Abstract
Cefazolin is commonly used as an alternative to antistaphylococcal penicillins (ASPs) in treating methicillin-susceptible Staphylococcus aureus (MSSA) infections; however, no study has compared these agents in MSSA spinal epidural abscess (SEA). We describe our experience in managing MSSA SEA and compare the clinical efficacy of cefazolin with ASPs. This retrospective multicenter study reviewed 79 adult patients diagnosed with SEA between January 2006 and July 2020 using data collected from electronic health records and clinical microbiology laboratory databases. Forty-five patients received cefazolin, while 34 received ASPs. The total antibiotic duration was longer in the ASPs group but not statistically significant. There were no significant differences in treatment failure at week 6 vs week 12, 30-day vs overall mortality, or in 90-day recurrence rates between the treatment groups. Cefazolin was equally as effective as ASPs, and our findings suggest that it can be an alternative to ASPs in the treatment of MSSA SEA.
Keywords: cefazolin, epidural abscess, medical management, nafcillin, oxacillin
A spinal epidural abscess is an organized purulent collection situated between the spinal column and dura mater. Owing to its location, devastating complications such as permanent neurologic deficits, paralysis, and even death may occur. While uncommon, an increase in epidural abscess incidence has been reported and postulated to be related to the rise in the number of intravenous (IV) drug users, the aging population, and the increase in patients undergoing invasive spinal procedures [1–3].
A number of organisms can cause spinal epidural abscesses (SEAs), although the most commonly identified pathogen is Staphylococcus aureus [1, 3, 4].The isolation of methicillin-susceptible Staphylococcus aureus (MSSA) is common in community-acquired infections [5]; however, spinal epidural abscesses are relatively rare, and treatment recommendations have mostly been based on smaller studies and case series. Management often requires combining both medical and surgical interventions. Typically, antistaphylococcal penicillins (ASPs) are the recommended first-line agents for the treatment of patients with MSSA spinal epidural abscesses [5, 6]. As some β-lactamases efficiently hydrolyze cefazolin, this regimen is considered second-line or alternative treatment. Although this phenomenon’s clinical significance remains uncertain, a cefazolin inoculum effect (CIE) has been observed, potentially resulting in higher treatment failure rates in serious, deep-seated MSSA infections [7]. Clindamycin resistance has been associated with CIE positivity, possibly serving as a surrogate marker [8]. Another critical consideration for cefazolin is the supposed weak meningeal diffusion and lack of central nervous system (CNS) penetration [9]. On the contrary, a recent study showed that meningeal diffusion appeared sufficient with high-dose cefazolin [10]. Similarly, findings from 1 retrospective study suggested that patients who received cefazolin had a lower risk of mortality and a similar risk of recurrent infection than those who received ASPs [11].
Despite the potential disadvantages, the benefits of cefazolin include reduced dosage frequency in patients with normal kidney function and lower cost compared with ASPs [12]. There has been a recent increase in clinical experience of cefazolin in the treatment of MSSA infections, leading to it becoming the preferred agent in many institutions. However, the use of cefazolin remains controversial in treating MSSA spinal epidural abscesses, mostly for its unclear CNS penetration. While several studies have compared cefazolin with ASPs in treating MSSA bacteremia and MSSA infective endocarditis (IE), there have been no comparative studies examining patients with MSSA spinal epidural abscess. Therefore, we aimed to compare clinical outcomes associated with cefazolin vs ASPs to treat MSSA spinal epidural abscesses.
METHODS
Study Design
This was a single-center, multisite retrospective cohort study that included adult patients (≥18 years of age) diagnosed with MSSA spinal epidural abscesses from January 1, 2014, to July 31, 2020, at Mayo Clinic sites in Arizona, Florida, and Minnesota. Spinal epidural abscesses were ascertained from International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision–Clinical Modification (ICD-10-CM), codes, and data were collected and managed using Research Electronic Data Capture tools, hosted at the Mayo Clinic. Medical and surgical diagnostic approaches were performed at the discretion of the treating physicians. The Mayo Clinic Institutional Review Board approved the study.
