Structured Abstract
Objective:
To determine if short-term weight change among women with unexplained infertility (UI) and polycystic ovary syndrome (PCOS) undergoing ovulation induction is associated with live birth.
Design:
Secondary analysis of randomized trials
Setting:
Multicenter
Patients:
900 women with UI and 750 women with PCOS.
Main outcome measure(s):
Live birth
Intervention:
Weight assessment as enrollment and start of up to 4–5 cycles of clomiphene, letrozole or gonadotropins and intrauterine insemination for women with UI and clomiphene or letrozole with regular intercourse for women with PCOS.
Results:
Weight data were available for 856 women with UI and 697 women with PCOS. Mean weight change was −0.2 ± 0.3 kg among women with UI and +2.2 ± 0.2 kg among women with PCOS and did not differ based on treatment allocation. There were 115 women with PCOS (16.4%) who gained >3 kg. Increased body mass index (OR=1.04; 95% Cl 1.01–1.07, P=0.02) and ≥ 3 cycles (OR 16.0, 95% CI 1.79–143.6, P=0.013) was associated with weight gain in women with PCOS. There was no difference in live birth rate among women with PCOS and >3 kg weight gain and women with PCOS who did not gain weight (aOR= 0.54; 95% CI 0.26–1.13; P=0.10).
Conclusions:
Women with PCOS gained an average of 2.2 kg regardless of the medication received while women with UI experienced no short-term weight change during ovulation induction. Weight gain in women with PCOS was not associated with live birth rate.
Keywords: short-term weight change, ovulation induction, unexplained infertility, polycystic ovary syndrome
Capsule
Women with PCOS are at risk for weight gain during ovulation induction but weight gain does not reduce live birth rate.
Introduction
Obesity, defined as a body mass index (BMI) of 30 kg/m2 or greater, is associated with reduced fecundity and live birth. Women with obesity experience longer time to pregnancy and increased ovulatory dysfunction compared to women with normal weight (1). Once pregnant, women with obesity are less likely to achieve a live birth due to increased first trimester pregnancy loss, as well as stillbirth. This relationship may differ among women undergoing fertility treatment based on diagnosis. Women with polycystic ovary syndrome (PCOS) and obesity defined as BMI of 35 kg/m2 or greater undergoing ovulation induction had lower rates of ovulation, conception, clinical pregnancy and live birth compared to women with BMI less than 30 kg/m2, though when BMI was treated as a continuous variable the association was observed for ovulation and conception, but not for clinical pregnancy or live birth (2). In contrast, women with unexplained infertility (UI) undergoing ovarian stimulation with insemination, obesity defined as BMI of 30 kg/m2 was not associated with live birth (3).
While BMI is a valuable marker for obesity, weight gain provides additional insightful information (4). Women who gain more than 5 kg between adolescence and adulthood experience reduced fecundity and longer time to pregnancy compared to women who maintain their weight (5). In addition, every 5 kg increase in adult weight was associated with a 3% increase in pregnancy loss (6). Women who gained weight during the 12–18 month prior to a conception were also at increased risk of pregnancy loss (7). Women who became overweight or obese between pregnancies and women who remained obese between pregnancies were at increased risk for stillbirth compared to normal weight women who maintained a normal weight between pregnancies (8). While weight gain as an adult, between pregnancies and in the months leading up to pregnancy leads to longer time to pregnancy and increased risk of pregnancy loss, less is known regarding short-term weight change during fertility treatment and outcomes, particularly among women with different infertility diagnoses.
Our objective is to determine if short-term weight change among women with UI and PCOS undergoing ovulation induction is associated with live birth.
Materials and Methods
This is a secondary analysis of data from the Reproductive Medicine Network trials titled Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) (9) and Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) (10). AMIGOS was a multicenter trial conducted at 12 United States (U.S.) clinical sites that randomized 900 couples diagnosed with unexplained infertility to ovarian stimulation with clomiphene, letrozole and gonadotropins (Menopur) with intrauterine insemination (IUI) for up to 4 treatment cycles. Letrozole is not Food and Drug Administration (FDA) approved for infertility but has been used off-label for this indication. Women were between 18 and 40 years of age with regular menses (nine or more cycles per year), had a normal uterine cavity with at least one patent fallopian tube, and had a male partner with a semen specimen of at least 5 million sperm per milliliter. PPCOS II was a double blind, multicenter trial conducted at 11 U.S. clinical sites that randomized 750 women with polycystic ovary syndrome (PCOS) to receive clomiphene or letrozole for up to 5 treatment cycles with regular intercourse and the intent of conception. The women were between ages 18 and 40 years of age, diagnosed with PCOS based on modified Rotterdam criteria (anovulation with either hyperandrogenism or polycystic ovaries), had at least one patent fallopian tube and a normal uterine cavity, and had a male partner with a sperm concentration of at least 14 million per milliliter. Participants in both studies were followed for pregnancy and live birth. An Advisory Board and a Data Safety Monitoring Committee appointed by the National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) approved the study protocol prior to implementation. Institutional Review Board (IRB) approval was obtained at each participating site and each participant provided written informed consent for their participation. This secondary analysis is exempt based on the University of Rochester IRB criteria.
