Abstract
Background
Evidence suggests that repeated influenza vaccination may reduce vaccine effectiveness (VE). Using influenza vaccination program maturation (PM; number of years since program inception) as a proxy for population-level repeated vaccination, we assessed the impact on pooled adjusted end-season VE estimates from outpatient test-negative design studies.
Methods
We systematically searched and selected full-text publications from January 2011 to February 2020 (PROSPERO: CRD42017064595). We obtained influenza vaccination program inception year for each country and calculated PM as the difference between the year of deployment and year of program inception. We categorized PM into halves (cut at the median), tertiles, and quartiles and calculated pooled VE using an inverse-variance random-effects model. The primary outcome was pooled VE against all influenza.
Results
We included 72 articles from 11 931 citations. Across the 3 categorizations of PM, a lower pooled VE against all influenza for all patients was observed with PM. Substantially higher reductions were observed in older adults (≥65 years). We observed similar results for A(H1N1)pdm09, A(H3N2), and influenza B.
Conclusions
The evidence suggests that influenza VE declines with vaccination PM. This study forms the basis for further discussions and examinations of the potential impact of vaccination PM on seasonal VE.
Keywords: seasonal influenza, systematic review, test-negative design, vaccination program, vaccine effectiveness
Influenza is responsible for considerable morbidity and mortality every year worldwide. Following influenza vaccination, antibody titers to influenza antigens may persist for months. However, the changing nature of influenza viruses, particularly the influenza A type (antigenic drift) [1], warrants reformulation of vaccine each influenza season in an attempt to match vaccine with the circulating virus strains [2]. Vaccination is therefore recommended each season for better protection against circulating virus strains. However, vaccine seroresponse may be impaired with repeated vaccination [2].
Generally, seasonal influenza vaccination is recommended for individuals at least 6 months old, with an emphasis on those at higher risk of developing complications such as the very young (<5), older adults (≥65), pregnant women, and individuals with certain health conditions [3, 4]. Many countries have adopted annual influenza vaccination policies and have established annual vaccination programs. Many vaccination programs are not publicly funded (paid for from the public purse) at inception. Publicly funded vaccination in some countries is only available to some of the at-higher-risk population subgroups, whereas some countries (or regions within some countries) offer universal vaccination (free for all). In addition, recommended influenza vaccines in each season may vary slightly across countries. However, publicly funded vaccination programs, even in some countries that have universal vaccination policies, was initially for a few at-higher-risk population subgroups, before gradually being expanded to cover all eligible persons. Nevertheless, these programs have led to some increases in vaccination rates and, with the introduction of the test-negative design (TND) [5, 6] in influenza vaccine effectiveness (VE) estimations, have reignited interest in the potential impact of repeated influenza vaccination.
Studies in the late 20th century were either inconclusive [7] or found no evidence of a negative impact of repeated influenza vaccination [8]. In particular, a large randomized controlled trial in the United States of America found some variations in infection rates between groups given 1 or more influenza vaccinations but noted no consistent pattern of differences in relation to number of successive seasonal vaccinations [9]. Recent studies have found reduced influenza VE in individuals who received prior repeated influenza vaccinations [10, 11]. A systematic review reported lower influenza VE against A(H3N2) and influenza B but not for A(H1N1) in individuals vaccinated in both current and previous seasons compared with those vaccinated only in the current season [12].
While accumulating evidence suggests that repeated influenza vaccination may reduce VE at the individual level, the impact on overall annual program effectiveness is still not clear. Understanding this impact may influence policy regarding population-wide annual influenza vaccination. We assessed the impact of repeated influenza vaccination on vaccine program effectiveness using influenza vaccination program maturation (PM; number of years since program inception) as a proxy for population-level repeated vaccination.
METHODS
We conducted a systematic review and meta-analysis following the Cochrane Handbook for Systematic Reviews of Interventions guidelines [13]. Our findings are reported following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [14]. The systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42017064595). Details of our methods have been reported in a previous publication [15].
Literature Search Strategy
A methodologist designed a search strategy for the review in MEDLINE (Ovid). The search strategy was reviewed by a knowledge synthesis librarian using the PRESS checklist [16]. The final search strategy (Supplementary Table 1) was adapted for other bibliographic databases, and the following databases were searched for literature: MEDLINE (Ovid), Embase (Ovid), PubMed, Scopus (Elsevier), and Web of Science. Google Scholar and relevant websites were also searched for literature. The literature search was conducted in April 2017. Updated searches were carried out in July 2018 and February 2020.
Literature Selection
All retrieved unique citations were imported into a specially designed Microsoft (MS) Access 2016 database (Microsoft Corporation, Redmond, WA, USA) for screening. We were only interested in TND studies of seasonal influenza VE conducted in outpatient settings after the 2009/2010 influenza pandemic.
We considered for inclusion only country-specific studies published in a full-text manuscript, irrespective of language of publication. Influenza diagnosis/confirmation was by a reverse transcriptase polymerase chain reaction (RT-PCR) assay or viral culture of a respiratory specimen. Study participants must have received seasonal influenza vaccine at least 14 days before onset of influenza-like symptoms. The symptoms must not have started more than 7 days before presentation for medical consultation. We included only multivariable-adjusted end-season VE estimates against all influenza, influenza A subtypes A(H1N1)pdm09 and A(H3N2), and influenza B. We excluded studies on hospitalized patients and mixed hospitalized and outpatient data that could not be separated. We also excluded studies conducted in care homes, schools, military barracks, prisons, and within unique subgroups such as individuals with chronic diseases.
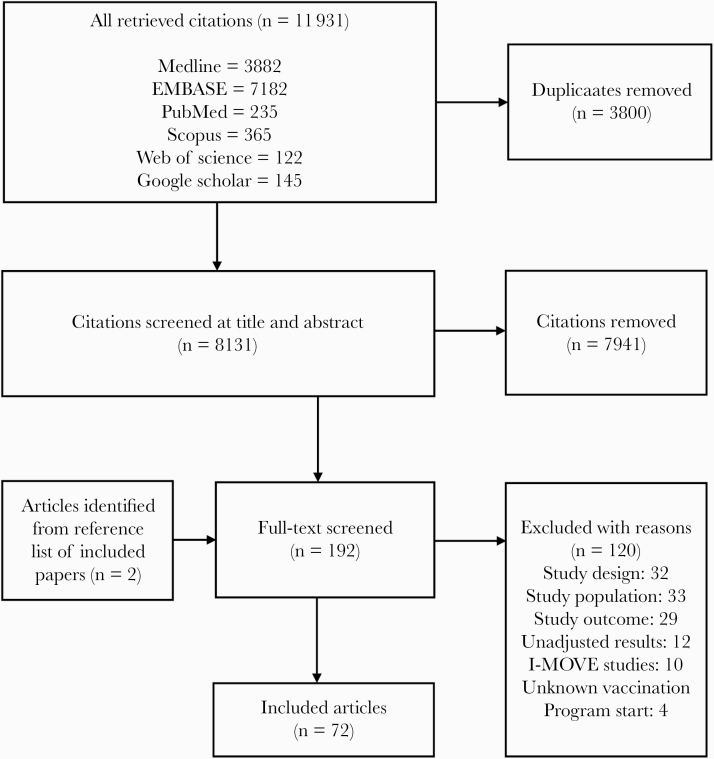
Two systematic reviewers independently screened the identified unique citations against the eligibility criteria using a 2-stage sifting approach to screen titles/abstracts and full-text articles. All included studies were examined for overlap or duplication of data. Disagreements between the reviewers were resolved through discussion or involvement of a third reviewer. The number of ineligible citations at the title/abstract screening stage and both the number and reasons for ineligibility at the full-text article screening stage were documented and are presented graphically as per PRISMA guidelines.
Data Extraction
One reviewer extracted data from the included studies using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA), and a second reviewer independently checked the extracted data for errors. We extracted basic study details, participants’ characteristics (sample size, mean age, age range, sex distribution), and vaccine information (method of vaccination status confirmation). We also extracted respiratory specimen (type and swab time), influenza diagnostic/confirmatory test, adjusted covariates in VE analysis, and outcome/results (multivariable-adjusted VE against all influenza, influenza A subtypes A(H1N1)pdm09 and A(H3N2), and influenza B; and their associated 95% CIs). We determined vaccine antigenic similarity with circulating virus strains using reports from the World Health Organization (WHO), national influenza centers, and region/country-specific centers for disease control.