Patient Population and Selection
Seventy-nine adult patients with MSSA spinal epidural abscesses were identified at our institution during the study period. Patient medical records were manually reviewed to ensure correct categorization. Demographic, microbiologic, management, and outcome data from each patient were extracted from the electronic health record.
Definitions
We defined a spinal epidural abscess as a well-localized collection of necrotic material between the dura mater and the bone, visible on imaging with either a computed tomography (CT) scan or magnetic resonance imaging (MRI). The diagnosis was associated with an MSSA epidural fluid culture result and at least 1 of the following antibiotic treatments: cefazolin 2 g every 8 hours, oxacillin 2 g every 4 hours, or nafcillin 2 g every 4 hours. Community-associated infection was defined as a spinal epidural abscess diagnosed within 48 hours of admission without prior health care involvement. Health care–associated infection was defined as a spinal epidural abscess diagnosed within 48 hours of admission in a patient with prior health care encounters in the last 3 months. Nosocomial-associated infection was defined as a spinal epidural abscess that occurred ≥48 hours after hospitalization. Prior health care involvement was defined as any IV therapy at home, wound care, specialized nursing care, hemodialysis patient, and chemotherapy within the past 30 days; also, any hospitalization in an acute care hospital for 2 or more days in the last 3 months [13]. Malignancy included hematologic and solid tumors. History of spinal/epidural procedure included any preceding back surgery, epidural injection, or lumbar puncture. Surgical management included aspiration, drain placement, and debridement for therapeutic purposes only. Only the first episode of MSSA bacteremia was included in our analysis. There were patients who received empiric antibiotic coverage on initial presentation, but patients were allocated to either the cefazolin or ASP group only if they received cefazolin or an ASP for >50% of the total treatment duration. Patients who received another antibiotic in addition to cefazolin or ASP for >50% of the total treatment duration were excluded from the study. Chronic suppression therapy was recorded separately and was not incorporated as part of the total antibiotic duration.
Normal inflammatory markers were defined as an erythrocyte sedimentation rate (ESR) of ≤22 mm/h for men, ≤29 mm/h for women, and a C-reactive protein (CRP) of ≤10 mg/L [14]. Treatment failure was defined as antibiotic extension beyond 6 weeks of therapy [15], relapse or recurrence of SEA within 3 months after discontinuing antibiotic therapy, and/or spinal epidural abscess–related mortality.
Statistical Analysis
Categorical variables were reported as counts and percentages using the chi-square or Fisher exact test for comparison, as appropriate. Continuous variables were reported as medians and interquartile ranges (IQRs) from the 25th to the 75th percentiles and compared using the t test or Wilcoxon rank-sum test, as deemed appropriate. Multivariable logistic regression analysis was used to evaluate the effect of cefazolin and nafcillin/oxacillin on treatment failure after adjustment by potential confounders. A P value of <.05 was considered significant. All analyses were performed using JMP software (SAS Institute Inc., Cary, NC, USA).