Participants self-reported age, race, ethnicity, duration of infertility, history of pregnancy, prior infertility and number of months attempting conception. Participants underwent an enrollment examination consisting of height (cm), weight (kg) and waist circumference (cm). Participants were weighed while dressed in light clothing and without shoes and were weighted at the same clinic for enrollment and baseline weight assessments. Height was measured without shoes. BMI (kg/m2) was calculated using enrollment height and weight. Fasting serum samples were obtained at the enrollment visit, frozen at −80°C, and shipped on dry ice for analysis at the University of Virginia Ligand Assay and Analysis Core Laboratories. Homeostatic model assessment (HOMA) score, a measure of insulin resistance, was calculated as fasting insulin x fasting glucose/405. Live birth was defined as the delivery of a viable infant. Multi-gestation pregnancies with a live birth of one infant and a loss or reduction of a second gestation was classified as a live birth.
Weight change was calculated based on cycle outcome and data availability. If the participant achieved a pregnancy, weight change was defined as the weight at the cycle that the participant was confirmed to be pregnant minus her weight at the first baseline visit. If the participant did not achieve pregnancy, weight change was defined as the weight at the cycle 4 visit minus the first baseline weight. If there was no weight data at cycle 4, weight change was deemed missing. Weight change was evaluated up to cycle 4 in order to compare parallel data for the women with UI and women with PCOS as women with UI completed up to 4 cycles and women with PCOS completed up to 5 cycles. For the analyses limited to women with PCOS, weight change was evaluated up to cycle 5. All cycles were consecutive in both trials and were complete within 24 weeks of randomization. In women with PCOS, the first baseline visit occurred 2–28 days after randomization and the next treatment cycle occurred 24–54 days after the first baseline visit in women depending on whether or not ovulation occurred. The 14 unique study sites for AMIGOS and PPCOS II were categorized into 5 geographic regions for analysis: Northeast, Midwest, Southeast, Southwest and West when comparing participants between studies. Similarly, the 11 study sites for PPCOS II was categorizing into 5 geographic regions for the analyses confined to women with PCOS.
We considered 3 kg of weight change to be significant given that short term fluctuations in body weight generally range from 1 to 2 kg and adults gain on average 0.5 to 1 kg per year (11, 12). Parametric continuous data such as weight change are presented as mean ± standard deviation, nonparametric continuous data are presented as median (interquartile range (IQR)) and categorical data are presented as frequencies and percentages. Differences in weight change between women who received letrozole, clomiphene and gonadotropins were tested using Chi-square tests and one-way ANOVA tests and the results were stratified by diagnosis (UI or PCOS). Differences in continuous baseline characteristics between women with PCOS who experienced no weight gain ≤0 kg, women with PCOS who experienced minimal weight gain, defined as ≥0 kg but ≤3 kg, and women with PCOS who experienced significant weight gain, defined as ≥ 3 kg, were tested using Student t-tests. Differences in categorical baseline characteristics were tested using Chi-square or Fisher’s exact tests. Pearson’s correlation coefficient test was used to test for an association with BMI and weight gain. Multivariable logistic regression modeling was performed to identify variables associated with significant weight gain and live birth among women with PCOS. The models were adjusted for weight gain, age (years), BMI (kg/m2), treatment allocation, history of pregnancy, number of months attempting conception, duration of infertility, ovulation, maximum dose of clomiphene or letrozole, number of treatment cycles, site region, HOMA-IR and total testosterone level. Associations are presented as odds ratios (OR) with 95% confidence intervals. Statistical significance was defined as a two-sided p-value less than 0.05. Statistical analysis was performed with SAS, version 9.4 (SAS Institute).
Results
There were 1650 women eligible for the secondary analysis including 900 with UI and 750 with PCOS. 44 women with UI and 53 women with PCOS were excluded from the analysis due to missing weight data. Of the 856 women with UI, 288 received clomiphene, 283 received letrozole and 285 received gonadotropins. Of the 697 women with PCOS, 345 received clomiphene and 352 received letrozole. Women with PCOS were younger (P<0.001) and had a higher BMI (<0.001), waist circumference (<0.001) and total testosterone (<0.001) (Table 1) (13).
