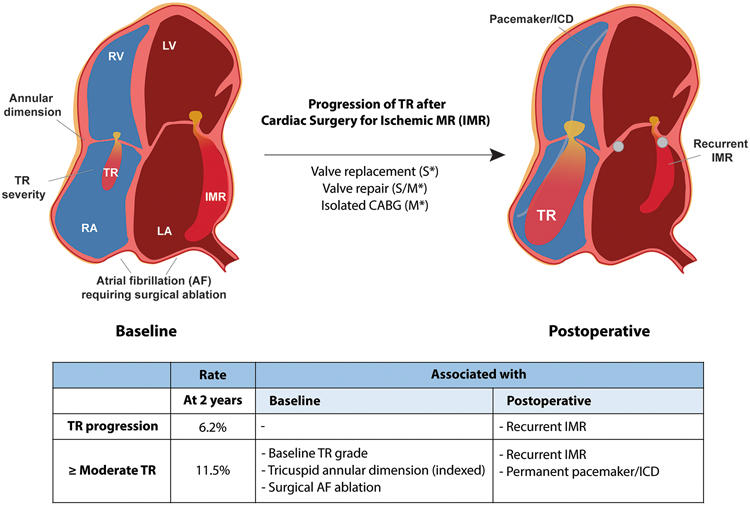
At 2 years after surgery for ischemic mitral regurgitation (IMR) progression of Tricuspid Regurgitation (TR) is not as common as generally expected. Postoperative TR depends not only on preoperative risk factors (baseline TR, tricuspid annular size, or atrial fibrillation), but is associated with postoperative factors such as MR recurrence and permanent pacemaker/defibrillator as well. *S, severe IMR trial; *M, moderate IMR trial
CABG, coronary artery bypass graft; ICD, implantable cardioverter defibrillator; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Perspectives.
Competency in medical knowledge: After surgery for ischemic mitral regurgitation progression of TR is not as common as previously reported. Postoperative TR depends not only on preoperative risk factors such as annular size or atrial fibrillation, but also on postoperative factors such as MR recurrence and permanent pacemaker/defibrillator.
Competency in patient care: The poor discriminative value of the tricuspid annular dimension in this study does not support the routine application of concomitant tricuspid valve repair based on tricuspid annular dimension alone.
Translational outlook: Further studies are needed to explore the natural history of tricuspid regurgitation after surgery for ischemic MR, and to identify patients that might benefit most from concomitant tricuspid valve intervention.
Acknowledgments
Sources of Funding: The CTSN is supported by a cooperative agreement (U01 HL088942) funded by the National Heart, Lung, and Blood Institute and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH), Bethesda, MD, and the Canadian Institutes for Health Research (CIHR), Ottawa, ON, Canada. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung and Blood Institute, the National Institute of Neurological Disorders and Stroke, the National Institutes of Health, or the US Department of Health and Human Services.
Abbreviations list
- CABG
coronary artery bypass grafting
- CTSN
Cardiothoracic Surgical Trials Network
- ICD
implantable cardioverter defibrillator
- IMR
ischemic mitral regurgitation
- MACE
major adverse clinical events
- MR
mitral regurgitation
- MV
mitral valve
- NYHA
New York Heart Association
- RV
right ventricle
- TR
tricuspid regurgitation
Footnotes
Disclosures:
P.B. Bertrand: None. J.R. Overbey: None. X. Zeng; None. R.A. Levine: None. G. Ailawadi: Honoraria; Modest; Medtronic, Edwards, Abbott, Admedus, Gore. M.A. Acker: None. P.K. Smith: None. V.H. Thourani: Other – advisory board; Modest; Gore Vascular, Research Grant; Modest; Abbott Vascular, Boston Scientific, Edwards Lifesciences, Jenavalve. E. Bagiella: None. M.A. Miller: None. L. Gupta: None. M.J. Mack: Honoraria; Modest; Gore, Research Grant; Modest; Edwards Lifesciences, Medtronic, Abbott. A.M. Gillinov: Other – Consultant; Significant; Medtronic, AtriCure, Edwards Lifesciences, Abbott, CryoLife, ClearFlow, Ownership Interest; Significant; ClearFlow, G. Giustino: Honoraria; Modest; Bristol-Myers Squibb. A.J. Moskowitz: None. A.C. Gelijns: None. M.E. Bowdish: None. P.T. O’Gara: Other – Member, Executive Committee, Apollo Trial (TMVR); Modest; Medtronic, Other – Member, Executive Committee, Early TAVR trial; Modest; Edwards Lifesciences, Other – Member, SAB; Modest; Medtrace. J.S. Gammie: Other – Consultant; Modest; Edwards Lifesciences. J. Hung: None.
