Editor
Scalp, face and neck involvement in psoriasis negatively impact patient quality of life. 1 , 2 , 3 In the phase 3 double‐blind, randomized, placebo‐controlled reSURFACE 1 trial (NCT01722331), Psoriasis Area and Severity Index (PASI) response rates in patients with moderate to severe plaque psoriasis were high and durable following treatment with interleukin (IL)‐23p19 inhibitor tildrakizumab, with acceptable safety. 4
This post hoc reSURFACE 1 analysis evaluated scalp, head and neck psoriasis over 28 weeks in adult patients with moderate to severe chronic plaque psoriasis receiving tildrakizumab 100 mg or placebo. Patients were randomized 1:2:2 to subcutaneous placebo or tildrakizumab 100 or 200 mg, respectively, at weeks 0 and 4 and every 12 weeks thereafter. At week 12, patients receiving placebo were re‐randomized to tildrakizumab 100 or 200 mg. Head involvement—including neck, scalp and face—was evaluated using PASI head component (PASIh) (range 0.0–7.2). To assess true clearance (PASIh = 0.0), values ≤0.5 were not rounded to 0. Efficacy was assessed for patients with baseline PASIh ≥ median baseline PASIh, ≥75th percentile of baseline PASIh and all patients using nonresponder imputation.
In total, 309 patients receiving tildrakizumab 100 mg and 154 receiving placebo were included. For 163 patients receiving tildrakizumab 100 mg with baseline PASIh ≥ 1.4, median (interquartile range [IQR]) baseline PASIh was 2.4 (1.8, 3.5); for 94 patients with baseline PASIh ≥ 2.4, median (IQR) baseline PASIh was 3.5 (2.8, 4.0). For all 309 patients receiving tildrakizumab 100 mg, median (IQR) baseline PASIh was 1.4 (0.8, 2.4). With placebo, median (IQR) baseline PASIh was 1.2 (0.6, 2.1). Among all patients, 23 (7.4%) receiving tildrakizumab 100 mg and 12 (7.8%) receiving placebo had baseline PASIh of 0.0.
For patients receiving tildrakizumab 100 mg with baseline PASIh ≥ 1.4, median (IQR) PASIh was 1.3 (0.6, 2.0) at week 4, 0.3 (0.0, 0.9) at week 12 and 0.3 (0.0, 1.2) at week 28. For patients with baseline PASIh ≥ 2.4, median (IQR) PASIh was 1.8 (1.0, 2.7) at week 4, 0.5 (0.0, 1.0) at week 12 and 0.3 (0.0, 1.2) at week 28. By week 12, 59 (36.2%) and 29 (30.9%) patients with baseline PASIh ≥ 1.4 and ≥2.4, respectively, achieved PASIh = 0.0 following tildrakizumab 100 mg treatment; 66 (40.5%) and 32 (34.0%) achieved PASIh = 0.0 by week 28 (Fig. 1).
Figure 1.
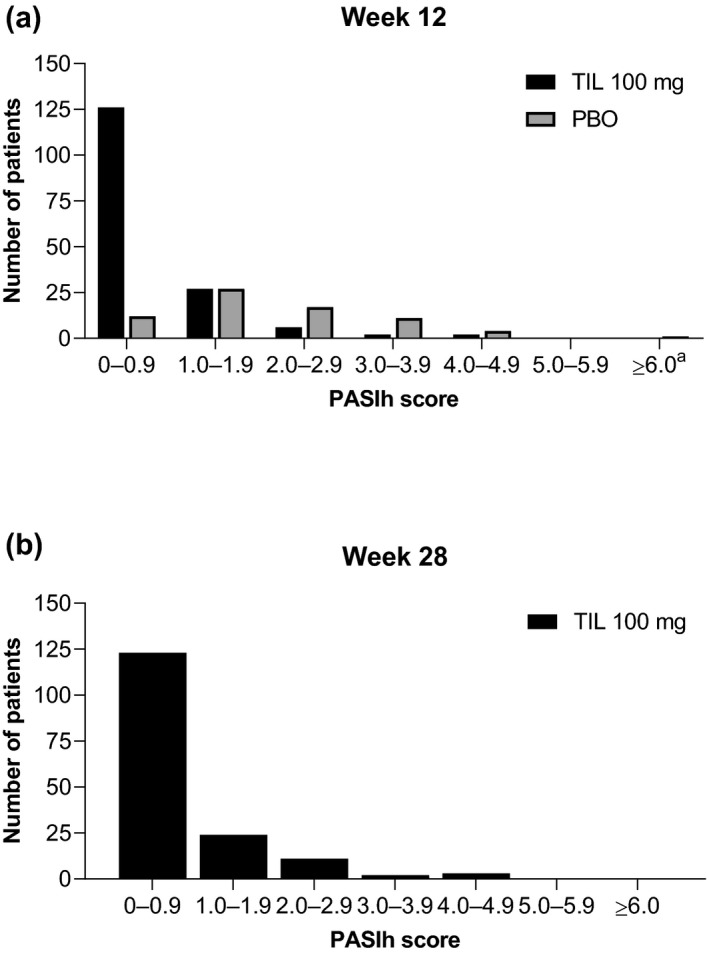
Distribution of PASIh scores of patients with baseline PASIh scores ≥ 1.4 at (a) week 12 and (b) week 28. Median baseline PASIh score was 1.4 for TIL 100 mg and 1.2 for PBO. TIL 100 mg, n = 163; PBO, n = 72. a n = 1. PBO‐randomized patients received PBO to week 12, followed by TIL 100 or 200 mg to week 28. PASIh, Psoriasis Area and Severity Index head component; PBO, placebo; TIL, tildrakizumab.