We contacted the WHO, national departments of health/public health agencies, and national centers for disease control for annual influenza vaccination program inception year for each country irrespective of program rollout plans, public funding of programs, and within-country regional differences in program inception (Supplementary Table 2). In countries with decentralized provincial/state health authorities where there was no single, countrywide inception year, we considered the earliest regional program inception year to be the program inception year for the country.
Study Quality Assessment
In the absence of a validated quality assessment tool for TND studies, we improvised quality assessment by examining relevant study characteristics that could introduce bias, such as the methods of determination of vaccination status, participants’ enrollment, and inclusion of age and/or medical condition, among other covariates, in the logistic regression model for VE analysis. We synthesized quality assessment in a tabular form for visualization.
Data Synthesis and Analysis
Relevant characteristics of the included studies were synthesized in a tabular form. Data management and analysis were implemented in STATA (version 13; StataCorp LP, TX, USA). Our primary outcome was pooled influenza VE against all influenza across categories of vaccination PM. Our secondary outcome was pooled influenza VE against influenza A subtypes A(H1N1)pdm09 and A(H3N2) and influenza B across categories of vaccination PM. We determined seasonal influenza vaccination PM by calculating the number of years from the year of program inception for each country to the beginning of each reported influenza season. We then grouped vaccination PM into categories: 2 (Q2, cut at the median), 3 (Q3, tertiles), and 4 (Q4, quartiles). We explored study variation (excess heterogeneity) using random-effects meta-regression [17].
We repeated the above PM categorization across levels of vaccine antigenic similarity with circulating virus strains after identifying vaccine antigenic similarity as a potential source of heterogeneity across the studies. We pooled adjusted VE estimates and associated 95% CIs using an inverse-variance random-effects model. We assessed and quantified statistical heterogeneity between pooled VE using I2 [18]. We utilized the χ 2 test to assess the statistical significance (P value) of the difference between pooled VE across categories of vaccination PM [19]. Where appropriate (≥10 studies), we assessed for publication bias statistically using Egger’s regression test [20]. We conducted subgroup analysis using VE estimates reported specifically for older adults (>65 years), an important subgroup for influenza vaccination. We also conducted subgroup analysis by study country geographical region (hemisphere) for only the primary outcome.
RESULTS
We identified 11 931 citations, from which we included 72 full-text articles that met our inclusion criteria (Figure 1) [21–92]. Relevant study characteristics are summarized in Supplementary Table 3, and a geographic heat map and graphical representation of the included articles are presented in Supplementary Figure 1. Overall, there were 59 articles from the Northern hemisphere and 13 articles from the Southern hemisphere. PM ranged from 1 to 64 years. Study quality assessment is summarized in Supplementary Table 4.
Figure 1.
Modified Preferred Reporting Items for Systematic Reviews and Meta-Analysis flowchart (study selection process).
Pooled VE Against all Influenza (All Patients)
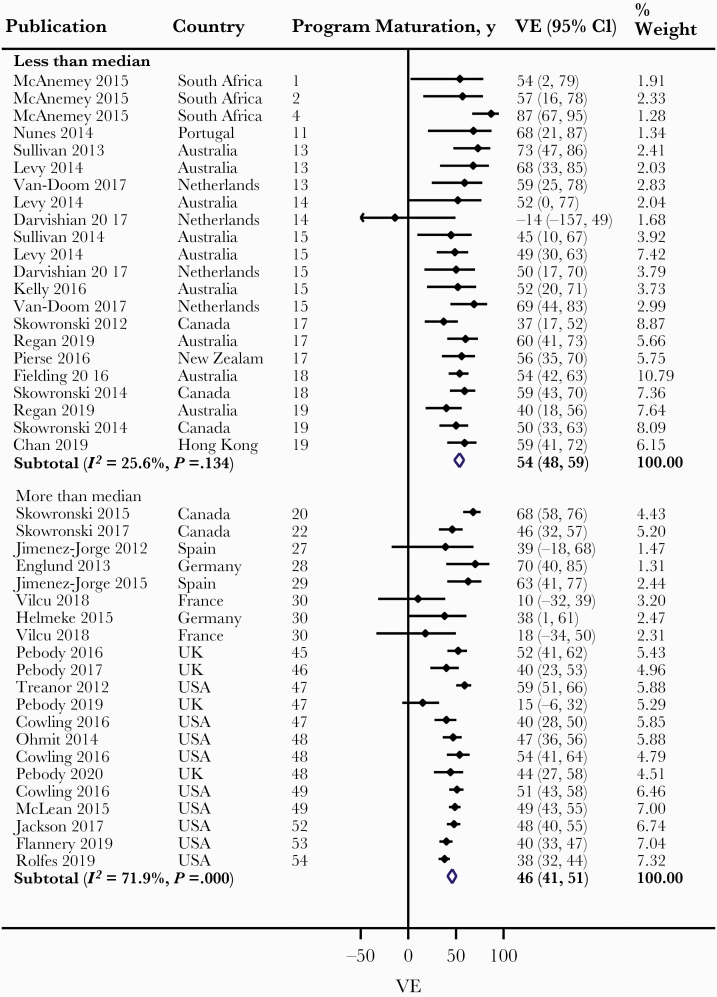
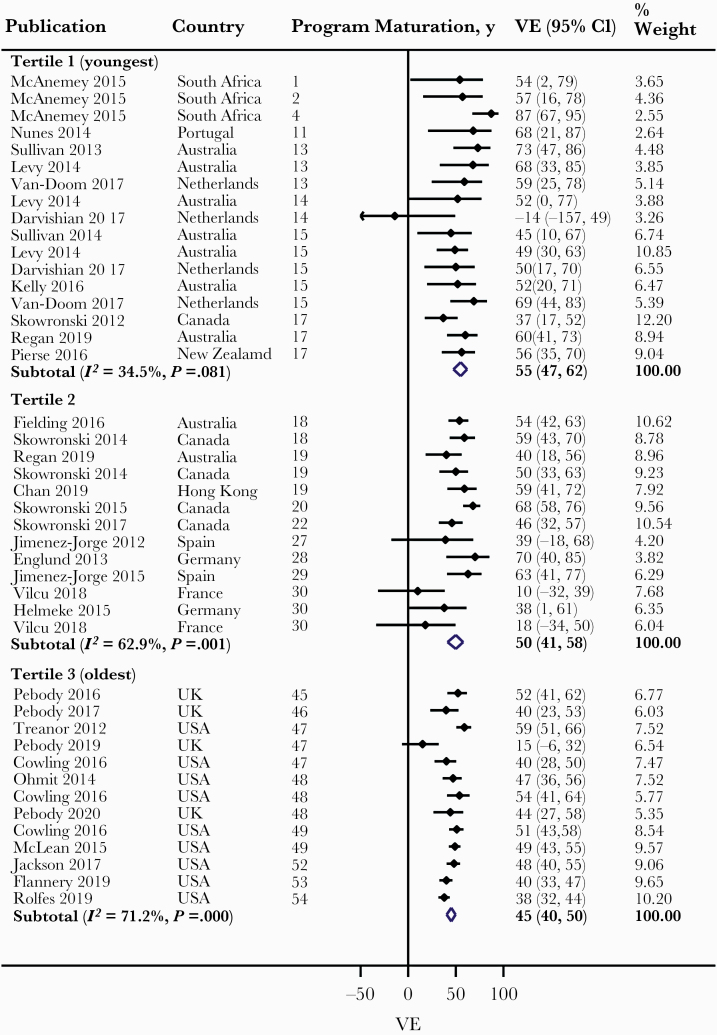
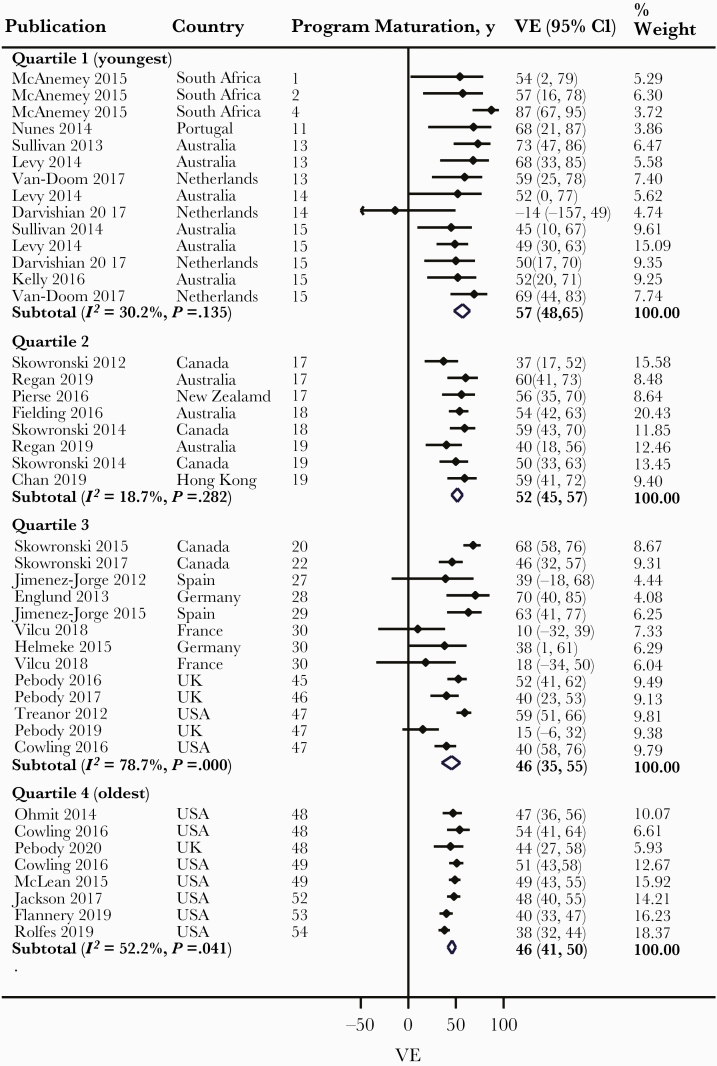
Overall, we observed a lower pooled VE with PM across levels of Q2 and Q3 categories and across the first 3 levels of Q4, albeit with high heterogeneity (Table 1). Meta-regression revealed vaccine antigenic similarity with circulating virus strains as a possible explanation for the observed heterogeneity (P < .001). Therefore, we conducted meta-analysis within levels of vaccine antigenic similarity for this and other assessed outcomes. Among studies with antigenically similar vaccines, we observed a lower pooled VE with PM across levels of Q2 category, from 54% (48%–59%) for less than median to 46% (41%–51%) for more than median, and the difference in VE was statistically significant (P = .035) (Figure 2). We observed a lower pooled VE with PM across levels of Q3 category, from 55% (47%–62%) for tertile 1 (youngest PM) to 50% (41%–58%) for tertile 2 and to 45% (40%–50%) tertile 3 (oldest PM), although the differences in VE between tertiles 1 and 2 and