References
- 1.Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 2005;79:127–32. [DOI] [PubMed] [Google Scholar]
- 2.David TE, David CM, Manhiolt C. When is tricuspid valve annuloplasty necessary during mitral valve surgery? J Thorac Cardiovasc Surg 2015;150:1043–4. [DOI] [PubMed] [Google Scholar]
- 3.Dion RA. Is the air in Toronto, Rochester, and Cleveland different from that in London, Monaco, Leiden, Genk, Milan, and New York? J Thorac Cardiovasc Surg 2015;150:1040–3. [DOI] [PubMed] [Google Scholar]
- 4.Gillinov M, Mick S, McCurry K, Navia J. The tricuspid valve: If it's not broken, don't fix it. J Thorac Cardiovasc Surg 2017;154:108–109. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura RA, Otto CM, Bonow RO et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner H, Falk V, Bax JJ et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 7.Matsunaga A, Duran CM. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation 2005;112:I453–7. [DOI] [PubMed] [Google Scholar]
- 8.Navia JL, Elgharably H, Javadikasgari H et al. Tricuspid Regurgitation Associated With Ischemic Mitral Regurgitation: Characterization, Evolution After Mitral Surgery, and Value of Tricuspid Repair. Ann Thorac Surg 2017;104:501–509. [DOI] [PubMed] [Google Scholar]
- 9.De Bonis M, Lapenna E, Pozzoli A et al. Mitral Valve Repair Without Repair of Moderate Tricuspid Regurgitation. Ann Thorac Surg 2015;100:2206–12. [DOI] [PubMed] [Google Scholar]
- 10.Acker MA, Parides MK, Perrault LP et al. Mitral-Valve Repair versus Replacement for Severe Ischemic Mitral Regurgitation. N Engl J Med 2014;370:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith PK, Puskas JD, Ascheim DD et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2014;371:2178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein D, Moskowitz AJ, Gelijns AC et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med 2016;374:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michler RE, Smith PK, Parides MK et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med 2016;374:1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 15.Zoghbi WA, Enriquez-Sarano M, Foster E et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 16.Zoghbi WA, Adams D, Bonow RO et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–371. [DOI] [PubMed] [Google Scholar]
- 17.Antunes MJ, Rodriguez-Palomares J, Prendergast B et al. Management of tricuspid valve regurgitation: Position statement of the European Society of Cardiology Working Groups of Cardiovascular Surgery and Valvular Heart Disease. Eur J Cardiothorac Surg 2017;52:1022–1030. [DOI] [PubMed] [Google Scholar]
- 18.Rudski LG, Lai WW, Afilalo J et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713; quiz 786-8. [DOI] [PubMed] [Google Scholar]
- 19.De Bonis M, Lapenna E, Sorrentino F et al. Evolution of tricuspid regurgitation after mitral valve repair for functional mitral regurgitation in dilated cardiomyopathy. Eur J Cardiothorac Surg 2008;33:600–6. [DOI] [PubMed] [Google Scholar]
- 20.Topilsky Y, Tribouilloy C, Michelena HI, Pislaru S, Mahoney DW, Enriquez-Sarano M. Pathophysiology of tricuspid regurgitation: quantitative Doppler echocardiographic assessment of respiratory dependence. Circulation 2010;122:1505–13. [DOI] [PubMed] [Google Scholar]
- 21.Addetia K, Muraru D, Veronesi F et al. 3-Dimensional Echocardiographic Analysis of the Tricuspid Annulus Provides New Insights Into Tricuspid Valve Geometry and Dynamics. JACC Cardiovasc Imaging 2019;12:401–412. [DOI] [PubMed] [Google Scholar]
- 22.Volpato V, Lang RM, Yamat M et al. Echocardiographic Assessment of the Tricuspid Annulus: The Effects of the Third Dimension and Measurement Methodology. J Am Soc Echocardiogr 2019;32:238–247. [DOI] [PubMed] [Google Scholar]
- 23.Sordelli C, Lancellotti P, Carlomagno G et al. Tricuspid Annular Size and Regurgitation Progression After Surgical Repair for Degenerative Mitral Regurgitation. Am J Cardiol 2016;118:424–31. [DOI] [PubMed] [Google Scholar]
- 24.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405–9. [DOI] [PubMed] [Google Scholar]
- 25.Benfari G, Antoine C, Miller WL et al. Excess Mortality Associated with Functional Tricuspid Regurgitation Complicating Heart Failure with Reduced Ejection Fraction. Circulation 2019. [DOI] [PubMed] [Google Scholar]
- 26.Prihadi EA, van der Bijl P, Gursoy E et al. Development of significant tricuspid regurgitation over time and prognostic implications: new insights into natural history. Eur Heart J 2018;39:3574–3581. [DOI] [PubMed] [Google Scholar]
- 27.Kazum SS, Sagie A, Shochat T et al. Prevalence, Echocardiographic Correlations, and Clinical Outcome of Tricuspid Regurgitation in Patients with Significant Left Ventricular Dysfunction. Am J Med 2019;132:81–87. [DOI] [PubMed] [Google Scholar]
- 28.Matsuyama K, Matsumoto M, Sugita T, Nishizawa J, Tokuda Y, Matsuo T. Predictors of residual tricuspid regurgitation after mitral valve surgery. Ann Thorac Surg 2003;75:1826–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


