Abstract
Background
Antimicrobial stewardship program (ASP) surveillance at our hospital is supplemented by an internally developed surveillance database. In 2013, the database incorporated a validated, internally developed, prediction rule for patient mortality within 30 days of hospital admission. This study describes the impact of an expanded ASP review in patients at the highest risk for mortality.
Methods
This retrospective, quasi-experimental study analyzed adults who received antimicrobials with the highest mortality risk score. Study periods were defined as 2011–Q3 2013 (historical group) and Q4 2013–2018 (intervention group). Primary and secondary outcomes were assessed for confounders and analyzed using both unadjusted and propensity score weighted analyses. Interrupted time-series analyses also analyzed key outcomes.
Results
A total of 3282 and 5456 patients were included in the historical and intervention groups, respectively. There were significant reductions in median antimicrobial duration (5 vs 4 days; P < .001), antimicrobial days of therapy (8 vs 7; P < .001), antimicrobial cost ($96 vs $85; P = .003), length of stay (LOS) (6 vs 5 days; P < .001), intensive care unit (ICU) LOS (3 vs 2 days; P < .001), total hospital cost ($10 946 vs $9119; P < .001), healthcare facility-onset vancomycin-resistant Enterococcus (HO-VRE) incidence (1.3% vs 0.3%; P ≤ .001), and HO-VRE infections (0.6% vs 0.2%; P = .018) in the intervention cohort.
Conclusions
Reductions in antimicrobial use, hospital and ICU LOS, HO-VRE, HO-VRE infections, and costs were associated with incorporation of a novel mortality prediction rule to guide ASP surveillance and intervention.
Keywords: antimicrobial stewardship, assessment tool, mortality risk score
Incorporation of a mortality prediction rule to guide antimicrobial stewardship surveillance and intervention were associated with reductions in antimicrobial use, hospital and intensive care unit length of stay, antimicrobial and hospital costs., and healthcare facility-onset vancomycin-resistant Enterococcus incidence and infections.
The Centers for Disease Control and Prevention (CDC)’s Core Elements of Hospital antimicrobial stewardship programs (ASPs) provides best-practice guidance for optimal program implementation and provides a blueprint for practices; however, individual program structure and daily responsibilities and workload distribution vary by institution [1]. As healthcare facilities across the country continue to expand the scope of their ASPs, operational models that maximize pharmacy department and ASP resources toward goal-directed, patient-centered outcomes are increasingly necessary, especially in the community hospital setting where resources may be more limited.
The use of risk stratification tools to prioritize service delivery have been suggested as a strategy to improve efficient delivery of clinical services [2, 3]. Such examples have been developed and reported to prioritize pharmacy services. These tools utilize both drug-related (eg, high-risk medication, drugs requiring monitoring, polypharmacy) and patient-related risk factors (eg, age, renal impairment or hepatic impairment, comorbidity) with goals of identifying patients at the highest risk for adverse drug events and medication errors [2]. Tools that identify and stratify patients for ASP review include proprietary scoring and clinical decision support (CDS) tools within electronic medical records and those developed to identify patients at risk of developing Clostridioides difficile infection (CDI) [4–6]. These tools have been reported as beneficial in identifying at-risk patients and improving measures such as reducing antibiotic consumption and adherence to guidelines; however, further information on the impact of these tools on patient outcomes is needed [2, 6].
We describe the systematic implementation and impact on patient outcomes of an internally developed, validated, prediction rule for patient mortality within 30 days of hospital admission into a proprietary, web-based, patient identification tool to facilitate expanded ASP surveillance and intervention [7].