between tertiles 2 and 3 were both nonsignificant (Figure 3). We also observed a lower pooled VE with PM across levels of Q4 category: from 57% (48%–65%) for quartile 1 (youngest PM) to 52% (45%–57%) for quartile 2, and 46% (35%–55%) and 46% (41%–50%) for quartiles 3 and 4, respectively. However, the differences in VE between quartiles 1 and 2, between quartiles 2 and 3, and between quartiles 3 and 4 were all nonsignificant (Figure 4). Largely similar observations were made among studies with antigenically dissimilar/partially similar vaccines (Supplementary Figures 2–4), and when limited to the Northern and Southern hemispheres, particularly with high antigenic match (Supplementary Figures 5–10).
Table 1.
Results of Pooled Vaccine Effectiveness Against All Influenza
| All Patients | ||||
|---|---|---|---|---|
| Influenza Type, Analyzed Subgroups/PM Categories | No. of Studies | Pooled VE (95% CI) | I 2, % | Publication Bias, Egger’s Test P Value |
| All influenza | ||||
| Overall | ||||
| Q2 | ||||
| Less than median | 36 | 50 (42–57) | 74.7 | .067 |
| More than median | 36 | 35 (29–40) | 78.8 | .239 |
| Q3 | ||||
| Tertile 1 (youngest) | 26 | 50 (37–60) | 74.7 | <.001 |
| Tertile 2 | 22 | 41 (30–50) | 78.6 | .742 |
| Tertile 3 (oldest) | 24 | 38 (32–43) | 80.0 | .571 |
| Q4 | ||||
| Quartile 1 (youngest) | 21 | 52 (39–62) | 67.3 | .029 |
| Quartile 2 | 15 | 49 (36–59) | 81.8 | .315 |
| Quartile 3 | 18 | 23 (10–34) | 66.7 | .678 |
| Quartile 4 (oldest) | 18 | 41 (36–47) | 81.3 | .432 |
| Antigenically similar vaccine | ||||
| Q2 | ||||
| Less than median | 22 | 54 (48–59) | 25.6 | .071 |
| More than median | 21 | 46 (41–51) | 71.9 | .564 |
| Q3 | ||||
| Tertile 1 (youngest) | 17 | 55 (47–62) | 34.5 | .042 |
| Tertile 2 | 13 | 50 (41–58) | 62.9 | .654 |
| Tertile 3 (oldest) | 13 | 45 (40–50) | 71.2 | .561 |
| Q4 | ||||
| Quartile 1 (youngest) | 14 | 57 (48–65) | 30.2 | .163 |
| Quartile 2 | 8 | 52 (45–57) | 18.7 | – |
| Quartile 3 | 13 | 46 (35–55) | 78.7 | .918 |
| Quartile 4 (oldest) | 8 | 46 (41–50) | 52.2 | – |
| Antigenically dissimilar/partially similar vaccine | ||||
| Q2 | ||||
| Less than median | 15 | 30 (12–44) | 67.5 | .006 |
| More than median | 14 | 20 (11–28) | 52.1 | .059 |
| Q3 | ||||
| Tertile 1 (youngest) | 12 | 37 (12–55) | 73.5 | .004 |
| Tertile 2 | 9 | 13 (–4 to 27) | 57.8 | – |
| Tertile 3 (oldest) | 8 | 25 (18–31) | 19.2 | – |
| Q4 | ||||
| Quartile 1 (youngest) | 12 | 37 (12–55) | 73.5 | .004 |
| Quartile 2 | 3 | 17 (3–29) | 0 | – |
| Quartile 3 | 7 | 10 (–18 to 31) | 66.2 | – |
| Quartile 4 (oldest) | 7 | 24 (17–31) | 30.5 | – |
| All Patients: Northern Hemisphere | ||||
| Influenza Type, Analyzed Subgroups/PM Categories | No. of Studies | Pooled VE (95% CI) | I 2, % | Publication Bias, Egger’s Test P Value |
| All influenza | ||||
| Overall | ||||
| Q2 | ||||
| Less than median | 30 | 38 (26–48) | 81.1 | .280 |
| More than median | 24 | 38 (32–43) | 80.0 | .571 |
| Q3 | ||||
| Tertile 1 (youngest) | 18 | 44 (28–57) | 84.2 | .309 |
| Tertile 2 | 18 | 23 (10–34) | 66.7 | .678 |
| Tertile 3 (oldest) | 18 | 41 (36–47) | 81.5 | .432 |
| Q4 | ||||
| Quartile 1 (youngest) | 15 | 39 (20–54) | 80.4 | .096 |
| Quartile 2 | 15 | 36 (20–49) | 82.1 | .810 |
| Quartile 3 | 12 | 32 (20–43) | 79.4 | .025 |
| Quartile 4 (oldest) | 12 | 41 (35–47) | 82.0 | .451 |
| Antigenically similar vaccine | ||||
| Q2 | ||||
| Less than median | 17 | 50 (41–58) | 61.2 | .979 |
| More than median | 13 | 45 (40–50) | 71.2 | .561 |
| Q3 | ||||
| Tertile 1 (youngest) | 10 | 55 (45–63) | 54.9 | .940 |
| Tertile 2 | 12 | 43 (32–52) | 73.7 | .813 |
| Tertile 3 (oldest) | 8 | 46 (41–50) | 52.2 | – |
| Q4 | ||||
| Quartile 1 (youngest) | 9 | 52 (43–61) | 38.7 | – |
| Quartile 2 | 8 | 48 (30–61) | 75.0 | – |
| Quartile 3 | 8 | 45 (36–53) | 75.9 | – |
| Quartile 4 (oldest) | 5 | 45 (40–50) | 67.3 | – |
| Antigenically dissimilar/partially similar vaccine | ||||
| Q2 | ||||
| Less than median | 12 | 12 (–9 to 29) | 66.0 | .163 |
| More than median | 12 | 25 (17–31) | 32.4 | .230 |
| Q3 | ||||
| Tertile 1 (youngest) | 8 | 23 (–8 to 45) | 74.5 | – |
| Tertile 2 | 8 | 11 (–10 to 27) | 62.3 | – |
| Tertile 3 (oldest) | 8 | 25 (18–31) | 19.2 | – |
| Q4 | ||||
| Quartile 1 (youngest) | 7 | 24 (–14 to 50) | 75.0 | – |
| Quartile 2 | 5 | 8 (–13 to 25) | 44.1 | – |
| Quartile 3 | 6 | 18 (–4 to 35) | 52.8 | – |
| Quartile 4 (oldest) | 6 | 26 (20–31) | 11.4 | – |
| All Patients: Southern Hemisphere | ||||
| Influenza Type, Analyzed Subgroups/PM Categories | No. of Studies | Pooled VE (95% CI) | I 2, % | |
| All influenza | ||||
| Overall | ||||
| Q2 | ||||
| Less than median | 9 | 62 (50–71) | 12.1 | |