Over 28 weeks, PASIh scores decreased rapidly for all 309 patients receiving tildrakizumab 100 mg (Fig. 2). At week 4, median (IQR) PASIh for all patients receiving tildrakizumab 100 mg was 0.5 (0.2, 1.4); by week 28, median (IQR) PASIh was 0.0 (0.0, 0.6). At weeks 12 and 28, 160 (20.8%) and 166 (21.5%) patients receiving tildrakizumab 100 mg, respectively, had PASIh = 0.0.
Figure 2.
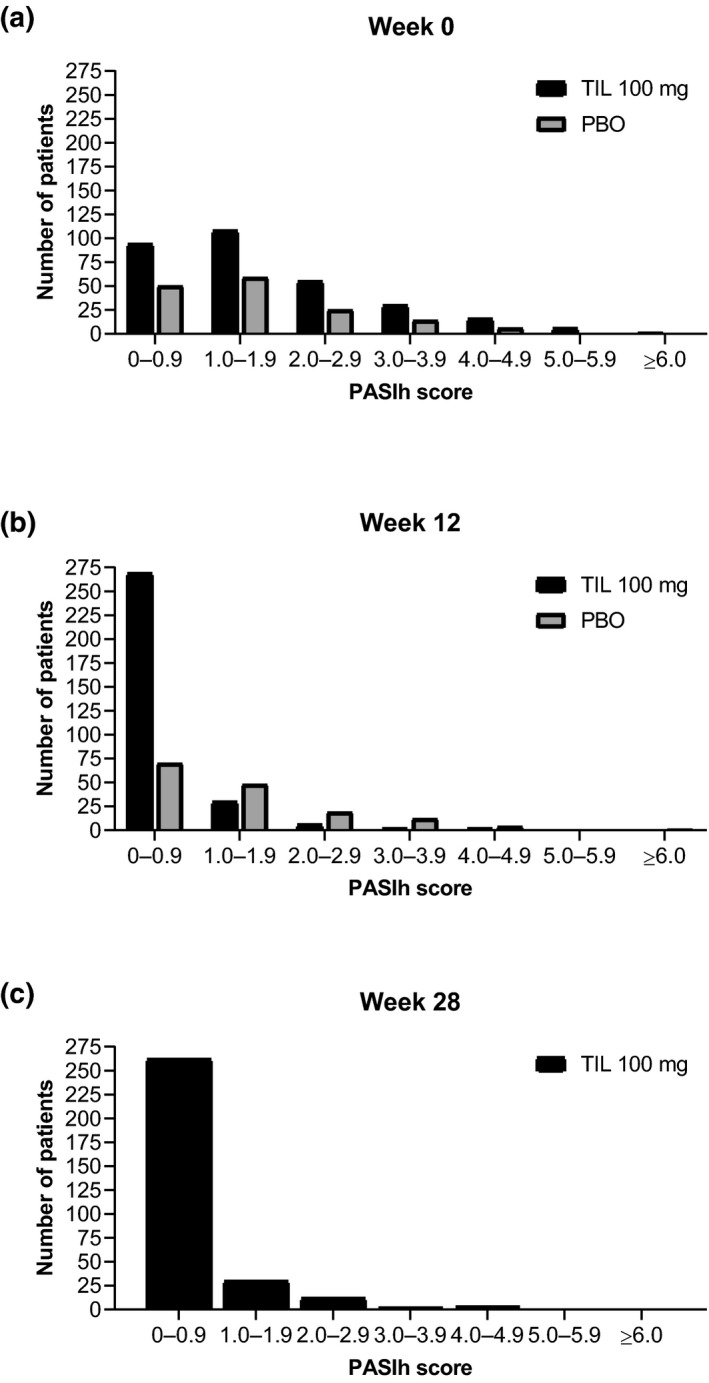
Distribution of PASIh scores of all patients receiving tildrakizumab 100 mg or placebo at (a) week 0, (b) week 12 and (c) week 28. TIL 100 mg, n = 309; PBO, n = 154. PBO randomized patients received PBO to week 12, followed by TIL 100 or 200 mg to week 28. PASIh, Psoriasis Area and Severity Index head component; PBO, placebo; TIL, tildrakizumab.