METHODS
Development and Integration
An ASP was established in 2009 at our 548-bed community teaching hospital [8]. As our program continued to evolve, we began to explore novel ways in which ASPs can improve the overall care of patients while improving and enhancing more traditional ASP surveillance efforts. Antimicrobial stewardship program surveillance and intervention at our hospital is supplemented by a proprietary, web-based, patient identification tool, referred to internally as the Quality Portal. Historically, daily antimicrobial surveillance by dedicated ASP personnel was limited to a select list of restricted antimicrobials identified by the Quality Portal (Appendix Table 1). Antimicrobial stewardship program personnel reviewed prescribing, assessed for compliance with institutional criteria, and provided feedback to prescribers, if necessary, through prospective audit and feedback. Additional antimicrobial review by clinical pharmacy staff occurred in 2 ways: (1) restricted antimicrobials (eg, vancomycin, fluoroquinolones) were identified and monitored daily for compliance with institutional criteria facilitated by commercially available pharmacy surveillance software (Sentri7 [Pharmacy OneSource; Bellevue, WA] [2011–2017] and Medmined [CareFusion Corporation; San Diego, CA] [2017–2018]); and (2) pharmacists monitored unrestricted antimicrobials on patient care services during participation in interdisciplinary rounds. Review of unrestricted antimicrobials through interdisciplinary rounds occurred Monday–Friday; however, coverage may have varied based on staffing levels and coverage assignments. For example, dedicated intensive care unit (ICU) pharmacists participated in daily (Monday–Friday) interdisciplinary rounds, whereas variable participation may have occurred on medicine services. This practice model left gaps in dedicated ASP personnel oversight when an antimicrobial was not identified in the Quality Portal.
A previously published, prediction rule for mortality within 30 days of hospital admission was developed at our facility [7]. The mortality prediction rule analyzes 24 validated patient risk factors to assign a risk-stratified score between 1 and 5 (Appendix Table 2) [7, 9]. Patients with the highest risk for mortality are assigned a score of 1 indicating an approximate 30% risk for 30-day mortality [9]. Risk-stratified 30-day mortality risk ranges from 13% to 0.3% among those with scores of 2 and 5, respectively. Researchers determined that incorporation of coordinated care processes proportional to the level of predicted risk could be considered for care planning allowing for closer monitoring in patients at highest risk of mortality [9]. Given the continued need to optimally use existing ASP resources for the most benefit, this mortality prediction rule was integrated into the Quality Portal in October 2013 to identify antimicrobials prescribed in patients with scoring indicating the highest risk for mortality. Antimicrobial stewardship program oversight subsequently expanded to include Monday–Friday review of all antimicrobials prescribed in these patients.
Patient Consent Statement
The design of the work and waiver of patient consent was approved by our Institutional Review Board.
Study Design
A single-center, retrospective, quasi-experimental observational study analyzed adult patients with the highest mortality risk score (1) who received antimicrobials not assessed through our historical ASP processes. Patients were identified through health-system administrative and AST databases if they received at least 1 antimicrobial identified upon query during their inpatient stay (Appendix Table 1). Patients were excluded if they received antimicrobials historically reviewed by our ASP and no other antimicrobial prompting ASP surveillance based on mortality risk score. Study periods were defined as patients admitted and discharged between Q1 2011 and Q3 2013 (historical group) and those admitted and discharged between Q4 2013 and 2018 (intervention group) after incorporation of the mortality risk score into the Quality Portal and subsequent expanded ASP oversight. The overall goal of the study was to determine the impact of expanded ASP review on patients with the highest risk for mortality. In addition to the use of the mortality risk score that incorporates multiple patient risk factors, outcomes were assessed adjusting for confounders including demographic data, ICU stay during admission, acute and unspecified renal failure, hemodialysis, chronic kidney disease, and infectious-related diagnoses identified by Agency for Healthcare Research and Quality Clinical Classifications Software (CCS) scoring [10].
Prospective mortality risk scores were implemented for use within the institution in August 2012. A comparable, but retrospectively generated, validated, mortality risk score was used to populate a portion of the historical group when prospective scores were unavailable [9]. Study outcomes included the following: 30-day mortality and readmission; inpatient hospital and antimicrobial costs; hospital and ICU length of stay (LOS); the incidence of infectious diseases (ID) consultation and ASP interventions; incidence of healthcare facility-onset CDI (HO-CDI); incidence and infection rates for healthcare facility-onset vancomycin-resistant Enterococcus spp (HO-VRE), healthcare facility-onset extended-spectrum β-lactamase (HO-ESBL)-producing Enterobacteriaceae, and healthcare facility-onset carbapenem-resistant Enterobacteriaceae (HO-CRE); antimicrobial days of therapy (DOTs) per 1000 patient days; and antimicrobial duration of therapy per patient [11]. Thirty-day mortality and 30-day readmission were identified through an institutional database that analyzes national and statewide death indexes, along with mortality and readmission information from our statewide health-system database. Inpatient hospital cost was unavailable for patients beginning in June 2018 due to changes in hospital reporting. Patients with unavailable hospital cost information were excluded from analyses.