| More than median | 9 | 52 (46–57) | 0 | |
| Q3 | ||||
| Tertile 1 (youngest) | 7 | 65 (51–75) | 20.1 | |
| Tertile 2 | 5 | 49 (37–59) | 0 | |
| Tertile 3 (oldest) | 7 | 53 (46–59) | 0 | |
| Q4 | ||||
| Quartile 1 (youngest) | 5 | 62 (38–77) | 39.4 | |
| Quartile 2 | 4 | 62 (46–73) | 0 | |
| Quartile 3 | 5 | 51 (39–60) | 0 | |
| Quartile 4 (oldest) | 4 | 52 (44–59) | 4.3 | |
| Antigenically similar vaccine | ||||
| Q2 | ||||
| Less than median | 9 | 58 (47–67) | 28.5 | |
| More than median | 4 | 52 (44–59) | 4.3 | |
| Q3 | ||||
| Tertile 1 (youngest) | 5 | 69 (54–79) | 26.8 | |
| Tertile 2 | 4 | 49 (36–59) | 0 | |
| Tertile 3 (oldest) | 4 | 52 (44–59) | 4.3 | |
| Q4 | ||||
| Quartile 1 (youngest) | 5 | 69 (54–79) | 26.8 | |
| Quartile 2 | 4 | 49 (36–59) | 0 | |
| Quartile 3 | 2 | 58 (45–68) | 0 | |
| Quartile 4 (oldest) | 2 | 49 (34–60) | 45.6 | |
| Older Adults | ||||
| Influenza Type, Analyzed Subgroups/PM Categories | No. of Studies | Pooled VE (95% CI) | I 2, % | Publication Bias, Egger’s Test P Value |
| All influenza | ||||
| Overall | ||||
| Q2 | ||||
| Less than median | 12 | 43 (22–58) | 30.2 | .696 |
| More than median | 12 | 23 (12–33) | 0 | .536 |
| Q3 | ||||
| Tertile 1 (youngest) | 8 | 56 (37–69) | 0 | – |
| Tertile 2 | 8 | 17 (–6 to 35) | 9.9 | – |
| Tertile 3 (oldest) | 8 | 26 (14–36) | 0 | – |
| Q4 | ||||
| Quartile 1 (youngest) | 7 | 54 (32–69) | 0 | – |
| Quartile 2 | 5 | 35 (–3 to 59) | 53.2 | – |
| Quartile 3 | 6 | 21 (–4 to 39) | 0 | – |
| Quartile 4 (oldest) | 6 | 24 (11–35) | 0 | – |
| Antigenically similar vaccine | ||||
| Q2 | ||||
| Less than median | 9 | 50 (34–62) | 0 | – |
| More than median | 8 | 23 (10–35) | 0 | – |
| Q3 | ||||
| Tertile 1 (youngest) | 6 | 56 (36–69) | 0 | – |
| Tertile 2 | 7 | 30 (10–46) | 4.4 | – |
| Tertile 3 (oldest) | 4 | 24 (9–37) | 0 | – |
| Q4 | ||||
| Quartile 1 (youngest) | 5 | 54 (31–70) | 0 | – |
| Quartile 2 | 4 | 46 (23–62) | 0 | – |
| Quartile 3 | 4 | 22 (–9 to 44) | 3.8 | – |
| Quartile 4 (oldest) | 4 | 24 (9–37) | 0 | – |
| Antigenically dissimilar/partially similar vaccine | ||||
| Q2 | ||||
| Less than median | 4 | 5 (–46 to 38) | 0 | – |
| More than median | 3 | 21 (–1 to 39) | 0 | – |
| Q3 | ||||
| Tertile 1 (youngest) | 3 | 26 (–31 to 58) | 0 | – |
| Tertile 2 | 2 | –16 (–87 to 28) | 0 | – |
| Tertile 3 (oldest) | 2 | 24 (0–42) | 0 | – |
Q2, Q3, and Q4 = categories of seasonal influenza vaccination program maturation; less than median = lower half of the sorted data; more than median = higher half of the sorted data.
Abbreviations: PM, program maturation; VE, vaccine effectiveness.
Figure 2.
Forest plot of vaccine effectiveness (VE) against all influenza across Q2 category (all patients: studies with antigenically similar vaccine). Less than median = lower half of the sorted data; more than median = higher half of the sorted data.
Figure 3.
Forest plot of vaccine effectiveness (VE) against all influenza across Q3 category (all patients: studies with antigenically similar vaccine).
Figure 4.
Forest plot of vaccine effectiveness (VE) against all influenza across Q4 category (all patients: studies with antigenically similar vaccine).
Pooled VE Against All Influenza (Older Adults)
We made similar observations to the analyses with all patients across levels of Q2 and Q3 categories and across 3 levels of Q4 category, but with significantly lower heterogeneity (Table 1). Among studies with antigenically similar vaccines, we observed a lower pooled VE with PM across levels of Q2 category, from 50% (34%–62%) for less than median to a much lower 23% (10%–35%) for more than median, and the difference in VE was statistically significant (P = .005) (Supplementary Figure 11). We observed a lower pooled VE with PM across levels of Q3 category, from 56% (36%–69%) for tertile 1 (youngest PM) to a much lower 30% (10%–46%) for tertile 2 and to 24% (9%–37%) for tertile 3 (oldest PM). The difference in VE between tertiles 1 and 2 was statistically significant (P = .037), but the difference in VE between tertiles 2 and 3 was nonsignificant (Supplementary Figure 12). We also observed a lower pooled VE with PM across levels of Q4 category, from 54% (31%–70%) for quartile 1 (youngest PM) to 46% (23%–62%) for quartile 2 to 22% (–9% to 44%) and a slightly higher 24% (9%–37%) for quartiles 3 and 4, respectively, although the differences in VE between quartiles 1 and 2 and between quartiles 2 and 3 were nonsignificant (Supplementary Figure 13). There was a paucity of data to enable adequate assessment among studies with antigenically dissimilar/partially similar vaccines (Supplementary Figures 14–16).
