Abstract
Background
Characterizing productive language deficits following lesions to the right (RH) or left hemispheres (LH) is valuable in identifying appropriate therapeutic goals. While damage to the LH classically is associated with deficits in language, RH lesions also result in changed communication beyond prosody due to cognitive-linguistic effects. Cohesion, reference to introduced content across sentences within discourse, relies on a listener’s clear and unambiguous understanding that a reference has occurred. To date, we are not aware of any prior work that has compared patterns of cohesive strategy between RH and LH lesioned individuals with cohesion deficits.
Aims
The purpose of the present study is to determine whether individuals with communication deficits following RH and LH lesions differ in the inclusion and clarity of cohesive markers.
Methods & Procedures
Seventy-six RH samples and 145 LH samples were used for comparison of cohesion performance in a Cookie Theft picture description task. Cohesive ties were assessed following the protocol outlined in Liles and Coelho (1998). It was hypothesized that individuals with LH lesions would present a different pattern of cohesion behaviour than RH lesioned individuals when considered both acutely and chronically.
Outcomes & Results
Overall, samples from LH and RH groups did not differ in word counts or cohesive marker usage. However, the patterns of markers they chose to employ were different. LH samples used conjunctions and personal pronouns more frequently and used lexical cohesive markers less frequently than RH samples. Acutely, patterns of cohesive marker use between LH and RH samples were more similar. Chronically, LH samples contained more personal pronouns and the differences in lexical cohesive markers remained unchanged. When cohesion was unsuccessful, LH and RH damage were associated with different patterns of error. LH samples tended to omit information needed to clarify the intended referent, resulting in incomplete cohesion errors. RH samples tended to sustain breakdowns in cohesion from sentence to sentence, not resolving incorrectly chosen pronouns or ambiguities left in their samples.
Conclusions
LH and RH lesions resulted in differing patterns of chosen cohesive markers and errors when cohesion was unsuccessful. This was particularly true in lexical cohesion, which has been far less studied than closed-class cohesive markers like referential pronouns. It was also noted that cohesive behavior did not appear to “recover” for either group, suggesting spontaneous recovery is minimal and present strategies for language therapy may not effectively address this linguistic function.
Keywords: cohesion, Cookie Theft
Introduction
Characterizing productive language deficits following lesions to the right (RH) or left hemispheres (LH) is valuable in identifying appropriate therapeutic goals. While damage to the LH classically is associated with deficits in language and has received extensive attention in the literature, patients with RH lesions also experience difficulties in communication. Classically, these include prosodic changes (Bowers, Blonder, Feinberg, & Heilman, 1991; Pell, 2007; Pell & Baum, 1997; Ross & Monnot, 2008); however, deficits may extend to other areas of language due to cognitive-linguistic effects that are less thoroughly understood.
Discourse, multiple sequentially- or logically-related sentences, is a high-level cognitive-linguistic task. It typically is elicited using single-picture description, as in the frequently used Cookie Theft task from the Boston Diagnostic Aphasia Examination (Goodglass, Kaplan, & Barressi, 1983). Cohesion, reference to introduced content across sentences within discourse, relies on a listener’s clear and unambiguous understanding that a reference has occurred (e.g., “Mary is in the kitchen. She is making a snack.”). When a word is used to reference a previously introduced piece of information, but is inscrutable without that referent, that is called a cohesive tie (Coelho, Liles, & Duffy, 1995; Liles & Coelho, 1998). Cohesion can be accomplished using conjunctions, demonstrative referents (e.g., “That is the cookie.”), personal referents (e.g., “She is washing a dish.”; together, closed-class markers) and lexical markers (e.g., “Mom is wearing a dress. She loves comfortable clothing.”; open-class markers). In order to use cohesion masterfully, the speaker must not only produce the correct words that clearly reference previously introduced content, but also monitor a listener’s understanding and resolve apparent confusion or ambiguity in real time. It is no surprise then that cohesion performance relates to both cognitive and linguistic abilities. Language cohesion is correlated with measures of executive function (Mozeiko, Le, Coelho, Krueger, & Grafman, 2011) and word-finding (Coelho, Grela, Corso, Gamble, & Feinn, 2005). It also has strong implications for social behaviour, as difficulty in recounting events in a way understood by others results in social barriers.
The literature on cohesion in stroke (specifically) is relatively limited, and early papers frequently combined either lesion location or etiology, or both, to achieve larger sample sizes. Despite these limitations, the general observation is that deficits in cohesion can occur in individuals regardless of whether the lesion is in the LH (Clark, 2018; Ellis, Rosenbek, Rittman, & Boylstein, 2005) or RH (Davis, O’Neil-Pirozzi, & Coon, 1997; Marini, 2012; Sherratt & Bryan, 2012). Both Uryase, Duffy, and Liles (1991) and Barker, Young and Robinson (2017) found significantly poorer cohesion performance regardless of lesion hemisphere among those in the chronic phase compared to typical speakers, with Uryase, Duffy, and Liles (1991) finding that 12 patients with LH damage appeared to have less success in their cohesive attempts than 22 patients with RH damage.