Antimicrobial use was further differentiated by antimicrobial class and spectrum of activity. Antipseudomonal agents and narrow-spectrum β-lactams were defined as agents with and without activity against Pseudomonas aeruginosa, respectively. Restricted antimicrobials were defined as antimicrobials included on the institution’s list of restricted antimicrobials (Appendix Table 1). Inpatient antimicrobial cost was determined by multiplying antimicrobial DOTs for each patient’s respective antimicrobial by the daily wholesale acquisition cost of the agent adjusted to 2019 dollars [12]. Antimicrobial DOTs, duration, and cost were analyzed per patient for their entire hospital stay. Antimicrobial duration per patient was defined as the sum of unique calendar days in which the patient received at least 1 antimicrobial. Antimicrobial DOTs per 1000 patient days and cost per patient day were also analyzed between cohorts in the aggregate by calendar quarter. Patient days were limited to the study cohort population and determined by utilizing patient-specific LOS from admission dates attributed to the admit quarter.
Secondary analyses reviewed outcomes in cohorts of patients with 3 common infectious diagnoses: septicemia (except in labor), pneumonia (except that caused by tuberculosis or a sexually transmitted disease), and urinary tract infections (UTIs) [10]. The incidence of HO-CDI was determined from institutional reports by trained infection preventionists to the CDC’s National Healthcare Safety Network (NHSN) beginning in 2013 [11]. Institutional diagnostic testing changed in November 2012 from glutamine dehydrogenase plus enzyme immunoassay (EIA) with cytotoxicity assay (CTA) for discordant results to the use of polymerase chain reaction confirmation for discordant results. There were no other changes to HO-CDI diagnostic testing during the study time period. A query of the electronic medical record for any positive EIA or CTA results were used to identify patients before NHSN reporting. In addition, retrospective chart review was used to identify positive CDI testing in patients in which queries of EIA or CTA testing results were unavailable. Identification of CDI diagnostic testing was unavailable before 2012; therefore, 2011 was excluded from HO-CDI analyses. The incidence of HO-VRE, HO-ESBL, and HO-CRE was also obtained using NHSN reporting definitions and further classified through retrospective chart review as infection or colonization. Infection was defined as treatment deemed necessary by the primary medical team and/or ID consultation. Identification of HO-VRE and HO-ESBL was unavailable for patients in 2011. These patients were excluded from analyses. Healthcare facility-onset vancomycin-resistant Enterococcus was not included in the cohort analysis of patients with pneumonia.
Antimicrobial interventions and ID consultation were obtained from Quality Portal documentation by ASP team members. Interventions were tracked during daily surveillance throughout the course of the intervention time period. A pharmacy or ASP intervention was defined as any documented change in antimicrobial therapy in patients without ID consultation. Interventions and ID consultation were reported as a percentage of the total cohort population.
Statistical Analyses
Continuous outcomes were evaluated using a t test or Wilcoxon rank-sum test to establish the effect of the expanded ASP review. The χ 2 test or Fisher’s Exact test was used to evaluate categorical outcomes. Statistical analysis was performed using R version 3.6.0 and Microsoft Excel 2016 (Redmond, WA). All P ≤ .05 were considered statistically significant.
Potential confounding variables listed in Table 1 were adjusted for using propensity score methods. Propensity scores were calculated from a Gradient Boosting Machine regression model with the intervention cohort as the outcome and baseline demographic and clinical variables of each participant as predictor variables. Propensity scores were converted to inverse probability of treatment weights, and outcomes were then compared using weighted versions of the χ 2 test or Fisher’s exact test for categorical outcomes or a weighted version of the t test or Wilcoxon rank-sum test for continuous outcomes. Separate propensity scores were calculated for secondary analyses involving disease state cohorts.
Table 1.