RESULTS
Clinical Characteristics
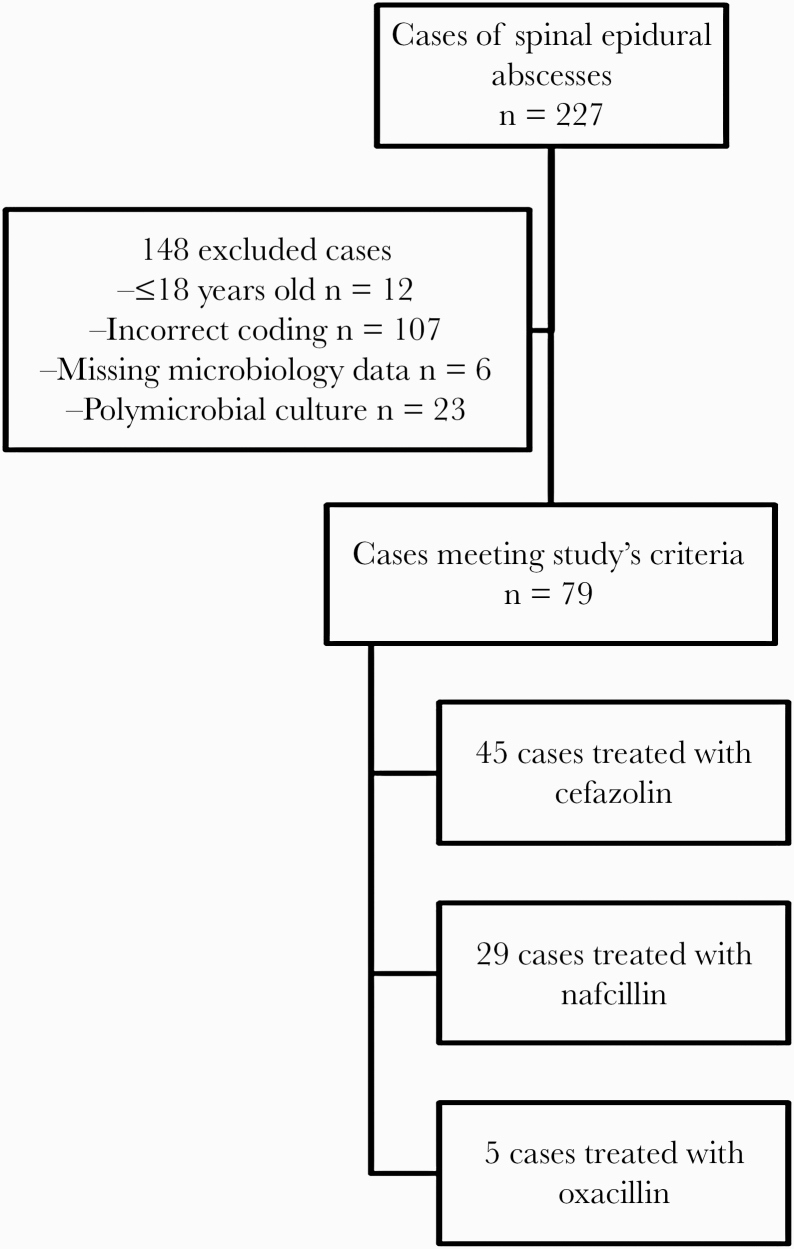
Among the 227 patients with spinal epidural abscesses at our institution during the study period, a total of 79 cases were secondary to MSSA. Of these, 45 were treated with cefazolin, 29 with nafcillin, and 5 with oxacillin (Figure 1).
Figure 1.
Patient population.
Patients’ demographics, medical, and laboratory data are summarized in Table 1. The median patient age for the cefazolin and ASPs groups (IQR) was 64.5 (55–73.2) years and 59.6 (47.2–64) years, respectively, and the majority were male (57.7% vs 55.9%).
Table 1.
Baseline Demographic and Characteristics of Patients Treated With Cefazolin Compared With Nafcillin/Oxacillin (n = 79)
| Characteristic | Cefazolin (n = 45) | Nafcillin or Oxacillin (n = 34) | P Value |
|---|---|---|---|
| Age, y | 64.5 [55–73.2] | 59.6 [47.2–64] | .0681 |
| Male | 26 (57.7) | 19 (55.9) | 1.0 |
| Body mass index, kg/m2 | 27.7 [23.4–33.8] | 30.6 [24.9–35.7] | .4228 |
| Charlson comorbidity index | 3 [2–4] | 2[1–4] | .0600 |
| Comorbidities | |||
| Corticosteroids | 3 (7) | 2 (5.9) | |
| Hypertension | 22 (49) | 15 (44.1) | |
| Intravenous drug use | 1 (2) | 0 | |
| Myocardial infarction | 3 (7) | 2 (5.9) | |
| Congestive heart failure | 3 (7) | 1 (2.9) | |
| Peripheral vascular disease | 2 (4) | 3 (8.8) | |
| Cerebrovascular accident | 2 (4) | 0 | |
| Chronic obstructive pulmonary disease | 5 (11) | 0 | |
| Connective tissue disease | 1 (2) | 1 (2.9) | |
| Peptic ulcer disease | 3 (7) | 5 (14.7) | |
| Liver disease | 0 | 1 (2.9) | |
| Diabetes mellitus | 8 (18) | 9 (26.5) | |
| Moderate to severe chronic kidney diseaseª | 0 | 0 | |
| Malignancy | 8 (18) | 3 (8.8) | |
| Community-onset infection | 27 (60) | 19 (55.9) | .82 |
| ICU admission | 5 (11) | 10 (29.4) | .048 |
| Duration of symptoms >7 d | 34 (76) | 27 (79.4) | .79 |
| History of spinal/epidural procedure or instrumentation | 21 (47) | 15 (44.1) | 1 |
| Time from procedure to positive epidural culture, d | 34 [23–160] | 28 [13–101] | .2952 |
| Concurrent Staphylococcus aureus bacteremia | 31 (69) | 24 (70.6) | 1 |
| Duration of BSI, d | 3 [2–5] | 4.5 [2–8] | .099 |