Table 1:
Baseline Characteristics by Diagnosis/Study
| Variable | PCOS/PPCOS II (N=697) |
UI/AMIGOS (N=856) |
P value* |
|---|---|---|---|
| Age | 29.0 (26.0, 32.0) | 32.0 (29.0, 35.0) | <0.001 |
| Race | 0.004 | ||
| White | 544/697 (78.1) | 695/856 (81.2) | |
| Black | 95/697 (13.6) | 73/856 (8.5) | |
| Other | 58/697 (8.3) | 88/856 (10.3) | |
| Ethnic group | <0.001 | ||
| Not Hispanic or Latino | 574/697 (82.3) | 767/856 (89.6) | |
| Hispanic or Latino | 123/697 (17.7) | 89/856 (10.4) | |
| BMI (kg/m2) | 35.0 (27.5, 41.3) | 25.0 (21.9, 30.1) | <0.001 |
| Waist Circumference (cm) | 107.0 (90.0, 120.0), n=695 | 83.0 (74.0, 96.0), n=855 | <0.001 |
| Treatment | <0.001 | ||
| Clomiphene | 345/697 (49.5) | 288/856 (33.6) | |
| Letrozole | 352/697 (50.5) | 283/856 (33.1) | |
| Gonadotropins | 0/697 (0.0) | 285/856 (33.3) | |
| Hormone levels | |||
| FSH –mIU/ml | 6.0 (4.9, 7.3), n=696 | 6.6 (5.6, 7.9), n=839 | <0.001 |
| Estradiol – pg/ml | 46.2 (35.4, 60.8), n=696 | 28.7 (22.3, 36.4), n=839 | <0.001 |
| Anti-Mullerian Hormone – ng/mL | 6.2 (3.6, 10.4), n=696 | 2.2 (1.2, 3.5), n=839 | <0.001 |
| Total Testosterone – ng/dl | 48.9 (35.6, 67.9), n=696 | 22.2 (15.8, 30.2), n=837 | <0.001 |
| Fasting glucose – mg/dl | 86.1 (79.2, 92.5), n=696 | 85.3 (78.8, 91.6), n=839 | 0.105 |
| SHBG – nmol/L | 26.8 (19.3, 39.8), n=696 | 56.1 (38.9, 75.4), n=835 | <0.001 |
| Insulin- uIU/ml | 14.5 (6.1, 24.1), n=696 | 4.9 (2.0, 10.5), n=838 | <0.001 |
| HOMA IR score | 55.4 (21.2, 95.5), n=696 | 18.1 (8.0, 40.3), n=838 | <0.001 |
| TSH – uIU/ml | 1.7 (1.3, 2.5), n=696 | 1.8 (1.2, 2.4), n=836 | 0.906 |
| hsCRP – mg/l | 4.2 (1.5, 8.8), n=696 | 1.5 (0.6, 3.9), n=838 | <0.001 |
| Site Region | 0.001 | ||
| MW | 149/697 (21.4) | 186/856 (21.7) | |
| NE | 286/697 (41.0) | 331/856 (38.7) | |
| SE | 61/697 (8.8) | 41/856 (4.8) | |
| SW | 111/697 (15.9) | 194/856 (22.7) | |
| W | 90/697 (12.9) | 104/856 (12.1) |
Abbreviations: PCOS, polycystic ovary syndrome, UI, unexplained infertility, NA non applicable, SHBG, sex hormone binding globulin, HOMA IR, Homeostatic Model Assessment of Insulin Resistance, TSH, thyroid stimulating hormone, hsCRP high sensitivity C-reactive protein, MW Midwest, NE Northeast, SE Southeast, SW Southwest, W West
Data are presented as median (interquartile range) or no./total no. (%). Wilcoxon’s rank-sum test, Chi-square, or Fisher’s exact test were used where appropriate.