Demographics and Clinical Characteristics by Cohort
| Variablea | Label | Historical (N = 3282) | Intervention (N = 5456) | Unadjusted P Value | PS Weighted P Value |
|---|---|---|---|---|---|
| Categorical Variables | |||||
| Gender | .015 | .599 | |||
| Male | 1801 (54.9) | 2846 (52.2) | |||
| Female | 1481 (45.1) | 2610 (47.8) | |||
| Age, yearsb | 76.4 (12.8) | 77.7 (12.7) | <.001 | .887 | |
| Race | .492 | .661 | |||
| White | 2870 (87.5) | 4742 (86.9) | |||
| Other | 412 (12.6) | 714 (13.1) | |||
| ICU stay | 1302 (39.7) | 1908 (35) | <.001 | .639 | |
| Acute and unspecified renal failure | 1429 (43.5) | 2550 (46.7) | .004 | .449 | |
| Chronic kidney disease | 1385 (42.2) | 2594 (47.5) | <.001 | .411 | |
| Hemodialysis | 796 (24.3) | 1700 (31.2) | <.001 | .399 | |
| Infections | |||||
| Septicemia | 1355 (41.3) | 2146 (39.3) | .075 | .96 | |
| Pneumonia | 1130 (34.4) | 1736 (31.8) | .013 | .478 | |
| Urinary tract infection | 792 (24.1) | 1136 (20.8) | <.001 | .588 | |
| Intestinal infection | 296 (9) | 342 (6.3) | <.001 | .469 | |
| Bacterial infection (unspecified site) | 281 (8.6) | 622 (11.4) | <.001 | .253 | |
| Skin and subcutaneous tissue infections | 173 (5.3) | 296 (5.4) | .795 | .579 | |
| Peritonitis and intestinal abscess | 62 (1.9) | 84 (1.5) | .251 | .832 | |
| Other Infections (including parasitic) | 45 (1.4) | 83 (1.5) | .636 | .576 | |
| Other upper respiratory infections | 24 (0.7) | 78 (1.4) | .005 | .311 |
Abbreviations: ICU, intensive care unit; PS, propensity score.
aData are no. (%) unless otherwise specified.
bData are summarized with mean (standard deviation).
Interrupted time series (ITS) analyses were performed on infection and antimicrobial utilization variables. Plots of the quarterly rate of each outcome were produced to visualize the trends before and after the expanded ASP review was implemented. Segmented least squares regression models were fit to the quarterly series for each outcome. The models assumed linearity of the trend lines within each intervention period and included parameters for an intercept, the preintervention trend, and the change in both level and trend after the intervention. If there was evidence of autocorrelation within the series from a Durbin Watson test, an autoregressive correlation structure was used when fitting the model, otherwise an unstructured correlation matrix was specified.
RESULTS
A total of 3282 and 5456 patients were included in the historical and intervention groups, respectively (Table 1; Appendix Figure 1). The groups were adequately balanced on demographic and clinical confounding variables after propensity score weighting. The LOS (6 vs 5 days; P < .001), ICU LOS (3 vs 2 days; P < .001), and total hospital cost ($10 946 vs $9119; P < .001) were significantly reduced in the intervention cohort (Table 2). There were significant reductions in median antimicrobial duration per patient (5 vs 4 days; P < .001), antimicrobial DOTs per patient (8 vs 7; P < .001), and antimicrobial cost per patient ($96 vs $85; P = .01). There were no significant differences observed in 30-day mortality or 30-day readmissions.
Table 2.
Outcomes by Cohort (Unadjusted and Propensity Score-Weighted)
| Variablea,b | Historical (N = 3282) | Intervention (N = 5456) | Unadjusted P Value | PS Weighted P Value |
|---|---|---|---|---|
| Categorical Outcomes | ||||
| 30-day mortality | 1191 (36.3) | 1919 (35.2) | .302 | .812 |
| 30-day readmission | 676 (20.6) | 1098 (20.1) | .614 | .684 |
| HO-CDIc | 52 (2.6) | 121 (2.2) | .357 | .43 |
| HO-VREc | 26 (1.3) | 16 (0.3) | <.001 | <.001 |
| HO-VRE infectionsc | 12 (0.6) | 8 (0.2) | .002 | .018 |
| HO-CRE | 6 (0.2) | 3 (0.1) | .089 | .18 |
| HO-CRE infections | 5 (0.2) | 1 (<0.1) | .031 | .075 |
| HO-ESBLc | 9 (0.5) | 13 (0.2) | .205 | .217 |
| HO-ESBL infectionsc | 7 (0.4) | 8 (0.2) | .138 | .146 |
| Continuous Outcomesd | ||||
| Hospital LOS, days | 6 (3–9) | 5 (3–8) | <.001 | <.001 |
| ICU LOS, days | 3 (2–6) | 2 (1–4) | <.001 | <.001 |
| Inpatient hospital cost, $ | 10 946 (6329–19 750) | 9119 (5603–15 371) | <.001 | <.001 |
| Antimicrobial cost, $ | 96 (28–237) | 85 (28–189) | <.001 | .01 |
| Antimicrobial duration, days | 5 (3–8) | 4 (2–7) | <.001 | <.001 |
| Antimicrobial days of therapy | 8 (4–14) | 7 (3–12) | <.001 | <.001 |
Abbreviations: CRE, healthcare facility-onset carbapenem-resistant Enterobacteriaceae; HO-CDI, healthcare facility-onset Clostridioides difficile infection; HO-HO-ESBL, healthcare facility-onset extended-spectrum beta-lactamase-producing Enterobacteriaceae; HO-VRE, healthcare facility-onset vancomycin-resistant Enterococci spp; ICU, intensive care unit; LOS, length of stay; PS, propensity score.