| Infective endocarditis | 0 | 2 (5.9) | .18 |
| Surgical procedure | |||
| Aspiration or IR guided | 13 (29) | 5 (14.7) | .18 |
| Surgical debridement | 32 (71) | 28 (82.4) | .30 |
| Single level | 15 (33) | 8 (23.5) | .45 |
| Multilevel | 30 (67) | 26 (76.5) | .45 |
| Clindamycin susceptible | 37 (82) | 31 (91.2) | .33 |
| Oxacillin MIC <1 | 44 (97.7) | 20 (58.8) | 0 |
| Vancomycin MIC <2 | 39 (87) | 24 (70.6) | .095 |
| Inpatient IV antimicrobial duration, d | 9 [5.5–15.5] | 12.5 [5.75–25] | .0723 |
| Outpatient IV antimicrobial duration, d | 38 [30–47] | 37 [25–51] | .7814 |
| Total antibiotic duration, d | 55.5 [42.2–96] | 67.5 [45–93.75] | .44 |
Data are presented as median [interquartile range] or No. (%).
Abbreviations: BSI, bloodstream infection; ICU, intensive care unit; IR, interventional radiology; IV, intravenous; MIC, minimal inhibitory concentration.
ªModerate = creatinine >3 mg/dL (0.27 mmol/L). Severe = on dialysis, status post–kidney transplant, uremia.
Compared with the cefazolin group, patients receiving ASPs had a higher prevalence of diabetes mellitus (26.5% vs 18%), peptic ulcer disease (14.7% vs 7%), and peripheral vascular disease (8.8% vs 4%). On the contrary, the cefazolin group had a higher prevalence of corticosteroid use (7% vs 5.9%), hypertension (49% vs 44.1%), and malignancy (18% vs 8.8%) compared with the ASP group.
A community onset of infection was more frequently observed in the cefazolin group than the ASPs group (60% vs 55.9%). However, a significant higher number of patients receiving ASPs were admitted to the intensive care unit (ICU; 29.4% vs 11%).
Thirty-six patients (46%) had a preceding spinal or epidural procedure/instrumentation. There was no significant difference in the time from procedure to positive epidural culture result between the antimicrobial groups.
Only 29.1% of patients had single level of epidural involvement, while 70.9% had multilevel involvement (Table 2). Those with multilevel involvement had a relapse rate of 13%, compared with 9% in those with only single-level involvement, though these patients received longer antibiotic therapy (85 days vs 41 days; P < .01) (Supplementary Table 1).
Table 2.
