Abstract
Background
Corynebacterium species are often dismissed as contaminants in blood cultures, but they can also cause infective endocarditis (IE), which is a severe condition. Antibiotic resistance of corynebacteria is increasing making treatment challenging. Reports on IE caused by Corynebacterium species are scarce and more knowledge is needed.
Methods
Cases of IE caused by Corynebacterium species were identified through the Swedish Registry of Infective Endocarditis. Isolates were collected for species redetermination by matrix-assisted laser desorption ionization-time of flight and for antibiotic susceptibility testing using Etests.
Results
Thirty episodes of IE due to Corynebacterium species were identified between 2008 and 2017. The median age of patients was 71 years (interquartile range, 60–76) and 77% were male. Corynebacterium striatum (n = 11) was the most common IE causing pathogen followed by Corynebacterium jeikeium (n = 5). Surgery was performed in 50% and in-hospital mortality rate was 13%. Patients with IE caused by Corynebacterium species were significantly more likely to have prosthetic valve endocarditis (70%), compared with patients with IE due to Staphylococcus aureus or non-beta-hemolytic streptococci (14% and 26%, respectively) (P < .0001). Vancomycin was active towards all Corynebacterium isolates, whereas resistance towards penicillin G was common.
Conclusions
Corynebacterium species cause IE, where prosthetic valves are mainly affected and surgery is often performed. Corynebacterium striatum is an important causative agent of IE within the genus. Antibiotic resistance of corynebacteria is relatively common but resistance towards vancomycin could not be detected in vitro.
Keywords: antibiotic, Corynebacterium, infective endocarditis
Corynebacterium species are facultative anaerobic nonsporulating Gram-positive bacilli. In recent years, nondiphterial Corynebacterium (diphtheroids) have been increasingly acknowledged for causing both localized and systemic infections. [1, 2]. Species-determination of corynebacteria is difficult, because there are many species with similar characteristics. However, matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) allows rapid species determination within the genus [3].
Findings of corynebacteria in blood cultures are often regarded as a potential contamination from skin flora, although invasive infections with corynebacteria such as sepsis and infective endocarditis (IE) have increasingly been reported [4–6]. A recent retrospective study described true infection in 30 (8.8%) of 339 episodes of Corynebacterium species bacteremia. The focus of infection was often unknown, but IE was recognized in 8 episodes, all of which were prosthetic valve endocarditis (PVE) [4]. Corynebacterium pseudodipthteriticum, Corynebacterium jeikeium, and Corynebacterium striatum have been acknowledged as important pathogens causing IE [5]. Patients with IE due to C jeikeum were more frequently in need of valve replacement, and C striatum has been associated with nosocomial origin of infection [5]. Case reports of IE caused by corynebacteria have described the condition as subacute, often occurring in elderly patients with comorbidities, immunosuppressive treatment, and/or with prosthetic devices [6–8]. The mortality rate is substantial and many patients are in need of surgery [5].
Increased antimicrobial resistance of Corynebacterium species has been described; in particular, C striatum has shown resistance to several antibiotics including beta-lactams, quinolones, daptomycin, and gentamicin [9–12]. Vancomycin is often used due to good activity in vitro and vancomycin-resistance is uncommon. Overall, reports of IE due to corynebacteria are few and knowledge of the clinical presentation and optimal antibiotic treatment is sparse.
MATERIALS AND METHODS
In 1995, the Swedish Society for Infectious Diseases introduced the Swedish National Registry of Infective Endocarditis (SRIE). All 30 departments of infectious diseases (ID) in Sweden have participated in the registry since its inception. These ID departments have regional responsibility for the care of patients with severe infections, and patients requiring acute surgery for IE are, in most cases, treated in ID departments during the pre- and/or postoperative period. All cases are reported on a standardized questionnaire at the time of discharge and a second questionnaire after follow-up (mean: 3 months after treatment). The origin of the etiologic agent is verified using, for example, blood cultures, cultures from valves during surgery, and polymerase chain reaction from tissue samples of valves. The registry has been used to present clinical data regarding various pathogens [13–16] and has made contributions to numerous studies within the framework of the International Collaboration on Endocarditis with approximately 30 publications [17, 18].