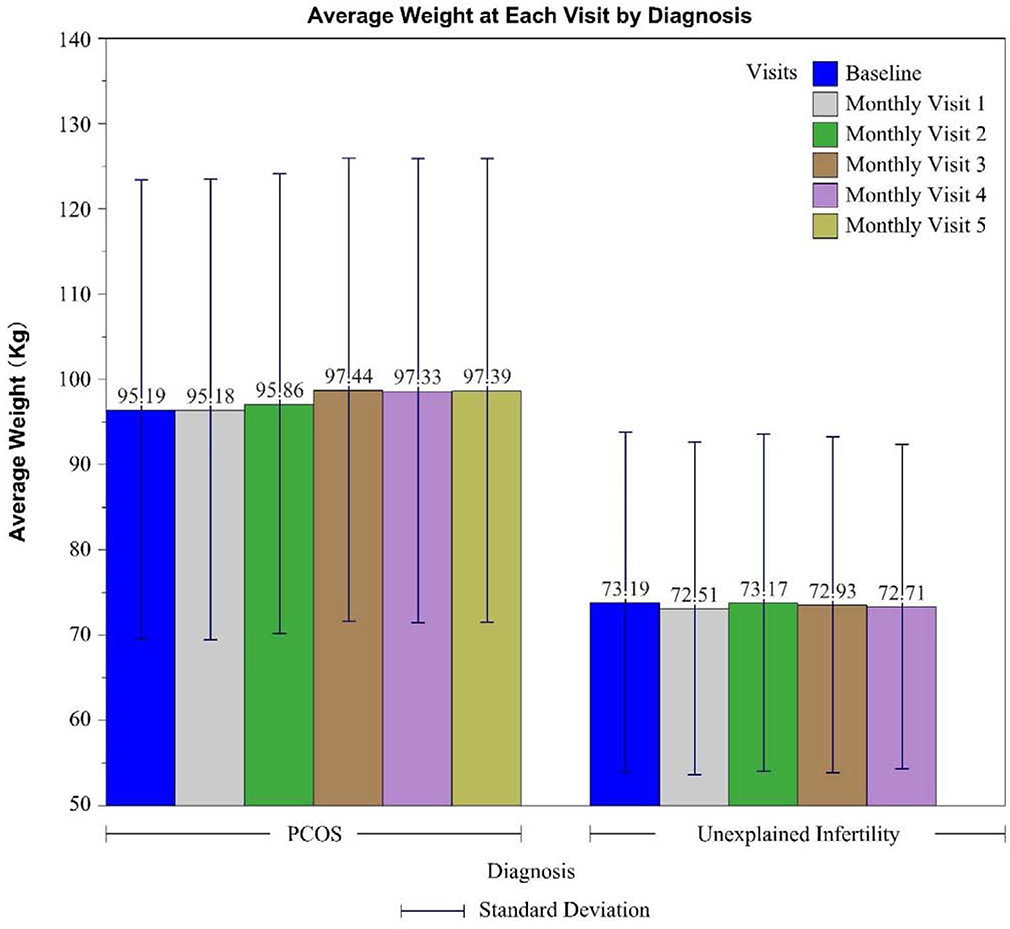
The mean weight change for women with UI and PCOS stratified by treatment cycle is shown in Figure 1. Mean weight change from enrollment weight to weight at the initiation of the 4th ovulation induction cycles was −0.2 ± 0.3 kg among women with UI and +2.2± 0.2 kg among women with PCOS. Mean weight change did not differ among women with UI who received clomiphene, letrozole or gonadotropins (P=0.22). Mean weight change did not differ among women with PCOS who received clomiphene or letrozole (P=0.52). Progressive weight gain with each cycle was observed among women with PCOS (Figure 1).
Figure 1: Mean weight (kg) with standard deviations at enrollment and cycle baseline visits by diagnosis/study.
Abbreviations: PCOS, polycystic ovary syndrome
Given that women with UI experienced minimal weight change during ovulation induction, further analysis of predictors of weight gain and outcome was focused on women with PCOS. There were 115 women with PCOS (16.4%) who experienced significant weight gain, 296 women (42.4%) who experienced minimal weight gain and 286 women (41.0%) who maintained their weight (Table 2). There was no difference in weight change when stratified by ovulation and weight was treated as a continuous variable (P=0.05) or as a categorical variable (P=0.42) (Supplemental Figure 1 and Supplemental Table 1). Women who had significant weight gain had increased body mass index (P<0.001), waist circumference (P<0.001) at enrollment and were more likely to receive the maximum dose of clomiphene or letrozole (P<0.001) and complete more treatment cycles (P<0.001) compared to women who maintained their weight or gained minimal weight (Table 2). In the adjusted model, increased BMI (OR=1.04; 95% confidence interval 1.01–1.07, P=0.02) and 3 treatment cycles (OR-16.0, 95% CI 1.79–143.6, P=0.01) were associated with ≥3 kg weight gain in women with PCOS (Supplemental Table 2). There was no statistical association between baseline BMI and weight gain using Pearson’s correlation coefficient test (Correlation: −0.03 P= 0.39).