Comparison of Treatment Outcomes With Cefazolin vs Oxacillin or Nafcillin
| Characteristic | Cefazolin (n = 45) | Nafcillin/Oxacillin (n = 34) | P Value |
|---|---|---|---|
| Hospital length of stay, d | 9 [5.5–15.5] | 13 [6–25] | .52 |
| Treatment failure at week 6 | |||
| Antibiotic extension due to clinical failure | 34 (75.6) | 28 (82.4) | .58 |
| Epidural abscess–related death | 2 (4) | 2 (5.9) | 1 |
| Treatment failure at week 12 | |||
| Antibiotic extension due to clinical failure | 15 (33.3) | 15 (44.1) | .35 |
| Epidural abscess–related death | 0 | 0 | 1 |
| Mortality | 7 (15.6) | 4 (11.8) | .75 |
| Overall, d | 290 [38–1301] | 114 [11.75–1348] | .52 |
| Overall at 6 wk | 2 (4.4) | 2 (5.9) | 1 |
| 30-d mortality | 1 (2) | 2 (5.9) | .57 |
| 90-d mortality | 0 | 0 | 1 |
| 90-d recurrence | 5 (11.4) | 3 (9.4) | 1 |
| Treatment interruption due to adverse drug event | 1 (2) | 1 (2.9) | 1 |
Data are presented as No. (%) or median [interquartile range].
Microbiology
The prevalence of concurrent Staphylococcus aureus bloodstream infection (SAB) at the time of spinal epidural abscess diagnosis was essentially equal in both groups (70.6% vs 69%). The duration of bacteremia in patients receiving ASP was longer at 4.5 days vs 3 days in those that received cefazolin, and complicated with IE in 5.9% vs 0% of the patients, respectively.
The majority of the isolates were clindamycin susceptible (n = 68, 86.1%), and had a vancomycin minimal inhibitory concentration (MIC) of <2 (n = 63, 79.7%). An oxacillin MIC <1 was significantly more frequent in the cefazolin group than the ASP group (97.7% vs 58.8%).
Management and Outcome
Comparisons of management, treatment, and outcomes in patients receiving cefazolin vs ASPs are summarized in Table 1. All but 1 patient underwent aspiration or debridement, with surgical debridement being more common overall. The median time from diagnosis to the first surgical procedure for the entire study population (IQR) was 1 (0–2.25) day. The median time to aspiration or surgery (IQR) was 1 (0–3) day for both the ASP group and the cefazolin group (0–2). All patients received IV antibiotics at treatment initiation. The total duration of inpatient IV antibiotic therapy (IQR) was 12.5 (5.75–25) days in the ASPs group vs 9 (5.5–15.5) days (P = .07) in the cefazolin group. The total duration of outpatient IV therapy (IQR) was 37 (25–51) days vs 38 (30–47) days (P = .78), respectively. There was no difference in the total duration of IV and oral antibiotic therapy in the ASPs group and the cefazolin group (median [IQR], 67.5 [45–93.7] days vs 55.5 [42.2–96] days; P = .44).The median hospital length of stay (IQR) was 13 (6–25) days for patients who received ASPs vs 9 (5.5–15.5) days for those who received cefazolin (P = .52).
Sixty-one patients (77.2%) received antibiotic therapy beyond 6 weeks, and the most common reasons for treatment extension were persistent symptoms (54%) and persistently elevated inflammatory markers (52%). A total of 26 (32.9%) patients had osteomyelitis or diskitis. Of those, 11 (42.3%) were on nafcillin, and 15 (57.7%) were on cefazolin (Supplementary Table 2). CRP normalized in 25 (41.6%) patients at week 6 of therapy, while ESR was normal in 15 (26.3%) patients. At week 6 of treatment, 82.4% of the ASPs cases had antibiotic extension due to clinical failure. Similarly, 75.6% of the patients receiving cefazolin required antibiotic extension (P = .58). At week 12 of treatment, 44.1% of the ASPs cases had antibiotic extension due to clinical failure, while 33.3% of the patients receiving cefazolin required antibiotic extension (P = .35).
At the time of treatment completion, 48 (81.4%) patients had normalization of CRP, while 36 (60%) patients had normal ESR. The median percentage reduction for ESR was 73% in the patients receiving cefazolin vs 71% in those receiving ASPs. The median percentage reduction of CRP was 97% vs 95%, respectively. Treatment with ASPs or cefazolin did not impact the odds of a normalized ESR or CRP.