Cases of IE due to corynebacteria, reported to the SRIE during the 10-year period 2008–2017, were identified. Clinical characteristics of cases of IE due to corynebacteria were compared with cases with IE due to other more common IE causing pathogens including Staphylococcus aureus, Enterococcus faecalis, and non-beta-haemolytic streptococci (defined as alpha-hemolytic streptococci, Streptococcus bovis, Granulicatella, or Abiotrophia). Data of cases of IE due to Corynebacterium species occurring in the county of Skåne between the years 2012 and 2017 was reported in a recent published study by Rasmussen et al [4]. The 8 cases of IE due to Corynebacterium species may have been reported to the SRIE and thus also presented herein.
Patient Consent Statement
Because of the retrospective nature of the study, obtaining individual patient consent was not applicable. This design of the study was approved by the regional ethical research committee in Lund (reference number 2017/1002).
Bacterial Isolates and Species Determination
Bacterial isolates from cases of IE due to Corynebacterium species reported to the SRIE were collected from different departments of microbiology throughout Sweden. The isolates were cultivated on blood agar plates in 37°C and 5% CO2, and species were redetermined by Microflex MALDI-TOF MS (Bruker, Bremen, Germany) with the direct transfer protocol [19] and the software FlexControl and MBT Compass 4.1 with reference database DB-7854 MSP 2018 (MALDI Biotyper; Bruker) with a score value ≥2.0 for species determination. The species with the highest value were selected for species identification in each case. Those were compared with the species having the second highest value, which were <2.0 for all, because some Corynebacterium species may be difficult to differentiate from each other [1].
Antibiotic Susceptibility Testing
Isolates of corynebacteria were cultivated on blood agar plates in 37°C and 5% CO2. Mueller-Hinton agar plates, supplemented with 5% mechanically defibrinated horse blood and β-NAD, were inoculated with a suspension of Corynebacterium species adjusted to 0.5 McFarland turbidity standard. Etest strips ((BioMerieux, Marcy l’Etoile, France) of penicillin G, gentamicin, rifampicin, and vancomycin were applied for minimal inhibitory concentration (MIC) determination [20]. The plates were incubated in 37°C and 5% CO2. The MIC for each isolate towards every antibiotics was read after 16–20 hours or 40–44 hours, respectively. Clinical breakpoints of resistance for each antibiotic was set according to EUCAST (www.eucast.org).
Statistics
Fisher’s exact test was applied for categorical data when sample size was small and χ 2 test when applicable. Comparison of continuous variables was performed using Mann-Whitney U test. Significance was defined as P ≤ .05. Microsoft Excel 2016 (Microsoft Corporation) was used for data collection, and Graph Pad Prism, version 8.2.1 (Graph Pad Software) were used for statistical assays.
RESULTS
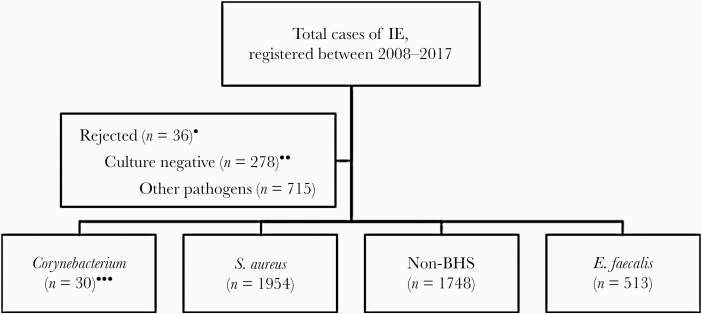
During the 10-year period 2008–2017, a total of 5275 episodes of IE were registered in the SRIE. From 2018, the design of the SRIE was changed, and some cases, occurring in 2018–2019, were reported in the old system instead of the new system designed in 2018. Episodes registered as “rejected IE” according to the modified Duke criteria, blood culture positive cases with pathogens other than corynebacteria, S aureus, non-beta-haemolytic streptococci (non-BHS) and E faecalis, or culture negative were excluded. Remaining 4246 IE cases were included in the study (Figure 1). Thirty of these cases were due to corynebacteria, 1954 were due to S aureus, 1748 were due to non-BHS, and 513 were due to E faecalis (Figure 1). Of these 30 cases of IE due to corynebacteria, all were isolated from blood, 1 isolate of which was also cultured from a heart valve. There was no apparent temporal or spatial clustering of episodes caused by specific species. The incidence of IE due to Corynebacterium species during that period was 0.032 IE/105 annually. Twenty isolates of Corynebacterium species were available for species redetermination. Corynebacterium striatum was the most commonly occurring pathogen followed by C jeikeium (Table 1).