aData are no. (%) unless otherwise specified.
b P values shown for the continuous outcomes are from the Wilcoxon rank-sum test.
cAnalysis conducted 2012–2018.
dData are presented as median (interquartile ratio).
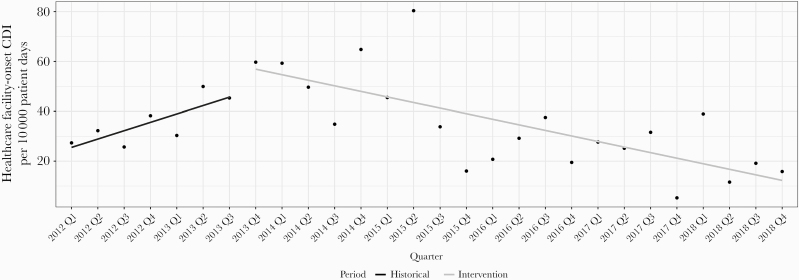
The incidence of HO-VRE (1.4% vs 0.3%; P ≤ .001) and HO-VRE infections (0.6% vs 0.2%; P = .018) were significantly decreased in comparisons between cohorts; however, when considering the quarterly trends within each cohort in the ITS analyses, there was not a significant difference in the rate of change of these outcomes (P = .531; P = .825) (Appendix Figure 2). There was no difference in overall HO-CDI (2.6% vs 2.2%; P = .43) between cohorts; however, the ITS analysis showed a significant change in the slope of HO-CDI per 10 000 patient days (P = .032), because the preintervention cohort showed an increasing trend in HO-CDI, whereas the postintervention cohort showed a decreasing trend (Figure 1). The HO-CRE, HO-CRE infections, HO-ESBL, and HO-ESBL infections were unchanged.
Figure 1.
Interrupted time-series analysis on healthcare facility-onset Clostridioides difficile infection per 10 000 patient days by calendar year quarter.
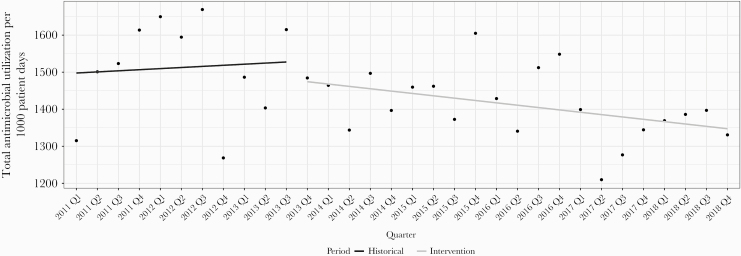
Unadjusted antimicrobial utilization between cohorts and ITS analyses between time periods are presented in Table 3. The overall average use of antimicrobials decreased 7% between time periods (1513 vs 1411 DOTs per 1000 patient days, P = .041) leading to 3648 DOTs avoided. The use of institutionally restricted antimicrobials decreased 11% (937 vs 831 DOTs per 1000 patient days, P = .009), and antipseudomonal agent utilization decreased 8% (478 vs 442 DOTs per 1000 patient days, P = .05). The prescribing of fluoroquinolones and intravenous vancomycin decreased 35% (84 vs 55 DOTs per 1000 patient days, P ≤ .001) and 23% (253 vs 194 DOTs per 1000 patient days, P < .001), respectively. The ITS analyses confirmed a significant trend in the decrease of intravenous (IV) vancomycin utilization (P = .012) (Appendix Figure 3); however, despite overall reductions, there were no significant decreases in slopes of any additional antimicrobial category, including total antimicrobial (Figure 2) and fluoroquinolone utilization (P = .709) (Appendix Figure 4). Clindamycin utilization decreased 44% (34 vs 19 DOTs per 1000 patient days, P = .008); however, the ITS slope indicated a leveling of decreases after the intervention (P = .017).