Patients’ mortality data are summarized in Table 2. A total of 11 (13.9%) patients in our study died, with a median (IQR) of 290 (38–1301) days in the cefazolin group and 114 (11.7–1348) days in the ASPs group (P = .52). Spinal epidural abscess–related death occurred in 4 patients at week 6, including 2 patients in each group. No further deaths were identified at week 12. There was no significant difference in mortality based on antibiotic treatment choice. Ninety-day recurrence was encountered in 8 patients (10.1%; P = 1).
DISCUSSION
In our cohort, we did not identify a difference in efficacy between cefazolin and ASPs in the treatment of MSSA spinal epidural abscess.
To our knowledge, this investigation is the first study comparing ASPs and cefazolin in patients with SEA. Several studies have already compared the efficacy of cefazolin and ASPs but in MSSA bacteremia [11, 12, 16–22] and IE [23], with outcomes either equivalent to or favoring cefazolin. Furthermore, previous studies have shown that cefazolin is better tolerated, less frequently interrupted, and has a more favorable safety profile [11, 17, 18]. Nevertheless, these studies may have had selection bias because physicians tend to select ASPs for the treatment of more serious infections, as our study demonstrated.
In our patient population, the most common comorbidities were hypertension and diabetes mellitus. A history of a preceding spinal or epidural procedure was seen in 46%, and concurrent bacteremia was seen in the majority of patients (69.6%). These findings are similar to what has been previously reported [1, 2, 24, 25].
There is a paucity of data regarding optimal duration of antimicrobial therapy, with experts recommending anywhere from 4 to 8 weeks of treatment. Treatment of SEA usually requires a surgical approach to drain the abscess combined with long-term antimicrobial therapy. Of note, a vast majority (92.4%) of our patient population had surgical management. A randomized controlled trial examined treatment duration for pyogenic vertebral osteomyelitis, showing that 6 weeks of treatment was noninferior to 12 weeks of treatment [15]. Park et al. [26] described the optimal duration of antibiotic therapy in patients with osteomyelitis and suggested that for high-risk patients an ≥8-week course was associated with a lower risk of recurrence.
For low-risk cases, defined as patients with drained paravertebral/psoas abscesses, those without end-stage kidney disease (ESKD), and those with MSSA infection, a shorter course of 6 to 8 weeks was sufficient. Our patient population was similar to the low-risk population described by Park et al. and received ~8 weeks of antibiotic therapy. Multilevel disease is usually associated with more complex management and less favorable outcomes [27], and as seen in our cohort, patients who had a multilevel abscess had longer antibiotic courses than those with single-level disease. We note that even at the time of treatment completion, 18.6% and 40% of patients still had elevated CRP and ESR, respectively. The recurrence rate in our patient population was 10.5%, which is higher than the 6.3% reported by Park et al. in those treated for 6–8 weeks, and 2.2% in those treated for 8 weeks or longer [26]. We hypothesize that the presence of persistent symptoms, multilevel disease, and elevated inflammatory markers prompted treatment extension beyond 6 weeks.
In our study, there were equal treatment interruptions due to adverse events in both groups. Interestingly, we did observe prolonged bacteremia in patients receiving ASPs when compared with the cefazolin group. Of note, 2 cases of IE were reported in the ASPs group.
CIE has been observed as a cause of treatment failure with cefazolin for MSSA infections in some studies [7, 28]. Song et al. [8] found that resistance to clindamycin and erythromycin was associated with CIE positivity; however, cefazolin remained a viable option in treating high-inoculum MSSA infections. In our cohort, despite 18% of the isolates in the cefazolin treatment group being resistant to clindamycin compared with 8.8% in the ASP group, we did not find any significant differences in the outcomes between the 2. The aforementioned finding may support the use of cefazolin even in cases of clindamycin resistance.
Reduced vancomycin susceptibility (RVS) was evaluated by Sullivan et al. [29], and while it did not have any significant impact on mortality, it was found to correlate with more complicated bloodstream infections, suggesting that the RVS phenotype is an indication of a more virulent strain. In our study, we noted that more isolates in the ASPs treatment group had MICs ≥2. More isolates in the ASPs group had oxacillin MICs ≥1. It remains unclear whether providers used this information in selecting treatment regimen and whether this played a role in overall outcomes.