Figure 1.
Flowchart of inclusion and exclusions of episodes of infective endocarditis (IE) reported to the Swedish Registry of Infective Endocarditis (SRIE). Cases of IE were identified through the SRIE. Cases of IE due to Corynebacterium, Staphylococcus aureus, non-beta hemolytic (Non-BHS ) streptococci, and Enterococcus faecalis were included in the study. Non-BHS comprised bacteria reported to the SRIE as alpha-hemolytic streptococci, Streptococcus bovis, Granulicatella, or Abiotrophia. *, Cases registered as rejected IE according to the modified Duke criteria. **, Cases registered as blood and/or valve culture negative (with or without polymerase chain reaction). ***, One case of IE due to Corynebacterium was registered twice.
Table 1.
Distribution of Corynebacterium Species Causing Infective Endocarditis
| Corynebacterium Species | n (%) |
|---|---|
| Corynebacterium striatum a | 11 (37) |
| Corynebacterium jeikeium b | 5 (17) |
| Corynebacterium amycolatum | 3 (10) |
| Corynebacterium coyleae c | 3 (10) |
| Corynebacterium propinquum d | 2 (7) |
| Corynebacterium pseudodiphteriticum | 2 (7) |
| Corynebacterium simulans | 1 (3) |
| Corynebacterium speciese | 3 (10) |
| Total | 30 |
NOTE: Isolates were subjected to renewed species determination using matrix-assisted laser desorption ionization time-of-flight mass spectrometry with exceptions as stated below.
aThree C striatum were not obtained for species redetermination.
bOne case had findings of coagulase negative staphylococci (CoNS) and C jeikeium in 2 separate blood cultures, and the isolate was not obtained for species redetermination.
cTwo C coyleae isolates were not obtained for species redetermination.
dOne of the C propinquum isolates was not available for species redetermination.
eThree isolates of Corynebacterium spp were not obtained for species redetermination. All the latter isolates (n = 10) mentioned were species determined according to routine protocol at respectively department of microbiology during the episode of IE.
Antibiotic Susceptibility Testing
The results of the antibiotic susceptibility testing with Etests are summarized in Table 2. Resistance to penicillin G was present in most isolates (14 of 20), comprising all isolates of C jeikeium (4 of 4). Resistance to rifampicin was detected in 50% (4 of 8) of the isolates of C striatum. All isolates were sensitive to vancomycin (MIC ranging from 0.5 to 2 μg/mL). Species-specific breakpoints for gentamicin are not established, but many corynebacteria had low MICs for gentamicin, with exception of some isolates of C striatum. Resistance to the tested antibiotics did not appear to change over time.
Table 2.