Pooled VE Against Influenza A Subtypes and Influenza B (All Patients)
When limited to studies with antigenically similar vaccines, we observed a lower pooled VE against A(H1N1)pdm09 with PM across levels of Q2 category (P = .023), Q3 category (mainly between tertile 1 [youngest PM] and tertile 2; P = .065), and, to some extent, Q4 category. Q4 category did not show a consistent reduction across the 4 levels, mostly due to quartile 4 (oldest PM) being driven by studies from the United States (80%) (Supplementary Table 5). This was also the case for A(H3N2): Q2 (P = .12) and Q3 (mainly between tertile 1 [youngest PM] and tertile 2; P = .15; with tertile 3 [oldest PM] driven by studies from the United States [86%]); and influenza B: Q2 (P = .38) and Q3 (mainly tertile 1 [youngest PM] and tertile 2; P = .33; with tertile 3 [oldest PM] driven by studies from the United States [87%]). No clear pattern was observed across the levels of Q4 category for both, mostly due to quartile 4 (oldest PM) being driven by studies from the United States (100% and 75% for A(H3N2) and influenza B, respectively). Similar observations were made with regard to A(H1N1)pdm09 among studies with antigenically dissimilar/partially similar vaccines. We observed a lower pooled VE against A(H3N2) and influenza B with PM across levels of Q2 category and Q4 category among studies with antigenically dissimilar/partially similar vaccines. The opposite observation was, however, made across levels of Q3 category with regard to influenza B (Supplementary Table 5).
Pooled VE Against Influenza A Subtypes and Influenza B (Older Adults)
Among studies with antigenically similar vaccines, we observed a lower pooled VE against A(H1N1)pdm09 with PM across only levels of Q2 category (Supplementary Table 6). There was not enough data to enable adequate assessment of A(H3N2). However, among studies with antigenically dissimilar/partially similar vaccines, we observed considerably lower pooled VE against A(H3N2) with PM across levels of Q2 category, Q3 category (mainly between tertiles 1 [youngest PM] and 2), and, to some extent, across levels of Q4 category (Supplementary Table 6). We also observed a lower pooled VE against influenza B with PM across levels of Q2 category, Q3 category (mainly between tertiles 1 [youngest PM] and 2), and levels of Q4 category among studies with antigenically similar vaccines (Supplementary Table 6). There was not enough data to enable assessment among studies with antigenically dissimilar/partially similar vaccines. None of the differences between VE across levels of the categories were statistically significant.
DISCUSSION
We assessed the association between seasonal influenza vaccination PM and influenza VE utilizing evidence from TND studies in outpatient settings after the 2009/2010 influenza pandemic. Irrespective of our categorization of PM, we observed a largely consistent trend. Among studies with antigenically similar vaccines, VE against all influenza declined with PM, with higher decline observed in older adults. Similar observations were made when limited to the Northern and Southern hemispheres. Overall, the difference in VE between the levels of PM categories was mostly statistically significant for the 2-level PM category (Q2). Considerably similar observations were made among studies with antigenically dissimilar/partially similar vaccines and with regard to VE against A(H1N1)pdm09, A(H3N2), and influenza B, except for a few inconsistencies (overall downward trend appears reversed) mainly due to higher VE in tertile 3 (oldest PM) compared with tertile 2 in some of the Q3 and Q4 categories. The inconsistencies were mainly driven by studies from the United States, which contributed 75% to 100% of the studies within these levels. Being from a more affluent country, this could reflect early adoption of quadrivalent, high-dose, adjuvanted, and recombinant vaccines in the United States, which have been shown to offer improved efficacy [93, 94], and may therefore have reversed or arrested any downward trends in VE. Examination of influenza VE over time in a large population of healthy people for whom vaccination is mandatory and vaccination and health care data are electronically available (for, eg, military and health care personnel) may help validate our findings.
There are currently no similar published studies to compare our findings against. However, our findings could be compared against what is currently known regarding repeat vaccination. Influenza vaccine remains the only vaccine regularly reformulated and administered every year due to influenza virus antigenic evolution. Whereas some studies have reported that repeated influenza vaccination may increase the risk of influenza infection, especially the A(H3N2) [80, 95, 96], others have reported no evidence of loss of protection including against A(H3N2) even when the circulating virus strains are antigenically dissimilar from the vaccine component [97]. A recent publication demonstrated that repeat seasonal influenza vaccination reduced antibody-affinity maturation to hemagglutinin 1 (HA1) domain of all 3 influenza virus strains irrespective of the vaccine platform [98]. The study highlighted an important influence of repeat vaccination on antibody-affinity maturation, which may contribute to lower influenza VE, as we observed. A recent systematic review and meta-analysis of 20 studies (including TND, cohort, and case–control) observed lower influenza VE against A(H3N2) and influenza B, but not against A(H1N1), in individuals vaccinated in both current and previous seasons compared with those vaccinated only in the current season [12]. These findings are similar to our findings, except for A(H1N1). However, it is not clear if data from hospitalized patients were included among the analyzed studies. Such inclusion may explain the observed lack of difference found with regard to A(H1N1). A study investigated the impact of repeated vaccination on VE against A(H3N2) and influenza B in the United States [11]. Utilizing 5 years of vaccination data, the authors found that current-season VE against A(H3N2) was significantly higher among vaccinated individuals with no prior vaccination history compared with those with a frequent vaccination history (P = .01). A similar observation was made with respect to influenza B (P = .05). These findings align largely with our findings in both all patients and older adults, and particularly within our Q2 category, although we observed an opposite trend within the Q3 category when data were limited to studies with antigenically dissimilar/partially similar vaccines for all patients. An explanation for such a trend may be differences in study characteristics, particularly patient age and comorbidity status. Another explanation could be the increasing use of quadrivalent influenza vaccines over trivalent vaccines in the older programs, which are in the more affluent countries. In recent years, seasonal influenza vaccines increasingly contain both influenza B strains (2 distinct lineages) in addition to the influenza A subtypes (quadrivalent vaccine) instead of just having a single component for influenza B in addition to 2 influenza A subtypes (trivalent vaccine), as was previously the case. This may have concealed the trend toward a reduced VE with repeated vaccinations, particularly for influenza B.
It has been suggested that the protection conferred by influenza vaccine in a season could prevent the natural immunity from exposure to circulating influenza viruses, and may therefore increase the risk of infection and impact VE in subsequent seasons [7]. The “antigenic distance” phenomenon has also been proposed, suggesting that negative interference from the previous seasonal influenza vaccine on the current season’s VE may occur when the previous and current season’s vaccines are antigenically closely related, but the previous season and the current circulating influenza virus strains are largely antigenically distinct [99]. Furthermore, evidence from studies on animals suggests that repeated vaccination could affect the development of cross-reactive immunity against influenza subtypes, suggestively facilitated by a decreased virus-specific CD8+ T-cell response [100]. Repeated seasonal vaccination has also been shown to affect development of virus-specific CD8+ T-cell immunity in children [101]. These studies suggest that repeated influenza vaccination may adversely affect VE, which could be a plausible biological explanation of our review findings. However, the issue of reduced influenza VE with repeated vaccination is multifaceted.
Even though we observed a trend that may suggest that VE declines with PM, cautious interpretation of our findings is necessary because of the limitations of our review and potential confounding that we could not explore. The studies reviewed differed by methods of participant enrollment, determination of influenza vaccination status, and respiratory specimen type. Sample size varied across studies, and statistical models differed significantly. However, in all of the studies, vaccination was at least 14 days before symptom onset, the respiratory specimen swab was collected within 7 days of symptom onset, and influenza diagnosis was made using gold standards (RT-PCR or viral culture), satisfying major conditions for TND study of influenza VE. A significant weakness of our review is the nature of the ecological data and the impact that differences in important characteristics, such as age, sex, comorbidity status, prior history of influenza vaccination, and “healthy vaccinee” effect (bias because of more healthier individuals vaccinated over time), across studies might have had on our findings. It was also not possible to assess the impact of differences in vaccination rates across studies. A lack of data resulted in a few data points for some outcomes, limited statistical power for some of the analyses, and precluded analysis in some cases. Nevertheless, findings from this review contribute significantly to the evidence base and provide population-level insights that may be of use to public health decision-making.
