Abstract
Background
Residual renal function is closely linked to quality of life, morbidity and mortality in dialysis patients. Beta-trace protein (BTP), a low molecular weight protein, has been suggested as marker of residual renal function, in particular in patients on hemodialysis. We hypothesized that BTP also serves as a marker of residual renal function in pertioneal dialysis patients.
Methods
In this study 34 adult patients on peritoneal dialysis were included. BTP, creatinine, cystatin C and urea concentrations were analyzed simultaneously in serum and dialysate to calculate renal and peritoneal removal of the analytes.
Results
In peritoneal dialysis patients with residual diuresis, mean serum BTP was 8.16 mg/l (SD ± 4.75 mg/l). BTP correlated inversely with residual diuresis (rs = − 0.58, p < 0.001), residual creatinine clearance (ClCr) (rs = − 0.69, p < 0.001) and total urea clearance (Clurea) (rs = − 0.56, p < 0.001). Mean peritoneal removal of BTP was 3.36 L/week/1.73m2 (SD ± 1.38) and mean renal removal 15.14 L/week/1.73m2 (SD ± 12.65) demonstrating a significant renal contribution to the total removal. Finally, serum BTP inversely correlated with alterations in residual diuresis (r = − 0.41, p = 0.035) and renal creatinine clearance over time (r = − 0.79, p = p < 0.001).
Conclusion
BTP measurement in the serum may be a simple tool to assess residual renal function in peritoneal dialysis patients.
Keywords: Beta-trace protein, Low-molecular weight proteins, Peritoneal dialysis, Residual diuresis
Introduction
In patients with chronic kidney failure requiring dialysis, quality of life and survival are linked to residual renal function (RRF) [1–5]. To assess RRF, timed urea clearances or urea/creatinine clearances are considered the gold standard, but they rely on sampling both urine and serum. Commonly used serum markers, such as creatinine, cystatin C and urea do not reflect RRF accurately, because they are subject to clearance by the dialysis procedure. Moreover, all these low weight molecular proteins (LWMP) may be influenced by external factors. For instance, creatinine production depends on individual muscle mass, and is related to race, sex and age [6], while cystatin C concentration may be altered by smoking [7], obesity [8], diabetes [9], certain types of cancer [6], inflammation [10] or treatment with prednisone [11]. In addition, there is substantial evidence for a non-renal clearance of cystatin C [12].
Despite significant efforts, no reliable serum biomarker mirroring renal function has been identified yet. However Beta-trace protein (BTP) may be a promising candidate. BTP, with a molecular weight of 23- to 29-kDa, is a small molecule expressed in nearly all tissues involved in prostaglandin metabolism, such as the brain, retina, melanocytes, male reproductive organs, heart and kidney [13–16]. BTP is filtered freely in the kidneys with little if any tubular reabsorption and excreted in the urine. BTP serum levels correlate with GFR [17–19] and with residual kidney function (RRF) in end-stage renal disease (ESRD) [20, 21]. Due to its molecular weight, BTP is generally not removed by hemodialysis, but its elimination increases considerably in hemodiafiltration. In addition, elimination is largely depending on the applied dialysis membrane [22–25].
Little data is available concerning BTP in patients undergoing peritoneal dialysis (PD) [26, 27]. Recently, it was suggested that BTP, unlike other markers for glomerular filtration, is not cleared peritoneally at all [27].
The study presented here aims to characterize BTP removal in PD patients as well as to describe the relationship between serum BTP concentration, residual diuresis and residual creatinine clearance over time. We propose BTP as a useful tool to assess residual function in patients on maintenance peritoneal dialysis.
Patients and methods
Patients and treatment description
This prospective study included a total of 34 consecutive adult dialysis patients undergoing maintenance peritoneal dialysis. Of those, 29 had residual diuresis > 200 ml/24 h.
Dialysis efficiency was estimated using Kt/V ratio. All maintenance dialysis patients were on regular dialysis treatment. PD patients were on automated PD (APD, n = 2) or continuous PD (CAPD, n = 32). Evaluation of peritoneal membrane characteristics was quarterly performed by using the modified peritoneal equilibrium test (PET). Patients were then categorized into a fast and slow transport type. Patients with a diuresis less than 200 ml were assigned to the anuria group.
Parameters and assays
All patients were routinely subjected to blood sample collections to quantify PD treatment adequacy and to a peritoneal adequacy test.
BTP, creatinine, cystatin C and urea concentrations were analyzed simultaneously in serum, dialysate and urine. Levels of BTP and cystatin C were analyzed by immunonephelometry, levels of urea by photometry, and creatinine concentration by a modification of the Jaffé method. Creatinine Clearance (ClCr) was adjusted to a standard body surface area of 1,73 m2.
Serum creatinine was measured using a modified Jaffé method; urea by a standard clinical analyzer. Serum cystatin C and BTP were analyzed by fully automated, latex-enhanced immunonephelometry (N latex Cystatin C, N latex BTP, respectively, on a Nephelometer II; Siemens, Erlangen, Germany).
Data on the residual diuresis were documented quarterly. Volumes of residual diuresis were determined by means of a 24-h urine collection as routinely performed at home quarterly.
Renal and peritoneal elimination of LWMP
The calculation of residual renal creatinine clearance was performed and normalized to body surface area (BSA) as follows:
In PD patients, weekly renal and peritoneal elimination of low molecular weight proteins were calculated and normalized to BSA as follows:
Weekly peritoneal clearance of LWMP:
Weekly renal clearance:
Total clearance was calculated as the sum of the peritoneal and the renal clearance.