Antibiotic Susceptibility of Corynebacteria (n = 20)
| Isolate | MIC GM (μg/mL) | MIC PcG (μg/mL) (S≤0.125 R>0.125) | MIC RIF (μg/mL) (S≤0.06 R>0.5) | MIC VAN (μg/mL) (S≤ 2 R>2) |
|---|---|---|---|---|
| Corynebacterium striatum | 0.032 | 1 a | >32 | 1 |
| C striatum | 0.032 | 16 | >32 | 1 |
| C striatum | ≤0.016 | 1 | ≤0.002 | 1 |
| C striatum | 2 | 0.125 | ≤0.002 | 0.5 |
| C striatum | ≤0.016 | 0.5 | ≤0.002 | 1 |
| C striatum | 16 | 8 | >32 | 1 |
| C striatum | 0.032 | >32 | >32 | 1 |
| C striatum | 0.5 | 1 | ≤0.002 | 1 |
| Corynebacterium jeikeium | 0.5 | >32 | ≤0.002 | 2 |
| C jeikeium* | 0.064 | >32 | 0.008 | 1 |
| C jeikeium | 0.125 | >32 | ≤0.002 | 1 |
| C jeikeium | 0.125 | >32 | ≤0.002 | 2 |
| Corynebacterium amycolatum | 0.25 | 0.5 | ≤0.002 | 1 |
| C amycolatum | 0.5 | 0.125 | ≤0.002 | 1 |
| C amycolatum | 0.125 | 0.5 | ≤0.002 | 1 |
| Corynebacterium pseudodiphtheriticum** | 0.032 | 0.004 | ≤0.002 | 0.5 |
| C pseudodiphtheriticum | ≤0.016 | 0.008 | ≤0.002 | 0.5 |
| Corynebacterium coyleae | 0.5 | 2 | 0.004 | 0.5 |
| Corynebacterium propinquum** | 0.032 | 0.004 | ≤0.002 | 0.5 |
| Corynebacterium simulans | ≤0.016 | 0.125 | ≤0.002 | 1 |
Abbreviations: GM, gentamicin; MIC, minimal inhibitory concentration; PcG, penicillin; RIF, rifampicin; S and R, refer to Eucast breakpoints, no Eucast breakpoints available for gentamicin; VAN, vancomycin.
aValues in bold are, according to Eucast breakpoints, resistant to tested antibiotic. All isolates were read after 16–20 hours incubation with exceptions;
*, Growth was recorded at 90 hours.
**, Plates were read after 40–44 hours.
Clinical Presentation of Infective Endocarditis Caused by Corynebacteria
The median age of patients with IE due to corynebacteria was 71 years (interquartile range, 60–76), a majority were male (77%), and in-hospital mortality rate was 13% (Table 3). The 4 patients who died at hospital were infected with C striatum. The mortality in IE caused by C striatum was statistically significantly higher compared with the mortality of patients infected with other species of corynebacteria (Fisher’s exact test, P = .01). Patients with IE caused by Corynebacterium species were significantly more likely to have PVE (70%) compared with patients with IE due to the other pathogens (14%–39%) (P < .0001 and P < .01). Surgery was performed in 50% of the patients with IE due to corynebacteria, which was significantly more often compared with the other pathogens (22%–26%) (P < .001, P < .01, and P < .05) (Table 3).
Table 3.
Clinical Features of IE Caused by Corynebacteria Compared to Other Organisms
| Demographics | Corynebacteria | Staphylococcus aureus | Non-BHS | Enterococcus faecalis |
|---|---|---|---|---|
| n = 30 | n = 1954 | n = 1478 | n = 513 | |
| Definite IE (%) | 87 | 93 | 87 | 90 |
| Age (years, median) | 71 (60–76)a | 66 (46–79)a | 70 (58–80)a | 73 (60–81)a |
| Gender (%male) | 77 | 62 | 72 | 77 |
| Underlying disease (%) | ||||
| Diabetes | 39 | 18 | 16 | 18 |
| Cancerb | 9 | 9 | 10 | 14 |
| IVDU | 0 | 24*** | 4 | 13 |
| Underlying heart disease (%) | ||||
| Native valve disease | 33 | 14** | 35 | 20 |
| Prosthetic heart valve | 70 | 14**** | 26**** | 39** |
| Previous IE | 23 | 9* | 9* | 16 |
| Pacemaker/ICD | 27 | 16 | 8** | 17 |
| Type of infection (%) | ||||
| NVE, left isolated | 23 | 46* | 57*** | 46* |
| NVE, right isolated | 0 | 21** | 2 | 5 |
| PVE | 70 | 14**** | 26**** | 39** |
| Isolated PME | 7 | 6 | 1* | 4 |
| Aortic valve | 70 | 30**** | 37*** | 53 |
| Mitral valve | 17 | 31 | 30 | 32 |
| Nosocomial | 23 | 12 | 4 | 12 |
| Course of Disease | ||||
| Onset to hospitalization (days) | 7 | 2**** | 14 | 7 |
| Length of stay (days) | 42 (32–46)a | 33* (27–44)a | 29**** (18–37)a | 39 (29–46)a |
| Treatment length (days) | 42 (30–46)a | 30*** (28–40)a | 28**** (26–33)a | 37 (28–42)a |
| Embolization (%) | 10 | 44*** | 22 | 23 |
| Onset of admission to operation (days) | 8 (3–18)a | 13 (7–25)a | 11 (6–21)a | 14 (6–25)a |
| Surgery (%)c | 50 | 24** | 22*** | 26* |
| Mortality (%)d | 13 | 15 | 5 | 11 |
Abbreviations: ICD, intracardiac device; IE, infective endocarditis; IVDU, intravenous drug use; non-BHS, non-beta-hemolytic streptococci; NVE, native valve endocarditis; PME, pacemaker endocarditis; PVE, prosthetic valve endocarditis.