Table 2:
Baseline characteristics of women with PCOS stratified by weight gain
| Variable | Weight Gain ≤ 0 kg (N=286) |
< 0 kg Weight Gain < 3 kg (N=296) |
Weight Gain ≥ 3 kg (N=115) |
P value* |
|---|---|---|---|---|
| Age (years) | 29.2 ± 4.4 | 28.5 ± 4.0 | 29.1 ± 4.2 | 0.142 |
| BMI (kg/m2) | 36.5 (28.5, 42.1) | 33.0 (25.7, 39.9) | 37.2 (29.7, 42.8) | <0.001 |
| Waist circumference (cm) | 109.0 (94.0, 122.0) | 102.0 (86.0, 117.0), n=295 | 110.0 (97.0, 123.0) | <0.001 |
| Race | 0.119 | |||
| White | 215/286 (75.2) | 237/296 (80.1) | 92/115 (80.0) | |
| Black | 49/286 (17.1) | 30/296 (10.1) | 16/115 (13.9) | |
| Other | 22/286 (7.7) | 29/296 (9.8) | 7/115 (6.1) | |
| Ethnic group | 0.043 | |||
| Not Hispanic or Latino | 241/286 (84.3) | 232/296 (78.4) | 101/115 (87.8) | |
| Hispanic or Latino | 45/286 (15.7) | 64/296 (21.6) | 14/115 (12.2) | |
| Duration of Infertility | 0.154 | |||
| <2 years | 93/276 (33.7) | 109/280 (39.0) | 46/110 (41.8) | |
| 2–4 years | 86/276 (31.2) | 97/280 (34.6) | 36/110 (32.7) | |
| >4 years | 97/276 (35.1) | 74/280 (26.4) | 28/110 (25.5) | |
| History of pregnancy | 108/286 (37.8) | 108/296 (36.5) | 39/115 (33.9) | 0.769 |
| History of infertility diagnosis | 248/286 (86.7) | 252/296 (85.1) | 99/115 (86.1) | 0.860 |
| History of prior therapy for infertility | 170/286 (59.4) | 161/296 (54.4) | 58/115 (50.4) | 0.210 |
| Number of months attempting conception | 36.0 (15.0, 64.5), n=276 | 24.0 (12.0, 60.0), n=280 | 24.0 (12.0, 60.0), n=110 | 0.068 |
| Ovulation | 250/286 (87.4) | 252/296 (85.1) | 90/115 (78.3) | 0.068 |
| Hormone at Baseline | ||||
| Total Testosterone -– ng/dl | 48.9 (35.5, 67.9) | 49.6 (36.0, 70.9) | 46.7 (35.0, 63.6), n=114 | 0.343 |
| Estradiol – pg/ml | 46.0 (35.1, 63.3) | 48.6 (36.0, 63.4) | 43.2 (34.5, 52.7), n=114 | 0.063 |
| Fasting glucose– mg/dl | 88.0 (79.9, 93.2) | 85.7 (78.6, 92.1) | 85.6 (79.9, 91.3), n=114 | 0.038 |
| SHBG – nmol/L | 27.0 (19.3, 39.2) | 26.2 (19.3, 41.1) | 27.4 (19.0, 39.3), n=114 | 0.968 |
| Insulin- uIU/ml | 17.0 (7.4, 24.7) | 13.1 (5.0, 24.5) | 13.6 (5.8, 21.0), n=114 | 0.051 |
| FSH - mIU/ml | 6.0 (4.9, 7.2) | 6.0 (4.9, 7.3) | 6.0 (5.0, 7.4), n=114 | 0.839 |
| TSH - uIU/ml | 1.8 (1.3, 2.5) | 1.6 (1.3, 2.4) | 1.8 (1.3, 2.5), n=114 | 0.306 |
| hsCRP – mg/l | 4.3 (1.5, 8.8) | 3.7 (1.3, 8.1) | 5.7 (1.7, 10.2), n=114 | 0.131 |
| Anti-Mullerian hormone – ng/mL | 5.7 (3.0, 10.1) | 6.5 (4.2, 10.2) | 6.5 (3.4, 10.9), n=114 | 0.253 |
| HOMA IR Score | 64.1 (27.2, 102.1) | 47.8 (17.5, 94.5) | 52.8 (20.6, 86.3), n=114 | 0.020 |
| Treatment | 0.137 | |||
| Clomiphene | 148/286 (51.8) | 134/296 (45.3) | 63/115 (54.8) | |
| Letrozole | 138/286 (48.2) | 162/296 (54.7) | 52/115 (45.2) | |
| Maximum Dose | <0.001 | |||
| 1 Tablets | 105/286 (36.7) | 90/296 (30.4) | 18/115 (15.6) | |
| 2 Tablets | 78/286 (27.3) | 83/296 (28.0) | 27/115 (23.5) | |
| 3 Tablets | 103/286 (36.0) | 123/296 (41.6) | 70/115 (60.9) | |
| Site Region | 0.314 | |||
| MW | 66/286 (23.1) | 55/296 (18.6) | 28/115 (24.4) | |
| NE | 116/286 (40.6) | 118/296 (39.9) | 52/115 (45.2) | |
| SE | 30/286 (10.5) | 24/296 (8.1) | 7/115 (6.1) | |
| SW | 42/286 (14.7) | 56/296 (18.9) | 13/115 (11.3) | |
| W | 32/286 (11.2) | 43/296 (14.5) | 15/115 (13.0) | |
| Achieved Pregnancy | <0.001 | |||
| Yes | 110/286 (38.5) | 113/296 (38.2) | 18/115 (15.7) | |
| No | 176/286 (61.5) | 183/296 (61.8) | 97/115 (84.3) | |
| Treatment Cycles | <0.001 | |||
| 1 | 40/286 (14.0) | 33/296 (11.1) | 1/115 (0.9) | |
| 2 | 37/286 (12.9) | 42/296 (14.2) | 4/115 (3.5) | |
| 3 | 33/286 (11.5) | 44/296 (14.9) | 11/115 (9.6) | |
| 4 | 27/286 (9.5) | 30/296 (10.1) | 17/115 (14.8) | |
| 5 | 149/286 (52.1) | 147/296 (49.7) | 82/115 (71.3) |
Abbreviations: PCOS, polycystic ovary syndrome, SHBG, sex hormone binding globulin, HOMA IR, Homeostatic Model Assessment of Insulin Resistance, TSH, thyroid stimulating hormone, hsCRP high sensitivity C-reactive protein, MW Midwest, NE Northeast, SE Southeast, SW Southwest, W West
Data are presented as median (interquartile range) or no./total no. (%). Wilcoxon’s rank-sum test, Chi-square, or Fisher’s exact test were used where appropriate.