Calculation of correlation between BTP and diuresis over time
To analyze a correlation between loss of diuresis and increase in serum LWMP over time all patients with residual diuresis and a minimum follow-up of 12 months were included.
Differences between the last and the first BTP serum value as well as the last and first diuresis volume were calculated and divided by the amount of follow-up quarters.
In addition, we also investigated likewise the correlation between decline in creatinine clearance and increase in serum BTP over time.
Afterwards, correlation between the values was calculated.
Statistics
Statistical analysis was performed with GraphPad Prism 6 (La Jolla, USA). Results are given as mean with standard deviation (SD) for quantitative variables. T-tests or one-way ANOVA were used for the comparison of quantitative values.
Spearman rank coefficient was used to test the correlation between two variables. By plotting a receiver operating characteristic (ROC) curve, we determined the best cut-off value for a residual renal creatinine clearance of 7 ml / min / 1.73m2. Two-sided p-levels < 0.05 were considered statistically significant.
Ethics approval
The study was approved by the local ethics committee, the ethics committee of the medical faculty of the university of Bonn – number 159/17, and informed consent was obtained from all patients enrolled into the study. The study was performed in accordance with the relevant guidelines and regulations.
Results
General characteristics of patients and treatment modality
In total, 34 patients (15 female and 19 male) were included into the study. Patients clinical and biochemical characteristics are listed in Table 1. Major causes of ESRD were glomerulonephritis, renovascular disease and interstitial nephritis. Of the patients with residual diuresis (n = 29), mean serum BTP was 8.16 mg/l (SD ± 4.75 mg/l). Serum BTP level correlated with serum creatinine (rs = 0.8, p < 0.001), serum cystatin C (rs = 0.79, p < 0.001) and serum urea concentration (rs = 0.12, p = 0.15). There was no significant correlation between serum levels of BTP and sex (r = 0.11; p = 0.6), body mass index (r = − 0.24; p = 0.34) or age (rs = − 0.3, p = 0.17).
Table 1.
General characteristics of patients
| patients in total | 29 |
|---|---|
| sex (male / female) | 18/11 |
| age (years) | 52.7 ± 12.15 |
| body weight (kg) | 81.16 (SD ± 14.96) |
| body mass index | 26.88 (SD ± 4.55) |
| residual diuresis (ml) | 1374 (SD ± 913) |
| PD regime (CAPD) | 29 |
| serum BTP (mg/L) | 8.16 (SD ± 4.75) |
| serum Cystatin C (mg/L) | 5.78 (SD ± 1.6) |
| serum urea (mg/dL) | 123.5 (SD ± 70.3) |
| serum creatinine (mg/dL) | 8.83 (SD ± 3.59) |
| dialysate volume (L) | 8.86 (SD ± 1.9) |
| dialysate BTP (mg/L) | 0.54 (SD ± 0.34) |
| dialysate Cystatin C (mg/L) | 0.64 (SD ± 0.35) |
| dialysate urea (mg/dL) | 107.9 (SD ± 42.01) |
| dialysate creatinine (mg/dL) | 7.03 (SD ± 9.4) |
| urine BTP (mg/L) | 14.7 (SD ± 9.58) |
| urine Cystatin C (mg/L) | 5.44 (SD ± 4.53) |
| urine urea (mg/dL) | 382.89 (SD ± 232.2) |
| urine creatinine (mg/dL) | 51.12 (SD ± 36.81) |
| weekly Kt/V | 2.28 (SD ± 0.76) |
| weekly peritoneal Kt/V | 1.4 (SD ± 0.49) |
| weekly renal Kt/V | 0.86 (SD ± 0.65) |
| total creatinine clearance (L/week/1,73 m2) | 91 (SD ± 51.61) |
| renal creatinine clearance (L/week/1,73 m2) | 54.82 (SD ± 52.87) |
| peritoneal creatinine clearance (L/week/1,73 m2) | 34.29 (SD ± 12.49) |
General characteristics of patients and treatment modality. Data are given as mean and standard deviation
LWMP values in serum and dialysate in patients with and without residual diuresis
Serum BTP concentration was higher in anuric patients (Residual diuresis: mean 8.16 mg/L, SD ± 4.75 mg/L; anuria: mean 12.2 mg/L, SD ± 3.84 mg/L, p < 0.05). Anuric PD patients had a mean dialysate BTP concentration of 0.6 mg/l (SD ± 0.32), which was comparable to PD patients with residual diuresis (mean 0.54 mg/l (SD ± 0.34), but dialysis volume per day was considerably higher in the anuric group (Residual diuresis: mean 8.86 L/day, SD ±1.9 versus anuria: mean 13.97 L/day, SD ± 6.17 L/day). In contrast to creatinine, cystatin C and urea, only serum BTP was significantly higher in anuric patients compared to patients with residual diuresis. Anuric patients were excluded from the following analyses.
Renal and peritoneal clearance of LWMP
Mean peritoneal removal of BTP was 3.36 L/week/1.73m2 (SD ± 1.38) and mean renal removal 15.14 L/week/1.73m2 (SD ± 12.65; p < 0.01) demonstrating a minor peritoneal removal (Fig. 1, Table 2). Renal and peritoneal removal of cystatin C and creatinine showed no significant differences. BTP was the only solute demonstrating a higher renal than peritoneal elimination, which was reflected by a significantly higher ratio of BTP Serum to BTP Dialysate (Fig. 1 a-c).