A major strength of this systematic review is its uniqueness. To the best of our knowledge, it is the first review to assess the impact of seasonal influenza vaccination PM on influenza VE. The evidence considered in this review was based on influenza VE estimates from TND study type, widely credited with reducing biases due to differential health care–seeking behavior between vaccinated and unvaccinated persons, differential misclassification of infection status, and easy access to study controls who are more representative of the case source population [5]. Our analysis was particularly in-depth, covering 3 different categorizations of PM with a good spread of the data across levels of each category. We explored differences that may exist between influenza types/subtypes and compared the overall analyses with those for older adults, considering that this unique subpopulation is possibly the most adherent to influenza vaccination and, therefore, would likely present good insights with respect to the potential impact of PM on VE.
CONCLUSIONS
The evidence suggests that influenza VE declines with vaccination PM, with potentially higher reduction among older adults when compared with all patients. Our findings form the basis for further discussions and examinations of the potential impact of influenza PM on seasonal VE but do not justify the curtailment or cessation of national annual vaccination programs, which continue to offer substantial net public health benefit.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Drs. Iwona Paradowska-Stankiewicz, Romana Tandara Haček, Daniel Levy-Bruhl, Sabine Reiter, Josefa Masa Calles, Suzanne Cotter, AnnaSara Carnahan, Aurora Limia, Franz Allerberger, Martin Sprenger, Aharona Glatman-Freedman, Gerald Haidinger, and Nesrin Cilingiroglu for responding kindly to our request for information.
Financial support. No external funding was obtained for this study. G.N.O. is a recipient of the Manitoba Training Program Fellowship Award, the Centre on Aging Betty Havens Memorial Graduate Fellowship Award, and the Evelyn Shapiro Award, all for health services research.
Potential conflicts of interest. J.S.N.-V.-T. was an employee of SmithKline Beecham plc. (now GlaxoSmithKline) and Aventis-Pasteur MSD (now Sanofi-Pasteur MSD) before 2005, both of which manufacture influenza vaccines; he divested or relinquished all shareholdings, share options, or accrued pension rights in both companies in 2005. He is seconded to the Department of Health and Social Care, England (DHSC). The views in this manuscript are those of the authors and do not necessarily represent those of the DHSC. S.M.M. is supported, in part, by funding from the Canada Research Chairs Program. S.M.M. has received unrestricted research grants from GlaxoSmithKline, Merck, Sanofi Pasteur, Pfizer, and Roche-Assurex for unrelated studies and fees as an advisory board member for Sanofi Pasteur. C.H.R. has received an unrestricted research grant from Pfizer for an unrelated study. The other authors declare that they have no conflicts of interest.
Author contributions. Conception (J.S.N.-V.-T.); design (G.N.O., S.M.M., & J.S.N.-V.-T.); data collection (G.N.O., F.R., T.A., S.K.H., & L.L.); data analysis/interpretation (G.N.O., C.H.R., S.M.M., & J.S.N.-V.-T.); drafting the manuscript (G.N.O.); domain expert guidance (S.M.M. & J.S.N.-V.-T.); manuscript revisions (G.N.O., F.R., T.A., S.K.H., L.L., C.H.R., S.M.M., & J.S.N.-V.-T.); approval for submission (G.N.O., F.R., T.A., S.K.H., L.L., C.H.R., S.M.M., & J.S.N.-V.-T.).
Patient consent. This study does not include factors necessitating patient consent.
References
- 1. Boni MF. Vaccination and antigenic drift in influenza. Vaccine 2008; 26(Suppl 3):C8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petrie JG, Ohmit SE, Johnson E, et al. Persistence of antibodies to influenza hemagglutinin and neuraminidase following one or two years of influenza vaccination. J Infect Dis 2015; 212:1914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices - United States, 2017–18 influenza season. MMWR Recomm Rep 2017; 66:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Advisory Committee on Immunization (NACI). Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2017–2018. Ottawa: Public Health Agency of Canada; 2017. [Google Scholar]
- 5. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 6. Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol 2016; 184:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoskins TW, Davies JR, Smith AJ, et al. Assessment of inactivated influenza—a vaccine after three outbreaks of influenza A at Christ’s Hospital. Lancet 1979; 1:33–5. [DOI] [PubMed] [Google Scholar]
- 8. Beyer WE, de Bruijn IA, Palache AM, et al. Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Arch Intern Med 1999; 159:182–8. [DOI] [PubMed] [Google Scholar]
- 9. Keitel WA, Cate TR, Couch RB, et al. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997; 15:1114–22. [DOI] [PubMed] [Google Scholar]
- 10. Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011-2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McLean HQ, Thompson MG, Sundaram ME, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis 2014; 59:1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramsay LC, Buchan SA, Stirling RG, et al. The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis. BMC Med 2019; 17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cumpston M, Li TJ, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Db Syst Rev 2019; 10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg 2010; 8:336–41. [DOI] [PubMed] [Google Scholar]
- 15. Okoli GN, Racovitan F, Righolt CH, Mahmud SM. Variations in seasonal influenza vaccine effectiveness due to study characteristics: a systematic review and meta-analysis of test-negative design studies. Open Forum Infect Dis 2020; 7:ofaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGowan J, Sampson M, Salzwedel DM, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016; 75:40–6. [DOI] [PubMed] [Google Scholar]
- 17. Huizenga HM, Visser I, Dolan CV. Testing overall and moderator effects in random effects meta-regression. Br J Math Stat Psychol 2011; 64:1–19. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–58. [DOI] [PubMed] [Google Scholar]
- 19. McHugh ML. The chi-square test of independence. Biochem Med (Zagreb) 2013; 23:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 2001; 323:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andrews N, McMenamin J, Durnall H, et al. Effectiveness of trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2012/13 end of season results. Euro Surveill 2014; 19:5–13. [PubMed] [Google Scholar]
- 22. Bateman AC, Kieke BA, Irving SA, Meece JK, Shay DK, Belongia EA. Effectiveness of monovalent 2009 pandemic influenza A virus subtype H1N1 and 2010–2011 trivalent inactivated influenza vaccines in Wisconsin during the 2010–2011 influenza season. J Infects Dis 2013; 207:1262–9. [DOI] [PubMed] [Google Scholar]
- 23. Carville KS, Grant KA, Sullivan SG, et al. Understanding influenza vaccine protection in the community: an assessment of the 2013 influenza season in Victoria, Australia. Vaccine 2015; 33:341–5. [DOI] [PubMed] [Google Scholar]
- 24. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Eurosurveillance 2013; 18:20388. [DOI] [PubMed] [Google Scholar]
- 25. Castilla J, Navascués A, Fernández-Alonso M, et al. ; Primary Health Care Sentinel Network; Network for Influenza Surveillance in Hospitals of Navarra . Effectiveness of subunit influenza vaccination in the 2014-2015 season and residual effect of split vaccination in previous seasons. Vaccine 2016; 34:1350–7. [DOI] [PubMed] [Google Scholar]
- 26. Castilla J, Portillo ME, Casado I, et al. ; Primary Health Care Sentinel Network and Network for Influenza Surveillance in Hospitals of Navarre . Effectiveness of the current and prior influenza vaccinations in Northern Spain, 2018-2019. Vaccine 2020; 38:1925–32. [DOI] [PubMed] [Google Scholar]