Limitations
The retrospective nature of this study and its relatively small sample size are the primary limitations. The number of patients may have been too small to detect differences in treatment outcomes between cefazolin and ASPs using propensity scores. Similarly, we could not identify predictors of failure given the small sample size and the limited number of patients who developed adverse outcomes. Given the limited number of cases in the cohort who did not have surgical debridement, we cannot comment on the optimal duration of therapy. At baseline, more patients were admitted to the ICU in the ASPs group than in the cefazolin group, which is a potential confounding bias. Finally, we did not directly measure the CIE of the clinical isolates; therefore, we could not determine the possible association between cefazolin failure and an inoculum effect by type A β-lactamases.
CONCLUSIONS
Our study did not show any difference in efficacy between cefazolin and ASPs in the treatment of MSSA spinal epidural abscess. Most experts recommend 6 weeks of treatment duration; however, in our cohort, the median duration of total antibiotic therapy was ~8 weeks. The presence of persistent symptoms and elevated inflammatory markers often prompted treatment extension beyond 6 weeks. Further high-quality clinical trials are needed to define the most beneficial treatment for spinal epidural abscesses.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. None for all authors.
Patient consent. The design of the work was approved by the local institutional review board (IRB# 20-009299). The study was considered minimal risk, and patient consent requirements were waived.
References
- 1. Artenstein AW, Friderici J, Holers A, Lewis D, Fitzgerald J, Visintainer P. Spinal epidural abscess in adults: a 10-year clinical experience at a tertiary care academic medical center. Open Forum Infect Dis 2016; 3:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pradilla G, Ardila GP, Hsu W, Rigamonti D. Epidural abscesses of the CNS. Lancet Neurol 2009; 8:292–300. [DOI] [PubMed] [Google Scholar]
- 3. Krishnamohan P, Berger JR. Spinal epidural abscess. Curr Infect Dis Rep 2014; 16:436. [DOI] [PubMed] [Google Scholar]
- 4. Baker AS, Ojemann RG, Swartz MN, Richardson EP Jr. Spinal epidural abscess. N Engl J Med 1975; 293:463–8. [DOI] [PubMed] [Google Scholar]
- 5. Arko L 4th, Quach E, Nguyen V, et al. Medical and surgical management of spinal epidural abscess: a systematic review. Neurosurg Focus 2014; 37:E4. [DOI] [PubMed] [Google Scholar]
- 6. Nissen JL, Skov R, Knudsen JD, et al. Effectiveness of penicillin, dicloxacillin and cefuroxime for penicillin-susceptible Staphylococcus aureus bacteraemia: a retrospective, propensity-score-adjusted casecontrol and cohort analysis. J Antimicrob Chemoth 2013; 68:1894–900. [DOI] [PubMed] [Google Scholar]
- 7. Miller WR, Seas C, Carvajal LP, et al. The cefazolin inoculum effect is associated with increased mortality in methicillin-susceptible Staphylococcus aureus bacteremia. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song KH, Jung SI, Lee S, et al. ; Korea INfectious Diseases (KIND) study group . Characteristics of cefazolin inoculum effect-positive methicillin-susceptible Staphylococcus aureus infection in a multicentre bacteraemia cohort. Eur J Clin Microbiol Infect Dis 2017; 36:285–94. [DOI] [PubMed] [Google Scholar]
- 9. Radouane A, Péhourcq F, Tramu G, et al. Influence of lipophilicity on the diffusion of cephalosporins into the cerebrospinal fluid. Fundam Clin Pharmacol 1996; 10:309–13. [DOI] [PubMed] [Google Scholar]
- 10. Gregoire M, Gaborit B, Deschanvres C, et al. High-dosage cefazolin achieves sufficient cerebrospinal diffusion to treat an external ventricular drainage-related Staphylococcus aureus ventriculitis. Antimicrob Agents Ch 2019; 63:e01844-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McDanel JS, Roghmann MC, Perencevich EN, et al. Comparative effectiveness of cefazolin versus nafcillin or oxacillin for treatment of methicillin-susceptible Staphylococcus aureus infections complicated by bacteremia: a nationwide cohort study. Clin Infect Dis 2017; 65:100–6. [DOI] [PubMed] [Google Scholar]
- 12. Li J, Echevarria KL, Hughes DW, et al. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 2014; 58:5117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 14. Clinic M. Test and procedures. Available at: https://www.mayoclinic.org/tests-procedures. Accessed 12 February 2020.