The interrelated skills necessary to cohesion suggest individuals with LH and RH lesions may experience differing breakdowns in this skill leading to differing strategies of cohesive marker use. While individuals with LH damage may have difficult with accessing appropriate words, RH damage is associated with difficulties in interpretation (Myers, 1978), integration of concepts to a “main idea” and keeping to the topic (Hillis Trupe & Hillis, 1985), “course coding” (activation of broad semantic fields; Blake, Tompkins, Scharp, Meigh, & Wambaugh, 2015), and ability to infer the mental state of the listener (Weed, McGregor, Nielsen, Roepstorff, & Frith, 2010), which may lead to distinct challenges in generating cohesion. Unfortunately, reporting of more specific patterns of cohesive tie usage has been even more limited and generally appears in authors’ discussions of observations. Uryase, Duffy, and Liles (1991) found that among a small sample of RH and LH lesioned individuals in the chronic phase of recovery, the types of cohesive markers elicited during a movie summary and subsequent dialogue were consistent regardless of whether neurological damage was present or absent. However, their participants with chronic lesions may have recovered from earlier deficits. In contrast, other work has suggested referential ties are particularly impacted in individuals with RH lesions (Chantraine, Joanette, & Ska, 1998). Davis et al. (1997) described conjunctions being used more frequently among individuals with RH damage (particularly “and,” which appeared overly frequently as a filler or sentence initiator in RH samples). Ellis et al. (2005) found that, for 12 individuals within the first year after a left hemisphere stroke, attempted usage of cohesive ties did not change, despite improvements in clarity and ambiguity of the ties used. Clarke (2018) described a pattern of more frequent errors in personal pronouns following LH stroke, but lacked sufficient power to test differences in tie usage. Among persons with aphasia (PWA), beyond the cohesion literature, certain sub-types of cohesive markers have been studied. Personal and demonstrative pronouns often lack antecedents (Linnik, Bastiaanse, & Höhle, 2016; Piehler & Holland, 1984), and PWA tend to have difficulty with appropriate use of definite articles, associated with reference (Armstrong, 2000). These deficits are thought to improve or resolve over time (Coelho, Liles, Duffy, Clarkson, & Elia, 1994; Piehler & Holland, 1984).
We are not aware of any prior work that has compared patterns of proportional cohesive marker use between RH and LH lesioned individuals with cohesion deficits, particularly in the acute phase. The purpose of the present study was to determine whether individuals with communication deficits following RH and LH lesions differ in the inclusion and clarity of cohesive markers. It was hypothesized that individuals with LH lesions would present a different pattern of cohesion behaviour than RH lesioned individuals when considered both acutely and chronically. Although specific differences in pattern were not formally part of the hypothesis, it was predicted that RH lesioned individuals would have more difficulty using closed-class markers unambiguously, which was previously reported (Barker et al., 2017) and is a skill that relies heavily on both tracking referents and accommodating speakers. In contrast, it was anticipated that LH lesioned individuals would demonstrate less use and success with lexical markers, as word-finding deficits would impede this method of cohesion. Finally, it was hypothesized that use of cohesion, like other cognitive-linguistic functions, can improve during recovery, perhaps as undamaged brain regions assume the functions of the damaged areas. So, we expected greater deficits in acute than chronic stroke if such recovery occurs.
Materials & Methods
Participants
A total of 164 participants, including 106 with a LH lesion and 58 with a RH lesion were identified through ongoing longitudinal protocols investigating stroke recovery. All participants were right-handed native English speakers with no history of co-occurring neurological diagnoses affecting the brain and normal or corrected-to-normal vision and hearing (participants and overall sample statistics are described in Table 1). Participants with LH and RH lesions were similar in age at the time of their lesion and self-reported gender distribution. Participants in the acute phase of recovery were defined as those within the first week following stroke, often the same day as admission. Participants in the chronic phase of recovery were tested at least 6 months after injury.
Table 1:
Sample information
| Overall | Left Hemisphere | Right Hemisphere | U | Sig. |
|---|---|---|---|---|
| Participants | N = 106 | N = 58 | ||
| Age at CVA | 58.23(11.65) | 60.43(15.69) | 4179.5 | n.s. |
| Females | N = 40 | N=23 | ||
| Samples | N = 145 | N = 76 | ||
| Word count | 70.59 (47.92) | 71.92 (47.39) | 5416 | n.s. |
| Cohesive marker count | 9.05 (6.32) | 8.79 (5.79) | 4606 | n.s. |
| Acute: < 1 week after stroke (N = 119) | ||||
| Samples | N = 86 | N = 33 | ||
| Word count | 63.76 (37.32) | 76.67 (48.59) | 1228.5 | n.s. |
| Cohesive marker count | 7.29 (5.79) | 10.58 (6.32) | 832.5 | ** |
| Chronic: 6–12 months after stroke (N = 102) | ||||
| Samples | N = 59 | N = 43 | ||
| Word count | 80.54 (59.08) | 68.28 (46.70) | 1042 | n.s. |
| Cohesive marker count | 9.14 (6.91) | 7.48 (5.04) | 1063.5 | n.s. |
Statistics are reported as Mean(Standard Deviation) unless otherwise noted.
U: Mann-Whitney U
p < 0.05
p < 0.01
Although the parent studies are longitudinal, relatively few participants contributed a sample to both the acute and chronic timepoints: N = 24 or 23% of LH samples and N = 16 or 28% of RH samples. Samples are considered independently, not paired, for the purposes of analysis.
Analysis of samples
We chose to assess response to picture descriptions over narrative retellings because picture descriptions are more sensitive to cohesive deficits (Marini, Carlomagno, Caltagirone, & Nocentini, 2005). The Cookie Theft picture description task previously has been characterized as an “ecologically valid approximation of spontaneous discourse” (Giles, Patterson, & Hodges, 1996) and was the most frequently completed spontaneous speech elicitation prompt in both our left and right hemisphere longitudinal data sets. Initially, 175 Cookie Theft samples from LH lesioned patients and 95 samples from RH lesioned patients were identified that previously had been collected from participants.