- 27. Chan YD, Wong ML, Au KW, Chuang SK. Seasonal influenza vaccine effectiveness at primary care level, Hong Kong SAR, 2017/2018 winter. Hum Vaccin Immunother 2019; 15:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chon I, Saito R, Hibino A, et al. Effectiveness of the quadrivalent inactivated influenza vaccine in Japan during the 2015-2016 season: a test-negative case-control study comparing the results by real time PCR, virus isolation. Vaccine: X 2019; 1:100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cowling BJ, Feng S, Finelli L, et al. Assessment of influenza vaccine effectiveness in a sentinel surveillance network 2010-13, United States. Vaccine 2016; 34:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Darvishian M, Dijkstra F, van Doorn E, et al. Influenza vaccine effectiveness in the Netherlands from 2003/2004 through 2013/2014: the importance of circulating influenza virus types and subtypes. PLoS One 2017; 12:e0169528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Englund H, Campe H, Hautmann W. Effectiveness of trivalent and monovalent influenza vaccines against laboratory-confirmed influenza infection in persons with medically attended influenza-like illness in Bavaria, Germany, 2010/2011 season. Epidemiol Infect 2013; 141:1807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fielding JE, Grant KA, Tran T, Kelly HA. Moderate influenza vaccine effectiveness in Victoria, Australia, 2011. Eurosurveillance 2012; 17:20115. [PubMed] [Google Scholar]
- 33. Fielding JE, Levy A, Chilver MB, et al. Effectiveness of seasonal influenza vaccine in Australia, 2015: an epidemiological, antigenic and phylogenetic assessment. Vaccine 2016; 34:4905–12. [DOI] [PubMed] [Google Scholar]
- 34. Flannery B, Chung JR, Monto AS, et al. ; US Flu VE Investigators . Influenza vaccine effectiveness in the United States during the 2016-2017 season. Clin Infect Dis 2019; 68:1798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flannery B, Kondor RJG, Chung JR, et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018-2019 Season. J Infect Dis 2020; 221:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaglani M, Pruszynski J, Murthy K, et al. Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013-2014 in the United States. J Infect Dis 2016; 213:1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gherasim A, Pozo F, de Mateo S, et al. ; cycEVA team and the VEVA Working Group . Waning protection of influenza vaccine against mild laboratory confirmed influenza A(H3N2) and B in Spain, season 2014-15. Vaccine 2016; 34:2371–7. [DOI] [PubMed] [Google Scholar]
- 38. Gherasim A, Martinez-Baz I, Castilla J, Pozo F, Larrauri A; cycEVA working group. Effect of previous and current vaccination against influenza A(H1N1) pdm09, A(H3N2), and B during the post-pandemic period 2010–2016 in Spain. PLoS One 2017; 12:e0179160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Helmeke C, Gräfe L, Irmscher HM, et al. Effectiveness of the 2012/13 trivalent live and inactivated influenza vaccines in children and adolescents in Saxony-Anhalt, Germany: a test-negative case-control study. PLoS One 2015; 10:e0122910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jackson ML, Chung JR, Jackson LA, et al. Influenza vaccine effectiveness in the United States during the 2015–2016 season. New Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiménez-Jorge S, Savulescu C, Pozo F, et al. ; cycEVA Study Team; Spanish Influenza Sentinel Surveillance System . Effectiveness of the 2010-11 seasonal trivalent influenza vaccine in Spain: cycEVA study. Vaccine 2012; 30:3595–602. [DOI] [PubMed] [Google Scholar]
- 42. Jiménez-Jorge S, de Mateo S, Delgado-Sanz C, et al. ; Spanish Influenza Sentinel Surveillance System . Effectiveness of influenza vaccine against laboratory-confirmed influenza, in the late 2011-2012 season in Spain, among population targeted for vaccination. BMC Infect Dis 2013; 13:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jimenez-Jorge S, de Mateo S, Delgado-Sanz C, et al. Estimating influenza vaccine effectiveness in Spain using sentinel surveillance data. Euro Surveill 2015; 20:21187. [DOI] [PubMed] [Google Scholar]
- 44. Jiménez-Jorge S, Pozo F, Larrauri A; cycEVA Study Team . Interim influenza vaccine effectiveness: a good proxy for final estimates in Spain in the seasons 2010-2014. Vaccine 2015; 33:3276–80. [DOI] [PubMed] [Google Scholar]
- 45. Kelly HA, Sullivan SG, Grant KA, Fielding JE. Moderate influenza vaccine effectiveness with variable effectiveness by match between circulating and vaccine strains in Australian adults aged 20-64 years, 2007–2011. Influenza Other Resp 2013; 7:729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kelly HA, Lane C, Cheng AC. Influenza vaccine effectiveness in general practice and in hospital patients in Victoria, 2011–2013. Med J Aust 2016; 204:76.e71–5. [DOI] [PubMed] [Google Scholar]
- 47. Levy A, Sullivan SG, Tempone SS, et al. Influenza vaccine effectiveness estimates for Western Australia during a period of vaccine and virus strain stability, 2010 to 2012. Vaccine 2014; 32:6312–8. [DOI] [PubMed] [Google Scholar]
- 48. Lo YC, Chuang JH, Kuo HW, et al. Surveillance and vaccine effectiveness of an influenza epidemic predominated by vaccine-mismatched influenza B/Yamagata-lineage viruses in Taiwan, 2011-12 season. PLoS One 2013; 8:e58222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ma C, Pan Y, Zhang L, et al. Influenza vaccine effectiveness against medically attended influenza illness in Beijing, China, 2014/15 season. Hum Vaccin Immunother 2017; 13:2379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martínez-Baz I, Martínez-Artola V, Reina G, et al. Effectiveness of the trivalent influenza vaccine in Navarre, Spain, 2010–2011: a population-based test-negative case-control study. BMC Public Health 2013; 13:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martínez-Baz I, Navascués A, Pozo F, et al. ; Primary Health Care Sentinel Network and Network for Influenza Surveillance in Hospitals of Navarra . Influenza vaccine effectiveness in preventing inpatient and outpatient cases in a season dominated by vaccine-matched influenza B virus. Hum Vaccin Immunother 2015; 11:1626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Martínez-Baz I, Navascués A, Casado I, et al. Remaining effect of influenza vaccines received in prior seasons. J Infect Dis 2019; 220:1136–40. [DOI] [PubMed] [Google Scholar]
- 53. McAnerney JM, Walaza S, Cohen AL, et al. Effectiveness and knowledge, attitudes and practices of seasonal influenza vaccine in primary healthcare settings in South Africa, 2010–2013. Influenza Other Resp 2015; 9:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McAnerney JM, Walaza S, Tempia S, et al. Estimating vaccine effectiveness in preventing laboratory-confirmed influenza in outpatient settings in South Africa, 2015. Influenza Other Respir Viruses 2017; 11:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McLean HQ, Thompson MG, Sundaram ME, et al. Influenza vaccine effectiveness in the United States during 2012-2013: variable protection by age and virus type. J Infect Dis 2015; 211:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Möhl A, Gräfe L, Helmeke C, et al. Estimating vaccine effectiveness against laboratory-confirmed influenza among children and adolescents in Lower Saxony and Saxony-Anhalt, 2012-2016. Epidemiol Infect 2018; 146:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nunes B, Machado A, Guiomar R, et al. Estimates of 2012/13 influenza vaccine effectiveness using the case test-negative control design with different influenza negative control groups. Vaccine 2014; 32:4443–9. [DOI] [PubMed] [Google Scholar]
- 58. Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011-2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pebody RG, Andrews N, Fleming DM, et al. Age-specific vaccine effectiveness of seasonal 2010/2011 and pandemic influenza A(H1N1) 2009 vaccines in preventing influenza in the United Kingdom. Epidemiol Infect 2013; 141:620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pebody RG, Andrews N, McMenamin J, et al. Vaccine effectiveness of 2011/12 trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: evidence of waning intra-seasonal protection. Eurosurveillance 2013; 18:20389. [DOI] [PubMed] [Google Scholar]
- 61. Pebody R, Warburton F, Andrews N, et al. Effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 end of season results. Eurosurveillance 2015; 20. [PubMed] [Google Scholar]
- 62. Pebody R, Warburton F, Ellis J, et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Eurosurveillance. 2016; 21:30348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pebody R, Warburton F, Ellis J, et al. End-of-season influenza vaccine effectiveness in adults and children, United Kingdom, 2016/17. Eurosurveillance 2017; 22:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pebody R, Djennad A, Ellis J, et al. End of season influenza vaccine effectiveness in adults and children in the United Kingdom in 2017/18. Eurosurveillance 2019; 24:23–39. [Google Scholar]
- 65. Pebody RG, Whitaker H, Ellis J, et al. End of season influenza vaccine effectiveness in primary care in adults and children in the United Kingdom in 2018/19. Vaccine 2020; 38:489–97. [DOI] [PubMed] [Google Scholar]