Fig. 1.
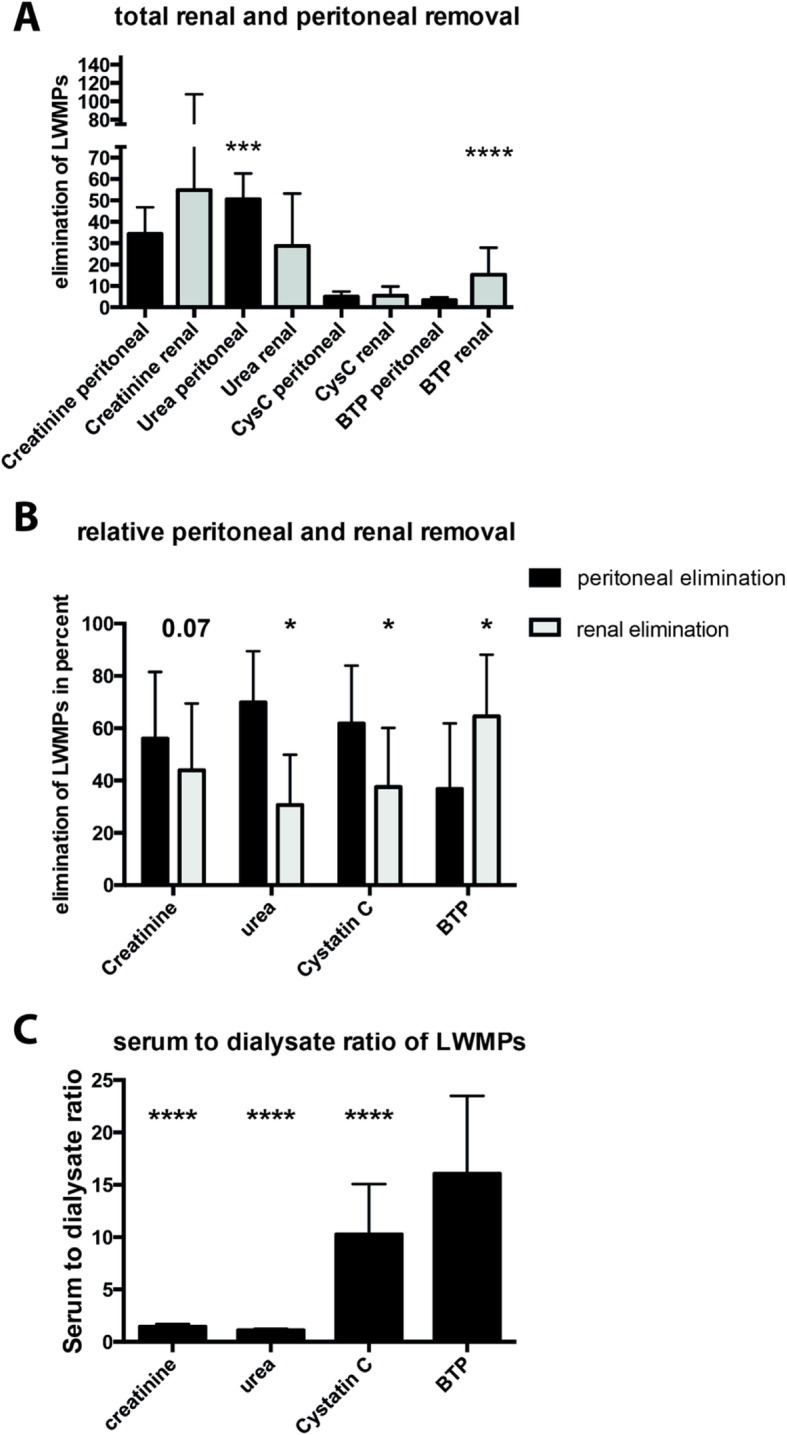
Renal and peritoneal elimination of analytes in patients with residual diuresis. a – total renal and peritoneal removal of analytes [(creatinine and urea in L / week / m2); (cystatin C (CysC) and BTP in L / week / m2)]; b – percentage expression of renal and peritoneal elimination. c – Quotient of serum and dialysate values for respective analyte. Data are given as mean and standard deviation. P-values < 0.05 were considered statistically significant. p < 0.05 = *; p < 0.001 = **; p < 0.0001 = ***; p < 0.00001 = ****
Table 2.
Renal and peritoneal removal of LWMPs. Values are given as mean and standard deviation. Only data of patients with residual diuresis were included
| Creatinine (L/week/1,73m2) | Urea (L/week/1,73m2) | Cystatin C (L/week/1,73m2) | BTP (L/week/1,73m2) | |
|---|---|---|---|---|
| patients with diuresis | ||||
| total clearance / week | 91 (± 51.61) | 76.83 (± 20.85.9) | 10.63 (± 5.68) | 18.35 (± 13.37) |
| renal removal / week | 54.82 (± 52.87) | 28.68 (± 24.51) | 5.43 (± 4.29) | 15.14 (± 12.65) |
| peritoneal removal / week | 34.29 (± 12.49) | 50.53 (± 12.1) | 4.95 (± 2.48) | 3.36 (± 1.38) |
LWMPs and residual renal function
Mean residual creatinine-clearance was 6.43 ml/min/1.73m2 (SD ± 0.41), mean residual urea clearance (KRU) 2.97 ml/min/1.73m2 (SD ± 2.42), mean residual creatinine-urea clearance (KRU/crea) 4.11 ml/min/1.73m2 (SD ± 3.65) and mean BTP clearance 1.89 ml/min/1.73 m2 (SD ± 1.56) in patients with residual diuresis.