To provide a reasonable basis for assessing cohesion, samples shorter than 20 words were removed from analysis, N = 27 LH samples and N = 10 RH samples. Remaining statistics are reported based on samples, not participants. As in prior literature, repeated words were counted toward the calculated word count; however, fillers for pauses and formulation (e.g., uh, um) were not (Nicholas & Brookshire, 1993). As our interest was specific to individuals with atypical cohesion performance, all samples were then compared to typical cohesion performance among a similarly aged population described in Glosser and Deser (1991). Subsequently, typical samples were excluded from analysis, N = 3 LH samples and N = 9 RH samples. The remaining 76 RH samples and 145 LH samples were used for comparison of cohesion performance (Table 1).
Sample sizes used for calculating cohesion measures did not differ between LH and RH groups overall1 or in either time point considered independently. Sample sizes also did not significantly increase between the acute and chronic time points for either those with LH lesions, Mann-Whitney U = 2054, p = 0.052, or those with RH lesions, Mann-Whitney U = 624, p = 0.373. While this is a minor point, it suggests the samples of patients’ speech used as a basis for examining cohesion was similar between the groups and phases of recovery that were examined.
Cohesive ties were assessed by the first author following the protocol outlined in Liles and Coelho (1998). Cohesive ties were analysed by first identifying words used as cohesive ties, then classifying the ties, and determining their adequacy (Liles & Coelho, 1998). Ties that are unambiguous are said to be “complete.” For example, “Mary is in the kitchen. She is making a snack.” Those that are not complete are either “incomplete” (information is not present; e.g., “Mary is in the kitchen. It is so delicious!”) or “erroneous” (information is ambiguous or misleading; e.g., “Mary is in the kitchen. He is making a snack.”). Successive references are called tied-errors or tied-incomplete attempts if an ambiguity is not reconciled. Conjunctions are measured as a simple proportion of overall cohesive ties and are not assessed for success. The systematic and convergent method of cohesion analysis presented in Liles and Coelho (1998) is considered a highly reliable adaptation of the original cohesion analysis described in Halliday and Hasan (1976) and has consistently resulted in high inter-examiner reliability (Liles, 1985; Liles, Coelho, Duffy, & Zalagens, 1989). Given the large number of samples, a randomly selected subset of the samples (20% = 44 samples) was selected for the calculation of intra- and inter-rater reliability, the latter of which was completed by the penultimate author. Intra-rater reliability resulted in strong agreement (McHugh, 2012), κ = 0.85 (95% CI, 0.79 to 0.90), p < 0.0001. Inter-rater reliability with a novel rater resulted in similarly strong agreement, κ = 0.84 (95% CI, 0.77 to 0.90), p < 0.0001.
Study-wide alpha was managed by first considering differences in the four cohesive marker proportions at α = 0.05/4 ≈ 0.01 across the phases of recovery. Marker types for which significant differences were observed were then considered at 0.01 recursively for each phase, acute and chronic. As there was minimal basis in the literature for determining effect size in error types across markers, these findings were also considered first overall and then separately for each phase of recovery. These results should be considered exploratory and are all reported for both α = 0.05 and α = 0.01.
Results
Overall use of cohesive markers
As anticipated, total cohesive markers use did not differ between LH and RH groups. LH samples taken during the acute phase after stroke had significantly fewer cohesive markers attempted than RH samples in the acute phase, but this difference was no longer significant in the chronic phase. This finding was not the apparent result of any change in the LH cohesion behavior, Mann-Whitney U = 1981.5, p = 0.074, but rather it reflected differences in overall cohesive marker usage from acute to chronic phases among the RH samples. Individuals with RH lesions used significantly fewer cohesive markers in the chronic phase than the acute phase, Mann-Whitney U = 453.5, p = 0.027. It is unclear whether this was an artifact of some kind related to our specific dataset, or perhaps, reflects a kind of adaptation to the challenges associated with keeping track of referents in conversation following a RH lesion.
Overall LH versus RH differences were observed in cohesive marker type (Table 2). LH samples used a significantly larger proportion of conjunctions (e.g., “and,” “or,” “else,” “however,” “then”) and personal referential markers (e.g., “his,” “her,” “them,” “it”), while RH samples used a significantly larger proportion of lexical or open-class markers. To control for the overall cohesive markers used when examining the strategies adopted by LH versus RH lesioned individuals when attempting to create cohesion in their samples, use of cohesive marker by type and accuracy was calculated and analysed as a proportion of total cohesive markers.
Table 2:
Proportionate use of cohesive markers by type
| Overall | Left Hemisphere | Right Hemisphere | U | Sig. |
|---|---|---|---|---|
| Conjunction | 0.351 (0.236) | 0.252 (0.204) | 4073.5 | ** |
| Demonstrative | 0.099 (0.156) | 0.095 (0.175) | 4846 | n.s. |
| Personal | 0.413 (0.263) | 0.304 (0.238) | 3788.5 | ** |
| Lexical | 0.136 (0.211) | 0.349 (0.272) | 2637 | ** |
| Acute: < 1 week after stroke (N = 119) | ||||
| Conjunction | 0.360 (0.256) | 0.219 (0.136) | 988 | * |
| Demonstrative | 0.104 (0.144) | 0.113 (0.180) | 1272 | n.s. |
| Personal | 0.378 (0.274) | 0.283 (0.196) | 1028 | n.s. |
| Lexical | 0.159 (0.222) | 0.386 (0.258) | 602.5 | ** |
| Chronic: 6–12 months after stroke (N = 102) | ||||
| Conjunction | 0.339 (0.205) | 0.276 (0.241) | 988 | n.s. |
| Demonstrative | 0.092 (0.172) | 0.082 (0.172) | 1128.5 | n.s. |
| Personal | 0.464 (0.238) | 0.320 (0.266) | 776.5 | ** |
| Lexical | 0.104 (0.190) | 0.323 (0.282) | 621.5 | ** |
Statistics are reported as Mean(Standard Deviation) unless otherwise noted. U: Mann-Whitney U
p < 0.05
p < 0.01
In addition to considering the types of cohesive markers selected by groups, it is also important to consider their relative success in using their chosen strategies. Individuals with RH lesions were significantly more accurate (defined as clear and unambiguous usage resulting in complete cohesion) in their use of both demonstrative and lexical cohesive markers but did not differ in their accuracy when using personal cohesive markers (see Table 3). When they made errors, LH samples contained a significantly higher proportion of incomplete cohesive attempts, in which the tie is ambiguous or underspecified, in demonstrative and lexical markers. RH samples contained a significantly higher proportion of unresolved, or tied, problems with cohesion. They had a significantly higher proportion of tied errors when using personal cohesive markers; they selected the wrong pronoun for a target and then did not remedy this error efficiently. They also had a significantly higher proportion of tied incomplete attempts at lexical cohesion.