- 15. Bernard L, Dinh A, Ghout I, et al. ; Duration of Treatment for Spondylodiscitis (DTS) study group . Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015; 385:875–82. [DOI] [PubMed] [Google Scholar]
- 16. Bai AD, Showler A, Burry L, et al. Comparative effectiveness of cefazolin versus cloxacillin as definitive antibiotic therapy for MSSA bacteraemia: results from a large multicentre cohort study. J Antimicrob Chemother 2015; 70:1539–46. [DOI] [PubMed] [Google Scholar]
- 17. Davis JS, Turnidge J, Tong S. A large retrospective cohort study of cefazolin compared with flucloxacillin for methicillin-susceptible Staphylococcus aureus bacteraemia. Int J Antimicrob Agents 2018; 52:297–300. [DOI] [PubMed] [Google Scholar]
- 18. Lee S, Choe PG, Song KH, et al. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob Agents Chemother 2011; 55:5122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee S, Song KH, Jung SI, et al. ; Korea INfectious Diseases (KIND) study group . Comparative outcomes of cefazolin versus nafcillin for methicillin-susceptible Staphylococcus aureus bacteraemia: a prospective multicentre cohort study in Korea. Clin Microbiol Infect 2018; 24:152–8. [DOI] [PubMed] [Google Scholar]
- 20. Paul M, Zemer-Wassercug N, Talker O, et al. Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia? Clin Microbiol Infect 2011; 17:1581–6. [DOI] [PubMed] [Google Scholar]
- 21. Pollett S, Baxi SM, Rutherford GW, et al. Cefazolin versus nafcillin for methicillin-sensitive Staphylococcus aureus bloodstream infection in a California tertiary medical center. Antimicrob Agents Chemother 2016; 60:4684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rao SN, Rhodes NJ, Lee BJ, et al. Erratum for Rao et al. , Treatment outcomes with cefazolin versus oxacillin for deep-seated methicillin-susceptible Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 2015; 59:7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lecomte R, Bourreau A, Deschanvres C, et al. Comparative outcomes of cefazolin versus antistaphylococcal penicillins in methicillin-susceptible Staphylococcus aureus infective endocarditis: a post hoc analysis of a prospective multicentre French cohort study [published online ahead of print September 17, 2020]. Clin Microbiol Infect. 2020; S1198-743X(20) 30564–4. [DOI] [PubMed] [Google Scholar]
- 24. Vakili M, Crum-Cianflone NF. Spinal epidural abscess: a series of 101 cases. Am J Med 2017; 130:1458–63. [DOI] [PubMed] [Google Scholar]
- 25. Darouiche RO. Spinal epidural abscess. N Engl J Med 2006; 355:2012–20. [DOI] [PubMed] [Google Scholar]
- 26. Park KH, Cho OH, Lee JH, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis 2016; 62:1262–9. [DOI] [PubMed] [Google Scholar]
- 27. Killen MC, Hernandez M, Berg A, Bhatia C. Nonoperative management of a multi-regional epidural abscess with neurological dysfunction. Int J Spine Surg 2015; 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loubet P, Burdet C, Vindrios W, et al. Cefazolin versus anti-staphylococcal penicillins for treatment of methicillin-susceptible Staphylococcus aureus bacteraemia: a narrative review. Clin Microbiol Infect 2018; 24:125–32. [DOI] [PubMed] [Google Scholar]
- 29. Sullivan SB, Austin ED, Stump S, et al. Reduced vancomycin susceptibility of methicillin-susceptible Staphylococcus aureus has no significant impact on mortality but results in an increase in complicated infection. Antimicrob Agents Chemother 2017; 61:e00316-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.