aInterquartile range.
bTreatment within treatment the past 5 years.
cSurgery during treatment.
dIn-hospital mortality. Continuous data are presented as median and categorical variables as count (percentage). The P values for difference between characteristics of patients with IE caused by corynebacteria compared with patients with S aureus, non-BHS streptococci, or E faecalis, respectively, are indicated as follows:
*, P < .05.
**, P < .01.
***, P < .001.
****, P < .0001.
Because it was obvious that corynebacteria mainly caused PVE, we compared episodes of PVE caused by corynebacteria to episodes of PVE caused by the other pathogens (Table 4). Median duration from of onset of disease to hospitalization was significantly longer for patients with PVE due to Corynebacterium species compared with patients with PVE due to S aureus (8 vs 2 days, P < .0001). The proportion of patients who underwent surgery was also significantly higher in patients with PVE due to corynebacteria compared with patients with PVE caused by the other pathogens (50% vs 15%–32%, P < .0001 and P < .05).
Table 4.
Features of Prosthetic Valve IE Due to Corynebacteria Compared With Other Pathogens
| Demographics | Corynebacteria | Staphylococcus aureus | Non-BHS | Enterococcus faecalis |
|---|---|---|---|---|
| n = 21 | n = 279 | n = 385 | n = 201 | |
| Definite IE (%) | 86 | 86 | 78 | 78 |
| Age (years, median) | 71 (60–77)a | 66 (56–81)a | 70 (62–82)a | 73 (67–81)a |
| Gender (%male) | 81 | 72 | 72 | 78 |
| Underlying disease (%) | ||||
| Diabetes | 24 | 16 | 16 | 23 |
| Cancerb | 9 | 13 | 14 | 21 |
| IVDU | 0 | 10 | 4 | 8 |
| Underlying Heart Disease (%) | ||||
| Native valve disease | 24 | 15 | 18 | 14 |
| Previous IE | 24 | 22 | 17 | 22 |
| Pacemaker/ICD | 33 | 25 | 14* | 19 |
| Type of Infection (%) | ||||
| Aortic valve | 70 | 56 | 53 | 56 |
| Mitral valve | 14 | 23 | 19 | 24 |
| Nosocomial | 19 | 15 | 4 | 15 |
| Course of Disease | ||||
| Onset to hospitalization (days) | 8 | 2**** | 9 | 7 |
| Length of stay (days) | 43 (33–46)a | 39 (27–50)a | 33** (28–43)a | 42 (27–46)a |
| Treatment length (days) | 38 (29–47)a | 38 (28–43)a | 33 (28–42)a | 42 (30–43)a |
| Embolization (%) | 10 | 35* | 16 | 20 |
| Onset of symptoms to operation (days) | 19 (10–35)a | 15 (9–28)a | 40** (21–53)a | 39* (15–66)a |
| Onset of admission to operation (days) | 8 (4–17)a | 13 (8–25)a | 15* (9–28)a | 15* (9–34)a |
| Surgery (%)c | 57 | 32* | 17**** | 15**** |
| Mortality (%)d | 5 | 25* | 7 | 11 |
Abbreviations: ICD, intracardiac device; IE, infective endocarditis; IVDU, intravenous drug use; non-BHS, non-beta-hemolytic streptococci; NVE, native valve endocarditis; PME, pacemaker endocarditis; PVE, prosthetic valve endocarditis.