Table 3:
Error patterns in cohesive markers by type
| Acute | Chronic | Overall | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LH | RH | U | Sig | LH | RH | U | Sig | LH | RH | U | Sig | |
|
Demonstrative referential cohesive markers (closed-class) | ||||||||||||
| Acc. | 26.0%(43.3%) | 39.4%(47.6%) | 258 | n.s. | 17.9%(38.6%) | 50%(48%) | 104 | n.s. | 23.1%(41.5%) | 44.5%(47.3%) | 702 | * |
| Inc. | 68.1%(44.5%) | 58.3%(46.4%) | 267 | n.s | 82.1%(38.6%) | 29.2%(41.4%) | 70 | ** | 73.1%(42.7%) | 44.3%(45.8%) | 623 | ** |
| Inc. tie | 3.5%(13.4%) | 2.2%(8.6%) | 299 | n.s. | 0% | 4.8%(17.8%) | 149.5 | n.s. | 2.2%(10.8%) | 3.4%(12.6%) | 920 | n.s. |
| Err. | 2.4%(15.6%) | 0% | 300 | n.s. | 0% | 16.1%(36.2%) | 126.5 | n.s. | 1.6%(12.5%) | 7.8%(26.0%) | 847 | n.s. |
| Err. tie | 0% | 0% | - | - | 0% | 0% | - | - | 0% | 0% | - | - |
|
Personal referential cohesive markers (closed-class) | ||||||||||||
| Acc. | 54.2%(43.5%) | 60.3%(42.2%) | 795 | n.s. | 71.2%(37.3%) | 68.0%(39.8%) | 851.5 | n.s. | 61.6%(41.6%) | 64.6%(40.7%) | 3351 | n.s. |
| Inc. | 23.1%(31.9%) | 12.4%(27.2%) | 688 | n.s. | 14.4%(23.7%) | 13.0%(25.7%) | 819 | n.s. | 19.3%(28.8%) | 12.7%(26.2%) | 3030 | n.s. |
| Inc. tie | 13.8%(27.2%) | 13.2%(27.1%) | 833.5 | n.s. | 11.6%(25.2%) | 9.3%(25.0%) | 836 | n.s. | 12.9%(26.3%) | 11.0%(25.8%) | 3470 | n.s. |
| Err. | 7.9%(17.8%) | 10.6%(23.0%) | 828.5 | n.s. | 2.8%(7.1%) | 4.5%(11.2%) | 839.5 | n.s. | 5.7%(14.3%) | 7.2%(17.4%) | 3440 | n.s. |
| Err. tie | 1%(5.8%) | 3.5%(11.1%) | 785.5 | n.s. | 0% | 5.1%(14.8%) | 765 | * | 0.6%(4.3%) | 4.4%(13.2%) | 3158.5 | ** |
|
Lexical cohesive markers (open-class) | ||||||||||||
| Acc. | 43.3%(47.7%) | 64.8%(40.7%) | 433 | n.s. | 43.6%(46.7%) | 62.0%(40.2%) | 291.5 | n.s. | 43.4%(47.0%) | 63.3%(40.1%) | 1480 | * |
| Inc. | 54.8%(46.8%) | 30.2%(36.0%) | 394.5 | * | 56.4%(46.7%) | 30.8%(35.1%) | 260 | n.s. | 55.4%(46.4%) | 30.5%(35.2%) | 1342 | ** |
| Inc. tie | 1.9%(8.7%) | 4.2%(12.5%) | 527.5 | n.s. | 0% | 4.1%(11.3%) | 310.5 | * | 1.2%(7.0%) | 4.1%(11.8%) | 1699.5 | * |
| Err. | 0% | 0.8% (3.1%) | 520 | n.s. | 0% | 3.1%(17.7%) | 356.6 | n.s. | 0% | 2.1% (13.0%) | 1795 | n.s. |
| Err. tie | 0% | 0% | - | - | 0% | 0% | - | 0% | 0% | - | - | |
Statistics are reported as Mean(Standard Deviation) unless otherwise noted. U: Mann-Whitney U
p < 0.05
p < 0.01
Acc.: Accuracy, proportion of complete cohesive ties to total ties. Inc.: Incomplete, proportion of ambiguous or underdefined ties to total ties of that type. Err.: Errors, proportion of incorrect referential items to total ties of that type. Ties: Incomplete or Errorful cohesive attempts that are inherited from previous sentences.