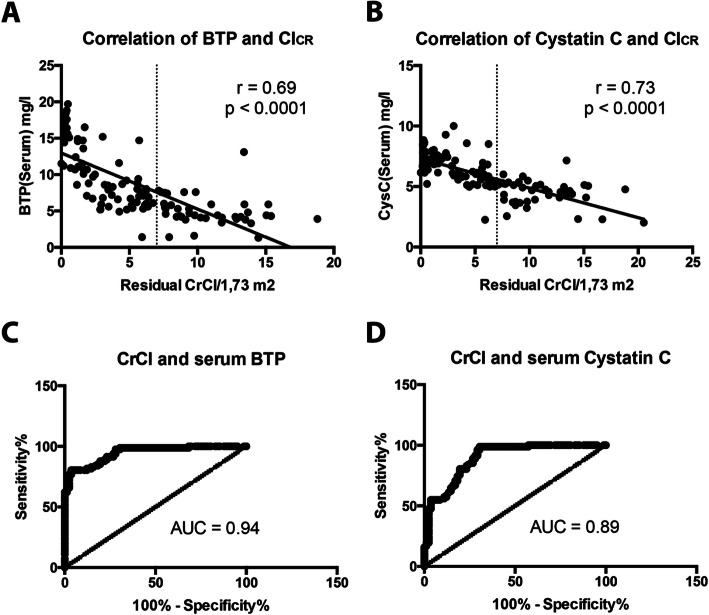
Serum BTP correlated inversely with residual diuresis (rs = − 0.58, p < 0.001), residual creatinine clearance (ClCr) (rs = − 0.69, p < 0.0001) and urea clearance (Clurea) (rs = − 0.56, p < 0.001). The correlation of the other investigated analytes with residual diuresis was lower (Creatinine: r = − 0.5, p < 0.001; Cystatin C: r = − 0.54, p = p < 0.001; Urea: r = − 0.08, p = 0.36). Cystatin C correlated equally well with ClCr in comparison to BTP [rs = − 0.73, p < 0.0001) (Fig. 2)].
Fig. 2.
Correlation of serum analytes and their predictive value. Figure 2: Correlation of serum analytes and residual renal creatinine clearance (ClCR). a – Serum BTP and ClCR; b - Serum Cystatin C and ClCR. Receiver operating characteristic (ROC) curve for prediction of a creatinine clearance < 7 ml / min based on serum level of BTP and Cystatin C. c – ROC analysis for BTP; d – ROC analysis for Cystatin C. AUC (Area under the curve): BTP: 0.94; Cystatin C: 0.89
By applying ROC analysis, a serum BTP concentration of 6.7 mg / l was identified as cut-off value to distinguish between a residual renal creatinine clearance of < 7 ml / min / 1.73 m2 and ≥ 7 ml / min / 1.73 m2 with a sensitivity of 76.8% (95% CI: 66.2–85.4) and a specificity of 97.6% (91.5–99.7)]. The positive and negative predictive value were calculated as 90.2 and 87.9%, respectively.
In comparison, BTP performed better than Cystatin C with an AUROC of 0.94 compared to 0.89. A serum cystatin C concentration of 5 mg / l was detected to differentiate between the clearance treshold of 7 ml / min / 1.73m2 with a sensitivity of 96.3% (95% CI: 89.7–99.2), a specificity of 70.3% (95% CI: 58.4–79.2), a positive predictive value of 81.4% and a negative predictive value of 46.2% (Fig. 2).
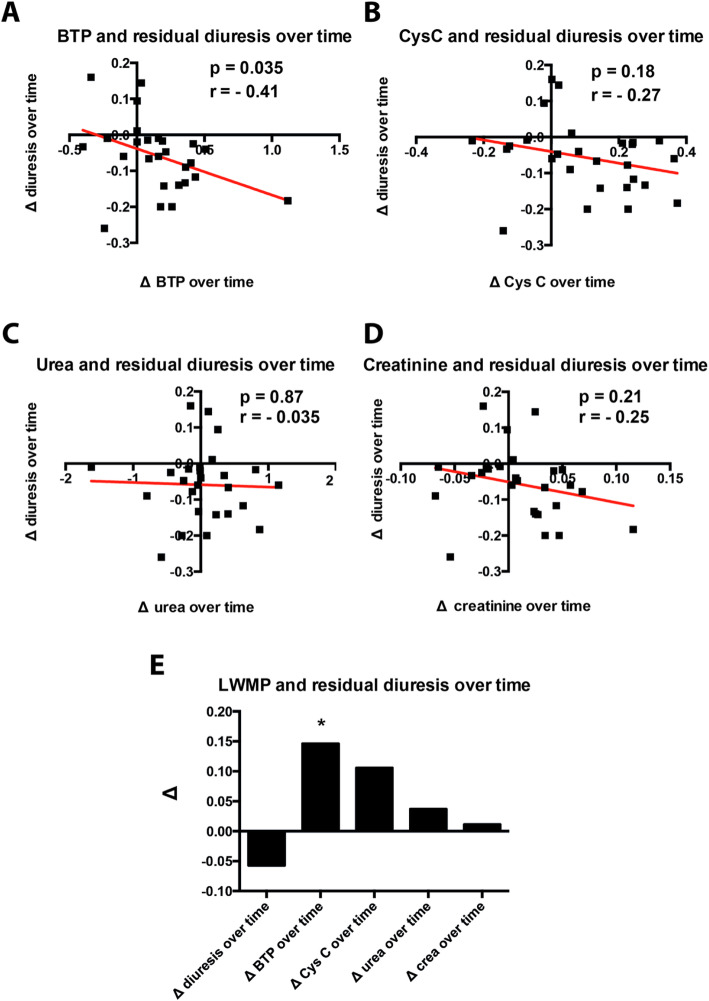
To assess changes of residual diuresis over time and corresponding serum levels of analytes, we calculated the difference between the last and first diuresis volume as well as BTP serum values and divided it by the amount of follow-up quarters. In contrast to the other markers, change in serum BTP correlated inversely with change in residual diuresis (r = − 0.41, p = 0.035) over time (Fig. 3).