There were 164 women with PCOS (25.1%) out of 697 subjects who achieved a live birth with 13 live births (11.3%) observed among the 115 women with significant weight gain and 74 live births (25.9%) among the 286 women with no weight gain (P < 0.001). In the adjusted model, women with lower BMI, total testosterone, shorter number of months attempting conception were more likely to achieve a live birth (Table 3). Weight gain ≥3 kg was associated not associated with live birth (OR 0.54, 95% CI 0.26–1.13, P=0.10). Completion of 5 treatment cycles was associated with a reduction in live birth (OR 0.10, 95% CI 0.05–0.18, P<0.001).
Table 3:
Odds ratios for predictive variables and live birth among women with PCOS
| Variables | Live Birth |
|||
|---|---|---|---|---|
| Adjusted OR (95% CL) | P-value | Unadjusted OR (95% CL) | P-value | |
| BMI (kg/m2) | 0.97 (0.95, 0.99) | 0.017 | 0.96 (0.94, 0.98) | <0.001 |
| Total Testosterone (ng/dL) | 0.99 (0.98, 0.99) | 0.008 | 0.99 (0.98, 0.99) | 0.002 |
| Number of months attempting conception | 0.99 (0.98, 0.99) | 0.016 | 0.99 (0.98, 0.99) | <0.001 |
| History of pregnancy | ||||
| Yes | 1 | 1 | ||
| No | 0.71 (0.46, 1.11) | 0.13 | 0.76(0.53, 1.09) | 0.14 |
| Treatment | ||||
| Clomiphene | 1 | 1 | ||
| Letrozole | 1.52 (0.99, 2.32) | 0.05 | 1.69 (1.18, 2.41) | 0.004 |
| Weight Gain | ||||
| Weight Gain ≤ 0 kg | 1 | 1 | ||
| < 0 kg Weight Gain < 3 kg | 0.84 (0.54, 1.32) | 0.46 | 1.01 (0.69, 1.46) | 0.97 |
| Weight Gain ≥ 3 kg | 0.54 (0.26, 1.13) | 0.10 | 0.37 (0.19, 0.69) | 0.002 |
| Site Region | ||||
| MW | 1 | 1 | ||
| NE | 0.95 (0.54, 1.67) | 0.87 | 1.20 (0.76, 1.91) | 0.44 |
| SE | 1.05 (0.44, 2.51) | 0.91 | 0.92 (0.45, 1.89) | 0.81 |
| SW | 0.67 (0.33, 1.35) | 0.26 | 0.88 (0.49, 1.61) | 0.69 |
| W | 0.67 (0.31, 1.46) | 0.32 | 0.91 (0.48, 1.71) | 0.76 |
| Treatment Cycles | ||||
| 1 | 1 | 1 | ||
| 2 | 1.21 (0.62, 2.35) | 0.58 | 1.09 (0.58, 2.04) | 0.79 |
| 3 | 1.25(0.64, 2.45) | 0.520 | 0.85 (0.46, 1.58) | 0.60 |
| 4 | 0.76 (0.37, 1.57) | 0.460 | 0.57 (0.29, 1.10) | 0.09 |
| 5 | 0.10 (0.05, 0.19) | <0.001 | 0.08 (0.04, 0.15) | <0.001 |
Abbreviations: PCOS, polycystic ovary syndrome, BMI, body mass index, MW Midwest, NE Northeast, SE Southeast, SW Southwest, W West
Potential adjustors: weight gain, age (years), BMI (kg/m2), ethnic group, treatment, site region, history of pregnancy, number of months attempting conception, ovulation, duration of Infertility and total testosterone levels
Discussion
While obesity is associated with reduced live birth among women trying to conceive, this relationship has not been consistently observed among women with PCOS and women with UI undergoing fertility treatment. In this secondary analysis, we found that women with PCOS undergoing ovulation induction are at risk for short-term weight gain, particularly if women enter treatment with a higher BMI and require 3 or more treatment cycles, whereas women with UI maintained their weight while treated with the same medications. Weight gain of 3 kg or greater among women with PCOS undergoing ovulation induction was not associated with live birth.