- 66. Pierse N, Kelly H, Thompson MG, et al. ; SHIVERS investigation team . Influenza vaccine effectiveness for hospital and community patients using control groups with and without non-influenza respiratory viruses detected, Auckland, New Zealand 2014. Vaccine 2016; 34:503–9. [DOI] [PubMed] [Google Scholar]
- 67. Powell L, Begue RE. Influenza Vaccine Effectiveness Among Children for the 2017-2018 Season. J Pediatric Infect Dis Soc 2020; 9:468–73. [DOI] [PubMed] [Google Scholar]
- 68. Redlberger-Fritz M, Kundi M, Popow-Kraupp T. Detailed report on 2014/15 influenza virus characteristics, and estimates on influenza virus vaccine effectiveness from Austria’s Sentinel Physician Surveillance Network. PLoS One 2016; 11:e0149916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Regan AK, Gibbs R, Bloomfield L, Effler PV. Estimating influenza vaccine effectiveness using data routinely available in electronic primary care records. Vaccine 2019; 37:755–62. [DOI] [PubMed] [Google Scholar]
- 70. Regan AK, Fielding JE, Chilver MB, et al. Intraseason decline in influenza vaccine effectiveness during the 2016 Southern hemisphere influenza season: a test-negative design study and phylogenetic assessment. Vaccine 2019; 37:2634–41. [DOI] [PubMed] [Google Scholar]
- 71. Rizzo C, Bella A, Alfonsi V, et al. Influenza vaccine effectiveness in Italy: age, subtype-specific and vaccine type estimates 2014/15 season. Vaccine 2016; 34:3102–8. [DOI] [PubMed] [Google Scholar]
- 72. Rolfes MA, Flannery B, Chung JR, et al. ; US Influenza Vaccine Effectiveness (Flu VE) Network, the Influenza Hospitalization Surveillance Network, and the Assessment Branch, Immunization Services Division, Centers for Disease Control and Prevention . Effects of influenza vaccination in the United States during the 2017-2018 influenza season. Clin Infect Dis 2019; 69:1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Savulescu C, Jiménez-Jorge S, Delgado-Sanz C, et al. ; Spanish Influenza Surveillance System . Higher vaccine effectiveness in seasons with predominant circulation of seasonal influenza A(H1N1) than in A(H3N2) seasons: test-negative case-control studies using surveillance data, Spain, 2003-2011. Vaccine 2014; 32:4404–11. [DOI] [PubMed] [Google Scholar]
- 74. Skowronski DM, Janjua NZ, De Serres G, et al. A sentinel platform to evaluate influenza vaccine effectiveness and new variant circulation, Canada 2010-2011 season. Clin Infect Dis 2012; 55:332–42. [DOI] [PubMed] [Google Scholar]
- 75. Skowronski DM, Janjua NZ, Sabaiduc S, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011-2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126–37. [DOI] [PubMed] [Google Scholar]
- 76. Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 2014; 9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Skowronski DM, Chambers C, Sabaiduc S, et al. Integrated sentinel surveillance linking genetic, antigenic, and epidemiologic monitoring of influenza vaccine-virus relatedness and effectiveness during the 2013-2014 influenza season. J Infect Dis 2015; 212:726–39. [DOI] [PubMed] [Google Scholar]
- 78. Skowronski DM, Chambers C, Sabaiduc S, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014-2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Skowronski DM, Chambers C, Sabaiduc S, et al. Beyond antigenic match: possible agent-host and immuno-epidemiological influences on influenza vaccine effectiveness during the 2015-2016 season in Canada. J Infect Dis 2017; 216:1487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Skowronski DM, Sabaiduc S, Leir S, et al. Paradoxical clade- and age-specific vaccine effectiveness during the 2018/19 influenza A(H3N2) epidemic in Canada: potential imprint-regulated effect of vaccine (I-REV). Eurosurveillance 2019; 24:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stein Y, Mandelboim M, Sefty H, et al. ; Israeli Influenza Surveillance Network (IISN) . Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016-2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66:1383–91. [DOI] [PubMed] [Google Scholar]
- 82. Sullivan SG, Kelly H. Late season interim estimates of influenza vaccine effectiveness reliably predict end of season estimates in Victoria, Australia, 2007 to 2012. Euro Surveill 2013; 18:20605. [DOI] [PubMed] [Google Scholar]
- 83. Sullivan SG, Komadina N, Grant K, et al. Influenza vaccine effectiveness during the 2012 influenza season in Victoria, Australia: influences of waning immunity and vaccine match. J Med Virol 2014; 86:1017–25. [DOI] [PubMed] [Google Scholar]
- 84. Suzuki M, Minh LN, Yoshimine H, et al. Vaccine effectiveness against medically attended laboratory-confirmed influenza in Japan, 2011–2012 season. PLoS One 2014; 9:e88813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Treanor JJ, Talbot HK, Ohmit SE, et al. ; US Flu-VE Network . Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012; 55:951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. van Doorn E, Darvishian M, Dijkstra F, et al. Influenza vaccine effectiveness estimates in the Dutch population from 2003 to 2014: the test-negative design case-control study with different control groups. Vaccine 2017; 35:2831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vilcu AM, Souty C, Enouf V, et al. Estimation of seasonal influenza vaccine effectiveness using data collected in primary care in France: comparison of the test-negative design and the screening method. Clin Microbiol Infect 2018; 24:431.e5–12. [DOI] [PubMed] [Google Scholar]
- 88. Wang Y, Zhang T, Chen L, et al. Seasonal influenza vaccine effectiveness against medically attended influenza illness among children aged 6-59 months, October 2011-September 2012: a matched test-negative case-control study in Suzhou, China. Vaccine 2016; 34:2460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wu S, Pan Y, Zhang X, et al. Influenza vaccine effectiveness in preventing laboratory-confirmed influenza in outpatient settings: a test-negative case-control study in Beijing, China, 2016/17 season. Vaccine 2018; 36:5774–80. [DOI] [PubMed] [Google Scholar]
- 90. Yang P, Thompson MG, Ma C, et al. Influenza vaccine effectiveness against medically-attended influenza illness during the 2012-2013 season in Beijing, China. Vaccine 2014; 32:5285–9. [DOI] [PubMed] [Google Scholar]
- 91. Yaron-Yakoby H, Sefty H, Pando R, et al. Effectiveness of influenza vaccine in preventing medically-attended influenza virus infection in primary care, Israel, influenza seasons 2014/15 and 2015/16. Eurosurveillance 2018; 23:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zimmerman RK, Nowalk MP, Chung J, et al. ; US Flu VE Investigators; U.S. Flu VE Investigators . 2014-2015 Influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis 2016; 63:1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–45. [DOI] [PubMed] [Google Scholar]
- 94. Mannino S, Villa M, Apolone G, et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in Northern Italy. Am J Epidemiol 2012; 176:527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Skowronski DM, Chambers C, De Serres G, et al. Serial vaccination and the antigenic distance hypothesis: effects on influenza vaccine effectiveness during A(H3N2) epidemics in Canada, 2010–2011 to 2014–2015. J Infects Dis 2017; 2015:1059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang A, Stacey HD, Mullarkey CE, Miller MS. Original antigenic sin: how first exposure shapes lifelong anti-influenza virus immune responses. J Immunol 2019; 202:335–40. [DOI] [PubMed] [Google Scholar]
- 97. Kim SS, Flannery B, Foppa IM, et al. Effects of prior season vaccination on current season vaccine effectiveness in the United States Flu Vaccine Effectiveness Network, 2012–2013 through 2017–2018. Clin Infect Dis 2020: ciaa706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Khurana S, Hahn M, Coyle EM, et al. Repeat vaccination reduces antibody affinity maturation across different influenza vaccine platforms in humans. Nat Commun 2019; 10:3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999; 96:14001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bodewes R, Fraaij PL, Kreijtz JH, et al. Annual influenza vaccination affects the development of heterosubtypic immunity. Vaccine 2012; 30:7407–10. [DOI] [PubMed] [Google Scholar]
- 101. Bodewes R, Fraaij PL, Geelhoed-Mieras MM, et al. Annual vaccination against influenza virus hampers development of virus-specific CD8+ T cell immunity in children. J Virol 2011; 85:11995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.