Fig. 3.
Serum analytes and their correlation with residual diuresis over time. All patients with residual diuresis who were followed up for at least 12 months were included. Correlation between the last and the first BTP serum value as well as the last and first diuresis volume were divided by the amount of follow-up quarters. a- BTP; b- Cystatin C (CysC); c- urea; d – creatinine; e – overview over all investigated parameters. Data are given as mean and standard deviation. P-values < 0.05 were considered statistically significant; p < 0.05 = *
Likewise, we further assessed the correlation between changes in renal creatinine clearance and serum levels of LWMPs.
The increase in serum BTP and the decline of renal creatinine clearance over time correlated well ((r = − 0.79, p = < 0.001). In contrast, changes of serum cystatin C as well as of serum urea did not correlate with residual creatinine clearance over time (Fig. 4).
Fig. 4.
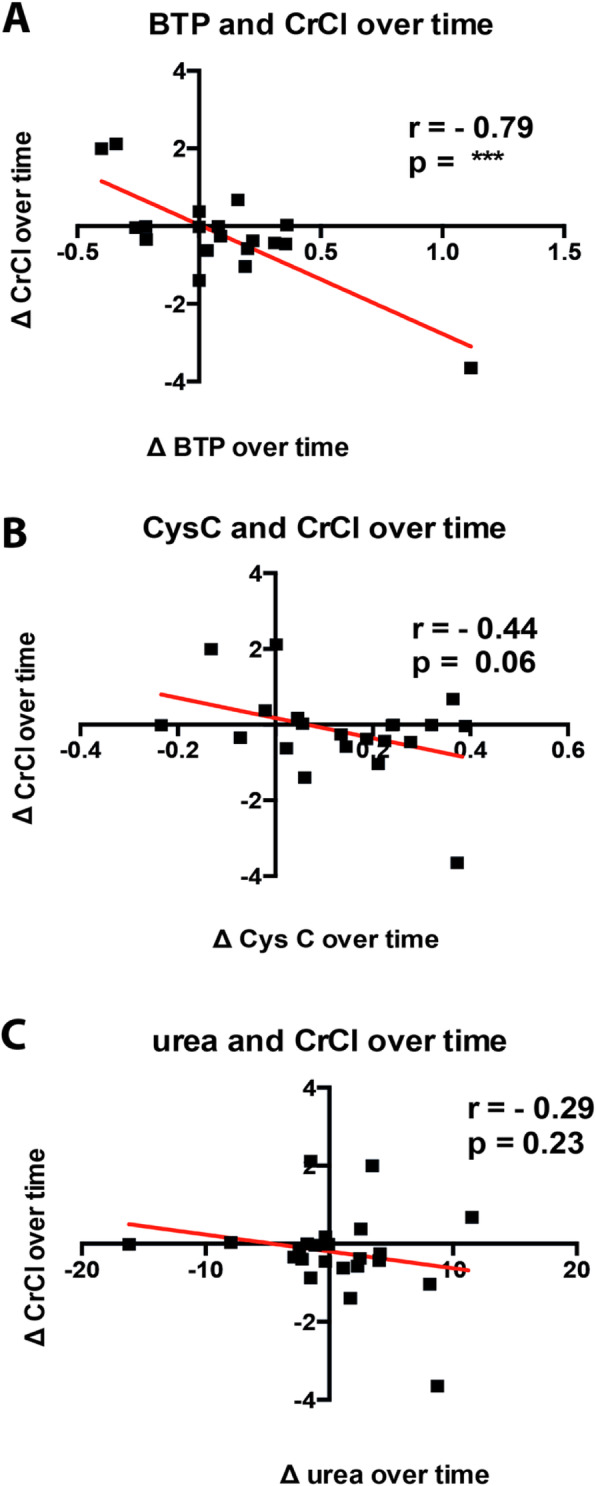
Serum analytes and their correlation with creatinine clearance over time. All patients with residual diuresis who were followed up for at least 12 months were included. Correlation between the last and the first BTP serum value as well as the last and first creatinine clearance were divided by the amount of follow-up quarters. a- BTP; b- Cystatin C (CysC); c- urea. Data are given as mean and standard deviation. P-values < 0.05 were considered statistically significant; p < 0.05 = *; p < 0.001 = **; p < 0.0001 = ***; p < 0.00001 = ****
Peritoneal clearance of LWMP in fast and slow transporters
Fast transporters (n = 16) exhibited a higher relative peritoneal clearance for BTP compared to slow transporters (n = 5) (fast: 41% SD ± 22% versus slow 19.9% SD ± 5.4%, p = 0.06), We did not note similar differences for creatinine and urea, whereas relative peritoneal elimination of cystatin C showed similar results as for BTP elimination (p = 0.06; Fig. 5).