For patients receiving placebo, PASIh distribution at week 12 was similar to baseline (Fig. 1). Median (IQR) PASIh was 1.1 (0.3, 1.8) at week 12. The proportion of patients with PASIh = 0.0 on placebo remained steady; only 8.4%, 12.3% and 12.3% of patients had PASIh = 0.0 at week 4, 8 and 12, respectively. In total, 3 patients with baseline PASIh ≥ 1.4 receiving placebo had PASIh = 0 at week 12.
Tildrakizumab 100 mg treatment through 28 weeks resulted in rapid, progressive reduction in psoriasis scalp, head and neck involvement for patients with severe disease at baseline. PASIh comprehensively assesses face, neck and scalp, capturing high impact areas. Systemic IL‐17 inhibitor ixekizumab and IL‐23 inhibitor guselkumab were effective for scalp clearance; face and neck have not been assessed. 5 , 6 Tildrakizumab efficacy for scalp, head and neck clearance was similar to IL‐17 inhibitor secukinumab and the tumour necrosis factor‐α inhibitor adalimumab. 7 , 8 , 9 A phase 3b study of tildrakizumab 100 mg for scalp psoriasis is underway.
Conflicts of interest
MAM has received grants and/or honoraria as a consultant, investigator and/or speaker for Abbott Labs, AbbVie, Amgen, Anacor, Boehringer Ingelheim, Celgene, Eli Lilly and Co., Janssen Biotech, LEO Pharma, Merck & Co., Novartis, Sienna, and UCB; and has been on an advisory board for AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly and Co., Janssen Biotech, LEO Pharma, and Sienna. GJM has received grants and/or honoraria as an investigator and/or speaker for Astellas Pharma US; Janssen Pharmaceuticals; Regeneron Pharmaceuticals; Merck & Co.; Eli Lilly and Co.; AbbVie; Pfizer; Demira; Bristol‐Myers Squibb; UCB; Qurient; Sun Pharmaceutical Industries, Inc.; ChemoCentryx; Galderma Research & Development; Sanofi Genzyme; and Kiniksa Pharmaceuticals; and may have stock in Valeant Pharmaceuticals North America and Astellas Pharma US. HG has received honoraria as a member of an advisory board and/or speaker from Aqua, Galderma, Ortho Dermatologics and Pfizer. AMM is an employee of Sun Pharmaceutical Industries, Inc., and has individual shares in Johnson and Johnson and as part of retirement account/mutual funds. JP has served as statistical consultant for Sun Pharmaceutical Industries, Inc., and is a statistical consultant for Kyowa Kirin Pharmaceutical Development. SJR and DD are employees of Sun Pharmaceutical Industries, Inc. AG has been an investigator for Sun Pharmaceutical Industries, Inc.
Funding sources
These studies were funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Analyses were funded by Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA. Medical writing support was provided by Kathleen Pieper, PhD, of AlphaBioCom, LLC, and funded by Sun Pharmaceutical Industries, Inc.
References
- 1. Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 645–648. [DOI] [PubMed] [Google Scholar]
- 2. Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: Nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther 2018; 31: e12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lakuta P, Marcinkiewicz K, Bergler‐Czop B, Brzezinska‐Wcislo L, Slomian A. Associations between site of skin lesions and depression, social anxiety, body‐related emotions and feelings of stigmatization in psoriasis patients. Postepy Dermatol Alergol 2018; 35: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reich K, Warren RB, Iversen L et al. Long‐term efficacy and safety of tildrakizumab for moderate‐to‐severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br J Dermatol 2020; 182: 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foley P, Gordon K, Griffiths CEM et al. Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol 2018; 154: 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reich K, Leonardi C, Lebwohl M et al. Sustained response with ixekizumab treatment of moderate‐to‐severe psoriasis with scalp involvement: results from three phase 3 trials (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). J Dermatolog Treat 2017; 28: 282–287. [DOI] [PubMed] [Google Scholar]
- 7. Navarini AA, Poulin Y, Menter A, Gu Y, Teixeira HD. Analysis of body regions and components of PASI scores during adalimumab or methotrexate treatment for patients with moderate‐to‐severe psoriasis. J Drugs Dermatol 2014; 13: 554–562. [PubMed] [Google Scholar]
- 8. Kircik L, Fowler J, Weiss J, Meng X, Guana A, Nyirady J. Efficacy of secukinumab for moderate‐to‐severe head and neck psoriasis over 52 weeks: pooled analysis of four phase 3 studies. Dermatol Ther (Heidelb) 2016; 6: 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bagel J, Duffin KC, Moore A et al. The effect of secukinumab on moderate‐to‐severe scalp psoriasis: Results of a 24‐week, randomized, double‐blind, placebo‐controlled phase 3b study. J Am Acad Dermatol 2017; 77: 667–674. [DOI] [PubMed] [Google Scholar]