Cohesion strategies in the acute phase
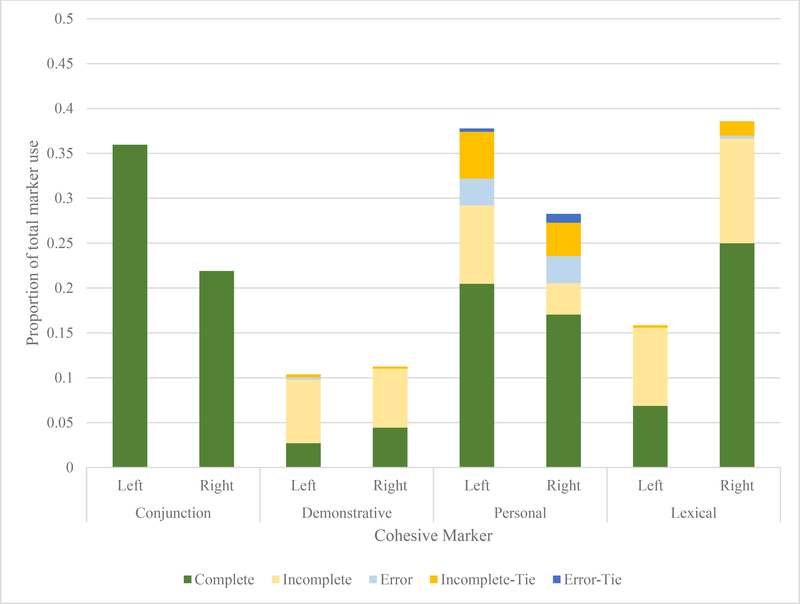
Individuals with LH lesions used a higher proportion of conjunctions to create cohesion than those with RH lesions. This finding was driven by their use of additive conjunctions, primarily seen in their use of the conjunction “and,” which approached significance, Mann-Whitney U = 981.5, p = 0.051. This pattern was often observed in samples in which “and” was used instead of independent clauses constituting individual sentences. Groups did not differ in their use of demonstrative (e.g., “this,” “that”) or personal referential cohesive markers in this phase. Individuals with LH lesions also used a significantly lower proportion of lexical or open-class cohesive markers.
There were no significant differences in success of cohesive attempts between those with LH and RH lesions in using closed-class markers. The only significant difference observed at this phase was that those with LH lesions had a higher proportion of incomplete cohesive attempts at lexical cohesion. It is possible that this was due to overall depression in word-finding abilities among those with LH lesions.
Cohesion strategies in the chronic phase
Among those samples taken in the chronic phase of recovery, patterns of cohesive marker use differed from the acute phase but were no less informative in distinguishing LH and RH lesioned participants. Differences in conjunction usage at the chronic phase were no longer significant, and differences in use of additive conjunctions disappeared, Mann-Whitney U = 1075.5, p = 0.316. The diminishing of this group difference appeared to be driven by two changes in conjunction use between the acute and chronic phase. Proportionate usage of temporal conjunctions (e.g., “then,” “next”) among those with LH lesions increased significantly between the acute and chronic phases, Mann-Whitney U = 2122, p = 0.014, while proportionate usage of causal conjunctions (e.g., “because,” “therefore,” “so”) among those with RH lesions increased significantly between the acute and chronic phases, Mann-Whitney U = 546, p = 0.040. These changes likely contributed to the change in overall conjunction usage observed between the two groups at the two phases of recovery.
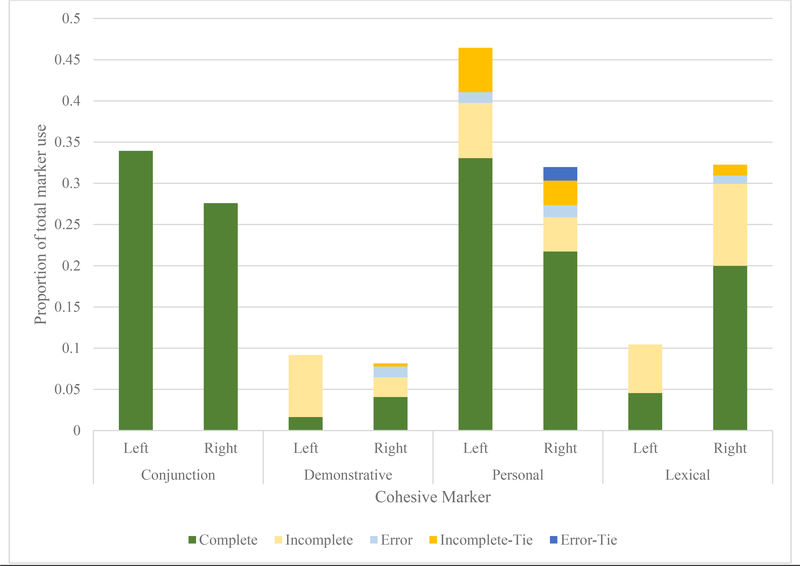
LH lesioned samples taken in the chronic phase also used a significantly larger proportion of personal referential cohesive markers in the chronic phase when compared to RH samples from the chronic phase. This change appeared driven by a significantly higher proportion of personal pronoun usage in the acute versus chronic phase, Mann-Whitney U = 1910, p = 0.037. Differences in demonstrative pronoun use were not significant. Differences in lexical cohesive marker usage remained significant in the chronic phase.
Successful usage of cohesive markers differed considerably between LH and RH samples in the chronic phase of recovery. Although overall accuracy across cohesive marker types remained comparable between LH and RH samples, the two groups behaved differently in the types of errors they tended to demonstrate. All errors made by LH lesioned individuals when using demonstrative cohesive markers were errors of under-specification, incomplete cohesive attempts. In contrast, under-specification, incomplete cohesive attempts made up around 30% of the errors using this marker among RH samples (a significant difference). Five percent of errors made by RH lesioned individuals when using personal pronouns for cohesion were error ties – situations where the wrong pronoun had been used for a target, and then continued to be used. This never happened among our LH chronic samples. When using lexical cohesion, errors in RH samples also contained around 4% incomplete cohesion ties, in which an ambiguous renaming of a target was not resolved by the next sentence. This also never happened among our LH chronic samples and constituted a significant difference.