Table 3.
Antimicrobial Utilization by Cohort (Unadjusted and Interrupted Time Series Analyses)
| Variablea | Historical (N = 3282) | Intervention (N = 5456) | Unadjusted P Value | ITS P Value |
|---|---|---|---|---|
| Antimicrobial Utilizationb | ||||
| Total antimicrobial utilization | 1513 (129) | 1411 (90) | .041 | .4 |
| Restricted antimicrobials | 937 (99) | 831 (81) | .009 | .099 |
| Unrestricted antimicrobials | 575 (56) | 580 (43) | .819 | .32 |
| β-lactam antibiotics | 646 (49) | 657 (41) | .547 | .169 |
| Antipseudomonal antibiotics | 478 (48) | 442 (35) | .05 | .574 |
| Narrow-spectrum β-lactams | 272 (31) | 276 (23) | .701 | .119 |
| Intravenous vancomycin | 253 (21) | 194 (46) | <.001 | .012 |
| Fluoroquinolones | 84 (18) | 55 (18) | <.001 | .709 |
| Clindamycin | 34 (14) | 19 (8) | .008 | .017 |
| Cost per patient day, $ | 27 (4) | 23 (2) | .015 | .127 |
Abbreviations: IQR, interquartile range; ITS, interrupted time-series analysis.
aData are presented as mean (standard deviation).
bData are presented as days of therapy per 1000 patient days unless noted.
Figure 2.
Interrupted time-series analysis on total antimicrobial utilization defined as days of therapy per 1000 patient days by calendar year quarter.
Interventions were documented on 13% of patients and 18% had documentation indicating ID consultation. Common interventions included discontinuing antimicrobials (7%) and changing antimicrobial selection (5%).
Analyses of cohorts with septicemia, pneumonia, and UTIs revealed similar results (Appendix Tables 3–5). After adjusting for demographic and clinical characteristics, hospital cost, LOS, ICU LOS, and antimicrobial DOTs per 1000 patient days were significantly improved in each disease-specific cohort. Antimicrobial duration and cost were improved in both the septicemia and pneumonia cohorts. The HO-VRE and HO-VRE infections were decreased in the septicemia cohort.
DISCUSSION
We present in this report results of an ASP’s incorporation of a mortality prediction rule into daily antimicrobial surveillance. Use facilitated targeted delivery of care for personnel with specialized expertise in ID and antimicrobial stewardship to a population at high risk for mortality. Results demonstrated improvements in several patient outcomes and costs including LOS, ICU LOS, hospital costs, antimicrobial cost, duration, and DOTs, with additional improvements in common disease states in hospitalized patients in the United States [13].
To our knowledge, this is the first report of the use of a mortality prediction rule to drive ASP surveillance and intervention; however, the concept of utilizing risk-adjusted tools to guide ASP efforts has been reported in various forms [4–6]. For example, Nault et al [14] described their experience with use of an ASP-developed CDS system that accounted for patient clinical information to guide pharmacist-led prospective audit and feedback. They showed reductions in antimicrobial use and spending, inappropriate prescriptions, and a sustained reduction in LOS. More recently, Ridgway et al [15] reported mixed results with utilization of a CDS tool incorporating patient characteristics to guide ASP recommendations for empiric prescribing. Guidelines-based interventions were associated with decreased LOS for cellulitis and decreased mortality for community-acquired pneumonia, but not with improved outcomes for UTI and abdominal-biliary infections. Results of our analysis and others suggest ASPs may benefit from incorporation of risk-adjustment tools into their daily ASP surveillance tailored to individual program and institution goals.
There are a few limitations inherent to this analysis including the single-center, retrospective nature of the analysis. Retrospective mortality scoring was used to identify a subset of patients in our historical group. Internal analyses of the prediction rule indicate a high degree of correlation between retrospective and prospective scoring; however, the potential for variability among groups is an acknowledged limitation [9]. It is likely that many improvements are multifactorial and not solely due to improved antimicrobial use. Likewise, this study was not designed to find a causal relationship between targeted monitoring and infection rates. Although we were able to attribute DOTs by calendar quarter, our use of patient LOS from quarterly admit date to define cohort-specific quarterly patient days limited accuracy in patients’ whose stay crossed quarterly lines. This was likely mitigated by similar numbers of patients crossing quarterly thresholds; however, this is an acknowledged limitation of the ITS analyses.