Fig. 5.
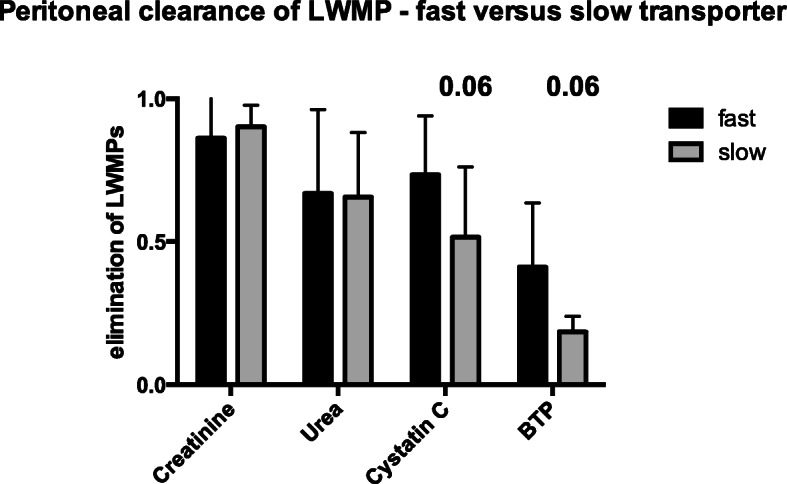
Peritoneal clearance in fast and slow peritoneal transporter. The cohort was divided into fast (n = 16) and slow peritoneal transporter (n = 5). Mean and standard deviation of peritoneal elimination are given
Discussion
RRF is associated with better survival in dialysis patients [4]. To date, RRF is generally expressed as urinary clearance of urea (United States) or the average of urea and creatinine clearance (European best practice guidelines) calculated from interdialytic urine collection and then corrected to 1.73m2 [28]. These methods need a timed urine collection, which is regarded a burden for patients and staff and therefore prone to failure or unsound results. Thus, there is a clinical need for simpler methods assessing RRF, such as by endogenous serum markers, in particular BTP and cystatin C [29–32]. Yet, studies of serum markers in PD are lacking. To the best of our knowledge, there are only a few studies investigating BTP as potential biomarker of RRF in PD patients [26, 27, 33].
In our study cohort, serum BTP levels correlated well with established biomarkers of renal function such as serum creatinine, serum cystatin C and total ClCr.
However, in contrast to the other parameters, only BTP showed a considerably high renal elimination during peritoneal dialysis. In fact, the renal elimination of BTP exceeds the peritoneal elimination, whereas all other investigated surrogate parameters are predominantly eliminated peritoneally (Fig. 1, Table 2). In line, serum BTP concentration was significantly higher in the anuric group than in patients with RRF, although the considerable difference in dialysis volume between the groups might be a confounding factor.
Serum BTP concentration has been reported to reflect RRF in patients undergoing hemodialysis [22, 23]. In a previous study, we demonstrated that, except for postdilution HDF, the applied dialysis procedures do not affect BTP levels significantly. However, with increasing permeability of the applied membrane, the reliability of BTP as a marker of RRF tends to decrease [23, 25]. For PD patients, peritoneal clearance of small molecules, such as creatinine, was described to be largely dependent on the number of dialysate exchanges. In contrast, peritoneal clearance of middle molecules, such as BTP, is mainly depending on the total dwell hours of PD [34]. Contrary to our findings a previous study reported no BTP removal by PD [27]. However, this study was comprised of only two patients on CAPD treatment, whereas in our study all patients with residual diuresis underwent CAPD. Summarizing our and previous studies’ findings on BTP in PD, peritoneal elimination of BTP is likely to depend on the PD modality and to increase with longer dwell time. Nevertheless we propose BTP as a good marker to describe RRF in PD patients due to its superiority over the established markers including creatinine, urea and cystatin C [27, 34].
In addition, our results indicate that peritoneal BTP clearance may depend on the transporter status, as peritoneal clearance was lower in the group of slow transporters. Nevertheless, BTP is a valuable marker of RRF independent of transporter statues because the majority of our cohort was classified as fast transporters.
We could not demonstrate any gender-associated difference in serum BTP concentrations, which is contrary to a study in kidney transplanted patients [20]. Probably, the effect of kidney function on BTP levels is much more pronounced than the effect of gender, so that gender differences do not become apparent in studies including patient with end-stage renal disease.
Although elimination ratio of LWMP depends on several factors, molecular mass is a factor of outstanding importance in the elimination over membranes. Molecular mass of BTP (23–29 kDa) is larger than cystatin C with a molecular weight of 13.3 kDa, which can partially explain why peritoneal clearance of cystatin C was higher compared to BTP. Cystatin C has shown a considerable non-renal clearance of 22.3 ml/min/1.73m2 that greatly exceeds its renal clearance in advanced renal failure [12, 35], which might also explain why BTP performed better as marker of RRF in our study.