We identified that increased BMI prior to initiating treatment and requiring 3 or more treatment cycles were variables associated with weight gain during ovulation induction among women with PCOS. In a study of short-weight change among women undergoing IVF, there was a trend towards weight gain of 1 kg or more among women who were overweight or obese based on baseline BMI, though short-term weight gain was not associated with live birth in this population nor in our population after adjusting for covariates such as the number of treatment cycles (14). Given that this is a secondary analysis, we are unable to speculate on mechanism of weight gain during ovulation induction for women with PCOS but did not see a difference in weight gain when we stratified by ovulation.
We identified differences in weight gain with ovulation induction based on diagnosis, but not by medication. The finding that weight gain differs by diagnosis mirrors the finding that short-term weight loss may improve live birth among women with obesity and PCOS but not women with obesity and other infertility diagnoses. The Lifestyle Trial randomized 577 women with obesity and infertility secondary to anovulation (46%), UI (28%), male factor (23%) and tubal factor (2%) to a 6-month lifestyle intervention followed by fertility treatment for 18 months or prompt fertility treatment for 24 months (15). The majority of women with anovulatory infertility (78%) were diagnosed with PCOS according to Rotterdam Criteria. Fertility treatment was based on the diagnosis and included clomiphene, with or without insemination, gonadotropins and in vitro fertilization with or without intracytoplasmic sperm injection. Though women who received the lifestyle intervention were more likely to have an ongoing pregnancy that was conceived naturally, there was no difference in the primary outcome, defined as the number of term vaginal births of healthy singletons at 24 months, between the two groups. In addition, post hoc subgroup analyses that were limited to women with anovulatory infertility or to women with UI showed no significant differences between the groups with respect to the rates of the primary outcome or of live births. An ongoing randomized controlled trial of women with obesity and UI comparing live birth after increased physical activity and intensive weight loss to increased physical activity with standard weight maintenance followed by clomiphene/IUI will provide additional data regarding the possible benefits of physical activity for women with UI undergoing ovulation induction (16). Additional randomized trials regarding short-term weight loss prior to ovulation induction for women with obesity and PCOS showed improved ovulation and live birth. The Treatment of Hyperandrogenism Versus Insulin Resistance in Infertile Polycystic Ovary Syndrome (OWL PCOS) trial randomized 216 women with obesity and PCOS to 16 weeks of continuous oral contraceptive pills, lifestyle modification or both followed by up to 4 cycles of ovulation induction with clomiphene (17). While ovulation was increased among women who received lifestyle modification, there was no difference in live birth, though the study was underpowered for this outcome. When comparing women who received lifestyle modification followed by clomiphene in OWL PCOS to women who received immediate clomiphene in PPCOS II, ovulation rates and live birth rates were significantly improved with lifestyle modification (18). While clinical guidelines recommend weight loss for women with obesity and infertility(19), perhaps recommendations should differ based on diagnosis as short-term weight loss appears to improve the odds of natural conception, ovulation and live birth among women with PCOS but not women with other infertility diagnosis.
The strengths of our secondary analysis are that the diagnoses and treatment of women with PCOS and UI was based on standardized protocols and that baseline and monthly visit weights were available for the majority of participants in the primary trials. While scales may differ between clinics, the participants were weighed at the same clinic for baseline and monthly visit weights. While women with obesity were counseled about the potential benefits of weight loss prior to conception, short term weight loss interventions were not offered in the primary trials and women who had undergone weight loss surgery could enroll if their weight was stable. Therefore, the weight change we observed was not related to weight cycling from recent weight loss. Finally, end of study weight was not included in this analysis as this data would reflect variable experiences that could influence weight such a recent pregnancy loss or delivery.
Conclusions
In conclusion, women with PCOS are at risk for short-term weight gain while undergoing ovulation induction with letrozole and clomiphene, while women with unexplained infertility appear to maintain their weight while undergoing ovarian stimulation with letrozole, clomiphene and gonadotropins. While short-term weight gain was not associated with a reduction in live birth among women with PCOS in this analysis, further research should evaluate if short-term weight gain is associated with an increased risk of miscarriage, preeclampsia and gestational diabetes in women undergoing fertility treatment.