aInterquartile range.
bMalignancy with treatment during the past 5 years.
cSurgery during treatment.
dIn-hospital mortality. Continuous data are presented as median and categorical variables as count (percentage). The P values for difference between characteristics of patients with IE caused by corynebacteria compared with patients with S aureus, non-BHS streptococci, or E faecalis, respectively, are indicated as follows:
*, P < .05.
**, P < .01.
***, P < .001.
****, P < .0001.
DISCUSSION
Infective endocarditis caused by Corynebacterium species is a rare disease and knowledge of clinical manifestations is limited. Our study, encompassing 30 cases of IE due to corynebacteria indicates that the condition mainly occurs in elderly patients with prosthetic valves. Our data confirms that C striatum is the most common cause of IE within the genus followed by C jeikeium. A similar impression has been conveyed in case reports in which C striatum, in particular, has been described as an important pathogen with different patterns of antibiotic resistance.
Increased antibiotic resistance of corynebacteria, mainly to beta-lactams and rifampicin, has been described. Our study investigated the frequency of antimicrobial resistance towards the most common types of antibiotics used in IE treatment, demonstrating a high proportion of isolates with resistance in particular to penicillin. Resistance to rifampicin was also frequent. Similar to our results, resistance in vitro to multiple antimicrobials have been recognized in several studies [21, 22]. Regarding therapy for pathogens such as C jeikeium or C striatum vancomycin may therefore be the initial choice of antibiotic therapy. In our study, a majority of the patients were treated with vancomycin (n = 21), whereas beta-lactam was given in 17 patients, rifampicin was given in 13 patients, and aminoglycoside was given in 11 patients.
This study has several limitations because it is a retrospective registry-based study, and the number of cases with IE due to corynebacteria is relatively low compared with the other more common IE pathogens. The national study design implies lower referral bias compared with studies from tertial centers. Findings from this study is overall based on the SRIE, and our results may therefore not be generalizable to other settings. Infective endocarditis caused by Corynebacterium species seems to be relatively uncommon, but it is possible that positive blood cultures with these pathogens might be regarded as a contamination and therefore IE can have been missed. Despite these limitations, the study is, to our knowledge, the largest on IE caused by Corynebacterium. Our study highlights the fact that IE due to Corynebacterium species occurs and that these bacteria are most often sensitive to vancomycin.
CONCLUSIONS
Most cases of IE due to Corynebacterium species occur in elderly patients with prosthetic heart valves. The course of infection is subacute and C striatum and C jeikeium are important causative agents. Resistance to several antibiotics were noted, but all Corynebacterium species were sensitive to vancomycin in vitro.
Acknowledgments
We acknowledge Gisela Hovold for important contribution with technical support and help. We also thank the Department of Clinical Microbiology, Office for Medical Services, Region Skåne, Lund, Sweden.
Author contributions. A. B. and M. R. drafted the study design and performed statistical analyses. A. B. and L. F. extracted and analyzed data. K. O. assisted in antibiotic susceptibility testing. L. O. contributed with information from the registry. All authors have contributed to, read, and approved of the final manuscript.