Patterns of cohesion across recovery
In addition to comparing left to right lesions within phases of recovery, it is also informative to consider how change over time may lead to unique profiles within the LH and RH groups. Minimal change was noted within LH and RH groups when acute and chronic phases were compared. Among those with LH lesions, only personal pronoun cohesion success improved between timepoints, Mann-Whitney U = 1340, p = 0.047). Individuals with RH lesions showed no change in accuracy between timepoints on any accuracy measure. That is, RH lesioned individuals, on average, did not “recover” accuracy in any demonstrable way. The lack of apparent “recovery” may be due to the minimal contribution of paired samples in this study and the overwhelming individual differences captured by high standard deviations across measures.
Summary
Combining the information gathered about which strategies LH and RH lesioned individuals employed with the findings of their relative success in using these strategies provides interesting insight into the different overall profiles of cohesive behaviour exhibited by these groups. Acute LH samples (Figure 1) rely heavily on conjunctions and personal pronouns (“He’s on the stool and she’s cleaning”), whereas RH samples tended toward shorter sentences and renaming referential targets (“The woman is cleaning. The lady has a dress. The mom isn’t paying attention.”). Chronic LH samples tended to be characterized by heavy, overwhelmingly accurate personal pronoun use (“He’s on the chair. She’s reaching. She’s scrubbing”), though this did still occasionally lead to under-specification for the listener (e.g., there are two females in the picture, the mother at the sink and the daughter assisting in the confectionary heist; Figure 2). Of note, although the two groups look different from one another, patterns do not appear to shift over time. Significant changes from acute to chronic phase are limited to greater use, Mann-Whitney U = 1910, p = 0.037, and accuracy, Mann-Whitney U = 1340, p = 0.047, in personal pronouns among those with LH lesions. Those with RH lesions do not improve in accuracy over time across any cohesive marker type, nor do their error types significantly change.
Figure 1:
Proportional use of cohesive markers and success in the acute phase
Figure 2:
Proportional use of cohesive markers and success in the chronic phase
Discussion
Overall, samples from LH and RH groups did not differ in word counts or cohesive marker usage. However, the patterns of which markers they chose to employ were different. LH samples used conjunctions, particularly “and,” about 10% more frequently, as defined by the difference in proportionate usage. They used personal pronouns about 10% more frequently, though they were not more accurate in doing so. They used demonstrative pronouns about as often as RH samples, but were about half as accurate in doing so, and they used lexical cohesive markers less than half as frequently, and about 20% less accurately.
When individuals with LH lesions made errors, their patterns of errors also differed from RH lesioned individuals. RH individuals had 8 times as many error ties in personal pronoun usage; that is, they used the incorrect pronoun when referring to a person in the image, and then continued to use that same pronoun without recognizing and addressing the error. LH individuals had about 30% more incomplete demonstrative pronouns, in which they used “that” or “this,” for example, ambiguously, though those pronouns were appropriate if one were looking at the image with them. Lexical marker errors also were very different between groups. LH individuals were 20% more likely to introduce lexical markers ambiguously and carry that ambiguity from sentence to sentence without resolution.
When acute samples were considered independently, samples from LH lesioned individuals used fewer cohesive markers overall, despite a similar overall word count. The pattern of conjunction use seen overall was also captured acutely; acute LH samples also used conjunctions about 10% more frequently. However, other closed class marker usage was similar at this phase of recovery. In contrast, acute LH individuals also demonstrated the same patterns of lexical marker usage seen overall; they used lexical cohesive markers less than half as frequently, and about 20% less accurately. These errors tended to be incomplete cohesion.
When chronic samples were considered independently, samples did not differ in word count or number cohesive markers overall. LH samples contained about 15% more personal pronouns. While chronic LH samples in our dataset contained no tied-errors in personal pronouns, 5% of errors in RH samples were the continued use of incorrect pronouns from sentence to sentence. LH samples used demonstrative pronouns about as often as RH samples but were about a third as accurate. When LH samples contained errors in demonstrative pronoun use, they were nearly always incomplete ties, which appeared 50% more frequently in LH samples than RH samples. RH samples had a significantly higher proportion of errorful ties, where the demonstrative pronoun was not appropriate to the context (e.g., “those” for a single item). Samples from RH individuals used about 3 times as many lexical markers than LH samples and were around 25% less likely to introduce lexical references ambiguously.
It also is interesting to consider the difference between the two groups’ trajectories over time. While LH samples in the acute and chronic phases did not show differences in word count or cohesive marker count, they nearly quadrupled their proportionate use of temporal conjunctions over time. Their personal pronoun usage became a larger part of their cohesive strategy, and they were about 20% more accurate when using personal pronouns. Meanwhile, RH samples had fewer cohesive marker attempts between the acute and chronic phases, despite no differences in word count. Despite this, their pattern of use, accuracy, and errors remained nearly identical over the course of recovery, with only causal conjunctions being used significantly less frequently, though not conjunctions overall.