The impact of ASP initiatives on reductions in incidence of resistant infections is limited. Baur et al [16] performed a systemic review and meta-analysis investigating the effect of antimicrobial stewardship on the incidence of infection and colonization with antibiotic-resistant bacteria. They found that implementation of ASPs was associated with a 48% reduction in the incidence of ESBL-producing Gram-negative bacteria [16]. Citing 3 studies reporting results, they found that VRE was not significantly changed after implementation of ASPs. De Angelis et al [17] in their meta-analysis found that hygiene measures were associated with a 47% decrease in VRE acquisition rates. Reductions in HO-VRE incidence and infections between cohorts were not confirmed via ITS analyses leaving us unable to attribute whether results were due to our intervention or simply from secular trends in changing resistance patterns and infection prevention practices. Variation in infection rates are impacted by a host of ancillary factors including improved infection prevention practices. Some of these factors changed during the study period. For instance, environmental cleaning practices and products were altered, isolation orders became automated system orders, CDI diagnostic stewardship practices changed, and large-scale educational provider campaigns for hand washing and donning and doffing personal protective equipment were implemented.
Our ASP also had several initiatives running concurrently that likely impacted results. Initiatives impacting all patients regardless of mortality scoring included extensive facility-specific guideline development, promotion of β-lactam prescribing utilizing allergy cross-reactivity guidelines, and targeted syndrome-specific interventions including diagnostic stewardship with focus on common infectious diseases such as pneumonias and UTIs [18–22].
The variation in results between propensity score weighted analyses and ITS analyses indicates that, although there was a difference between cohorts, outside of IV vancomycin and HO-CDI, there was no difference in trends between time periods. Knowing this, it is difficult to attribute results solely to enhanced surveillance and intervention facilitated by the mortality prediction rule. However, the use of a mortality prediction rule, in combination with a suite of infection prevention and additional ASP initiatives (described previously), contributed to improved outcomes over a multiyear timeframe. It should be noted, however, that many surveillance targets and expectations for pharmacist monitoring remained unchanged before and after implementation of the mortality prediction score to guide ASP review. Utilization of restricted antimicrobials with expectations for daily surveillance and intervention by pharmacy personnel were significantly improved, whereas unrestricted antimicrobials with less consistent monitoring expectations were unchanged. This may speak to the benefit of targeted expertise and enhanced surveillance on areas and antimicrobials that ASPs deem high priority; however, this does come at a cost. Increased resource use (ie, ASP personnel time) should be factored in because ASPs are considering where to best devote efforts to achieve program goals. The time-trade-off calculation for ASPs is often institution-specific and multifactorial. One area of future consideration is whether a combination of incorporation of mortality scoring with more sophisticated CDS or risk-stratification tools further streamlines surveillance while retaining optimal benefits. Defining optimal populations most likely to benefit from enhanced ASP surveillance and ongoing evaluations to achieve maximal return on time investment is warranted, particularly in resource-limited settings.
We believe results demonstrate a novel way in which ASPs may target expertise to improve medication use and optimize patient management. The combination of our mortality scoring index into a web-based, patient identification tool may not be generalizable to all institutions; however, the concept of focusing expertise on the oversight of patients with increased mortality-risk is generalizable to ASPs in all practice settings. The ASPs that seek to focus resources and prioritize target populations may benefit from this approach.
CONCLUSIONS
In summary, significant reductions in antimicrobial use, HO-VRE incidence and infections, inpatient and ICU LOS, and hospital and antimicrobial costs were associated with incorporation of a novel mortality prediction rule to guide and expand ASP surveillance and intervention.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Dr. Mark Cowen and Kishore Anam for assistance with this project at various points throughout the development, implementation, and review.