In addition to the established association of BTP to residual diuresis in end-stage renal disease, we present longitudinal data on the association of residual diuresis during a follow-up period of at least 12 months. In contrast to the other serum markers, change in BTP serum concentration correlated inversely and significantly with the change of diuresis over time.
Similar results could be demonstrated for the correlation between the increase in serum BTP and the decline of residual creatinine clearance over time, strengthening the concept that BTP might serve as surrogate marker for RRF and suggesting that rising levels of serum BTP over time indicate worsening kidney function.
Our data should be interpreted with a note of caution since our investigation was limited to a rather small study population consisting mostly of Caucasians. However, two other studies indicated that serum BTP might be superior to other serum markers of RRF [33, 34]. Clearly, replication in a larger cohort of PD patients with longer follow up time is needed to define BTP cut-off values for clinical practice.
In addition, our cohort size precludes reliable assessment of clinical outcomes. In a larger cohort, a BTP-based eGFR should be determined and associated with all-cause mortality, cardiovascular mortality and hospitalization, which might reveal if the statistical superiority of BTP over cystatin C translates into a better clinical prognostic prediction.
In summary, our study suggests BTP as a reliable marker of RRF in patients PD. Based on the demonstrated elimination rates of the investigated analytes, BTP seems superior to other markers since it shows a relatively moderate peritoneal elimination. In addition, changes in serum BTP over time reflect changes in residual diuresis. Further investigations are warranted to elucidate the predictive value of testing serum levels of BTP to assess RRF.
Acknowledgements
Not applicable.
Disclosures
RPW received honoraria from Siemens, Marburg, Germany.
Abbreviations
- BSA
Body surface area
- BTP
Beta-trace protein
- ESRD
End stage renal disease
- LWMP
Low weight molecular protein
- kDa
Kilo dalton
- GFR
Glomerular filtration rate
- RRF
Residual renal function
- KRU
Urea clearance
- KRU/crea
Creatinine-urea clearance
- SD
Standard deviation
Reference intervals of analytes in our laboratory
- BTP
0.39–0.74 mg/l
- cystatin
C 0.61–0.95 mg/l
- creatinine
0.7–1.3 mg/dl
- urea
16.6–48.5 mg/dl
Authors’ contributions
Dr. Sebastian Schwab: analysis and interpretation of data, drafting of the manuscript. Dr. Carola Ellen Kleine: acquisition, analysis and interpretation of data. Dr. Dominik Bös: acquisition, analysis and interpretation of data. Sylvie Bohmann: acquisition of data, analysis and interpretation of data. Prof. Dr. Christian Strassburg: administrative support, reviewing the manuscript. Dr. Philipp Lutz: analysis and interpretation of data, drafting of the manuscript. Prof. Dr. Rainer Peter Woitas: study concept and design, interpretation of data, obtaining funding, administrative support, study supervision. The author (s) read and approved the final manuscript.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee, the ethics committee of the medical faculty of the university of Bonn – number 159/17, and informed consent was obtained from all patients enrolled into the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest that could be perceived as prejudicing the impartiality of the research reported.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Philipp Lutz and Rainer Peter Woitas contributed equally to this work.
References
- 1.Bargman JM et al., Relative Contribution of Residual Renal Function and Peritoneal Clearance to Adequacy of Dialysis: A Reanalysis of the CANUSA Study. JASN 2001, 12 (10) 2158–2162. [DOI] [PubMed]
- 2.Cheung AK, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17(2):546–555. doi: 10.1681/ASN.2005020132. [DOI] [PubMed] [Google Scholar]
- 3.Rocco M, et al. Peritoneal dialysis adequacy and risk of death. Kidney Int. 2000;58(1):446–457. doi: 10.1046/j.1523-1755.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- 4.Termorshuizen F, et al. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands cooperative study on the adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15(4):1061–1070. doi: 10.1097/01.ASN.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 5.Wang AY, et al. Lai, The importance of residual renal function in dialysis patients. Kidney Int. 2006;69(10):1726–1732. doi: 10.1038/sj.ki.5000382. [DOI] [PubMed] [Google Scholar]
- 6.McMahon GM, et al. Biomarkers in nephrology: Core curriculum 2013. Am J Kidney Dis. 2013;62(1):165–178. doi: 10.1053/j.ajkd.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight EL, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65(4):1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 8.Muntner P, et al. Overweight, obesity, and elevated serum cystatin C levels in adults in the United States. Am J Med. 2008;121(4):341–348. doi: 10.1016/j.amjmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens LA, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hokari R, et al. Increased expression and cellular localization of lipocalin-type prostaglandin D synthase in helicobacter pylori-induced gastritis. J Pathol. 2009;219(4):417–426. doi: 10.1002/path.2615. [DOI] [PubMed] [Google Scholar]