Findings from this study build upon a rich literature examining cohesion in individuals with aphasia. The sheer number of samples available for analysis in the acute and chronic phase has allowed for a rich analysis of cohesive strategy and success. The authors acknowledge that these samples were not necessarily of ideal length for analysis, and it is possible that other methods of elicitation (e.g., conversation samples, narrative discourse) may have provided differing opportunities for cohesive marker usage. However, even if cohesive marker usage differs as task demands differ, it seems unlikely that such limitations would have disproportionately impacted one group of lesioned individuals’ cohesion usage over the other. Overarching metrics, like total cohesive marker usage in the acute phase, are consistent with recent work (Barker et al., 2017). However, our findings suggest this discrepancy resolves for LH lesioned individuals in the weeks following injury. The primary focus of this work was to examine the groups’ samples qualitatively, as highlighted when comparing closed- and open-class cohesive markers.
Prior work has focused on differing usage of closed-class cohesive markers among individuals with aphasia, with varying results. The primary observation has been that patients with aphasia tend to omit antecedents (e.g., Andreetta, Cantagallo, & Marini, 2012; Armstrong, 2000; Glosser & Deser, 1991; Marini, Andreetta, Del Tin, & Carlomagno, 2011; Ulatowska, North, & Macaluso-Haynes, 1981), as captured by the high rate of incomplete referential cohesive markers observed here. However, our findings do not confirm the observation found by some authors that RH lesions result in greater difficulty in closed-class cohesive markers (Barker et al., 2017; Marini et al., 2005). Where groups differed in closed-class marker accuracy, RH samples performed cohesion more successfully. Our only evidence of their greater difficulty was limited and taken from the chronic samples. Although RH and LH samples were similarly accurate, when personal referential cohesion was unsuccessful, 5% of errors in RH samples were the continued use of incorrect pronouns from sentence to sentence (tied errors). RH samples also had a significantly higher proportion of errorful ties, where the demonstrative pronoun was not appropriate to the context (e.g., “those” for a single item). It is the quality of errors that appears to distinguish the two groups. They also more frequently selected inappropriate or errorful referential markers and less frequently remedied these errors over time (e.g., once the mother was referred to with “he,” this would continue consistently throughout the remainder of the sample), suggesting perhaps they struggled with monitoring and self-correction commonly needed in agile, typical conversation.
LH lesioned samples tended to omit information, resulting in ambiguous reference. This is perhaps reflective of word-finding difficulties, but also may be indicative of an overly heavy reliance on perceived shared knowledge (i.e., the testing environment of a patient and clinician jointly attending to a picture) rather than navigating the appropriate pragmatics for the situation (i.e., that the purpose of the picture description is to acquire a rich sample of the patient’s language ability). In contrast, RH individuals tended to select and replace labels for the picture’s objects readily, often creating more work for the listener and seeming unnatural, even if not strictly wrong from the perspective of cohesive completeness. This may have been deliberate or may have been driven by deficits in attention and working memory (i.e., not recalling that they had previously presented information).
Our findings also support the ongoing investigation of open-class or lexical markers in these populations, as they constituted one of the key differences between groups. Open-class markers have been far less studied than pronouns and conjunctions, but resulted in the most significant difference between groups, both acutely and overall. RH individuals produced more lexical cohesion and were less ambiguous when doing so. This may reflect differences in underlying profiles of deficits and should be explored further in conversational and narrative samples in order to assess cognitive-linguistic implications of these different lesion locations.
Supplementary Material
Acknowledgements
We are grateful to the individuals with stroke for their participation in this study.
Funding
This study was supported by research grants from the National Institutes of Health / National Institute on Deafness and Other Communication Disorders (NIDCD): R01DC05375 (PI: Hillis), R01 DC015466, and P50 DC011739
Footnotes
Declaration of Interest
The authors report no conflict of interest.
1 As cohesive markers represent low proportions of words used, data were not anticipated to be normally distributed (and primary dependent variables, indeed, violated this assumption for parametric statistics). Thus, Mann-Whitney U test results are reported for all comparisons.
References
- Andreetta S, Cantagallo A, & Marini A (2012). Narrative discourse in anomic aphasia. Neuropsychologia, 50(8), 1787–1793. [DOI] [PubMed] [Google Scholar]
- Armstrong E (2000). Aphasic discourse analysis: The story so far. Aphasiology, 14(9), 875–892. [Google Scholar]
- Barker MS, Young B, & Robinson GA (2017). Cohesive and coherent connected speech deficits in mild stroke. Brain and Language, 168, 23–36. [DOI] [PubMed] [Google Scholar]
- Blake ML, Tompkins CA, Scharp VL, Meigh KM, & Wambaugh J (2015). Contextual Constraint Treatment for coarse coding deficit in adults with right hemisphere brain damage: Generalisation to narrative discourse comprehension. Neuropsychological Rehabilitation, 25(1), 15–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers D, Blonder L, Feinberg T, & Heilman K (1991). Differential impact of right and left hemisphere lesions on facial emotion and object imagery. Brain, 114(6), 2593–2609. [DOI] [PubMed] [Google Scholar]