Financial support. This work was funded by an institutional research grant from the St. Joseph Mercy Health System Research Committee (R-16-1723).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Centers for Disease Control and Prevention. Core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/core-elements/index.html. Accessed 6 July 2020. [DOI] [PMC free article] [PubMed]
- 2. Alshakrah MA, Steinke DT, Lewis PJ. Patient prioritization for pharmaceutical care in hospital: a systematic review of assessment tools. Res Social Adm Pharm 2019; 15:767–79. [DOI] [PubMed] [Google Scholar]
- 3. Lewis P. Right patient, right time, right pharmacist: the time for clinical prioritisation tools? Eur J Hosp Pharm 2017; 24:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuntz JL, Smith DH, Petrik AF, et al. Predicting the risk of Clostridium difficile infection upon admission: a score to identify patients for antimicrobial stewardship efforts. Perm J 2016; 20:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kullar R, Goff DA, Schulz LT, et al. The “epic” challenge of optimizing antimicrobial stewardship: the role of electronic medical records and technology. Clin Infect Dis 2013; 57:1005–13. [DOI] [PubMed] [Google Scholar]
- 6. Rittmann B, Stevens MP. Clinical decision support systems and their role in antibiotic stewardship: a systematic review. Curr Infect Dis Rep 2019; 21:29. [DOI] [PubMed] [Google Scholar]
- 7. Cowen ME, Czerwinski JL, Posa PJ, et al. Implementation of a mortality prediction rule for real-time decision making: feasibility and validity. J Hosp Med 2014; 9:720–6. [DOI] [PubMed] [Google Scholar]
- 8. Malani AN, Richards PG, Kapila S, et al. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control 2013; 41:145–8. [DOI] [PubMed] [Google Scholar]
- 9. Cowen ME, Strawderman RL, Czerwinski JL, et al. Mortality predictions on admission as a context for organizing care activities. J Hosp Med 2013; 8:229–35. [DOI] [PubMed] [Google Scholar]
- 10. Healthcare Cost and Utilization Project. Tools and Software. Available at: https://www.hcup-us.ahrq.gov/. Accessed 6 July 2020. [Google Scholar]
- 11. Centers for Disease Control and Prevention. Multidrug-resistant organism & Clostridioides difficile infection (MDRO/CDI) module. Available at: https://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf. Accessed 6 July 2020.
- 12. Red Book Online. Greenwood Village, CO: Truven Health Analytics. Available at: https://www.micromedexsolutions.com/home/dispatch. Accessed 31 July 2019. [Google Scholar]
- 13. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nault V, Pepin J, Beaudoin M, et al. Sustained impact of a computer-assisted antimicrobial stewardship intervention on antimicrobial use and length of stay. J Antimicrob Chemother 2017; 72:933–40. [DOI] [PubMed] [Google Scholar]
- 15. Ridgway JP, Robicsek A, Shah N, et al. A randomized controlled trial of an electronic clinical decision support tool for inpatient antimicrobial stewardship. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1048. [DOI] [PubMed] [Google Scholar]
- 16. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17:990–1001. [DOI] [PubMed] [Google Scholar]
- 17. De Angelis G, Cataldo MA, De Waure C, et al. Infection control and prevention measures to reduce the spread of vancomycin-resistant enterococci in hospitalized patients: a systematic review and meta-analysis. J Antimicrob Chemother 2014; 69:1185–92. [DOI] [PubMed] [Google Scholar]
- 18. Collins CD, Scheidel C, Anam K, et al. Impact of an antibiotic side chain-based cross-reactivity chart combined with enhanced allergy assessment processes for surgical prophylaxis antimicrobials in patients with beta-lactam allergies. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa232. [DOI] [PubMed] [Google Scholar]
- 19. Vaughn VM, Flanders SA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: a multihospital cohort study. Ann Intern Med 2019; 171:153–63. [DOI] [PubMed] [Google Scholar]
- 20. Petty LA, Vaughn VM, Flanders SA, et al. Risk factors and outcomes associated with treatment of asymptomatic bacteriuria in hospitalized patients. JAMA Intern Med 2019; 179:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collins CD, Kabara JJ, Michienzi SM, Malani AN. Impact of an antimicrobial stewardship care bundle to improve the management of patients with suspected or confirmed urinary tract infection. Infect Control Hosp Epidemiol 2016; 37:1499–501. [DOI] [PubMed] [Google Scholar]
- 22. Brumley PE, Malani AN, Kabara, et al. Effect of an antimicrobial stewardship bundle for patients with Clostridium difficile infection. J Antimicrob Chemother 2016; 71:936–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.