- 11.Poge U, et al. Time course of low molecular weight proteins in the early kidney transplantation period--influence of corticosteroids. Nephrol Dial Transplant. 2004;19(11):2858–2863. doi: 10.1093/ndt/gfh341. [DOI] [PubMed] [Google Scholar]
- 12.Vilar E, et al. Removal and rebound kinetics of cystatin C in high-flux hemodialysis and hemodiafiltration. Clin J Am Soc Nephrol. 2014;9(7):1240–7. doi: 10.2215/CJN.07510713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han F, et al. Induction of lipocalin-type prostaglandin D synthase in mouse heart under hypoxemia. Biochem Biophys Res Commun. 2009;385(3):449–453. doi: 10.1016/j.bbrc.2009.05.092. [DOI] [PubMed] [Google Scholar]
- 14.Eguchi Y, et al. Expression of lipocalin-type prostaglandin D synthase (β-trace) in human heart and its accumulation in the coronary circulation of angina pectoris. Proc Natl Acad Sci USA. 1997;94:14689–14694. doi: 10.1073/pnas.94.26.14689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata N, et al. De novo synthesis, uptake and proteolytic processing of lipocalin-type prostaglandin D synthase, β-trace, in the kidneys. FEBS J. 2009;276(23):7146–7158. doi: 10.1111/j.1742-4658.2009.07426.x. [DOI] [PubMed] [Google Scholar]
- 16.Orenes-Pinero E, et al. Beta-trace protein: from GFR marker to cardiovascular risk predictor. Clin J Am Soc Nephrol. 2013;8(5):873–881. doi: 10.2215/CJN.08870812. [DOI] [PubMed] [Google Scholar]
- 17.White CA, et al. Estimating GFR using serum beta trace protein: accuracy and validation in kidney transplant and pediatric populations. Kidney Int. 2009;76(7):784–791. doi: 10.1038/ki.2009.262. [DOI] [PubMed] [Google Scholar]
- 18.Benlamri A, et al. Development of a beta-trace protein based formula for estimation of glomerular filtration rate. Pediatr Nephrol. 2010;25(3):485–490. doi: 10.1007/s00467-009-1355-y. [DOI] [PubMed] [Google Scholar]
- 19.Spanaus KS, et al. Serum creatinine, cystatin C, and beta-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clin Chem. 2010;56(5):740–749. doi: 10.1373/clinchem.2009.138826. [DOI] [PubMed] [Google Scholar]
- 20.White CA, et al. Beta-trace protein: a marker of GFR and other biological pathways. Am J Kidney Dis. 2015;65(1):131–146. doi: 10.1053/j.ajkd.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 21.Shafi T, et al. Serum beta-trace protein and risk of mortality in incident hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(9):1435–1445. doi: 10.2215/CJN.02240312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhardt T, et al. Serum levels of beta-trace protein and its association to diuresis in haemodialysis patients. Nephrol Dial Transplant. 2008;23(1):309–314. doi: 10.1093/ndt/gfm510. [DOI] [PubMed] [Google Scholar]
- 23.Schwab S, et al. Beta-trace protein in hemodialysis - comparison of different therapy modalities and high flux dialyzers. Clin Chem Lab Med. 2018;56(11):265–267. doi: 10.1515/cclm-2018-0178. [DOI] [PubMed] [Google Scholar]
- 24.Lindstrom V, et al. Different elimination patterns of beta-trace protein, beta2-microglobulin and cystatin C in haemodialysis, haemodiafiltration and haemofiltration. Scand J Clin Lab Invest. 2008;68(8):685–691. doi: 10.1080/00365510802047693. [DOI] [PubMed] [Google Scholar]
- 25.Donadio C, et al. Beta-trace protein is highly removed during haemodialysis with high-flux and super high-flux membranes. BMC Nephrol. 2017;18(1):68. doi: 10.1186/s12882-017-0489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melegos D, et al. Highly elevated levels of prostaglandin D synthase in the serum of patients with renal failure. Urology. 1999;53(1):32–37. doi: 10.1016/S0090-4295(98)00453-1. [DOI] [PubMed] [Google Scholar]
- 27.Bargnoux AS, et al. Estimation of residual renal function using beta-trace protein: impact of dialysis procedures. Artif Organs. 2020;44(6):647–654. doi: 10.1111/aor.13641. [DOI] [PubMed] [Google Scholar]
- 28.White CA, et al. The estimation, measurement, and relevance of the glomerular filtration rate in stage 5 chronic kidney disease. Semin Dial. 2011;24(5):540–549. doi: 10.1111/j.1525-139X.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- 29.Filler G, et al. β-Trace protein, cystatin C, β2-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem. 2002;48(5):729–736. doi: 10.1093/clinchem/48.5.729. [DOI] [PubMed] [Google Scholar]
- 30.Juraschek SP, et al. Comparison of serum concentrations of beta-trace protein, beta2-microglobulin, cystatin C, and creatinine in the US population. Clin J Am Soc Nephrol. 2013;8(4):584–592. doi: 10.2215/CJN.08700812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhavsar NA, et al. Comparison of measured GFR, serum creatinine, cystatin C, and beta-trace protein to predict ESRD in African Americans with hypertensive CKD. Am J Kidney Dis. 2011;58(6):886–893. doi: 10.1053/j.ajkd.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poge U, et al. Beta-trace protein-based equations for calculation of GFR in renal transplant recipients. Am J Transplant. 2008;8(3):608–615. doi: 10.1111/j.1600-6143.2007.02117.x. [DOI] [PubMed] [Google Scholar]
- 33.See YP, et al. Utility of serum beta-trace protein as a tool for estimating residual kidney function in peritoneal dialysis patients. Perit Dial Int. 2021;41(2):089686082094546. [DOI] [PubMed]
- 34.Kim DJ, et al. Dissociation between clearances of small and middle molecules in incremental peritoneal dialysis. Perit Dial Int. 2001;21(5):462–6. [PubMed]
- 35.Sjostrom P, et al. Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest. 2005;65(2):111–124. doi: 10.1080/00365510510013523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.