- Chantraine Y, Joanette Y, & Ska B (1998). Conversational abilities in patients with right hemisphere damage. Journal of Neurolinguistics, 11(1–2), 21–32. [Google Scholar]
- Clark A (2018). Changes in Discourse Production Following Left Hemisphere Stroke. (Master of Arts Thesis). University of Houston, Houston. [Google Scholar]
- Coelho CA, Grela B, Corso M, Gamble A, & Feinn R (2005). Microlinguistic deficits in the narrative discourse of adults with traumatic brain injury. Brain Injury, 19(13), 1139–1145. [DOI] [PubMed] [Google Scholar]
- Coelho CA, Liles BZ, & Duffy RJ (1995). Impairments of discourse abilities and executive functions in traumatically brain-injured adults. Brain Injury, 9(5), 471–477. [DOI] [PubMed] [Google Scholar]
- Coelho CA, Liles BZ, Duffy RJ, Clarkson JV, & Elia D (1994). Longitudinal assessment of narrative discourse in a mildly aphasic adult. Clinical Aphasiology, 22, 145–155. [Google Scholar]
- Davis GA, O’Neil-Pirozzi TM, & Coon M (1997). Referential cohesion and logical coherence of narration after right hemisphere stroke. Brain and Language, 56(2), 183–210. [DOI] [PubMed] [Google Scholar]
- Ellis C, Rosenbek JC, Rittman MR, & Boylstein CA (2005). Recovery of cohesion in narrative discourse after left-hemisphere stroke. Journal of Rehabilitation Research and Development, 42(6), 737. [DOI] [PubMed] [Google Scholar]
- Giles E, Patterson K, & Hodges JR (1996). Performance on the Boston Cookie Theft picture description task in patients with early dementia of the Alzheimer’s type: missing information. Aphasiology, 10(4), 395–408. [Google Scholar]
- Glosser G, & Deser T (1991). Patterns of discourse production among neurological patients with fluent language disorders. Brain and Language, 40(1), 67–88. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, & Barressi B (1983). Cookie Theft picture. Boston diagnostic aphasia examination. Philadelphia, PA: Lea & Febiger. [Google Scholar]
- Halliday MAK, & Hasan R (1976). Cohesion in english. London, UK: Longman. [Google Scholar]
- Hillis Trupe E, & Hillis A (1985). Paucity vs. verbosity: Another analysis of right hemisphere communication deficits. Clinical aphasiology, 15, 83–96. [Google Scholar]
- Liles BZ (1985). Cohesion in the narratives of normal and language-disordered children. Journal of Speech, Language, and Hearing Research, 28(1), 123–133. [DOI] [PubMed] [Google Scholar]
- Liles BZ, & Coelho CA (1998). Cohesion Analysis. In Cherney LR, Coelho CA, & Shadden BB (Eds.), Analyzing discourse in communicatively impaired adults (pp. 65–83). Gaithersburg, MD: Aspen Publishers. [Google Scholar]
- Liles BZ, Coelho CA, Duffy RJ, & Zalagens MR (1989). Effects of elicitation procedures on the narratives of normal and closed head-injured adults. Journal of Speech and Hearing Disorders, 54(3), 356–366. [DOI] [PubMed] [Google Scholar]
- Linnik A, Bastiaanse R, & Höhle B (2016). Discourse production in aphasia: A current review of theoretical and methodological challenges. Aphasiology, 30(7), 765–800. [Google Scholar]
- Marini A (2012). Characteristics of narrative discourse processing after damage to the right hemisphere. Paper presented at the Seminars in Speech and Language. [DOI] [PubMed] [Google Scholar]
- Marini A, Andreetta S, Del Tin S, & Carlomagno S (2011). A multi-level approach to the analysis of narrative language in aphasia. Aphasiology, 25(11), 1372–1392. [Google Scholar]
- Marini A, Carlomagno S, Caltagirone C, & Nocentini U (2005). The role played by the right hemisphere in the organization of complex textual structures. Brain and Language, 93(1), 46–54. [DOI] [PubMed] [Google Scholar]
- McHugh ML (2012). Interrater reliability: the kappa statistic. Biochemia medica: Biochemia medica, 22(3), 276–282. [PMC free article] [PubMed] [Google Scholar]
- Mozeiko J, Le K, Coelho CA, Krueger F, & Grafman J (2011). The relationship of story grammar and executive function following TBI. Aphasiology, 25(6–7), 826–835. [Google Scholar]
- Myers PS (1978). Analysis of right hemisphere communication deficits: Implications for speech pathology. Paper presented at the Clinical Aphasiology: Proceedings of the Conference 1978. [Google Scholar]
- Nicholas LE, & Brookshire RH (1993). A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. Journal of Speech, Language, and Hearing Research, 36(2), 338–350. [DOI] [PubMed] [Google Scholar]
- Pell MD (2007). Reduced sensitivity to prosodic attitudes in adults with focal right hemisphere brain damage. Brain and Language, 101(1), 64–79. [DOI] [PubMed] [Google Scholar]
- Pell MD, & Baum SR (1997). Unilateral brain damage, prosodic comprehension deficits, and the acoustic cues to prosody. Brain and Language, 57(2), 195–214. [DOI] [PubMed] [Google Scholar]
- Piehler MF, & Holland AL (1984). Cohesion in aphasic language. Paper presented at the Clinical Aphasiology: Proceedings of the Conference 1984. [Google Scholar]
- Ross ED, & Monnot M (2008). Neurology of affective prosody and its functional–anatomic organization in right hemisphere. Brain and Language, 104(1), 51–74. [DOI] [PubMed] [Google Scholar]
- Sherratt S, & Bryan K (2012). Discourse production after right brain damage: Gaining a comprehensive picture using a multi-level processing model. Journal of Neurolinguistics, 25(4), 213–239. [Google Scholar]
- Ulatowska HK, North AJ, & Macaluso-Haynes S (1981). Production of narrative and procedural discourse in aphasia. Brain and Language, 13(2), 345–371. [DOI] [PubMed] [Google Scholar]
- Uryase D, Duffy RJ, & Liles BZ (1991). Analysis and description of narrative discourse in right-hemisphere-damaged adults: A comparison with neurologically normal and left-hemisphere-damaged aphasic adults (Vol. 19): Pro-Ed. [Google Scholar]
- Weed E, McGregor W, Nielsen JF, Roepstorff A, & Frith U (2010). Theory of Mind in adults with right hemisphere damage: What’s the story? Brain and Language, 113(2), 65–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.