Supplementary Material
Acknowledgement
The authors would like to thank Esther Eisenberg, MD for feedback on the concept and analysis and Mr. Zehua Pan for assistance with data analysis. The authors would also like to thank the members and principal investigators of the Reproductive Medicine Network for their contributions to the original trials upon which this research is based. In addition to the authors, other members of the National Institute of Child Health and Human Development Reproductive Medicine Network were: University of Pennsylvania: C. Coutifaris; University of Florida: G. Christman; University of Texas Health Science Center at San Antonio: R. Robinson, R. Brzyski; University of Colorado: W. Schlaff; University of Vermont: P. Casson; SUNY Upstate Medical University: J. C. Trussell; and Eunice Kennedy Shriver National Institute of Child Health and Human Development: E. Eisenberg, C. Lamar, L. DePaolo. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
Supported by grants (R25HD075737) CREST Program (Clinical Research/Reproductive Scientist Training), U10 HD38992, U10HD055925 and U10 HD39005, Eunice Shriver National Institute of Child Health and Human Development
Financial Disclosure: MPD is a member of the board of directors and stockholder at Advanced Reproductive Care. RSL reports consulting fees from Ferring, Abbvie, Bayer and Fractyl and research funding from Guerbet. The remaining author have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Legro RS. Effects of obesity treatment on female reproduction: results do not match expectations. Fertil Steril 2017;107:860–7. [DOI] [PubMed] [Google Scholar]
- 2.Kuang H, Jin S, Hansen KR, Diamond MP, Coutifaris C, Casson P et al. Identification and replication of prediction models for ovulation, pregnancy and live birth in infertile women with polycystic ovary syndrome. Hum Reprod 2015;30:2222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen KR, He AL, Styer AK, Wild RA, Butts S, Engmann L et al. Predictors of pregnancy and live-birth in couples with unexplained infertility after ovarian stimulation-intrauterine insemination. Fertil Steril 2016;105:1575–83 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaskins AJ. Recent advances in understanding the relationship between long- and short-term weight change and fertility. F1000Res 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaskins AJ, Rich-Edwards JW, Missmer SA, Rosner B, Chavarro JE. Association of Fecundity With Changes in Adult Female Weight. Obstet Gynecol 2015;126:850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaskins AJ, Rich-Edwards JW, Colaci DS, Afeiche MC, Toth TL, Gillman MW et al. Prepregnancy and early adulthood body mass index and adult weight change in relation to fetal loss. Obstet Gynecol 2014;124:662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radin RG, Mumford SL, Sjaarda LA, Silver RM, Wactawski-Wende J, Lynch AM et al. Recent attempted and actual weight change in relation to pregnancy loss: a prospective cohort study. BJOG 2018;125:676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteman VE, Crisan L, McIntosh C, Alio AP, Duan J, Marty PJ et al. Interpregnancy body mass index changes and risk of stillbirth. Gynecol Obstet Invest 2011;72:192–5. [DOI] [PubMed] [Google Scholar]
- 9.Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P et al. Letrozole, Gonadotropin, or Clomiphene for Unexplained Infertility. N Engl J Med 2015;373:1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014;371:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhutani S, Kahn E, Tasali E, Schoeller DA. Composition of two-week change in body weight under unrestricted free-living conditions. Physiol Rep 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutfless S, Gudzune KA, Maruthur N, Wilson RF, Bleich SN, Lau BD et al. Strategies to prevent weight gain in adults: a systematic review. Am J Prev Med 2013;45:e41–51. [DOI] [PubMed] [Google Scholar]
- 13.Santoro N, Eisenberg E, Trussell JC, Craig LB, Gracia C, Huang H et al. Fertility-related quality of life from two RCT cohorts with infertility: unexplained infertility and polycystic ovary syndrome. Hum Reprod 2016;31:2268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavarro JE, Ehrlich S, Colaci DS, Wright DL, Toth TL, Petrozza JC et al. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril 2012;98:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutsaerts MA, van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WK, Perquin DA et al. Randomized Trial of a Lifestyle Program in Obese Infertile Women. N Engl J Med 2016;374:1942–53. [DOI] [PubMed] [Google Scholar]
- 16.https://clinicaltrials.gov/ct2/show/NCT02432209. In. Vol. 2019. https://clinicaltrials.gov/ct2/show/NCT02432209.
- 17.Legro RS, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI et al. Randomized Controlled Trial of Preconception Interventions in Infertile Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab 2015;100:4048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legro RS, Dodson WC, Kunselman AR, Stetter CM, Kris-Etherton PM, Williams NI et al. Benefit of Delayed Fertility Therapy With Preconception Weight Loss Over Immediate Therapy in Obese Women With PCOS. J Clin Endocrinol Metab 2016;101:2658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Practice Committee of the American Society for Reproductive M. Obesity and reproduction: a committee opinion. Fertil Steril 2015;104:1116–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.