Financial support. This work was funded by the Royal Physiographic Society of Lund, the Swedish Government Funds for Clinical Research (ALF), and the foundations by Österlund and Lundgren.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Leal SM Jr, Jones M, Gilligan PH. Clinical significance of commensal Gram-positive rods routinely isolated from patient samples. J Clin Microbiol 2016; 54:2928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chandran R, Puthukkichal DR, Suman E, Mangalore SK. Diphtheroids-important nosocomial pathogens. J Clin Diagn Res 2016; 10:DC28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alatoom AA, Cazanave CJ, Cunningham SA, et al. Identification of non diphtheriae corynebacterium by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2012; 50:160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rasmussen M, Mohlin AW, Nilson B. From contamination to infective endocarditis-population-based retrospective study of Corynebacterium isolated from blood cultures. Eur J Clin Microbiol Infect Dis 2020; 39:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belmares J, Detterline S, Pak JB, Parada JP. Corynebacterium endocarditis species-specific risk factors and outcomes. BMC Infect Dis 2007; 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong HL, Koh HI, Lee AJ. Native valve endocarditis due to Corynebacterium striatum confirmed by 16S ribosomal RNA sequencing: a case report and literature review. Infect Chemother 2016; 48:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rezaei Bookani K, Marcus R, Cheikh E, et al. Corynebacterium jeikeium endocarditis: a case report and comprehensive review of an underestimated infection. IDCases 2018; 11:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JY, Lee SH, Kim WH. Three-valve endocarditis caused by Corynebacterium striatum. Korean Circ J 2018; 48:861–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMullen AR, Anderson N, Wallace MA, et al. When good bugs go bad: epidemiology and antimicrobial resistance profiles of Corynebacterium striatum, an emerging multidrug-resistant, opportunistic pathogen. Antimicrob Agents Chemother 2017; 61:e01111–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hahn WO, Werth BJ, Butler-Wu SM, Rakita RM. Multidrug-resistant Corynebacterium striatum associated with increased use of parenteral antimicrobial drugs. Emerg Infect Dis 2016; 22:1908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McElvania TeKippe E, Thomas BS, Ewald GA, et al. Rapid emergence of daptomycin resistance in clinical isolates of Corynebacterium striatum… acautionary tale. Eur J Clin Microbiol Infect Dis 2014; 33:2199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Werth BJ, Hahn WO, Butler-Wu SM, Rakita RM. Emergence of high-level daptomycin resistance in Corynebacterium striatum in two patients with left ventricular assist device infections. Microb Drug Resist 2016; 22:233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olaison L, Schadewitz K; Swedish Society of Infectious Diseases Quality Assurance Study Group for Endocarditis . Enterococcal endocarditis in Sweden, 1995-1999: can shorter therapy with aminoglycosides be used? Clin Infect Dis 2002; 34:159–66. [DOI] [PubMed] [Google Scholar]
- 14. Bläckberg A, Nilson B, Özenci V, et al. Infective endocarditis due to Streptococcus dysgalactiae: clinical presentation and microbiological features. Eur J Clin Microbiol Infect Dis 2018; 37:2261–72. [DOI] [PubMed] [Google Scholar]
- 15. Nilson B, Olaison L, Rasmussen M. Clinical presentation of infective endocarditis caused by different groups of non-beta haemolytic streptococci. Eur J Clin Microbiol Infect Dis 2016; 35:215–8. [DOI] [PubMed] [Google Scholar]
- 16. Bjursten H, Rasmussen M, Nozohoor S, et al. Infective endocarditis after transcatheter aortic valve implantation: a nationwide study. Eur Heart J 2019; 40:3263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murdoch DR, Corey GR, Hoen B, et al. ; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators . Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009; 169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lalani T, Cabell CH, Benjamin DK, et al. ; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators . Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121:1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bizzini A, Durussel C, Bille J, et al. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J Clin Microbiol 2010; 48:1549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pankey GA, Ashcraft DS, Dornelles A. Comparison of 3 Etest(®) methods and time-kill assay for determination of antimicrobial synergy against carbapenemase-producing Klebsiella species. Diagn Microbiol Infect Dis 2013; 77:220–6. [DOI] [PubMed] [Google Scholar]
- 21. Soriano F, Zapardiel J, Nieto E. Antimicrobial susceptibilities of Corynebacterium species and other non-spore-forming Gram-positive bacilli to 18 antimicrobial agents. Antimicrob Agents Chemother 1995; 39:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldner NK, Bulow C, Cho K, et al. Mechanism of high-level daptomycin resistance in Corynebacterium striatum. mSphere 2018; 3:e00371–18. [DOI] [PMC free article] [PubMed] [Google Scholar]